Published online Aug 15, 2020. doi: 10.4251/wjgo.v12.i8.877
Peer-review started: May 12, 2020
First decision: June 15, 2020
Revised: June 20, 2020
Accepted: July 26, 2020
Article in press: July 26, 2020
Published online: August 15, 2020
Processing time: 91 Days and 22.3 Hours
Kras mutant colon cancer shows abnormal activation of the nuclear factor kappa-B (NF-κB) pathway, resulting in the proliferation of tumor cells. Treatment with fluorouracil (5-FU) might not achieve the expected inhibition of proliferation of malignant cells based on the fluorouracil-induced activation of the NF-κB pathway.
To detect whether interleukin (IL)-1 receptor antagonist (IL-1RA) could increase the chemosensitivity to 5-FU by decreasing the activation of the NF-κB pathway and reducing the proliferation of colon cancer cells.
Western blot analysis was performed to detect the persistent activation of the NF-κB pathway in colon cancer cell lines. Reverse transcription-polymerase chain reaction was used to detect the IL-1RA-reduced expression levels of IL-6, IL-8, IL-17, IL-21 and TLR4 in colon cancer cell lines. We used a xenograft nude mouse model to demonstrate the downregulation of the NF-κB pathway by blocking the NF-κB-regulated IL-1α feedforward loop, which could increase the efficacy of chemotherapeutic agents in inhibiting tumor cell growth.
IL-1 receptor antagonist could decrease the expression of IL-1α and IL-1β and downregulate the activity of the NF-κB pathway in Kras mutant colon cancer cells. Treatment with 5-FU combined with IL-1RA could increase the chemosensitivity of the SW620 cell line, and decreased expression of the TAK1/NF-κB and MEK pathways resulted in limited proliferation in the SW620 cell line.
Adjuvant chemotherapy with IL-1RA and 5-FU has a stronger effect than single chemotherapeutic drugs. IL-1RA combined with fluorouracil could be a potential neoadjuvant chemotherapy in the clinic.
Core tip: A feedback loop between the upregulated nuclear factor kappa-B (NF-κB) pathway and interleukin (IL)-1 lads to the proliferation of cancer cells. Fluorouracil (5-FU), a chemotherapy drug used to treat colon carcinoma cells, can activate the NF-κB pathway and lead to chemotherapy resistance. IL-1 receptor antagonist combined with 5-FU has a stronger inhibitory effect on the proliferation of colon cancer cells than single 5-FU treatment due to the blockade of IL-1. This report could provide an adjuvant chemotherapy strategy for the clinic and provide a theoretical basis for neoadjuvant chemotherapy.
- Citation: Yan Y, Lin HW, Zhuang ZN, Li M, Guo S. Interleukin-1 receptor antagonist enhances chemosensitivity to fluorouracil in treatment of Kras mutant colon cancer. World J Gastrointest Oncol 2020; 12(8): 877-892
- URL: https://www.wjgnet.com/1948-5204/full/v12/i8/877.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i8.877
Colonicrectal cancer is the primary cause of death among gastrointestinal cancers and the third most common cancer worldwide[1,2]. Nearly 50% of patients with recurrent colon cancer undergo colon cancer procedures and chemotherapy[3], and it has been indicated that the current chemotherapy regimen may not be effective in controlling the recurrence and metastasis of the tumor[4]. Systemic toxicity and drug resistance of tumor cells are two major problems in cancer chemotherapy[5]. Therefore, various studies have explored how to reduce the toxicity of conventional chemotherapeutic drugs and increase the chemical sensitivity to achieve better curative effects of chemotherapy and gain more benefits for patients with colon cancer[6,7].
Fluorouracil (5-fluorouracil, 5-FU) is the first-choice drug for various chemotherapy regimens of colon cancer in recent decades[8]. Even the current classic chemotherapy plans, including the FOLFOX regimen and FOLFIRI regimen, contain 5-FU as a component for colon cancer treatment. This drug can inhibit the synthesis of adenylate synthetase and interfere with the synthesis of DNA in tumor cells. The growth of cells remained at a low level, and cell apoptosis was increased[9]. However, the effect of 5-FU is not ideal due to the chemoresistance in colon carcinoma patients treated with 5-FU[10]. The clinical benefit of colon cancer patients is considered to be limited, especially for those with advanced tumors[11]. Adjuvant chemotherapy has been studied to overcome the chemoresistance to 5-FU in colon cancer.
Kras mutant colon carcinoma shows persistent activation of the nuclear factor kappa-B (NF-κB) pathway, which promotes the proliferation and metastasis of tumor cells[12]. The persistently activated NF-κB pathway could promote chemoresistance to 5-FU in colon cancer treatment[13]. NF-κB is a transcription factor protein that includes five subunits: Rel (cRel), p65 (RelA, NF-κB3), RelB, and p50 (NF-κB1)[14]. The high expression of NF-κB is related to inflammatory factors and is closely related to cell growth and proliferation[15-17]. In tumor biology, the NF-κB pathway is highly active with high expression in various tumor cells[18]. It was found that 5-FU could increase the phosphorylation of P65 in colon cancer cells, which increased the chemotherapy resistance to 5-FU in clinical treatment[19,20]. However, downregulating the NF-κB pathway increased the chemosensitivity to 5-FU in colon cancer chemotherapy[21].
Our previous studies have shown that NF-κB remains persistently activated in Kras mutant pancreatic cancer[22,23], which is closely related to the high expression of interleukin (IL)-1α[24]. IL-1α can increase the activity of the NF-κB pathway by upregulating AP-1 in pancreatic cancer cells[25]. Similarly, the inhibition of NF-κB activity also decreased the expression of IL-1 in pancreatic cancer cells. IL-1 and NF-κB show a cyclic relationship, which leads to persistent activation of NF-κB in tumor cells[26]. In Kras and p53 mutant mice, we found that the NF-κB activity was downregulated by inhibiting the IL-1 receptor, which could effectively slow tumor growth. Other studies have shown that an NF-kB inhibitor had proapoptotic effects on colon cancer cells following IL-6 stimulation[27]. The aim of this study was to assess whether treatment with 5-FU combined with IL-1 receptor antagonist can increase the chemosensitivity to 5-FU by decreasing the activation of the NF-κB pathway and reducing the proliferation of colon cancer cells. The results obtained will provide a theoretical basis for clinical adjuvant chemotherapy.
The normal epithelial cell line (NCM460 cell line) and the human colon carcinoma cell lines (including COLO205, SW480, HT-29, LoVo, HCT116, DLD1, SW620, LS174T, and SW1116) were purchased from Nanjing Purisi Biotechnology Company (Jiangsu, China). All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM Caisson Laboratories, Inc.).
TRIzol (American Invitrogen 15596-026); ethanol, chloroform, isopropanol (National Drug Group); cDNA first chain synthesis kit (United States Thermo Fisher K1622); and SYBR Premix Ex Taq II (Japanese TaKaRa RR820A) were used in this study. Primer design was performed by Nanjing Golden Srey Technology Co., Ltd. Substance synthesis and PAGE primer purification were also performed. The drug 5-FU was purchased from Thermo Biocompany. IL-1RA was purchased from Nanjing Purisi Biotechnology Company.
Thirty male athymic nude mice (NCI-nu), which were 4-6 weeks old and weighed approximately 24.9-33.0 g, were purchased from Nanjing Puruisi Biological Company. All mice were housed and treated at Shandong University in accordance with the guidelines of The Animal Care and Use Committee, which provided the license number SYNK (Lu) 2019-0005, and the scope of application: Barrier environment and SPF level (dogs, rabbits, rats, and mice). SW620 colon cancer cells were harvested in PBS with 20% Matrigel (Fisher Scientific). Then, all nude mice were subcapsularly injected with SW620 colon cancer cells (1.0 × 106 cells in 50 μL of PBS) in the subcutaneous tissue of the back. The effect of chemotherapy was observed in 15 nude mice with tumor loads that were euthanized by carbon dioxide inhalation (the flow rate of CO2 used for euthanasia increased from 0% to 20% of the chamber volume per minute). Lack of breath and discoloration of the eyes were observed in all nude mice. The flow of carbon dioxide was maintained for a minimum of 1 min after respiratory arrest, and the tumor tissues were dissected (cervical dislocation was used for the approved secondary physical method of euthanasia). All mice were evaluated every 3 d to observe tumor growth during the 3-wk treatment. Tumor volume was determined as follows: V = (length × width2)/2. If multiple tumors were present, the final result was the sum of the measured results of each single tumor. The limited diameter of the tumor was 3 cm, which measured a single tumor or the sum of multiple tumors.
The survival time was observed in the other 15 nude mice, which died due to cachexia or overloaded tumors more than 3 cm in diameter. The groups were as follows: Control group (5 nude mice with PBS treatment), 5-FU group (5 nude mice with 5-FU treatment), and 5-FU and IL-1RA group (5 nude mice with 5-FU and IL-1RA treatment). For in vivo studies, 1.5 mg of intraperitoneal rhIL-1RA diluted with PBS was used to treat the nude mice (daily, 3 wk), and 20 mg/kg of intraperitoneal 5-FU diluted by PBS was used to treat the nude mice (twice a week, 3 wk).
Cell lysates were extracted from cells with radioimmunoprecipitation assay protein lysate buffer. The cellular extracts were boiled for 5 min to denature the protein. A total of 30 µg of protein was loaded into each well and separated on a gel. Then, the protein samples were transferred to a polyvinylidene fluoride (PVDF) membrane for 1 h at 300 mA. The PVDF membrane was blocked with 5% skim milk powder in 0.1% TBST for 1 h and incubated overnight with primary antibodies at 4°C. The primary antibodies against phosphorylated p65, p65, phosphorylated TAK1, TAK1, phosphorylated MEK, and MEK were purchased from Nanjing Puruisi Biotechnology Company and diluted 1:500. The primary antibody against IL-1α was purchased from ABcam Biotechnology Company and diluted 1:200. The secondary antibodies and β-actin antibody were purchased from ABcam Biotechnology Company.
The optical density (OD) values of RNA samples extracted from cells were measured at 260 nm and 280 nm. RNA concentration was calculated as OD260 × dilution factor × 0.04 µg/µL. The range of OD260/280 was 1.8 to 2.1, indicating a high purity of the extracted RNA. Then, the samples were mixed with nuclease-free enzyme, oligo dT (18), and nuclease-free double-distilled water to the total volume. Mixed RNase inhibitor, reaction buffer, dNTPs, DTT (1 M), reverse transcriptase (AMV), and nuclease-free double-distilled water were added in reverse transcription-polymerase chain reaction (RT-PCR) tubes. After the cDNA reaction, the samples were subjected to amplification. The cycle conditions were as follows: Denaturation for 30 s at 95 °C, annealing for 30 s at 55 °C, and extension for 35 s at 72 °C. A total of 32 cycles were performed. The sequence information is as follows: hIL-1α-F, 3'-TCCCCAGGGACCTCTCTCTA-5' and hIL-1α-R, 3'-GAGGGTTTGCTACAACATGGG-5'; hIL-1β-F, 3'-TCGCCAGTGAAATGATGGCT-5' and hIL-1β-R, 3'-TGGAAGGAGCACTTCATCTG; hIL-6-F, 3'-TCAATATTAGAGTCTCAACCCCCA-5 and hIL-6-R 3'-GAAGGCGCTTGTGGAGAAGG-5; hIL-8-F, 3'-GCTCTGTGTGAAGGTG CAGTT-5' and hIL-8-R, 3'-ACCCAGTTTTCCTTGGGGTC-5'; hIL-17-F, 3'-TGGAATCTCCACCGCAATGA-5' and hIL-17-R, 3'-GCTGGATGGGGACAGAGTTC-5'; hIL-21-F, 3'-ACACAGACTAACATGCCCTTCA-5' and hIL-21-R, 3'-ACCGTGAGTAACTAAGAAGCAA-5'; TLR4-F, 3'-GGTCAGACGGTGATAGCGAG-5' and hTLR4-R, 3'-TTTAGGGCCAAGTCTCCACG-5'; hP65-F, 3'-ACAACAACCCCTTCCAAGAAGA-5' and hP65-R, 3'-TCACTCGGCAGATCTTGTTG-5'.
After being treated with P65-siRNA for 48 h for interference, the SW4690 cell line was assessed for the RNA and protein levels of the target gene by RT-PCR and/or Western blot assays. The P65-siRNA oligo package was purchased from Suzhou Gemma Gene Biotechnology Company. The information of two basic P65-siRNAs is as follows: (1) siRNA1: Sense, 5'-GGCGAGAGGAGCACAGAUACC-3' and antisense, 5'-UAUC UGUGCUCCUCUCGCCUG-3'; and (2) siRNA2: Sense, 5'-CCCACGAGCUU GUAGGAAAGG-3' and antisense, 5'-UUUCCUACAAGCUCGUGGGGG-3'. The sequence of siRNA scramble (GenePharma Company, Shanghai) is: Sense, 5’-UUCUCCGAACGUGUCACGUTT-3’ and antisense, 5’–ACGUGACACG UUCGGAGAATT–3’. The information of two basic P65-siRNAs is as follows: Sense: 5’-UUCUCCGAACGUGUCACGUTT-3’; antisense: 5’–ACGUGACACGUUCG GAGAATT–3’.
For the MTT assay, the cell suspension was seeded in each well of 96-well plates. After 12 h, attached cells were treated with various doses of 5-FU or/and IL-1RA. The cells were incubated in 4.5% CO2 at 37 °C for 1, 2, 3, 4, and 5 d, 10 µL of MTT solution (5 mg/mL) was added to crystallize the cells for 4 h, and 150 µL of DMSO was added for 10 min to oscillate the cells. The absorbance value was measured at 490 nm. In the colony formation assay, DMEM-diluted cell suspension was inoculated in a 6-well culture dish containing 10 mL of 37 °C incubation medium at a density of 300 cells per well. After 12 h, attached cells were treated with various doses of 5-FU or/and IL-1RA. The cells were incubated in a cell incubator for 2 wk, and then, the colonies were immobilized with formalin (Sigma-Aldrich) within 30 min and stained with crystal violet (Sigma-Aldrich) within 1 h.
Commercially available SPSS version 19.0 software (Chicago, IL, United States) and GraphPad Prism software (La Jolla, CA, United States) were used for statistical analyses. An unpaired t-test (one-tailed) was used to analyze the differences between groups. One-way ANOVA was used to analyze the differences among multiple groups. The log-rank test was used to analyze the differences in survival time between groups. The Bonferroni test was used following ANOVA for multiple comparisons. P < 0.05 was considered statistically significant.
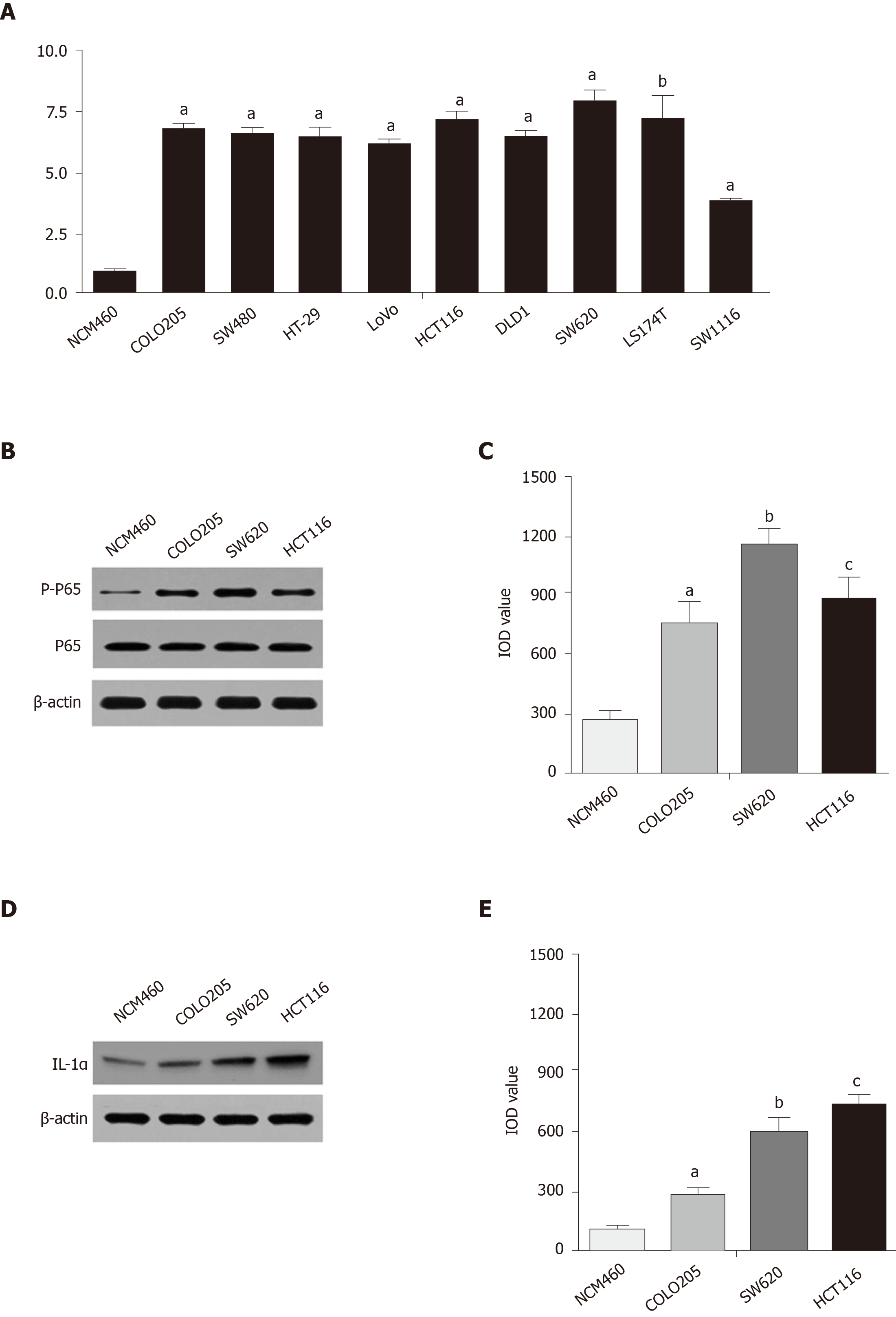
The abnormal activity of cancer cells was determined by the NF-κB pathway, which led to the proliferation of malignant cells, and this pathway could be persistently activated by the Kras mutant gene targeting TAK1 and AP1[26]. In colon carcinoma cell lines, including COLO205, SW480, HT-29, LoVo, HCT116, DLD1, SW620, LS174T, and SW1116, the activity of the Kras gene remained high compared with that in NCM460, a normal epithelial cell line (Figure 1A). Kras gene mutation in colon cancer also resulted in excessive proliferation of malignant cells through persistent activation of the NF-κB pathway[28]. The expression of phosphorylated P65 in the COLO205, SW620, and HCT116 cell lines was significantly higher than that in the NCM460 cell line, as shown by Western blot assays (Figure 1B and C). The expression of IL-1α in the COLO205, SW620, and HCT116 cell lines remained at a high level compared with that in the NCM460 cell line (Figure 1D and E).
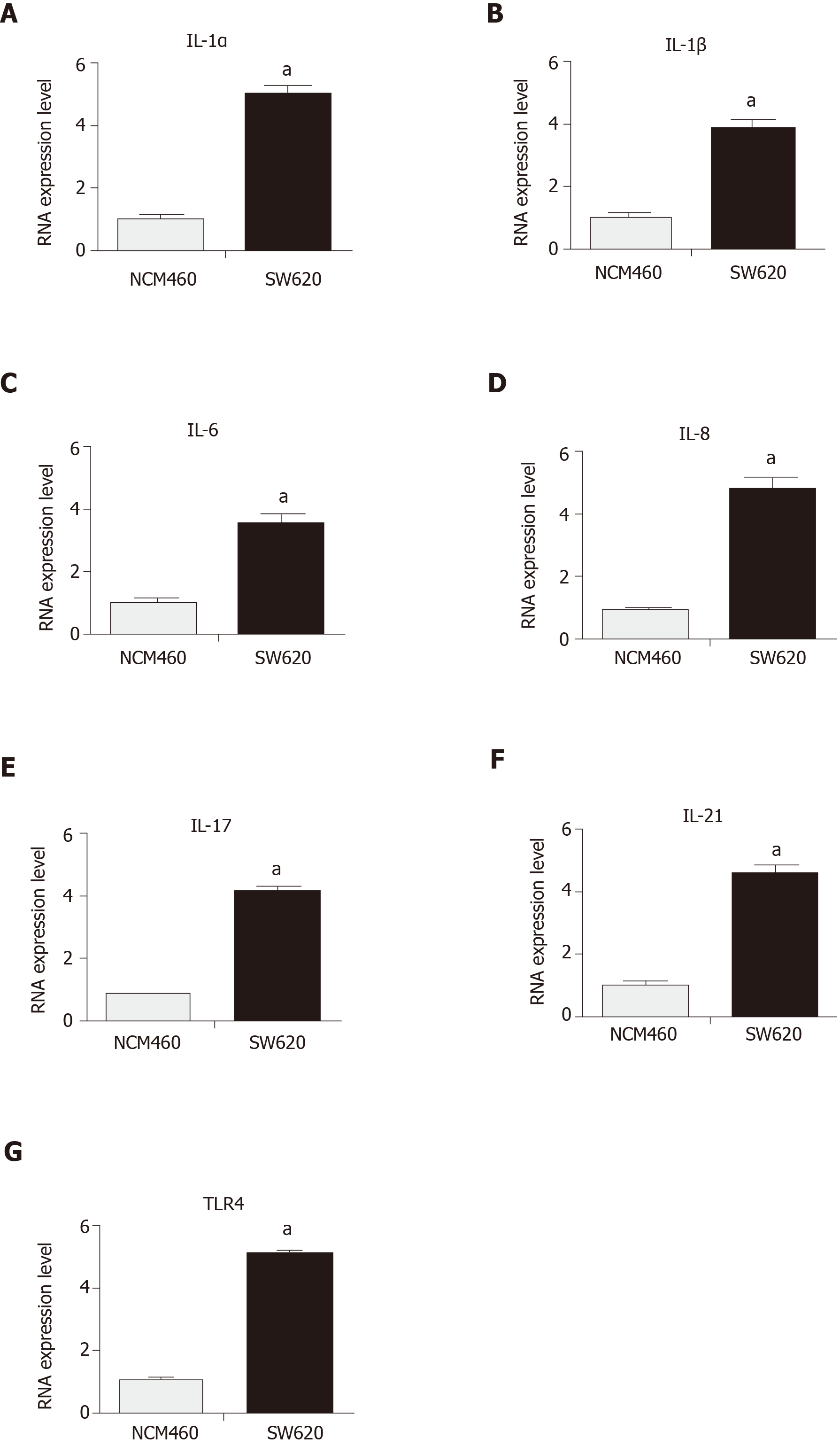
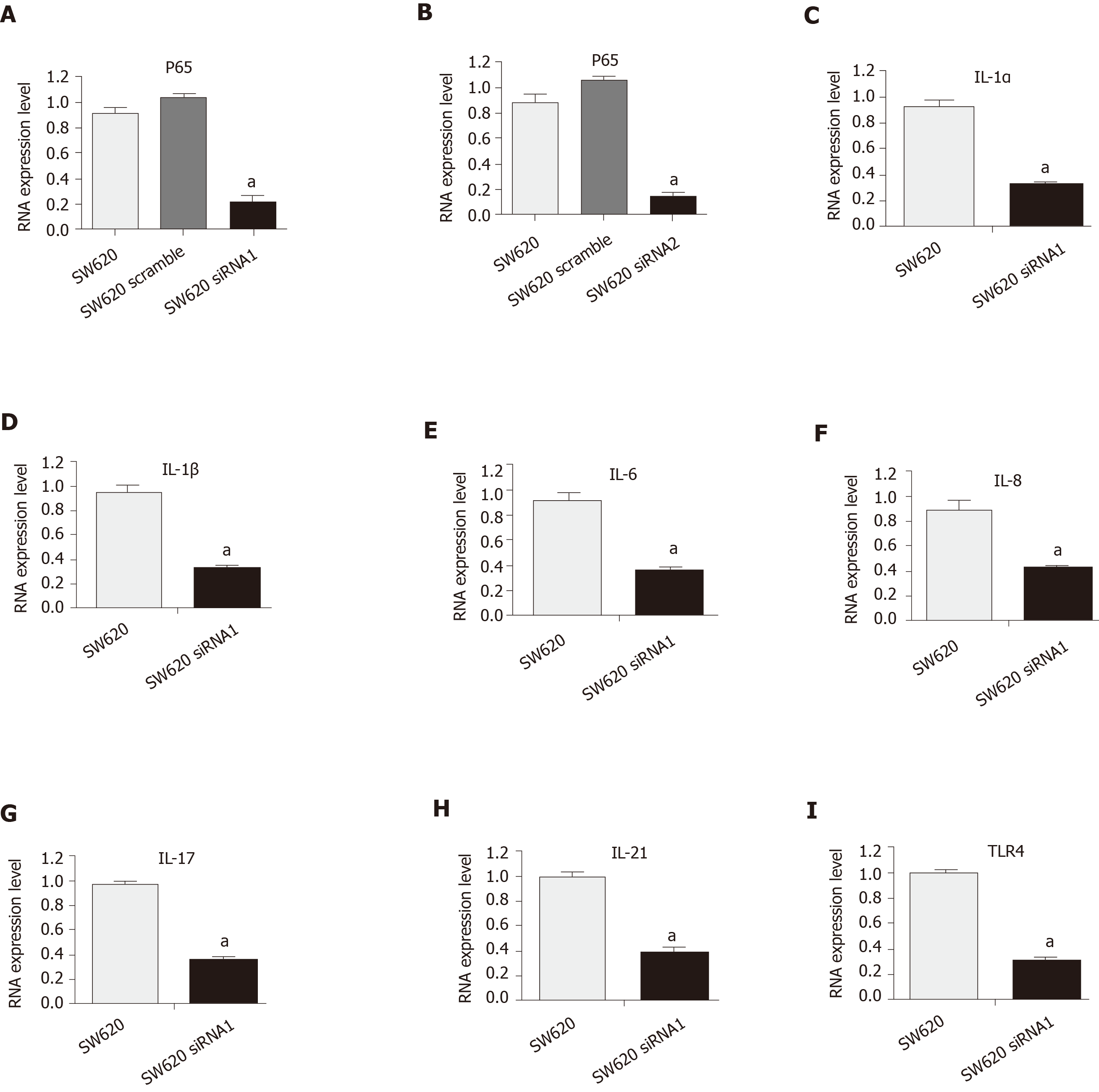
Previous studies focused on IL-6 for growth inhibition of colon cancer cells[29]. In this study, IL-1α and IL-1β were targeted to detect their expression, which remained at a high level in colon carcinoma cells with persistent activation of the NF-κB pathway (Figure 2A and B). The continuous activation of the NF-κB pathway was confirmed to increase the expressions of IL-6, IL-8, IL-17, IL-21, and TLR4 in colon carcinoma cell lines (Figure 2C-G). The activity of the NF-κB pathway was inhibited to observe whether it could decrease IL-1α and IL-1β in the colon carcinoma cell line. The SW620 cells were treated with siRNA to downregulate the activity of the NF-κB pathway. The mRNA level of P65 was decreased by interference with siRNA1 and siRNA2 in the SW620 cell line, as shown by RT-PCR assays, compared with that of the untransfected SW620 cells (Figure 3A and B). The mRNA levels of IL-1α and IL-1β were also significantly decreased with siNF-κB interference in the SW620 cell line (Figure 3C and D). The expression levels of IL-6, IL-8, IL-17, IL-21, and TLR4 were reduced in the colon cancer cell lines after siRNA interference in the NF-κB pathway (Figure 3E-I). The results suggested that IL-1 is closely related to the NF-kappa B pathway in the SW620 cell line of Kras mutant colon carcinoma.
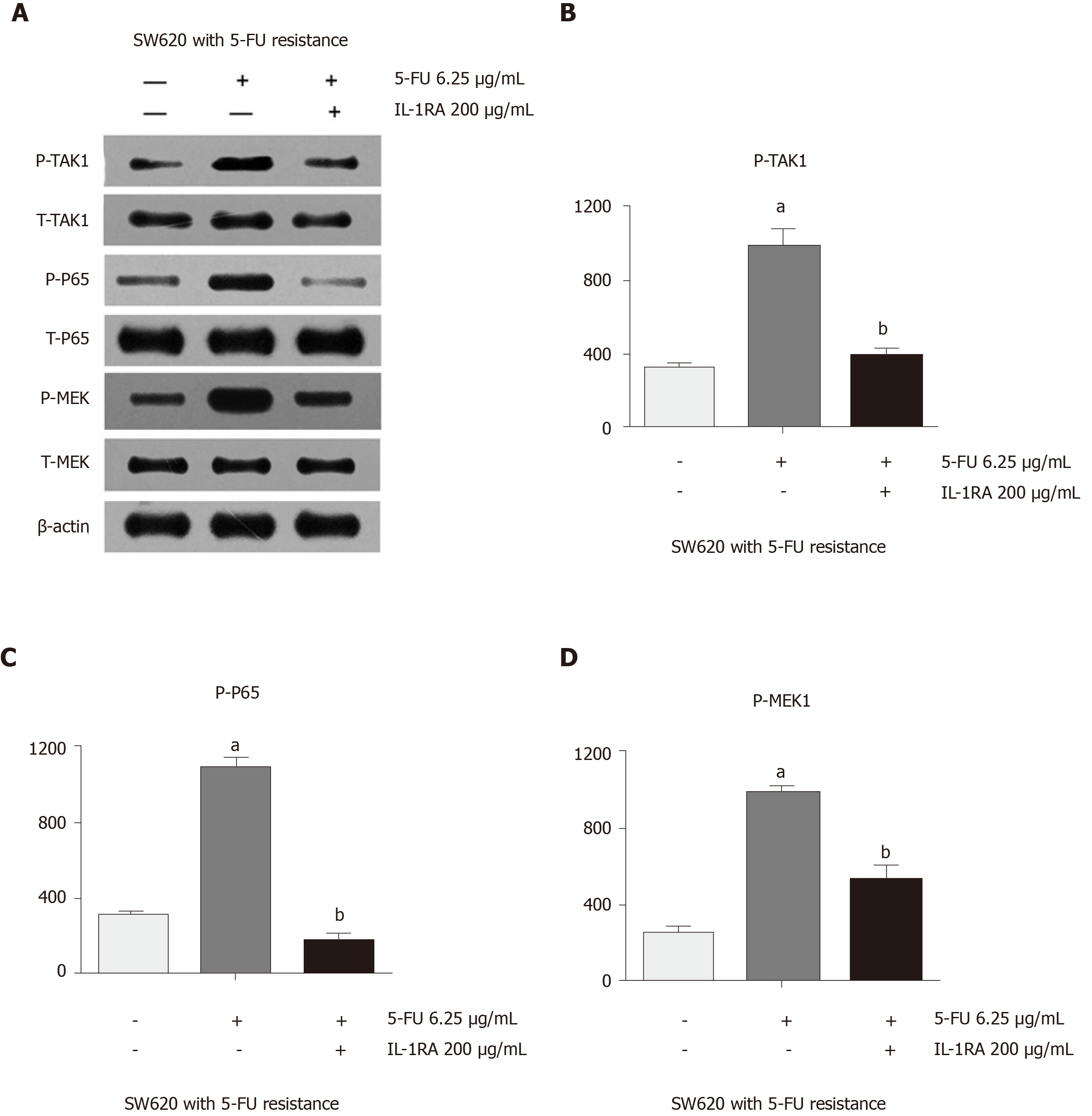
Abnormal activity of the NF-κB pathway was found in SW620 cells when they were treated with 5-FU chemotherapy. After 5-FU treatment, SW620 cells expressed high levels of phosphorylated TAK1 and phosphorylated MEK, which could explain the unexpected proliferation of drug-resistant malignant cells after chemotherapy (Figure 4A). Western blot assays showed that the expression of phosphorylated TAK1 and phosphorylated MEK was significantly higher in the SW620 cell line treated with 5-FU than in the untreated SW620 cell line (Figure 4B and D). This phenomenon in the SW620 cell line could be inhibited by 5-FU combined with IL-1 receptor antagonist, which decreased the phosphorylation of P65 and had an inhibitory effect on the phosphorylation of TAK1 and MEK. Western blot assays showed that the expression levels of phosphorylated TAK1 and phosphorylated MEK were significantly lower in the 5-FU and IL-1 receptor antagonist treatment groups than in the 5-FU treatment group (Figure 4B-D).
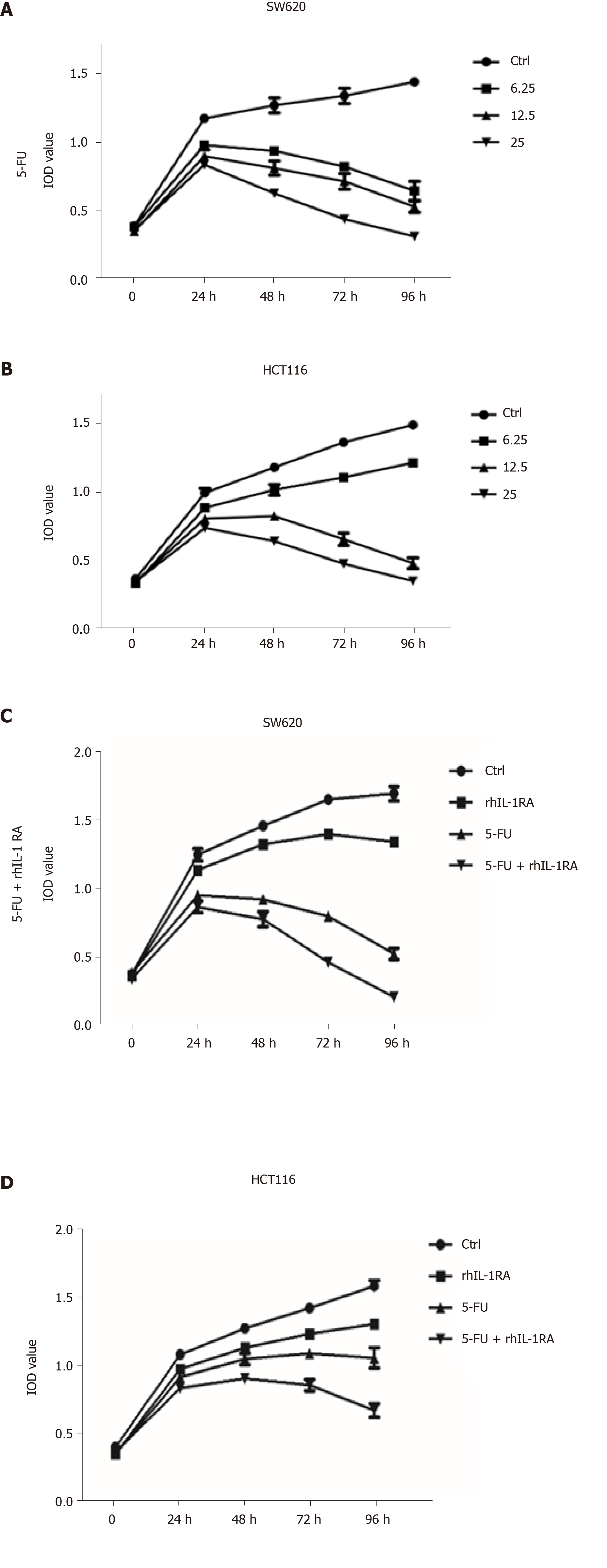
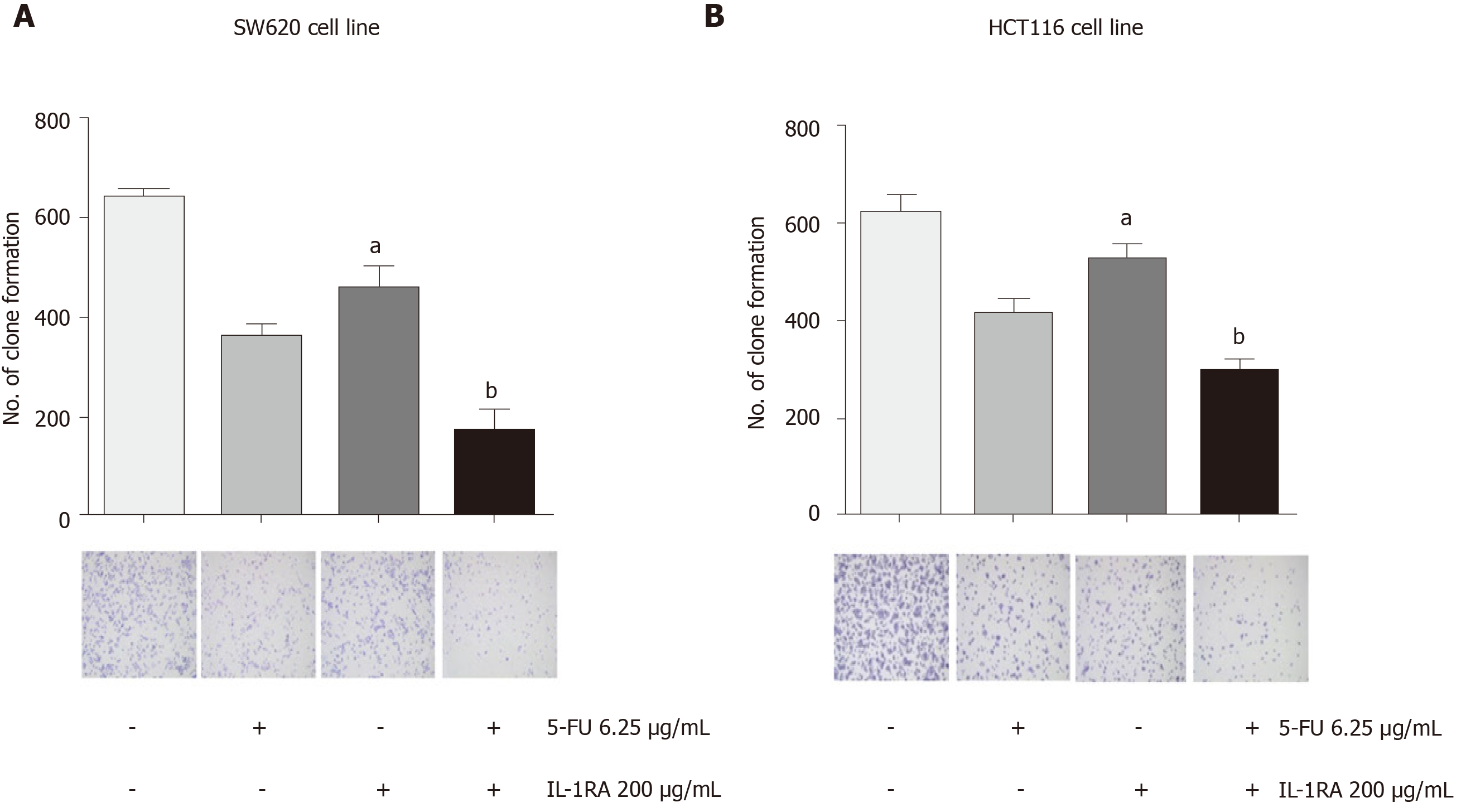
As a chemotherapeutic agent for colon cancer, 5-FU is widely used in clinical treatment, but side effects and drug resistance can occur. First, 6.25, 12.5, and 25 mg/mL of 5-FU was used to treat the SW620 and HCT116 cell lines, and the trend of the cell growth curve was observed at 96 h by MTT assays. IL-1RA combined with 5-FU was more effective in inhibiting the proliferation of the SW620 and HCT116 cell lines than 5-FU alone. The results showed that 200 mg/mL of IL-1RA combined with 12.5 mg/mL of 5-FU could significantly inhibit cell growth (Figure 5). IL-1RA combined with 5-FU had a greater inhibitory effect on the monoclonal formation than single treatment. In clonogenicity assays, we used 200 mg/mL of IL-1RA and 6.25 mg/mL of 5-FU to treat the colon cancer cell line for 3 d. We found that the inhibitory effect of 200 mg/mL IL-1RA alone on the colony formation of tumor cells was weak, but IL-1RA combined with 5-FU had an obvious inhibitory effect on colony formation compared with 6.25 mg/mL 5-FU (Figure 6).
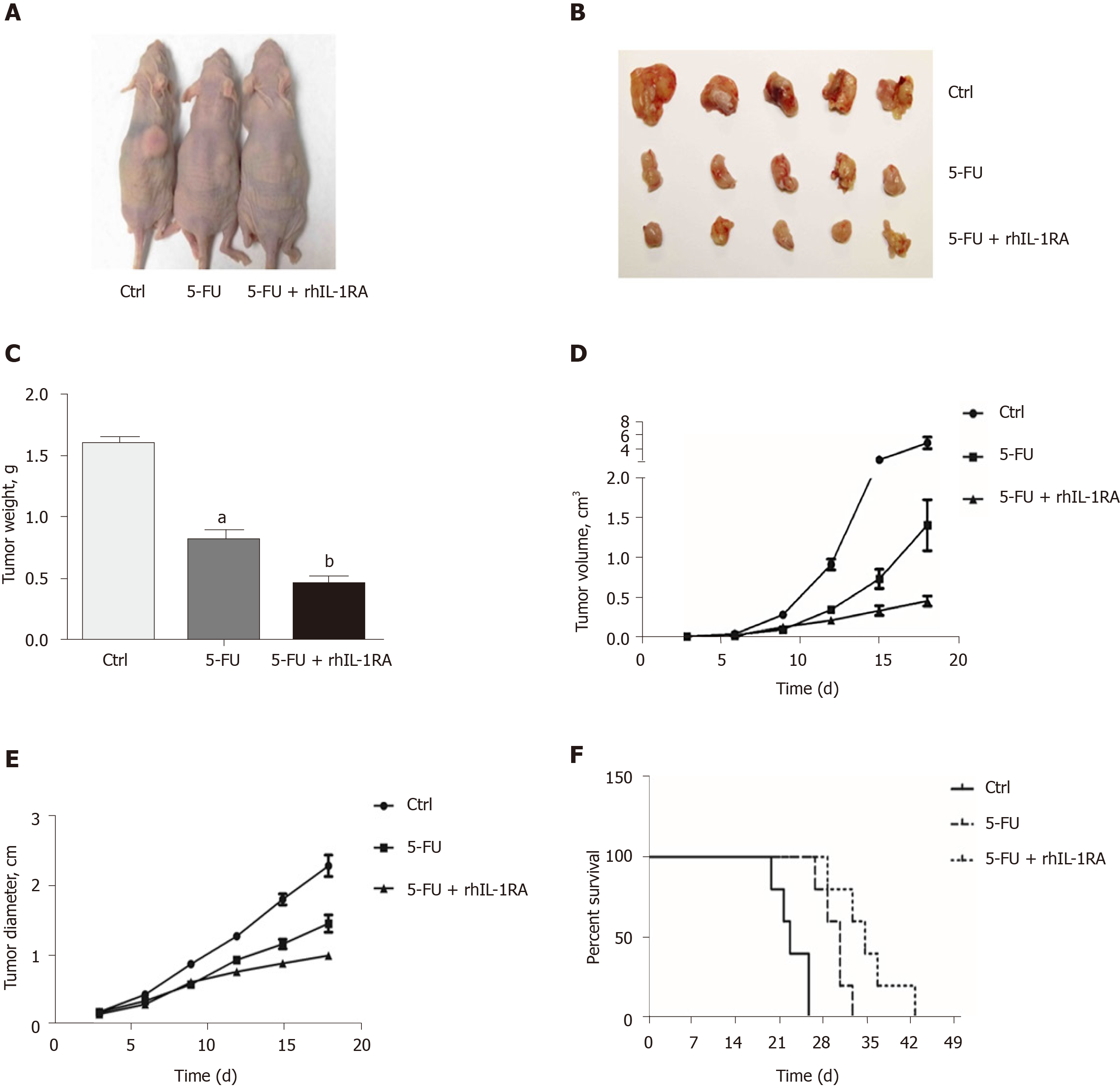
We used a xenograft nude mouse model to demonstrate the downregulation of the NF-κB pathway by blocking the NF-κB-regulated IL-1α feedforward loop, which could increase the efficacy of chemotherapeutic agents in inhibiting tumor cell growth. The tumor size of the control group treated with PBS (100 μL/mouse) for 3 wk significantly increased compared to that of the experimental groups (Figure 7A and B). After 3 wk of chemotherapy, the tumor weights of the nude mice treated with 5-FU and IL-1RA were significantly decreased compared with those in the single 5-FU treatment group (Figure 7C). In the 3-wk treatment, IL-1RA combined with 5-FU treatment limited the speed of tumor growth in the nude mice, according to the changes in tumor volume (Figure 7D) and diameter (Figure 7E). The survival time of the xenograft mouse model treated with chemotherapy was significantly longer than that of the control group treated with PBS (100 μL/mouse) (Figure 7F). IL-1RA combined with 5-FU treatment had a greater effect in extending the survival time of the xenograft tumor-bearing nude mice than 5-FU single therapy.
Previous studies have found that 30%-40% of colon cancers have Kras gene mutations, which can promote the activity of the NF-κB pathway in carcinoma cells[30]. Currently, only four of the drugs used to treat colorectal cancer are related to genetic mutations, and biomarkers must be detected. The detection-based biomarkers for treatment include EGFR, MSI-H/dMMR, BRAF + MEK, and NTRK fusion targets[31,32]. These patients must have wild-type KRAS/NRAS/BRAF and only left-sided tumors after gene testing. However, the 5-year survival rate of colorectal cancer is only 11%[33]. This study attempted to extend the detection of biomarkers to provide guidance for precise treatment of Kras mutant colon cancer, thus improving the patient survival rate.
IL-1 receptor antagonist targets IL-1 and competitively inhibits the activity of the NF-κB pathway in Kras mutant colorectal cancer, and it was combined with 5-FU as a neoadjuvant chemotherapy in this study. IL-1α and IL-1β, two types of IL-1, were associated with the NF-κB pathway and highly expressed in Kras mutant colon cancer cell lines, which suggested that IL-1 was closely related to the NF-κB pathway in colon carcinoma. The NF-kB pathway plays an essential role in the transcriptional regulation in response to various stimuli[30]. Consistent with other studies, high expression of phosphorylated P65 could activate downstream genes and promote the proliferation of colon cancer cell lines, such as COLO205, SW620, and HCT116[34]. This change persistently activated the NF-κB pathway, which was associated with the mutant Kras gene as an oncogene that promotes the proliferation of colon cancer cells[34]. IL-1 and other interleukins, such as IL-6, IL-8, IL-17, and IL-21, and TLR4 are also highly expressed in colon cancer cells and are associated with the NF-κB pathway in a feedback loop[34]. In Kras mutant colon cancer cells, the activation of P65 was inhibited by siRNA, and decreased expression of interleukins was detected. Among the interleukins, IL-1α and IL-1β were sensitive to changes in the NF-κB pathway, and a significant decrease was found in the si-P65 colon cancer cell line.
Inhibition of the interleukins associated with the NF-κB pathway could reduce the proliferation of tumor cells and promote their apoptosis[35,36]. A previous study found that inhibition of IL-6 could decrease the growth of cancer cells and promote cellular apoptosis[36,37]. Furthermore, IL-1 receptor antagonist combined with 5-FU chemotherapeutic drugs may achieve enhanced effects compared to 5-FU alone in treating the colon cancer cell lines SW620 and HCT116. IL-1 RA could counteract the abnormally high expression of P-TAK1, P-P65, and P-MEK caused by 5-FU in the SW620 cell line. The anti-pyrimidine chemotherapeutic drug 5-FU is currently one of the most commonly used drugs in the clinic[38]. Clinical studies have shown that 5-FU has certain therapeutic effects on many kinds of tumors, such as digestive tract tumors, breast cancer, ovarian cancer, chorionic carcinoma, cervical cancer, liver cancer, bladder cancer, skin cancer (local smear), and leukoplakia (local smear), and inhibits the synthesis of DNA[39]. This drug has been clinically used in various chemotherapy regimens for colon cancer. Studies have shown that 5-FU has notable side effects, including bone marrow suppression, gastrointestinal reaction, and hair loss[40]. A previous study found that 5-FU chemotherapy combined with IL-6 inhibitors could better inhibit the growth of colon cancer cells than single treatment[41], and a low dose of 5-FU combined with IL-6 inhibitors can achieve the same effect and reduce the side effects of chemotherapy[42].
In this study, the combination treatment of 5-FU and IL-1 receptor antagonist in SW620 and HCT116 cell lines significantly inhibited the cell proliferation compared with single 5-FU treatment. The activation of TAK1 and high expression of MEK may lead to the proliferation of malignant cells with drug resistance to 5-FU. The chemosensitivity to 5-FU could be enhanced by IL-1 receptor antagonist, which inhibits the expression of phosphorylated P65, TAK1, and MEK in SW620 and HCT116 cell lines. In the clonogenicity assay, IL-1 receptor antagonist combined with 5-FU had a stronger effect in inhibiting cell clone formation than 5-FU alone. Therefore, treatment with IL-1 receptor antagonist combined with 5-FU can reduce the 5-FU dose to achieve an inhibitory effect on the proliferation of colon cancer cells and reduce the side effects of chemotherapeutic drugs. Notably, treatment with IL-1 receptor antagonist alone in this study did not achieve a significant inhibitory effect on the proliferation of Kras mutant colon cancer cells. A low dose of IL-1 receptor antagonist (50 mg/mL, 100 mg/mL) combined with 5-FU did not have a strong inhibitory effect on the proliferation of colon cancer cells.
In summary, the mutant Kras gene can promote the activity of the NF-κB pathway in colon carcinoma cells. There is a feedback loop between the upregulated NF-κB pathway and IL-1, which leads to the proliferation of cancer cells. The chemotherapy drug 5-FU can activate the NF-κB pathway and lead to chemotherapy resistance in colon carcinoma cells. IL-1 receptor antagonist combined with 5-FU has a stronger inhibitory effect on the proliferation of colon cancer cells than single drug treatment due to the blockade of IL-1. More experiments are needed to confirm and explore the underlying mechanism to provide a potential adjuvant chemotherapy for the clinic and a theoretical basis for neoadjuvant chemotherapy.
The systemic toxicity and drug resistance of tumor cells are still two major problems in cancer chemotherapy. The chemotherapeutic drug 5-fluorouracil (5-FU) can inhibit the synthesis of adenylate synthetase and interfere with the synthesis of DNA in tumor cells. The effect of 5-FU is not ideal due to the chemoresistance in colon carcinoma patients treated with 5-FU.
Interleukin (IL)-1 and nuclear factor kappa-B (NF-κB) show a cyclic relationship, which leads to persistent activation of NF-κB in tumor cells. In Kras and p53 gene mutant mice, we found that the activity of NF-κB was downregulated by inhibiting the IL-1 receptor, which could effectively slow tumor growth.
The aim of this study was to determine whether treatment with 5-FU combined with IL-1 receptor antagonist can increase the chemosensitivity to 5-FU by decreasing the activation of the NF-κB pathway and reducing the proliferation of colon cancer cells. The results obtained provide a theoretical basis for clinical adjuvant chemotherapy.
The expression of phosphorylated P65 in the COLO205, SW620, and HCT116 cell lines was significantly higher than that in the NCM460 cell line, as shown by Western blot assays. We used a xenograft nude mouse model to demonstrate the downregulation of the NF-κB pathway by blocking the NF-κB-regulated IL-1α feedforward loop, which could increase the efficacy of chemotherapeutic agents in inhibiting colon tumor cell growth.
IL-1RA combined with 5-FU showed stronger inhibition of the proliferation of the SW620 and HCT116 cell lines than 5-FU treatment. IL-1RA combined with 5-FU treatment had a greater effect in extending the survival time of the tumor-bearing nude mice than 5-FU single therapy.
IL-1 receptor antagonist combined with 5-FU has a stronger inhibitory effect on the proliferation of colon cancer cells than 5-FU alone due to the blockade of IL-1.
These results could provide an adjuvant chemotherapy strategy for the clinic and provide a theoretical basis for neoadjuvant chemotherapy.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dustin LB, Shetty K S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25533] [Article Influence: 1823.8] [Reference Citation Analysis (7)] |
| 3. | Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1208] [Cited by in RCA: 1176] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 4. | Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 858] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 5. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8101] [Article Influence: 506.3] [Reference Citation Analysis (2)] |
| 6. | Xu GY, Tang XJ. Troxerutin (TXN) potentiated 5-Fluorouracil (5-Fu) treatment of human gastric cancer through suppressing STAT3/NF-κB and Bcl-2 signaling pathways. Biomed Pharmacother. 2017;92:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, Li Z, Feng X, Hao J, Zhang K, Xiao B, Chen M, Huang W, Xiong S, Wu X, Deng W. Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal Res. 2017;62:e12380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Kuhn JG. Fluorouracil and the new oral fluorinated pyrimidines. Ann Pharmacother. 2001;35:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Benson AB. New approaches to the adjuvant therapy of colon cancer. Oncologist. 2006;11:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Li Y, Fan L, Niu Y, Mian W, Zhang F, Xie M, Sun Y, Mei Q. An apple oligogalactan enhances the growth inhibitory effect of 5-fluorouracil on colorectal cancer. Eur J Pharmacol. 2017;804:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Polk A, Vistisen K, Vaage-Nilsen M, Nielsen DL. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Tahir AA, Sani NF, Murad NA, Makpol S, Ngah WZ, Yusof YA. Combined ginger extract & Gelam honey modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29 cells. Nutr J. 2015;14:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Shin JY, Kim JO, Lee SK, Chae HS, Kang JH. LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein-Barr virus-positive gastric cancer cells. BMC Cancer. 2010;10:425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Liu T, Liu D, Liu J, Song JT, Gao SL, Li H, Hu LH, Liu BR. Effect of NF-κB inhibitors on the chemotherapy-induced apoptosis of the colon cancer cell line HT-29. Exp Ther Med. 2012;4:716-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Kim HJ, Hawke N, Baldwin AS. NF-kappaB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 331] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 16. | Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, Greenberg AS, Mason JB. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012;23:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Ho GY, Wang T, Zheng SL, Tinker L, Xu J, Rohan TE, Wassertheil-Smoller S, Xue X, Augenlicht LH, Peters U, Phipps AI, Strickler HD, Gunter MJ, Cushman M. Circulating soluble cytokine receptors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2014;23:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Wang YW, Wang SJ, Zhou YN, Pan SH, Sun B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-κB and nuclear factor-κB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:785-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Samuel T, Fadlalla K, Gales DN, Putcha BD, Manne U. Variable NF-κB pathway responses in colon cancer cells treated with chemotherapeutic drugs. BMC Cancer. 2014;14:599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Sakamoto K, Maeda S, Hikiba Y, Nakagawa H, Hayakawa Y, Shibata W, Yanai A, Ogura K, Omata M. Constitutive NF-kappaB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res. 2009;15:2248-2258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1191] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 22. | Lee CH, Jeon YT, Kim SH, Song YS. NF-kappaB as a potential molecular target for cancer therapy. Biofactors. 2007;29:19-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Chang Z, Ju H, Ling J, Zhuang Z, Li Z, Wang H, Fleming JB, Freeman JW, Yu D, Huang P, Chiao PJ. Cooperativity of oncogenic K-ras and downregulated p16/INK4A in human pancreatic tumorigenesis. PLoS One. 2014;9:e101452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Melisi D, Niu J, Chang Z, Xia Q, Peng B, Ishiyama S, Evans DB, Chiao PJ. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7:624-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, Liu J, Lemischka IR, Hung MC, Chiao PJ. KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 419] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 26. | Niu J, Li Z, Peng B, Chiao PJ. Identification of an autoregulatory feedback pathway involving interleukin-1alpha in induction of constitutive NF-kappaB activation in pancreatic cancer cells. J Biol Chem. 2004;279:16452-16462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Sun D, Shen W, Zhang F, Fan H, Tan J, Li L, Xu C, Zhang H, Yang Y, Cheng H. α-Hederin Arrests Cell Cycle at G2/M Checkpoint and Promotes Mitochondrial Apoptosis by Blocking Nuclear Factor-κB Signaling in Colon Cancer Cells. Biomed Res Int. 2018;2018:2548378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Lehmann ML, Brachman RA, Listwak SJ, Herkenham M. NF-kappaB activity affects learning in aversive tasks: possible actions via modulation of the stress axis. Brain Behav Immun. 2010;24:1008-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Fang X, Hong Y, Dai L, Qian Y, Zhu C, Wu B, Li S. CRH promotes human colon cancer cell proliferation via IL-6/JAK2/STAT3 signaling pathway and VEGF-induced tumor angiogenesis. Mol Carcinog. 2017;56:2434-2445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9954] [Article Influence: 995.4] [Reference Citation Analysis (0)] |
| 31. | Fujiyoshi K, Yamamoto G, Takahashi A, Arai Y, Yamada M, Kakuta M, Yamaguchi K, Akagi Y, Nishimura Y, Sakamoto H, Akagi K. High concordance rate of KRAS/BRAF mutations and MSI-H between primary colorectal cancer and corresponding metastases. Oncol Rep. 2017;37:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Goto H, Shimauchi T, Fukuchi K, Yokota N, Koizumi S, Aoshima M, Endo Y, Masuda Y, Miyazawa H, Kasuya A, Nakamura K, Ito T, Tokura Y. Therapeutic Effectiveness of Immunoradiotherapy on Brain-metastatic BRAF/MEK Inhibitor-resistant Melanoma with Balloon Cell Change. Acta Derm Venereol. 2019;99:612-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Iversen LH. Aspects of survival from colorectal cancer in Denmark. Dan Med J. 2012;59:B4428. [PubMed] |
| 34. | Zhang R, Zhao J, Xu J, Jiao DX, Wang J, Gong ZQ, Jia JH. Andrographolide suppresses proliferation of human colon cancer SW620 cells through the TLR4/NF-κB/MMP-9 signaling pathway. Oncol Lett. 2017;14:4305-4310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Yao J, Zhao L, Zhao Q, Zhao Y, Sun Y, Zhang Y, Miao H, You QD, Hu R, Guo QL. NF-κB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014;5:e1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Yashiro M. Molecular Alterations of Colorectal Cancer with Inflammatory Bowel Disease. Dig Dis Sci. 2015;60:2251-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Li S, Tian J, Zhang H, Zhou S, Wang X, Zhang L, Yang J, Zhang Z, Ji Z. Down-regulating IL-6/GP130 targets improved the anti-tumor effects of 5-fluorouracil in colon cancer. Apoptosis. 2018;23:356-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Tang JC, Feng YL, Liang X, Cai XJ. Autophagy in 5-Fluorouracil Therapy in Gastrointestinal Cancer: Trends and Challenges. Chin Med J (Engl). 2016;129:456-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2950] [Cited by in RCA: 2821] [Article Influence: 112.8] [Reference Citation Analysis (1)] |
| 40. | Romano G, Santi L, Bianco MR, Giuffrè MR, Pettinato M, Bugarin C, Garanzini C, Savarese L, Leoni S, Cerrito MG, Leone BE, Gaipa G, Grassilli E, Papa M, Lavitrano M, Giovannoni R. The TGF-β pathway is activated by 5-fluorouracil treatment in drug resistant colorectal carcinoma cells. Oncotarget. 2016;7:22077-22091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Grothey A, Sargent DJ. Adjuvant Therapy for Colon Cancer: Small Steps Toward Precision Medicine. JAMA Oncol. 2016;2:1133-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Folprecht G, Pericay C, Saunders MP, Thomas A, Lopez Lopez R, Roh JK, Chistyakov V, Höhler T, Kim JS, Hofheinz RD, Ackland SP, Swinson D, Kopp M, Udovitsa D, Hall M, Iveson T, Vogel A, Zalcberg JR. Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: the AFFIRM study. Ann Oncol. 2016;27:1273-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |