Published online Aug 15, 2020. doi: 10.4251/wjgo.v12.i8.833
Peer-review started: January 31, 2020
First decision: April 18, 2020
Revised: May 1, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: August 15, 2020
Processing time: 193 Days and 10.1 Hours
Exocrine pancreatic neoplasms represent up to 95% of pancreatic cancers (PCs) and are widely recognized among the most lethal solid cancers, with a very poor 5-year survival rate of 5%-10%. The remaining < 5% of PCs are neuroendocrine tumors that are usually characterized by a better prognosis, with a median overall survival of 3.6 years. The most common type of PC is pancreatic ductal adenocarcinoma (PDAC), which accounts for roughly 85% of all exocrine PCs. However up to 10% of exocrine PCs have rare histotypes, which are still poorly understood. These subtypes can be distinguished from PDAC in terms of pathology, imaging, clinical presentation and prognosis. Additionally, due to their rarity, any knowledge regarding these specific histotypes is mostly based on case reports and a small series of retrospective analyses. Therefore, treatment strategies are generally deduced from those used for PDAC, even if these patients are often excluded or not clearly represented in clinical trials for PDAC. For these reasons, it is essential to collect as much information as possible on the management of PC, as assimilating it with PDAC may lead to the potential mistreatment of these patients. Here, we report the most significant literature regarding the epidemiology, typical presentation, possible treatment strategies, and prognosis of the most relevant histotypes among rare PCs.
Core tip: Due to their rarity and lack of consistent literature, rare subtypes of exocrine pancreatic cancer are often assimilated with the more frequent pancreatic ductal adenocarcinoma, even if they have peculiarities in their presentation and treatment strategy. The aim of this review is to summarize the most relevant literature regarding these rare subtypes of pancreatic cancers.
- Citation: Niger M, Prisciandaro M, Antista M, Monica MAT, Cattaneo L, Prinzi N, Manglaviti S, Nichetti F, Brambilla M, Torchio M, Corti F, Pusceddu S, Coppa J, Mazzaferro V, de Braud F, Di Bartolomeo M. One size does not fit all for pancreatic cancers: A review on rare histologies and therapeutic approaches. World J Gastrointest Oncol 2020; 12(8): 833-849
- URL: https://www.wjgnet.com/1948-5204/full/v12/i8/833.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i8.833
Traditionally, when speaking about pancreatic cancer (PC), we refer to exocrine pancreatic neoplasms, which represent up to 95% of all PCs and are widely recognized among the most lethal solid cancers, with a very poor 5-year survival rate of 10%[1]. Exocrine PCs account for 7.8 new cases every 100000 people and is the 11th most common cancer worldwide[2]. The remaining approximately < 5% of PCs are neuroendocrine tumors, which are characterized by a better overall survival (OS), with a median OS (mOS) of 3.6 years (ranging from 15 mo for grade 3 disease to 140 mo for grade 1 disease)[3].
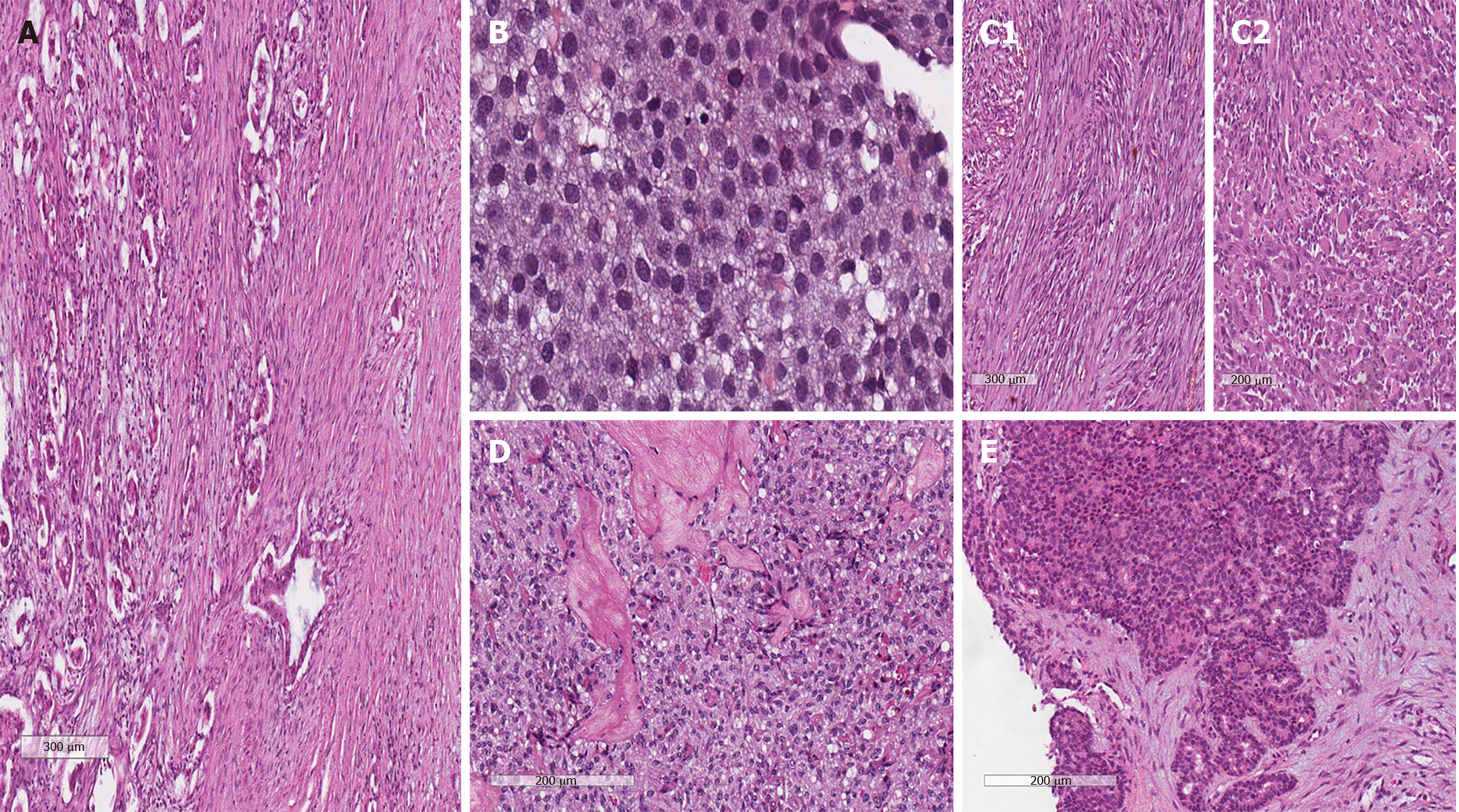
Since roughly 85% of exocrine PCs are pancreatic ductal adenocarcinomas (PDACs), the vast majority of literature and data from clinical trials and basic research are focused on this particular histotype. However, up to 10% of PCs have a rare histotype such as adenosquamous carcinoma, undifferentiated carcinoma (UC), acinar cell carcinoma (ACC), cystic tumors, papillary adenocarcinoma, and other exocrine variants[4]. As reported in Table 1, the World Health Organization (WHO) has identified more than 10 subtypes of PCs according to their anatomopathological characteristics[4] (Figure 1). Due to their very low incidence, the biological and clinical features of these rare types of PC are still poorly understood. Furthermore, since patients with rare histotypes of PC are often excluded or not well represented in clinical trials for PDAC, there are limited data regarding the best treatment strategy and most information is from case reports or small case series. The aim of this review was to discuss the most relevant rare subtypes and their peculiarities in terms of epidemiology, diagnosis, prognosis, and treatment.
| Histotype | Subtype | Frequency, % |
| Ductal adenocarcinoma | NOS | 85% |
| Carcinoma undifferentiated | 1%-7% | |
| Adenosquamous carcinoma | 1%-4% | |
| Undifferentiated carcinoma with osteoclast-like giant cell | < 1% | |
| Colloid carcinoma | 1%-3% | |
| Poorly cohesive carcinoma | Extremely rare | |
| Signet-ring cell carcinoma | Extremely rare | |
| Medullary carcinoma NOS | Extremely rare | |
| Hepatoid carcinoma | Extremely rare | |
| Large cell with rhabdoid phenotype | Extremely rare | |
| Acinar cell carcinoma | NOS | < 2% |
| Acinar cell cystadenocarcinoma | Extremely rare | |
| Mixed acinar-neuroendocrine carcinoma | Extremely rare | |
| Mixed acinar-endocrine-ductal carcinoma | Extremely rare | |
| Mixed acinar -ductal carcinoma | Extremely rare | |
| Pancreatoblastoma | Extremely rare | |
| Solid pseudopapillary neoplasm | NOS | 3% |
| With high-grade carcinoma | Extremely rare |
ACC of the pancreas represents < 2% of all PCs. As for most rare cancers, there are no prospective data and nearly all of the information is from small single-center series and the United States National Registry of PC[5-8]. Compared to patients with PDAC, those with ACC appear to be younger, with a median age of 60-67 years, and more frequently male. Additionally, patients with ACC tend to have larger tumors, with a median diameter ranging from 8 to 10 cm. Despite previous evidence stating that these tumors are more frequently located in the tail of the pancreas, the most recent WHO classification states that this specific histotype is more often located in the head of the pancreas. However, these cancers do not usually cause jaundice, and patients typically have several non-specific symptoms, such as weight loss and abdominal pain. Despite this, compared to PDAC, ACC is less likely to have distant disease at diagnosis and is more frequently diagnosed at an earlier stage, allowing for surgical resection in about 38% of patients[7,8].
These tumors seem to be less aggressive than the most common PDAC, even if their prognosis is still poor. In particular, in previously reported series, the mOS of ACC patients ranged from 17 to 19 mo, reaching 47 mo in those who underwent surgical resection[5-9]. Based on a more recent analysis of 57804 PC patients who underwent surgical resection, ACC achieved an mOS of 67.5 mo (51% 5-year OS)[10]. In a few case reports, OS reached up to 123 mo. To date, there are no variables that can help select these patients with long OS. These data might encourage a more aggressive approach for evaluating and treating these tumors[11,12].
Macroscopically, ACCs are large tumors, which are frequently well circumscribed and partially encapsulated. The cut surface is usually homogeneous, and pink to tan with a fleshy or friable consistency. Necrosis, cystic evolution, and hemorrhage are sometimes observed. Upon histological examination, the most frequent feature is an acinar pattern, with neoplastic cells arranged in small glandular units, followed by a trabecular, glandular, and solid architecture. Nonetheless, some case series have also shown that ACC can rarely show pleomorphic or spindle cells[4,13,14]. Furthermore, neoplastic cells have a moderate amphophilic to eosinophilic granular cytoplasm rich in zymogen granules, which are usually positive with periodic-acid Schiff and are resistant to diastase. When present, this feature is highly supportive of ACC diagnosis. However, the milestone for diagnosis of ACC is the immunohistochemical identification of pancreatic enzyme. Upon analysis with a specific antibody, trypsin and chymotrypsin can be useful for diagnosis, but B-cell lymphoma/leukemia 10 (BCL-10) shows high specificity[4]. Cytokeratin (CK) 19 and CK 7 can also be expressed, as in PDAC.
Recent studies with whole-exome sequencing, even with the limits of small numbers, have revealed specific molecular patterns of ACC that apparently differ from those known for PDAC[15-17]. First of all, ACC seems to be characterized by a higher mutational frequency than PDAC, comparable to those of other digestive tract cancer such as colorectal cancer. Additionally, typical PDAC mutations such as KRAS are not as frequent in these small series of ACC. In particular, Jiao et al[15] analyzed 17 ACCs, identifying somatic mutations in SMAD4 (23%), JAK1, RB1 or TP53 (17%) APC, ARID1A, GNAS, MLL3, PTEN, FAT4, and CTNNB1 (11%) as the most frequent alterations[15]. In addition, Furukawa et al[16] described somatic or germline mutations of BRCA2 (3 of 7 cases) and FAT genes (4 of 7 cases)[16]. Finally, Jäkel et al[17] showed that ACC is also characterized by chromosomal alterations with a numerous copy number variations, an aberrantly DNA methylation, and downregulation of the tumor suppressor genes ID3, ARIDI1A, APC, and CDKN2A, which may be related to the loss of function of DNA repair systems[17]. Chromosomal alterations were well explored in several other studies, which demonstrated BRAF (about 20%)[18,19] and RET (7.5%) rearrangements[20]. Although these alterations were found in very small series, some of them may be susceptible to targeted therapy, opening the door to possible new treatment developments in ACC.
On computed tomography (CT), ACC lesions tend to be large, partially, or completely exophytic and mostly hypodense due to relative hypovascularity in comparison to the surrounding pancreas, on both arterial and venous phase images. A sizeable proportion has an enhancing capsule, cystic, hemorrhage or necrotic component, which may be related to the digestive effect of the pancreatic enzymes released by neoplastic cells[21]. Moreover, lesions located in the pancreas head or uncinate process unfrequently cause severe pancreatic or biliary ductal dilatation[21-24].
On magnetic resonance imaging (MRI), ACC predominantly presents as an oval, large, well-marginated exophytic mass with moderate and heterogeneous enhancement after intravenous administration of contrast. It is frequently characterized by cystic and necrotic areas, and hemorrhage can be observed. Interestingly, ACC is clearly visible in diffuse-weighted MRI, which may be a promising useful tool for ACC diagnosis[23,24].
MRI yields excellent soft tissue contrast and appears to be superior to CT in showing the tumor margin, cystic and necrotic areas, peripancreatic extension, and vascular involvement even without the administration of contrast. Imaging of ACC is so characteristic that it may have a key role in suggesting the diagnosis of this rare histotype. However, some exceptions have been reported[23].
Overall, surgical resection for localized disease remains the only curative treatment. To date, the contribution of adjuvant chemotherapy and radiotherapy has not been well investigated. However, considering the available data, resection and adjuvant chemotherapy are associated with the longest rate of mOS, whereas in some cases, chemoradiation has shown activity in both the neoadjuvant and locally advanced settings[5,8,9,25-28].
In metastatic patients, various first-line treatments have been reported, even if only in small series and case reports. Most of the time, these patients are treated with regimens commonly used for PDAC, such as capecitabine or gemcitabine monotherapy or Gemcitabine- (gemcitabine/nab-paclitaxel, GEMOX) or fluoropyrimidine-based (FOLFOX, FOLFIRI, FOLFIRINOX) regimens. There are very few data available for the second-line setting. In 2002, Holen et al[5] published a retrospective monocentric series of 18 patients treated with various regimens: Only 2 patients had a partial response (PR) obtained respectively with FOLFIRI and the combination of cytarabine plus cisplatin and caffeine[5]. In 2011, Lowery et al[29] published a new retrospective study, from the same institution, describing the results obtained in 25 patients treated with various regimens in first- and/or second-line. The mOS of patients with metastatic disease was 19.6 mo, with survival up to 57 mo in the 11 patients who had PR or confirmed prolonged stable disease (SD). These patients were treated with GEMOX, gemcitabine-docetaxel-capecitabine, cisplatin plus gemcitabine, gemcitabine plus erlotinib and cisplatin plus irinotecan[29]. The potential activity of regimens containing fluoropyrimidine and/or platin-based compounds was confirmed by Kruger et al[30], who reported PR to first- and second-line treatments. In first-line setting, the authors reported PR with FOLFIRINOX in two patients (maintained for up to 14 mo) and with GEMOX and capecitabine in two other patients (maintained for up to about 6 and 13 mo, respectively). In the second-line setting, the objective response was reported in three patients treated with FOLFOX and FOLFIRINOX, with progression-free survival ranging from 9 to 12 mo[30]. This work is not the only report of FOLFIRINOX activity in this setting, as there are at least three case reports in which prolonged SD (time to progression [TTP] of 9 mo) and two PRs were observed[31-33]. Similarly, Yoo et al[28] observed a PR in three of four patients treated with FOLFOX in second-line, with 6.5 mo (95%CI, 2.8 to 10.2 mo) of progression-free survival[28]. Finally, in a recent multicenter Italian retrospective study including patients with rare pancreatic histotype tumors, Brunetti et al[26] showed that PR was achieved in first-line in 2 of 23 patients treated with GEMOX and gemcitabine-fluorouracil, and in second-line in 1 patient treated with FOLFIRINOX[26].
Regarding the use of single-agent gemcitabine, three small series reported poor activity[28,30,34]. Finally, there have been reports of the potential activity of S-1 in first and second-line settings[34-36].
In conclusion, the published literature shows promising activity of combination treatments, particularly regimens based on fluoropyrimidine and oxaliplatin. In addition, studies have also shown signs of activity for gemcitabine-based regimens, whereas no data support the efficacy of its use as a single agent in the first-line treatment of patients with ACC. As more data regarding molecular classification and alterations involving DNA repair genes arise such as BRCA2 mutations[16], there is interest in the development of novel therapeutic strategies that can exploit these characteristics.
Pseudopapillary tumors (PTs) account for about 3% of all exocrine PCs, with increasing prevalence due to improvements in imaging devices. PTs are more frequent in adolescent girls and young women, with a median age of 28 years at diagnosis, whereas less than 10% of PTs are diagnosed in slightly older men (median age 35). There is no known association with ethnic origin or clinical or genetic syndromes, although very rare cases have been reported in the setting of familial adenomatous polyposis[37]. At diagnosis, PT is characterized by a large round solid or mixed solid cystic lesion frequently located in the pancreatic tail, which may be the reason that patients infrequently experience jaundice. Additionally, there are no specific symptoms of PT and it often presents as a palpable abdominal mass, abdominal discomfort and pain, nausea, vomiting, asthenia, weight loss, back pain, or pancreatitis.
Despite being counted among malignant pancreatic neoplasms, PTs are considered to be a low-grade, indolent disease. Indeed, only 10% to 15% of cases recur or metastasize and the overall 5-year survival is about 97% even in the presence of metastasis. These survival rates are definitely better and more encouraging than those of PDACs or other aggressive histotypes. Furthermore, the more aggressive cases are often tumors that harbor an undifferentiated component or have peculiar pathological features usually associated with an aggressive behavior such as diffuse growth pattern, high mitotic activity, nuclear atypia, and tumor necrosis[4,38,39].
Grossly, PTs appear as large lesions with solid and cystic components, they are usually very soft, but may be firm and sclerotic. They are well-demarcated with a rim of fibrous capsule and adjacent organs invasion is rare[4,40,41]. Cut sections of PT show alternate solid and yellow areas with cystic, necrotic, and hemorrhagic zones which sometimes may be as large as a pseudocyst. Calcifications are also frequently observed[39].
Histologically, the solid component of PT consists of poorly cohesive epithelioid cells with oval nuclei, finely dispersed chromatin low nucleus and either eosinophilic or clear vacuolated cytoplasm and perivascular pseudo papillae. Some of the neoplastic cells contain eosinophilic, diastase-resistant PAS-positive globules of varying size, which may also occur extracellularly, sometimes in large amounts. Glycogen is not prominent and mucin is absent. Mitotic figures might be present, although they are usually rare[4,39,42].
Immunohistochemistry analysis has shown that PT has a low Mib1/Ki67 rate and is positive for beta-catenin (nuclear and cytoplasmic), vimentin, synaptophysin, progesterone receptor (nuclear), CD56, neuron-specific enolase, CD10, and nuclear E-cadherin. Extracellular expression of e-cadherin is lost due to mechanisms that are not clear yet[4,42,43], E-cadherin is a transmembrane protein that has a pivotal role in cell adhesion through interactions with catenins, and its extracellular loss may explain the loss of cell cohesiveness of pseudopapillary pattern[44], making it useful in distinguishing PT from other histotypes[45].
Immunohistochemical beta-catenin overexpression is strongly correlated with mutations of its gene which frequently occur in PT, mostly on exon-3. This mutation leads to hyperactivation of the beta-catenin/Wnt pathway and subsequent activation of the transcription of several oncogenic genes, such as cyclin D1. The consequent deregulation of cell cycle plays an important role in PT development[46-48]. However, several studies have shown that PTs are also characterized by the overexpression of cyclin-dependent kinase inhibitors p21 and p27, which have inhibitory effects on cyclin D1 and its cyclin-dependent kinases complex. Although the mechanism of p21 and p27 upregulation is unknown, it may explain the low growth rate of this rare histotype of PC[48]. By contrast, molecular changes often detected in PDAC, such as alterations of p53 and K-RAS, have not been detected in PT[46].
CT features of PT include both solid and cystic lesions without any internal septation, secondary to hemorrhagic degeneration, which are well demarcated by a surrounding capsule. At the margin of the mass, calcification and solid areas can be identified[49,50]. During the CT pancreatic phase, there is weak enhancement compared to the surrounding pancreatic parenchyma, which gradually increases in the hepatic venous phase[51]. Atypical PTs on CT have no surrounding capsule, solid or cystic component, with hyperattenuation during the pancreatic phase and dense internal calcification with no defined margin[51].
Otherwise, on MRI, PT is defined as an encapsulated lesion with both a solid and cystic component as well as hemorrhage without internal septation[52]. Interestingly, Yu et al[52] proposed an MRI classification in which PT lesions were separated in three main classes by specific MRI features on T1- and T2-weighted images related to the predominance of solid or hemorrhagic areas. According to their study, MRI may be considered superior to CT in terms of correlation of clinicopathological and radiological findings of PT[52].
For localized disease, surgery is the treatment of choice and more than 95% of patients can be cured after radical resection. Due to the favorable behavior of this disease, surgery with organ preservation is indicated when feasible[53]. Moreover, since evidence of lymph node metastasis is extremely rare[42,54] a formal lymphadenectomy should not be routinely performed[42,53]. Considering the excellent long-term prognosis even for metastatic disease, which is mostly confined to the liver, mesentery, and peritoneum, surgical approaches[53,55] or locoregional treatments[56] are reported to be potentially effective even in this setting, with a high 5-year survival rate with en bloc resection of locally progressed PTs or with synchronous or metachronous resection of distant metastases[57-60]. Furthermore, in highly selected cases, even orthotopic liver transplantation (OLT) may be taken into account for patients with unresectable liver metastases. To date, four cases of patients with liver metastatic PT who underwent OLT have been published. In the first two reports, young 14- and 21-year-old patients with PTs and multiple unresectable hepatic metastases were successfully treated with partial liver transplantation from a living donor without any sign of disease recurrence after 2 years of follow up[61,62]. Longer recurrence-free survival was later reported by Łągiewska et al[63], whose patient was free of disease after 5 years from cadaveric OLT. However, in two other patients, disease recurred within 1 and 4 years[64,65].
There are only anecdotal data on the role of systemic treatment in neoadjuvant, adjuvant and metastatic settings. Tumor response to preoperative chemotherapy using cisplatin in combination with 5-fluorouracil (5-FU) or gemcitabine were reported[66-68]. Similar results were observed in a patient treated with a combination of etoposide, cisplatin, cyclophosphamide, doxorubicin, and vincristine[54] and combination of cisplatin, ifosfamide, etoposide, and vincristine followed by intraoperative radiofrequency ablation[69]. By contrast, no response to multiple agents was seen in another case report[58]. Radiotherapy showed activity in a single case of a locally advanced unresectable disease[70].
Regarding the metastatic setting, in a small series, Brunetti et al[26] treated two patients with GEMOX, achieving 1 SD and 1 progressive disease (PD) with a TTP of 5 and 2 mo, respectively: One patient received first-line treatment with gemcitabine plus 5-FU with SD, and the last one interestingly achieved a 22-mo SD with single agent gemcitabine[26]. In the same study, those patients who progressed to GEMOX received second line with gemcitabine plus 5-FU with SD reaching a TTP of 9 and 8 mo, respectively. Similar results were also observed with capecitabine used as second line after progression to gemcitabine plus 5-FU with SD and a TTP of 6 mo[26]. Moreover, Morikawa et al[71] described a case of a patient treated with paclitaxel as second line after S1 and gemcitabine, who was alive and without progression after 20 mo of follow-up[71]. The low malignant potential of PT was also confirmed by an interesting case of a patient with long survival after several lines of treatment: gemcitabine alone (6 cycles), gemcitabine plus irinotecan (3 cycles), oxaliplatin plus irinotecan and capecitabine (8 cycles), gemcitabine plus capecitabine (6 cycles), weekly 5-FU and lastly, until publication of the data, capecitabine alone[72].
UC is a histological variant of PDAC that accounts for 1% to 7% of all exocrine PCs. Anaplastic, sarcomatoid, and carcinosarcomas are recognized morphological variants of UC composed of pleomorphic mononuclear cells admixed with bizarre giant cells or spindle and rhabdoid cells. UC of the pancreas has been defined as an epithelial neoplasm without a definitive direction of differentiation with a diffuse sheet-like growth pattern without overt glandular differentiation. UC with osteoclast like giant cells (OCGC) is another histologic subtype of PDAC, with different morphologic features and clinical behavior. UC is usually diagnosed at a median age of 61 years and tends to be more frequent in males and Caucasians. At diagnosis, it is observed as a bulky tumor, usually involving the pancreatic head. Its clinical presentation is similar to that of PDAC and is characterized by abdominal pain, jaundice, weight loss, and fatigue[73-76]. In addition, anemia and elevated leukocyte count have been reported, possibly related to hemorrhage and necrosis that follow the rapid and uncontrolled tumor growth[75]. Some authors also reported increased secretion of granulocyte colony-stimulating factor, which may contribute to leukocytosis and is correlated with a worse prognosis[75,77,78]. However, even with some discrepancies in the literature, cancer antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) levels are reportedly lower than those found in PDAC[74].
Considering the rarity and aggressive behavior of this histotype, survival data about UC are mostly represented by single case reports or small case series. To date, there are no large prospective clinical studies. The overall prognosis of patients affected by UC appears to be worse than those affected by PDAC, with a median OS of about 5.5 mo. There are reports of advanced patients dying within weeks from the onset of symptoms. However, patients who undergo surgical resection have a mOS similar to that of PDAC[74,75,79]. Several reports have shown that among UC patients, those with OCGC tend to have a longer survival. This may be related to the more indolent biological behavior of this specific subtype, which allows a higher rate of curative resections or slower disease progression in the metastatic setting[73,75,79,80].
Since the first description in 1954, UC has been described using various terms including anaplastic, spindle cell, giant cell, pleomorphic giant cell, and round cell. In 2019, the WHO classified this heterogeneous neoplasm as a subtype of PDAC under the name of UC[4]. As mentioned above, however, OCGC is described as a separate variant of PDAC due to its different biological and clinical behaviors.
Macroscopic examination of UC has shown that it can be a large solid or cystic lesion, or a mixture of both. Cystic evolution is consequent to the rapid uncontrolled growth of neoplastic cells that are highly prone to degeneration, hemorrhage, and necrosis. This feature may help to distinguish UC from PDAC, in which cystic components are infrequent (< 1%). The histological examination of UC is remarkably heterogeneous and characterized by the presence of pleomorphic and/or spindle cells, whose predominance differentiates the two main subtypes (anaplastic UC/sarcomatoid UC), which grow in poorly cohesive nests supported by scanty fibrous stroma. Pleomorphic cells are usually arranged in solid sheets without gland formation showing markedly pleomorphic nuclei and abundant eosinophilic cytoplasm. On the contrary, spindle cells tend to arrange in a storiform pattern and to be more uniform, ovoid to spindle shaped. Furthermore, areas of infiltrating ductal adenocarcinoma at the periphery of the lesions, squamous differentiation, and areas of phagocytic activity are frequently observed[73,81,82]. Although this bizarre morphology, electron microscopy, and above all, immunohistochemistry analysis have demonstrated the epithelial origin of UC neoplastic cells. In particular, similar to PDAC, these cells express CKs such as CK7, CK8, CK18 and CK19. Vimentin, CA 19.9, CEA, and p53 may also be expressed. In addition, similar to PDAC, KRAS point mutations are frequently observed upon molecular analysis[73,82]. Although further studies are still needed, these specific features support the hypothesis that these cells may be the result of a dedifferentiation process of a preceding ductal adenocarcinoma.
On the other end, UC with OCGC is defined by the abundance of non-neoplastic osteoclast-like multinucleated giant cells admixed with a mononuclear histiocytic component and a neoplastic mononuclear cell component[4]. OCGC is frequently located in the junction between necrotic hemorrhagic areas and viable areas of the tumor. Abundant hemosiderin pigment is scattered throughout the tumor; moreover, osteoid formation and foci of in situ or invasive adenocarcinoma may also be found. To date, the real origin of these cells is still not clear. In contrast to pleomorphic and spindle cells, OCGC is immunohistochemically negative for CKs, but positive for histiocytic markers, supporting the hypothesis of a histiocytic origin. Indeed, some authors have hypothesized that OCGC may result from the fusion of histiocyte/macrophages chemoattracted to the tumor site by factors released by neoplastic cells, and this may be the reason why phagocytic areas are commonly described[83]. Neoplastic cells may be spindle-shaped or epithelioid and can be very large and pleomorphic; these cells can show keratin positivity and have a high Ki-67 proliferation index.
In reported imaging series, on enhanced CT scan, UC is described as a large mass, usually in the pancreatic head, with relative hypodensity compared to normal parenchyma during the pancreatic and portal vein phases, with a peripheral contrast enhancement. Therefore, it may sometimes be difficult to distinguish UC from cystic lesions. Additionally, pancreatic duct dilatation and rare calcifications was observed[84-86].
At MRI, several authors have described UC as a well-defined lesion with low intensity on T1-, T2-, and diffusion-weighted images. This last feature, in particular, may be related to hemosiderin deposits on mononuclear histiocytic and OCGC and may be helpful for the differential diagnosis between UC and cystic lesions, in which hemorrhage and hemosiderin deposits are unusual. Calcifications are also occasionally described[85,86].
To date, there is no a standard treatment for UC. For patients with resectable disease, surgery represents the only curative option, with a survival rate that is comparable with that of patients with PDAC who undergo R0 resection. No significant benefits have been reported for neoadjuvant/adjuvant treatments or palliative surgery[74,87].
For advanced disease, only few case reports with various systemic treatment have been reported[80,87]. For example, Wong et al[88] treated a patient with GEMOX in combination with radiofrequency ablation of liver metastases, reaching SD with an OS of 15 mo[88]. Gemcitabine-base regimes was also explored by Brunetti et al[26], who reported PR with an OS of 14 mo and SD with OS of 8 mo in two patients treated with gemcitabine plus nab-paclitaxel. Furthermore, there are case reports showing benefits of first-line and second-line therapy with FOLFIRINOX[89] and FOLFIRI[90], respectively. Finally, an interesting approach presented by Wakatsuki et al[91]. In their case report, paclitaxel as a single agent was selected to treat the patient, using a chemosensitivity test with the adenosine triphosphate assay achieving a complete response after two cycles and a disease-free survival of 23 mo[91].
Pancreatoblastoma (PBL) presents more often in children, where it accounts for 25% of all pancreatic tumors[92], whereas it is an extremely rare histotype in adults, accounting for less than 1% of PC. In the few series available in English literature (accounting for < 100 patients in total), PBL typically appears as a large tumor (up to 20 cm), which is more frequently localized in the pancreatic head. It usually presents with nonspecific signs and symptoms such as abdominal pain, abdominal mass, jaundice, weight loss, chronic diarrhea and upper gastrointestinal bleeding[93-96]. The prognosis is poor, with an mOS of 5 mo in patients who cannot undergo surgery. About 40% of patients are metastatic at diagnosis, with the liver being the most common site of secondary involvement; local infiltration of surrounding tissues is also frequent[97]. Of note, even if PBL is considered to be a sporadic tumor, there have been reports of its association with familial adenomatous polyposis syndrome[93,98] and Beckwith-Weidemann syndrome[99].
There are two populations of cells with distinctive characteristics: blast-like tumor cells and squamous morules. In particular, blast-like cells are monotonous, round, and small (1.5-2.0 times the size of a red blood cell), with a high nuclear-to-cytoplasmic ratio, scant cytoplasm, and infrequent anisonucleosis. Focal nuclear molding and crushing resembling small-cell carcinoma are seen in all cases. Abnormal mitotic figures were occasionally described in a case with metastatic disease with a poor prognosis (< 10 d). The immunohistochemical staining was positive for trypsin, chymotrypsin, lipase, BCL10 and alpha-fetoprotein. Squamous morules were seen in 75% of patients. They were composed of whorling or streaming epithelioid cells with abundant, dense, granular cytoplasm; syncytial arrangement; low nuclear to cytoplasmic ratio; and elongated nuclei with blunted ends and vesicular chromatin. Morule overexpress estrogen receptor beta and have aberrant nuclear/cytoplasmatic B catenin staining. Aberrant Wnt pathway activation manifest as somatic CTNNB1 mutations (in 90% of cases) and loss of heterozygosity (LOH) of APC (in 10%). Other abnormalities include upregulation of the R-spondin/LGR5/RNF43 module, a progenitor-like pancreatic cell expression profile, and LOH of chromosome 11p. APC/β-catenin pathway alterations are seen in patients with and without familial adenomatous polyposis. Another syndrome seen in childhood PBL is Beckwith-Wiedemann syndrome, which is also associated with chromosome 11p LOH.
Previous data on nine case of pancreatoblastoma showed in 78% of cases strong nuclear and cytoplasmic accumulation of b-catenin protein, 86% had LOH for TH and D11S1984, microsatellite markers near the WT-2 locus on chromosome 11p15.5. There is frequent involvement of the APC/β-catenin pathway, with no evidence for KRAS mutations or TP53 tumor suppressor gene alterations[98,100].
On ultrasound, PBL appears as a solid inseparable pancreatic mass, with mixed echotexture[101]. On CT scan, it is usually a large, well-circumscribed, and heterogeneous mass, with both solid and cystic components[102]. MRI can be used to delineate intratumoral hemorrhage and necrotic areas.
Due to the rarity of this histology there is no guideline regarding treatment for this cancer. The only curative therapy remains surgical resection. The role of postoperative chemoradiotherapies is still unclear and there is no consensus on the best chemo regimen neither in adjuvant nor in palliative setting. There are clinical case reports describing the use of regimens containing platinum and/or doxorubicin and fluorouracil (e.g., cisplatin/vincristine/bleomycin, 5-FU/doxorubicin/mitomycin, doxorubicin/carboplatin, cisplatin/doxorubicin), with mixed results[103,104]. Furthermore, there are reports of long-term disease-free survival for patients with liver metastases undergoing surgical resection of primary and metastatic lesions, suggesting a role for aggressive surgical approach[105,106].
Adenosquamous pancreatic cancer (ASPC) accounts for about 1%-4% of all pancreatic cancer and it is defined as a mixture of the adenocarcinoma and the squamous cell carcinoma components[107]. Based on data from the National Cancer Database and Surveillance, Epidemiology and End Results database, ASPC tends to be larger, more frequently in body/tail, undifferentiated and in early stage at diagnosis time[108,109]. When compared to the 205328 PDAC present in the NCBD, overall prognosis of the 1745 ASPC is similarly poor, with a mOS of 5.7 mo. In patients who underwent surgery, ASCP had worse OS (14.8 mo vs 20.5 mo, P < 0.001) than PDAC, unless there was negative lymph node status, R0 surgical resection, and receipt of chemotherapy[109]. In surgical patients retrieved from the Surveillance, Epidemiology and End Results database between 2004 and 2015, mOS was 12 mo, with a median cancer specific survival of 16 mo.
Pure primary pancreatic squamous cell carcinoma is extremely rare and accounts for 0.5% to 5% of all exocrine PC. It is characterized by a worse prognosis than PDAC, with reported overall mOS of about 4-7 mo. Squamous cell carcinoma is more frequently located in the head of the pancreas, commonly presenting with pain, weight loss and jaundice. More than half of patients are diagnosed in stage IV, with liver being the most frequent site of metastasis, and the median age at diagnosis is 69 years[10,110,111].
Squamous differentiation in PC usually occurs in association with conventional PDAC, in which a squamous component of at least 30% of the neoplasm should be detected in order to classify it as an adenosquamous carcinoma[4].
On macroscopy, ASPC made of is yellowish-white to gray, firm masses. Common findings are central necrosis and cystic degeneration.
Histologically, the adenocarcinoma component forms glandular structures, while squamous differentiation is detected by sheets of cells with distinct cellular borders, prominent intercellular junctions, dense eosinophilic cytoplasm and keratinization, expressing p63, p40 and low molecular weight cytokeratins[4].
The immunohistochemistry analysis demonstrate loss of p16 protein expression and strong reactivity for p53, with a profile similar to PDAC[4].
Almost all cases harbor KRAS mutation at codon 12 and TP53 mutations. There are reports showing that the adenous and the squamous part of the adenosquamous cancer had similar molecular alterations, suggesting that the two components may results from the same progenitor cancer cell origin[112].
The angiogenic pattern appears to be more active in ASCP than PDAC[113], while there are data showing high PD-L1 expression mostly in the squamous cell carcinoma component of ASPC[114].
Of note, in the integrated evaluation about histopathological pancreatic cancer and whole-genome and deep-exome sequencing of PC, ASCPs were mostly represented in the squamous subtype, that was also an independent poor prognostic factor[115]. Squamous tumors are characterized by alterations in four core gene programs, including gene networks involved in inflammation, hypoxia response, metabolic reprogramming, TGF-β signaling, MYC pathway activation, autophagy and upregulated expression of TP63∆N and its target genes.
ASPC is a hyper-vascular tumor. On CT scan with contrast enhancement, it appears as a large well defined predominantly solid and lobulated mass with ring enhancement in the peripheral area and central necrosis. Patients with vessel invasion have a poor prognosis[116].
Usually ASPC presents as a large mass in the pancreatic tail; small adenosquamous lesions appear to be more frequently in the head of pancreas. Venous tumor thrombus was seen only in large masses[117].
At CT scan hypervascularity can be observed. Fajardo et al[118] reported the use of dynamic CT scan with a bolus injection of intravenous contrast to examine a patient with pancreatic squamous cell carcinoma: the attenuation of this tumor increased from 35 HU to 61 HU[118].
As per the other types of PC, surgical resection is the only curative approach for ASPC and SCC , that can significantly improve survival[107,110,119,120]. Patients affected by ASPC or SCC undergoing surgery and post-operative treatment with chemotherapy, radiotherapy or both appear to have a benefit[108,121-123], even though there is no consensus regarding the best regimen to use, that are commonly fluoropyrimidine-based, gemcitabine based or platinum-based. Data from Johns Hopkins Hospital show, in particular, benefit for ASPC patients treated with platinum-based chemotherapy, with a mOS of 19.1 mo[121].
In the metastatic setting, several chemotherapy regimens are reported to be active, including fluoropyrimidine-, gemcitabine- or platinum-based therapy[124-127]. For instance, Brunetti et al[26] and De Souza et al[128] reported 1 PR each in patients affected by ASPC treated with gemcitabine and GEMOX, respectively, and SD maintained for 10 mo, 9 mo and 8 mo in patients treated with gemcitabine, FOLFOXIRI and cisplatin + gemcitabine, respectively.
Despite the increase of retrospective case series and data regarding rare histological subtypes of PC that can help in their diagnosis, there are still many unanswered questions about the management of these cancers, due to the absence of prospective trials or guidelines. For clinicians facing these patients in their real-life routine, the key question is whether they have to be approached and treated in the same way as the more common PDAC or if they need to have a specific strategy. As described above, if taken singularly, these histotypes may differ significantly from PDAC, especially in terms of prognosis. While ASPC has a similar prognosis to PDAC and SCC appears in some reports to have even worse outcomes, the natural history of a patient with PT or ACC can be radically different, considering the unequivocal survival advantage showed in these subtypes[129]. Based on the analysis of 57804 PC patients who underwent surgical resection, the mOS of PDAC and ACC is 20.2 mo (22% 5-year OS) and 67.5 mo (51% 5-year OS), respectively, while the mOS of resected PT is not even reached (97% 5-year OS)[10]. This consideration alone may justify for a more aggressive surgical approach for these tumors than what we are used to consider for PDAC, with special interest to the possible benefit reported for the surgical resection of metastatic disease. On the contrary, the poor prognosis and aggressive behavior of UC, ASPC and SCC must be taken in consideration for the treatment strategy of these tumors.
Finally, for the majority of these subtypes, there are no clear data regarding chemosensitivity and the role of specific chemotherapy regimens both for locoregional and advanced disease. Nevertheless, we tried to summarize the most relevant data that, to the best of our knowledge, can give some inputs for clinical decision-making.
Even if the very low incidence of these malignancies makes it almost impossible to design and run prospective clinical trials, we believe that multi center collaborations are essential, in order to gather as much homogeneous information as possible leading to a more histotype-guided therapeutic approach to these cancers.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gazouli M, Luo HS, Tomizawa M S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li X
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15276] [Article Influence: 3055.2] [Reference Citation Analysis (4)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55686] [Article Influence: 7955.1] [Reference Citation Analysis (132)] |
| 3. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2463] [Article Influence: 307.9] [Reference Citation Analysis (4)] |
| 4. | International Agency for Research on Cancer. WHO classification of tumours of the digestive system: International Agency for Research on Cancer, 2019. 5th ed. In: Tumours of the pancreas. World Health Organization, 2019; 295-370. |
| 5. | Holen KD, Klimstra DS, Hummer A, Gonen M, Conlon K, Brennan M, Saltz LB. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673-4678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007;35:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Wisnoski NC, Townsend CM, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Glazer ES, Neill KG, Frakes JM, Coppola D, Hodul PJ, Hoffe SE, Pimiento JM, Springett GM, Malafa MP. Systematic Review and Case Series Report of Acinar Cell Carcinoma of the Pancreas. Cancer Control. 2016;23:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Pokrzywa CJ, Abbott DE, Matkowskyj KA, Ronnekleiv-Kelly SM, Winslow ER, Weber SM, Fisher AV. Natural History and Treatment Trends in Pancreatic Cancer Subtypes. J Gastrointest Surg. 2019;23:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Armstrong MD, Von Hoff D, Barber B, Marlow LA, von Roemeling C, Cooper SJ, Travis P, Campbell E, Paz-Fumagalli R, Copland JA, Colon-Otero G. An effective personalized approach to a rare tumor: prolonged survival in metastatic pancreatic acinar cell carcinoma based on genetic analysis and cell line development. J Cancer. 2011;2:142-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Suzuki A, Sakaguchi T, Morita Y, Oishi K, Fukumoto K, Inaba K, Takehara Y, Baba S, Suzuki S, Konno H. Long-term survival after a repetitive surgical approach in a patient with acinar cell carcinoma of the pancreas and recurrent liver metastases: report of a case. Surg Today. 2010;40:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 346] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 14. | La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne). 2015;2:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Jiao Y, Yonescu R, Offerhaus GJ, Klimstra DS, Maitra A, Eshleman JR, Herman JG, Poh W, Pelosof L, Wolfgang CL, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N, Wood LD. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J Pathol. 2014;232:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Furukawa T, Sakamoto H, Takeuchi S, Ameri M, Kuboki Y, Yamamoto T, Hatori T, Yamamoto M, Sugiyama M, Ohike N, Yamaguchi H, Shimizu M, Shibata N, Shimizu K, Shiratori K. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep. 2015;5:8829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Jäkel C, Bergmann F, Toth R, Assenov Y, van der Duin D, Strobel O, Hank T, Klöppel G, Dorrell C, Grompe M, Moss J, Dor Y, Schirmacher P, Plass C, Popanda O, Schmezer P. Genome-wide genetic and epigenetic analyses of pancreatic acinar cell carcinomas reveal aberrations in genome stability. Nat Commun. 2017;8:1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, Elvin J, Ali SM, Ross JS, Basturk O, Balasubramanian S, Lipson D, Yelensky R, Pao W, Miller VA, Klimstra DS, Stephens PJ. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Wang L, Basturk O, Wang J, Benayed R, Middha S, Zehir A, Linkov I, Rao M, Aryeequaye R, Cao L, Chmielecki J, Ross J, Stephens PJ, Adsay V, Askan G, Balci S, Klimstra DS. A FISH assay efficiently screens for BRAF gene rearrangements in pancreatic acinar-type neoplasms. Mod Pathol. 2018;31:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Chou A, Brown IS, Kumarasinghe MP, Perren A, Riley D, Kim Y, Pajic M, Steinmann A, Rathi V, Jamieson NB, Verheij J, van Roessel S, Nahm CB, Mittal A, Samra J, Gill AJ. RET gene rearrangements occur in a subset of pancreatic acinar cell carcinomas. Mod Pathol. 2020;33:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Liu K, Peng W, Zhou Z. The CT findings of pancreatic acinar cell carcinoma in five cases. Clin Imaging. 2013;37:302-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Raman SP, Hruban RH, Cameron JL, Wolfgang CL, Kawamoto S, Fishman EK. Acinar cell carcinoma of the pancreas: computed tomography features--a study of 15 patients. Abdom Imaging. 2013;38:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Hsu MY, Pan KT, Chu SY, Hung CF, Wu RC, Tseng JH. CT and MRI features of acinar cell carcinoma of the pancreas with pathological correlations. Clin Radiol. 2010;65:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, Silverman SG. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol. 2005;184:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | Matos JM, Schmidt CM, Turrini O, Agaram NP, Niedergethmann M, Saeger HD, Merchant N, Johnson CS, Lillemoe KD, Grützmann R. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg. 2009;13:1495-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Brunetti O, Aprile G, Marchetti P, Vasile E, Casadei Gardini A, Scartozzi M, Barni S, Delfanti S, De Vita F, Di Costanzo F, Milella M, Cella CA, Berardi R, Cataldo I, Scarpa A, Basile D, Mazzuca F, Graziano G, Argentiero A, Santini D, Reni M, Cascinu S, Silvestris N. Systemic Chemotherapy for Advanced Rare Pancreatic Histotype Tumors: A Retrospective Multicenter Analysis. Pancreas. 2018;47:759-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 27. | Seth AK, Argani P, Campbell KA, Cameron JL, Pawlik TM, Schulick RD, Choti MA, Wolfgang CL. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg. 2008;12:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Yoo C, Kim BJ, Kim KP, Lee JL, Kim TW, Ryoo BY, Chang HM. Efficacy of Chemotherapy in Patients with Unresectable or Metastatic Pancreatic Acinar Cell Carcinoma: Potentially Improved Efficacy with Oxaliplatin-Containing Regimen. Cancer Res Treat. 2017;49:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Lowery MA, Klimstra DS, Shia J, Yu KH, Allen PJ, Brennan MF, O'Reilly EM. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16:1714-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Kruger S, Haas M, Burger PJ, Ormanns S, Modest DP, Westphalen CB, Kleespies A, Angele MK, Hartwig W, Bruns CJ, Kirchner T, Werner J, Heinemann V, Boeck S. Acinar cell carcinoma of the pancreas: a rare disease with different diagnostic and therapeutic implications than ductal adenocarcinoma. J Cancer Res Clin Oncol. 2016;142:2585-2591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Callata-Carhuapoma HR, Pato Cour E, Garcia-Paredes B, Fernandez RM, Mendoza Fernandez ML, Fernandez AM, De La Rosa CA, Sotelo Lezama MJ, Cabezas-Camarero S, Sastre Varela J. Pancreatic acinar cell carcinoma with bilateral ovarian metastases, panniculitis and polyarthritis treated with FOLFIRINOX chemotherapy regimen. A case report and review of the literature. Pancreatology. 2015;15:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 32. | Schempf U, Sipos B, König C, Malek NP, Bitzer M, Plentz RR. FOLFIRINOX as first-line treatment for unresectable acinar cell carcinoma of the pancreas: a case report. Z Gastroenterol. 2014;52:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Pfrommer S, Weber A, Dutkowski P, Schäfer NG, Müllhaupt B, Bourquin JP, Breitenstein S, Pestalozzi BC, Stenner F, Renner C, D'Addario G, Graf HJ, Knuth A, Clavien PA, Samaras P. Successful Salvage Chemotherapy with FOLFIRINOX for Recurrent Mixed Acinar Cell Carcinoma and Ductal Adenocarcinoma of the Pancreas in an Adolescent Patient. Case Rep Oncol. 2013;6:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Seki Y, Okusaka T, Ikeda M, Morizane C, Ueno H. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or S-1 as a single agent. Jpn J Clin Oncol. 2009;39:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Yamamoto T, Ohzato H, Fukunaga M, Imamura H, Furukawa H. Acinar cell carcinoma of the pancreas: a possible role of S-1 as chemotherapy for acinar cell carcinoma. A case report. JOP. 2012;13:87-90. [PubMed] |
| 36. | Sumiyoshi T, Shima Y, Okabayashi T, Kozuki A, Iwata J, Saisaka Y, Tokumaru T, Nakamura T, Morita S. Long-term survival following pancreatectomy and s-1 chemotherapy for pancreatic acinar cell carcinoma with peritoneal dissemination: a case report and literature review. Medicine (Baltimore). 2015;94:e378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Ruo L, Coit DG, Brennan MF, Guillem JG. Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg. 2002;6:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Lanke G, Ali FS, Lee JH. Clinical update on the management of pseudopapillary tumor of pancreas. World J Gastrointest Endosc. 2018;10:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 39. | Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol. 2005;29:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 40. | Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131-136. [PubMed] |
| 41. | Cai H, Zhou M, Hu Y, He H, Chen J, Tian W, Deng Y. Solid-pseudopapillary neoplasms of the pancreas: clinical and pathological features of 33 cases. Surg Today. 2013;43:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Katz MH, Mortenson MM, Wang H, Hwang R, Tamm EP, Staerkel G, Lee JH, Evans DB, Fleming JB. Diagnosis and management of cystic neoplasms of the pancreas: an evidence-based approach. J Am Coll Surg. 2008;207:106-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Serra S, Chetty R. Revision 2: an immunohistochemical approach and evaluation of solid pseudopapillary tumour of the pancreas. J Clin Pathol. 2008;61:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 44. | Ohara Y, Oda T, Hashimoto S, Akashi Y, Miyamoto R, Enomoto T, Satomi K, Morishita Y, Ohkohchi N. Pancreatic neuroendocrine tumor and solid-pseudopapillary neoplasm: Key immunohistochemical profiles for differential diagnosis. World J Gastroenterol. 2016;22:8596-8604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 45. | Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008;39:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y, Kobayashi Y. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401-8404. [PubMed] |
| 47. | Müller-Höcker J, Zietz CH, Sendelhofert A. Deregulated expression of cell cycle-associated proteins in solid pseudopapillary tumor of the pancreas. Mod Pathol. 2001;14:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Tiemann K, Heitling U, Kosmahl M, Klöppel G. Solid pseudopapillary neoplasms of the pancreas show an interruption of the Wnt-signaling pathway and express gene products of 11q. Mod Pathol. 2007;20:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Choi JY, Kim MJ, Kim JH, Kim SH, Lim JS, Oh YT, Chung JJ, Yoo HS, Lee JT, Kim KW. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187:W178-W186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 193] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 51. | Baek JH, Lee JM, Kim SH, Kim SJ, Kim SH, Lee JY, Han JK, Choi BI. Small (< or =3 cm) solid pseudopapillary tumors of the pancreas at multiphasic multidetector CT. Radiology. 2010;257:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Yu CC, Tseng JH, Yeh CN, Hwang TL, Jan YY. Clinicopathological study of solid and pseudopapillary tumor of pancreas: emphasis on magnetic resonance imaging findings. World J Gastroenterol. 2007;13:1811-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (11)] |
| 53. | Romics L, Oláh A, Belágyi T, Hajdú N, Gyurus P, Ruszinkó V. Solid pseudopapillary neoplasm of the pancreas--proposed algorithms for diagnosis and surgical treatment. Langenbecks Arch Surg. 2010;395:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006;93:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 55. | de Castro SM, Singhal D, Aronson DC, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Management of solid-pseudopapillary neoplasms of the pancreas: a comparison with standard pancreatic neoplasms. World J Surg. 2007;31:1130-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Prasad TV, Madhusudhan KS, Srivastava DN, Dash NR, Gupta AK. Transarterial chemoembolization for liver metastases from solid pseudopapillary epithelial neoplasm of pancreas: A case report. World J Radiol. 2015;7:61-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000;17:66-80. [PubMed] |
| 58. | Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 283] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 59. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 533] [Article Influence: 26.7] [Reference Citation Analysis (1)] |
| 60. | Panieri E, Krige JE, Bornman PC, Graham SM, Terblanche J, Cruse JP. Operative management of papillary cystic neoplasms of the pancreas. J Am Coll Surg. 1998;186:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Sumida W, Kaneko K, Tainaka T, Ono Y, Kiuchi T, Ando H. Liver transplantation for multiple liver metastases from solid pseudopapillary tumor of the pancreas. J Pediatr Surg. 2007;42:e27-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Kocman B, Jadrijević S, Skopljanac A, Mikulić D, Gustin D, Buhin M, Matasić H, Gasparov S, Suknaić S, Ivanović D. Living donor liver transplantation for unresectable liver metastases from solid pseudo-papillary tumor of the pancreas: a case report. Transplant Proc. 2008;40:3787-3790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Łągiewska B, Pacholczyk M, Lisik W, Cichocki A, Nawrocki G, Trzebicki J, Chmura A. Liver transplantation for nonresectable metastatic solid pseudopapillary pancreatic cancer. Ann Transplant. 2013;18:651-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Dovigo AG, Díaz MB, Gütierrez MG, Selles CF, Grobas JP, Valladares M, Suárez F, Marini M. Liver transplantation as treatment in a massive metastasis from Gruber-Frantz pancreatic tumor: a case report. Transplant Proc. 2011;43:2272-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Wójciak M, Gozdowska J, Pacholczyk M, Lisik W, Kosieradzki M, Cichocki A, Tronina O, Durlik M. Liver Transplantation for a Metastatic Pancreatic Solid-Pseudopapillary Tumor (Frantz Tumor): A Case Report. Ann Transplant. 2018;23:520-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Strauss JF, Hirsch VJ, Rubey CN, Pollock M. Resection of a solid and papillary epithelial neoplasm of the pancreas following treatment with cis-platinum and 5-fluorouracil: a case report. Med Pediatr Oncol. 1993;21:365-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Maffuz A, Bustamante Fde T, Silva JA, Torres-Vargas S. Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas. Lancet Oncol. 2005;6:185-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 68. | Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 2008;143:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Hah JO, Park WK, Lee NH, Choi JH. Preoperative chemotherapy and intraoperative radiofrequency ablation for unresectable solid pseudopapillary tumor of the pancreas. J Pediatr Hematol Oncol. 2007;29:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Fried P, Cooper J, Balthazar E, Fazzini E, Newall J. A role for radiotherapy in the treatment of solid and papillary neoplasms of the pancreas. Cancer. 1985;56:2783-2785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 71. | Morikawa T, Onogawa T, Maeda S, Takadate T, Shirasaki K, Yoshida H, Ishida K, Motoi F, Naitoh T, Rikiyama T, Katayose Y, Egawa S, Unno M. Solid pseudopapillary neoplasms of the pancreas: an 18-year experience at a single Japanese Institution. Surg Today. 2013;43:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Sperti C, Berselli M, Pasquali C, Pastorelli D, Pedrazzoli S. Aggressive behaviour of solid-pseudopapillary tumor of the pancreas in adults: a case report and review of the literature. World J Gastroenterol. 2008;14:960-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Paal E, Thompson LD, Frommelt RA, Przygodzki RM, Heffess CS. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 91] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Strobel O, Hartwig W, Bergmann F, Hinz U, Hackert T, Grenacher L, Schneider L, Fritz S, Gaida MM, Büchler MW, Werner J. Anaplastic pancreatic cancer: Presentation, surgical management, and outcome. Surgery. 2011;149:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Hoshimoto S, Matsui J, Miyata R, Takigawa Y, Miyauchi J. Anaplastic carcinoma of the pancreas: Case report and literature review of reported cases in Japan. World J Gastroenterol. 2016;22:8631-8637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Molberg KH, Heffess C, Delgado R, Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 77. | Kitade H, Yanagida H, Yamada M, Satoi S, Yoshioka K, Shikata N, Kon M. Granulocyte-colony stimulating factor producing anaplastic carcinoma of the pancreas treated by distal pancreatectomy and chemotherapy: report of a case. Surg Case Rep. 2015;1:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Murata T, Terasaki M, Sakaguchi K, Okubo M, Fukami Y, Nishimae K, Kitayama Y, Hoshi S. A case of anaplastic carcinoma of the pancreas producing granulocyte-colony stimulating factor. Clin J Gastroenterol. 2009;2:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Clark CJ, Graham RP, Arun JS, Harmsen WS, Reid-Lombardo KM. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J Am Coll Surg. 2012;215:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Hartwig W, Denneberg M, Bergmann F, Hackert T, Hinz U, Strobel O, Büchler MW, Werner J. Acinar cell carcinoma of the pancreas: is resection justified even in limited metastatic disease? Am J Surg. 2011;202:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | El Hussein S, Khader SN. Cytopathology of anaplastic carcinoma of the pancreas: Review of a rare entity and description of a variant with signet ring cell features. Diagn Cytopathol. 2019;47:956-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Hoorens A, Prenzel K, Lemoine NR, Klöppel G. Undifferentiated carcinoma of the pancreas: analysis of intermediate filament profile and Ki-ras mutations provides evidence of a ductal origin. J Pathol. 1998;185:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Newbould MJ, Benbow EW, Sene A, Young M, Taylor TV. Adenocarcinoma of the pancreas with osteoclast-like giant cells: a case report with immunocytochemistry. Pancreas. 1992;7:611-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Ichikawa T, Federle MP, Ohba S, Ohtomo K, Sugiyama A, Fujimoto H, Haradome H, Araki T. Atypical exocrine and endocrine pancreatic tumors (anaplastic, small cell, and giant cell types): CT and pathologic features in 14 patients. Abdom Imaging. 2000;25:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 85. | Ishigami K, Nishie A, Yamamoto T, Asayama Y, Ushijima Y, Kakihara D, Fujita N, Morita K, Ohtsuka T, Kawabe K, Mochidome N, Honda H. Imaging features of undifferentiated carcinoma of the pancreas. J Med Imaging Radiat Oncol. 2019;63:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Fukukura Y, Kumagae Y, Hirahara M, Hakamada H, Nagano H, Nakajo M, Kamimura K, Nakajo M, Higashi M, Yoshiura T. CT and MRI features of undifferentiated carcinomas with osteoclast-like giant cells of the pancreas: a case series. Abdom Radiol (NY). 2019;44:1246-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Khashab MA, Emerson RE, DeWitt JM. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of anaplastic pancreatic carcinoma: a single-center experience. Pancreas. 2010;39:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Wong M, See JY, Sufyan W, Diddapur RK. Splenic infarction. A rare presentation of anaplastic pancreatic carcinoma and a review of the literature. JOP. 2008;9:493-498. [PubMed] |
| 89. | Shinagare AB, Ramaiya NH, Bellizzi AM, Mayer RJ. Locally advanced anaplastic pancreatic adenocarcinoma with initial response to FOLFIRINOX and rapid progression after five months. Pancreatology. 2012;12:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 90. | Ungaro A, Orsi F, Casadio C, Galdy S, Spada F, Cella CA, Tonno CD, Bonomo G, Vigna PD, Murgioni S, Frezza AM, Fazio N. Successful palliative approach with high-intensity focused ultrasound in a patient with metastatic anaplastic pancreatic carcinoma: a case report. Ecancermedicalscience. 2016;10:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Wakatsuki T, Irisawa A, Imamura H, Terashima M, Shibukawa G, Takagi T, Takahashi Y, Sato A, Sato M, Ikeda T, Suzuki R, Hikichi T, Obara K, Ohira H. Complete response of anaplastic pancreatic carcinoma to paclitaxel treatment selected by chemosensitivity testing. Int J Clin Oncol. 2010;15:310-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Shorter NA, Glick RD, Klimstra DS, Brennan MF, Laquaglia MP. Malignant pancreatic tumors in childhood and adolescence: The Memorial Sloan-Kettering experience, 1967 to present. J Pediatr Surg. 2002;37:887-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 93. | Reid MD, Bhattarai S, Graham RP, Pehlivanoglu B, Sigel CS, Shi J, Saqi A, Shirazi M, Xue Y, Basturk O, Adsay V. Pancreatoblastoma: Cytologic and histologic analysis of 12 adult cases reveals helpful criteria in their diagnosis and distinction from common mimics. Cancer Cytopathol. 2019;127:708-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Omiyale AO. Clinicopathological review of pancreatoblastoma in adults. Gland Surg. 2015;4:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 95. | Chen M, Zhang H, Hu Y, Liu K, Deng Y, Yu Y, Wu Y, Qi A, Li Y, Wen G. Adult pancreatoblastoma: A case report and clinicopathological review of the literature. Clin Imaging. 2018;50:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 96. | Klimstra DS, Wenig BM, Adair CF, Heffess CS. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol. 1995;19:1371-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 133] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 97. | Balasundaram C, Luthra M, Chavalitdhamrong D, Chow J, Khan H, Endres PJ. Pancreatoblastoma: a rare tumor still evolving in clinical presentation and histology. JOP. 2012;13:301-303. [PubMed] |
| 98. | Abraham SC, Wu TT, Klimstra DS, Finn LS, Lee JH, Yeo CJ, Cameron JL, Hruban RH. Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am J Pathol. 2001;159:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 171] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 99. | Kohda E, Iseki M, Ikawa H, Endoh M, Yokoyama J, Mukai M, Hata J, Yamazaki H, Miyauchi J, Saeki M. Pancreatoblastoma. Three original cases and review of the literature. Acta Radiol. 2000;41:334-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 100. | Yamaguchi S, Fujii T, Izumi Y, Fukumura Y, Han M, Yamaguchi H, Akita T, Yamashita C, Kato S, Sekiya T. Identification and characterization of a novel adenomatous polyposis coli mutation in adult pancreatoblastoma. Oncotarget. 2018;9:10818-10827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Lee JY, Kim IO, Kim WS, Kim CW, Yeon KM. CT and US findings of pancreatoblastoma. J Comput Assist Tomogr. 1996;20:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 102. | Montemarano H, Lonergan GJ, Bulas DI, Selby DM. Pancreatoblastoma: imaging findings in 10 patients and review of the literature. Radiology. 2000;214:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Zhang D, Tang N, Liu Y, Wang EH. Pancreatoblastoma in an adult. Indian J Pathol Microbiol. 2015;58:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Salman B, Brat G, Yoon YS, Hruban RH, Singhi AD, Fishman EK, Herman JM, Wolfgang CL. The diagnosis and surgical treatment of pancreatoblastoma in adults: a case series and review of the literature. J Gastrointest Surg. 2013;17:2153-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Benoist S, Penna C, Julié C, Malafosse R, Rougier P, Nordlinger B. Prolonged survival after resection of pancreatoblastoma and synchronous liver metastases in an adult. Hepatogastroenterology. 2001;48:1340-1342. [PubMed] |
| 106. | Charlton-Ouw KM, Kaiser CL, Tong GX, Allendorf JD, Chabot JA. Revisiting metastatic adult pancreatoblastoma. A case and review of the literature. JOP. 2008;9:733-738. [PubMed] |
| 107. | Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res. 2012;174:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 108. | Fang Y, Pu N, Zhang L, Wu W, Lou W. Chemoradiotherapy is associated with improved survival for resected pancreatic adenosquamous carcinoma: a retrospective cohort study from the SEER database. Ann Transl Med. 2019;7:522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Hester CA, Augustine MM, Choti MA, Mansour JC, Minter RM, Polanco PM, Porembka MR, Wang SC, Yopp AC. Comparative outcomes of adenosquamous carcinoma of the pancreas: An analysis of the National Cancer Database. J Surg Oncol. 2018;118:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 110. | Ntanasis-Stathopoulos I, Tsilimigras DI, Georgiadou D, Kanavidis P, Riccioni O, Salla C, Psaltopoulou T, Sergentanis TN. Squamous cell carcinoma of the pancreas: A systematic review and pooled survival analysis. Eur J Cancer. 2017;79:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 111. | Tella SH, Kommalapati A, Yadav S, Bergquist JR, Truty MJ, Durgin L, Ma WW, Cleary SP, McWilliams RR, Mahipal A. Survival and prognostic factors in patients with pancreatic squamous cell carcinoma. Eur J Surg Oncol. 2019;45:1700-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 112. | Fang Y, Su Z, Xie J, Xue R, Ma Q, Li Y, Zhao Y, Song Z, Lu X, Li H, Peng C, Bai F, Shen B. Genomic signatures of pancreatic adenosquamous carcinoma (PASC). J Pathol. 2017;243:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 113. | Silvestris N, Danza K, Longo V, Brunetti O, Fucci L, Argentiero A, Calabrese A, Cataldo I, Tamma R, Ribatti D, Tommasi S. Angiogenesis in adenosquamous cancer of pancreas. Oncotarget. 2017;8:95773-95779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 114. | Tanigawa M, Naito Y, Akiba J, Kawahara A, Okabe Y, Ishida Y, Ishikawa H, Hisaka T, Fujita F, Yasunaga M, Shigaki T, Sudo T, Mihara Y, Nakayama M, Kondo R, Kusano H, Shimamatsu K, Okuda K, Akagi Y, Yano H. PD-L1 expression in pancreatic adenosquamous carcinoma: PD-L1 expression is limited to the squamous component. Pathol Res Pract. 2018;214:2069-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 115. | Bailey P, Chang DK. Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2534] [Article Influence: 281.6] [Reference Citation Analysis (0)] |
| 116. | Zhao R, Jia Z, Chen X, Ren S, Cui W, Zhao DL, Wang S, Wang J, Li T, Zhu Y, Tang X, Wang Z. CT and MR imaging features of pancreatic adenosquamous carcinoma and their correlation with prognosis. Abdom Radiol (NY). 2019;44:2822-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Feng YF, Chen JY, Chen HY, Wang TG, Shi D, Lu YF, Pan Y, Shao CW, Yu RS. 110 Patients with adenosquamous carcinomas of the pancreas (PASC): imaging differentiation of small (≤ 3 cm) versus large (> 3 cm) tumors. Abdom Radiol (NY). 2019;44:2466-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 118. | Fajardo LL, Yoshino MT, Chernin MM. Computed tomography findings in squamous cell carcinoma of the pancreas. J Comput Tomogr. 1988;12:138-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Nikfam S, Sotoudehmanesh R, Pourshams A, Sadeghipour A, Sotoudeh M, Mohamadnejad M. Squamous cell carcinoma of the pancreas. Arch Iran Med. 2013;16:369-370. [PubMed] |
| 120. | Taibi A, Jacques J, Durand Fontanier S, Charissoux A, Bardet SM, Christou N, Fredon F, Valleix D, Mathonnet M. Long-term survival after surgery of pancreatic primary squamous cell carcinoma: A case report and literature review. Clin Case Rep. 2019;7:2092-2101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 121. | Wild AT, Dholakia AS, Fan KY, Kumar R, Moningi S, Rosati LM, Laheru DA, Zheng L, De Jesus-Acosta A, Ellsworth SG, Hacker-Prietz A, Voong KR, Tran PT, Hruban RH, Pawlik TM, Wolfgang CL, Herman JM. Efficacy of platinum chemotherapy agents in the adjuvant setting for adenosquamous carcinoma of the pancreas. J Gastrointest Oncol. 2015;6:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (1)] |
| 122. | Voong KR, Davison J, Pawlik TM, Uy MO, Hsu CC, Winter J, Hruban RH, Laheru D, Rudra S, Swartz MJ, Nathan H, Edil BH, Schulick R, Cameron JL, Wolfgang CL, Herman JM. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol. 2010;41:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 123. | Gruhl JD, Garrido-Laguna I, Francis SR, Affolter K, Tao R, Lloyd S. The impact of squamous cell carcinoma histology on outcomes in nonmetastatic pancreatic cancer. Cancer Med. 2020;9:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 124. | Del Arco H, Chakiba-Brugère C, Salabert L, Béchade D. Adenosquamous Carcinoma of the Pancreas. Clin Med Insights Oncol. 2019;13:1179554919886587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 125. | De Souza AL, Saif MW. Squamous cell carcinoma of the pancreas. JOP. 2014;15:630-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 126. | Al-Shehri A, Silverman S, King KM. Squamous cell carcinoma of the pancreas. Curr Oncol. 2008;15:293-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 127. | Kodavatiganti R, Campbell F, Hashmi A, Gollins SW. Primary squamous cell carcinoma of the pancreas: a case report and review of the literature. J Med Case Rep. 2012;6:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | De Souza AL, Saif MW. Platinum-based therapy in adenosquamous pancreatic cancer: experience at two institutions. JOP. 2014;15:144-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 129. | Luo G, Fan Z, Gong Y, Jin K, Yang C, Cheng H, Huang D, Ni Q, Liu C, Yu X. Characteristics and Outcomes of Pancreatic Cancer by Histological Subtypes. Pancreas. 2019;48:817-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |