Published online Jun 15, 2020. doi: 10.4251/wjgo.v12.i6.699
Peer-review started: January 18, 2020
First decision: April 16, 2020
Revised: May 7, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 15, 2020
Processing time: 149 Days and 4.6 Hours
Colitis is one of the immune-related side effects of immunotherapy. Usually, such type of side effect was reported to develop within a few weeks of treatment initiation, our case started within a few days.
We present a case of a 37-year-old gentleman with bright red loose stools, abdominal pain, and tenesmus. A diagnosis of colitis was made based on endoscopic and histologic findings. Treatment was thereafter continued with oral steroids and discontinuation of the immunotherapy medications. Symptoms resolved after starting the treatment and the patient continued to be symptom-free on subsequent follow-up. The unique about this case report is that the patient developed bloody diarrhea within five days of the 1st immunotherapy cycle, and the patient was on combined ipilimumab and nivolumab.
Immunotherapy related complications might occur within days from being on immunotherapy; we need more research to open the way for future pathological and clinical research to further understand the pathophysiology behind it.
Core tip: Immunotherapy is one of the novel treatments of this century, though it can cause many predicted and unpredicted immune-related side effects. This is a rare case of immunotherapy induced colitis in an otherwise healthy male who was recently diagnosed with melanoma and was started on appropriate immunotherapy treatment. Clinicians should keep in mind the possible immune side effects of immune checkpoint inhibitors as they can develop at any stage, which will help physicians in stopping these medications earlier and avoiding further serious harm.
- Citation: Abu Khalaf S, Albarrak A, Yousef M, Tahan V. Immune checkpoint inhibitors induced colitis, stay vigilant: A case report. World J Gastrointest Oncol 2020; 12(6): 699-704
- URL: https://www.wjgnet.com/1948-5204/full/v12/i6/699.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i6.699
Immunotherapy are classes of medications that can affect the immune system at different levels to be able to fight cancer cells[1], one of the Immunotherapy types is the immune checkpoint inhibitors (ICIs), their administration could be associated with various immune-related side effects; such as colitis[2]. Our patient is a 37-year-old gentleman who complained of the passage of bright red loose stools, abdominal pain, and tenesmus. His symptoms started five days after the first immunotherapy cycle for melanoma treatment; he was on combined ipilimumab and nivolumab. A diagnosis of colitis was made based on endoscopic and histologic findings. The primary core stone for the treatment of high-grade immunotherapy induced-gastrointestinal (GI) adverse events is the cessation of the immunotherapy and/or the introduction of anti-inflammatory therapy[3]. Our patient was treated with oral prednisone and discontinuation of immunotherapy.
A 37-year-old presented in August 2018 with chief complaints of bloody diarrhea of one-day duration which was accompanied by generalized abdominal pain, feeling of tenesmus, anorexia, and weight loss of 10 kg which were all noted along the previous few days prior to presentation. The temperature was maintained at 99-100 °F/37.2-37.8 °C.
He describes his recent bowel movements as irregular, loose, and bloody bowel movements, with a frequency of 10-15 times per day for one day.
He denied having chills, night sweats, arthralgia, eye pain, rash, ingestion of undercooked meat, sick contact, or recent travel history. patient denies any previous history of abdominal pain, bloody diarrhea, arthralgia, visual problems, or skin rash. Medical history was remarkable for a recent diagnosis of metastatic melanoma in left axillary lymph nodes with a positive BRAF marker with the staging of (T0, N3c, M0). No personal or family history of colitis. The symptoms started five days after starting nivolumab and ipilimumab treatment.
On medical exam, his vital signs were unremarkable. On general inspection of the abdomen, there was no distension, superficial palpation revealed generalized abdominal tenderness without rigidity. Otherwise, the physical examination was unremarkable.
Laboratory tests were significant for leukocytosis, and isolated elevation in alanine transaminase, cholestasis labs with alkaline phosphatase, and bilirubin were within the normal range, liver synthetic function tests showed slightly reduced albumin, inflammatory markers including Erythrocyte sedimentation rate was within the normal range whereas C-reactive protein was mildly elevated. Initial significant laboratory results values included a hemoglobin level of 14.0 g/dL, leukocytes of 12.39 × 109/L, and platelet count 475 × 109/L; other test results included a creatinine 1.19 mg/dL, blood urea nitrogen 16 mg/dL, alanine transaminase 124 U/L, aspartate aminotransferase 21 U/L, albumin 3.7 g/dL, C-reactive protein 2.13 mg/dL. Fecal pathogen panel and Clostridioides difficile test didn’t show any detected positive results.
Abdominal computed tomography scan revealed inflammatory changes involving the mildly thickened walls of the transverse and descending colon.
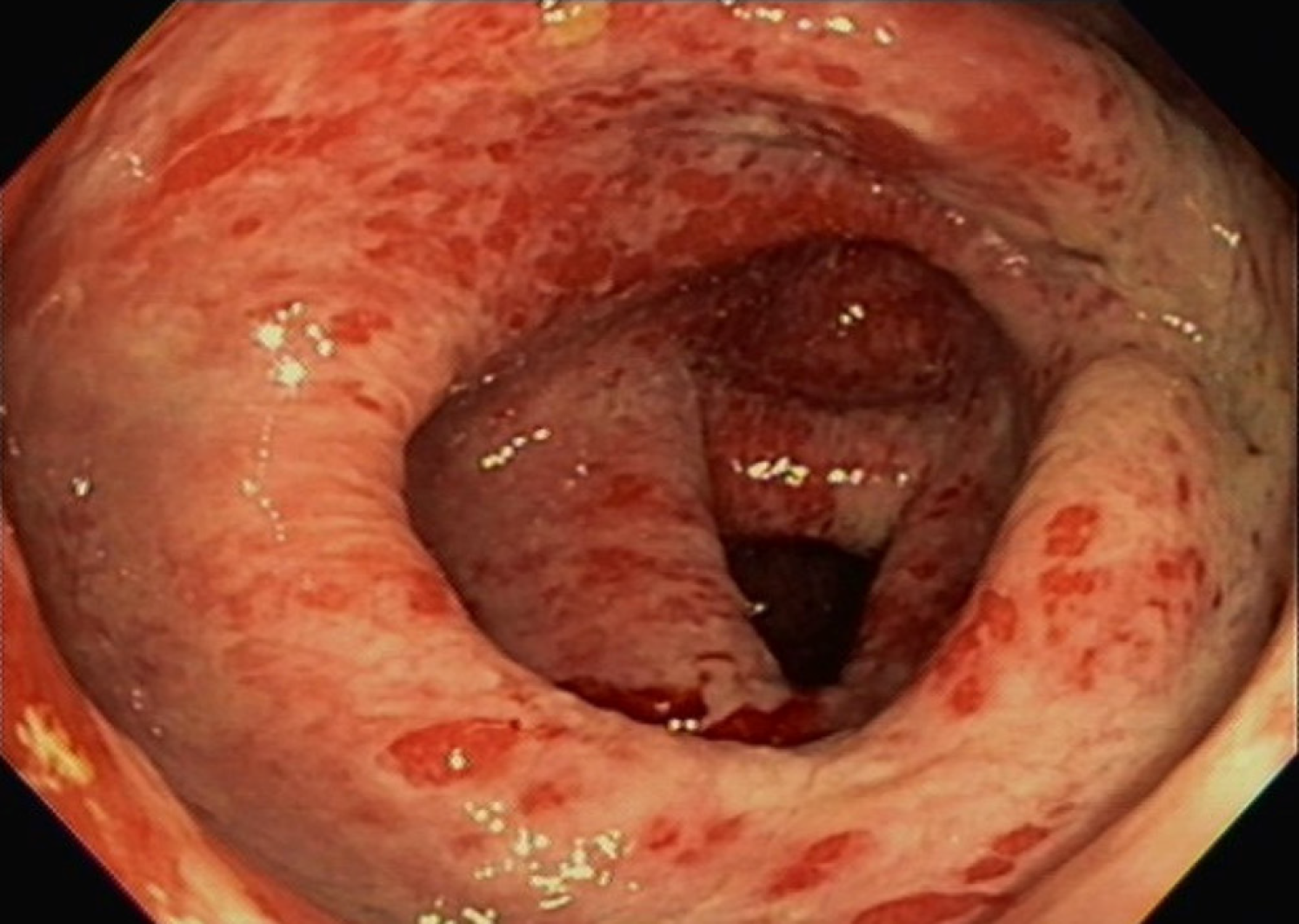
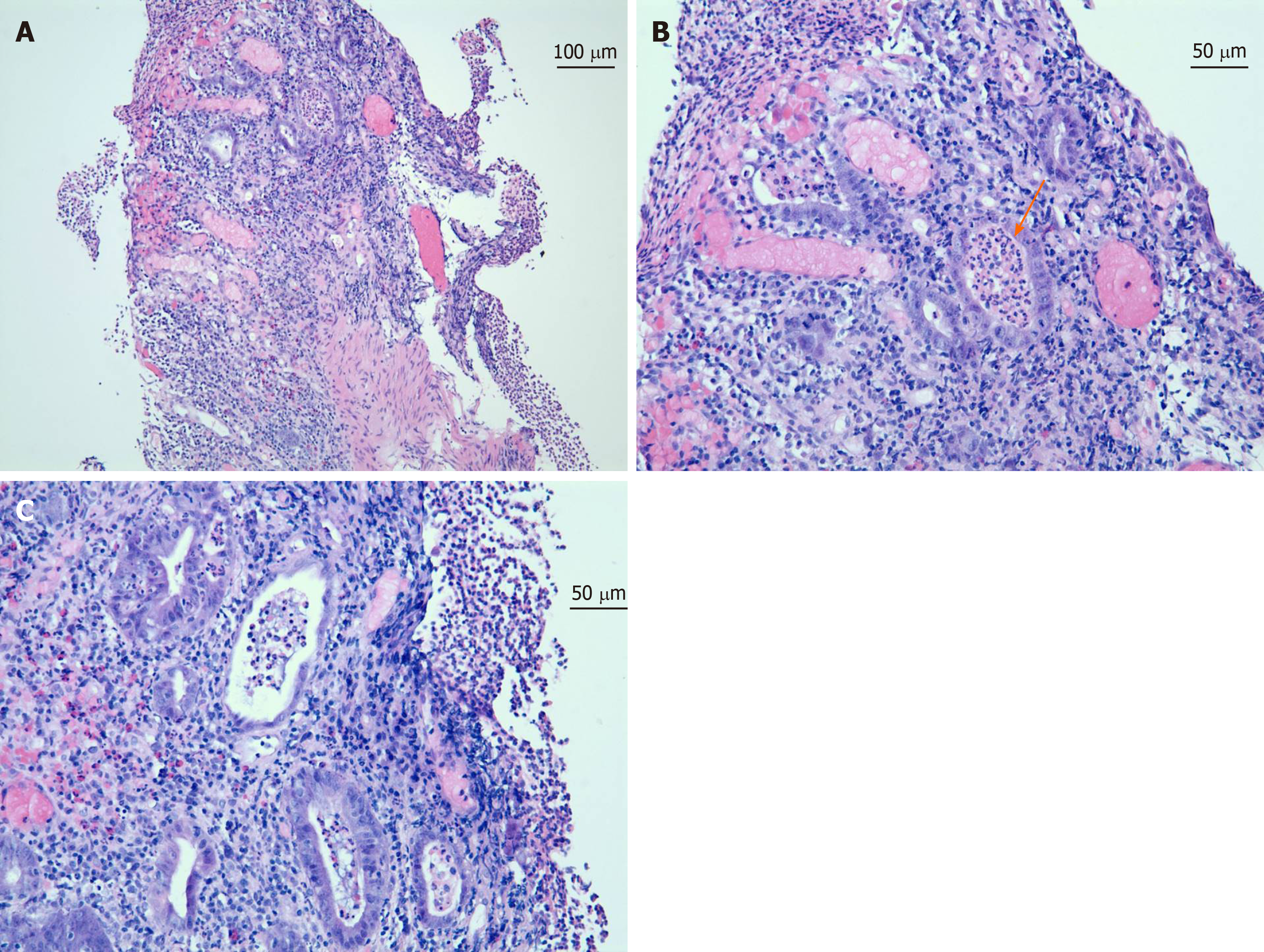
Gastroenterology, Infectious disease, and Hematology/Oncology were consulted. a colonoscopy was performed. Colonoscopy revealed widespread inflammatory findings with erythematous mucosa and diffuse ulcerative lesions involving the large intestine and rectum (Figure 1) but sparing the terminal ileum. Colon biopsies revealed acute ulcerative inflammation with formation of granulation tissue (Figure 2). Infectious workup was unremarkable for diarrhea so Infectious disease recommended discontinuation of antibiotics.
Based on the history, endoscopic and histologic exams, the patient was diagnosed with grade IV colitis induced by immunotherapy.
On admission, empiric antibiotic coverage with ciprofloxacin and metronidazole was started while waiting for cultures. In addition, a high dose prednisone of 80 mg daily was started empirically. Antibiotics were discontinued after one week. The dose of prednisone was increased to 120 mg daily after colonoscopy findings of grade IV colitis in the setting of persistent mildly improved diarrhea, continued on this dose for two weeks. He was transitioned to 80 mg daily with the tapering schedule for over one and a half months.
The patient was able to wean off the prednisone totally after two months, his symptoms have improved and currently denied any bloody diarrhea. The immunotherapy cycles were discontinued due to the colitis event. The patient received two months of prednisone treatment. His immunotherapy regimen was changed to dabrafenib and trametinib (BRAF/MEK inhibitors) for melanoma. The patient continued to report no complaints. He refused to undergo follow up colonoscopy.
Immunotherapies are very broad-spectrum medications that can be used in the treatment of different types of diseases including malignancies; one of the immunotherapy classes is the ICIs. To differentiate between normal and foreign cells, the immune system uses checkpoints, which function as regulators of the immune system; when stimulated, it slows down the immune response to the insult, and when inhibited/blocked the immune system is going to keep working, so the idea behind using the immunotherapy checkpoint inhibitors is to keep the cycle on without brakes. The drawback of this mechanism is the unspecific and unchecked activation of the immune system cells, which has resulted in overshooting immune response and autoimmune diseases. Scientists found that the cancer cells can protect themselves from immune attack by stimulating the checkpoints; Among these checkpoints are the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 which are receptors located on the T cells, their role is to inhibit T cells by various mechanisms[1].
Checkpoints inhibitors have achieved remarkable outcomes with different types of cancers. Since the time of approval of the ICIs, Advanced metastatic melanoma’s survival rate has improved significantly, which resulted in expanding their use in other malignancies at the expense of rising autoimmune-related side effects. Case reports have drawn attention towards various GI tract related autoimmune adverse effects, including but not limited to diarrhea, colitis, and hepatitis[2-4].
Diarrhea, colitis, and hepatitis are clinically graded using the Common Terminology Criteria for Adverse Events V.4 which was developed by the National Cancer Institute of the National Institutes of Health[5].
Parakh et al[6] found in a study of 45 patients who received a combination of nivolumab (anti- programmed cell death-1), and ipilimumab (anti-CTLA-4) that adverse events due to the immunotherapy were reported in the majority of the patients, and in more than 50% of the patients, they were high-grade adverse events. Forty-four percent of the patients had the treatment discontinued[6].
Of patients who have been treated with ipilimumab, high-grade adverse events related to the treatment have been reported in 27.3%, diarrhea was reported in 35%[2,6], only 5% of those taking ipilimumab experienced grade 3 or 4 colitis[2], it was reported that the average duration to develop diarrhea is 5-8 wk after starting ipilimumab[3]. Of patients who have been treated with nivolumab, high-grade adverse events were reported in 16.3%, diarrhea was reported in 17%-20%[6], notably nivolumab will take a longer time to cause diarrhea; in average 3-6 mo[3].
In patients who have been treated with nivolumab and ipilimumab combination, development of high-grade adverse events connected to the treatment were reported in 55%, and diarrhea was reported in 44%[6]. It is reported that a combination of therapy involving ipilimumab might cause diarrhea with a median onset of 5-8 wk after starting the treatment[3]. The patient was receiving nivolumab and ipilimumab therapy, and his symptoms developed acutely in five days following the first therapy period.
The combination therapy has a higher incidence of adverse effects in comparison with the individualized treatment, immune checkpoints inhibitors work through different pathways, to boost the effector T cell response which will lead to a robust immune-inflammatory response required for tumor-fighting[3,7].
No accurate statistics about the colitis as a side effect of immunotherapy, part of the reason is that the colonoscopy is not indicated for every case of colitis. Persistent diarrhea of grades two or more should be evaluated with colonoscopy. Macroscopic and microscopic findings of immunotherapy related-GI effects were reported, including exudates, granularity, erythema, loss of vascularity, and ulcerations. The main finding is pancolitis. However, normal findings were reported too. It is recommended to take biopsies even with normal-appearing mucosa, to rule out colitis[3,8].
The primary cornerstone for treatment of high-grade immunotherapy induced-GI adverse events is the cessation of the immunotherapy and/or the introduction of anti-inflammatory therapy, route, and duration of anti-inflammatory therapy are based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events grades of diarrhea and colitis[8,9].
In refractory cases, a single dose of infliximab might be beneficial[10]. Symptomatic improvement with mesalazine treatment was reported[8]. A combination of mycophenolate mofetil and high-dose corticosteroids may speed up the recovery[10]. Therapy with a vedolizumab was reported to be effective in a patient who was steroid-dependent[11].
It is becoming more apparent that immunotherapy induced colitis has a lot in common with inflammatory bowel disease (IBD), including the clinical presentation, macroscopic, microscopic, and serological findings. However, what can be a difference is the acuity of the symptoms, patients with immunotherapy induced colitis tend to have an accelerated clinical course, unlike IBD, which has a chronic relapsing course usually. Moreover, immunotherapy induced colitis is reversible with the cessation of the immunotherapy and the introduction of the steroid or other anti-inflammatory medications. Another aspect of the relation between immunotherapy-induced colitis and IBD is anti-CTLA-4 colitis which is associated with the production of serologies specific to Ulcerative colitis and Crohn’s diseases[2,12].
We are reporting this adverse event to raise clinicians’ awareness of this possible complication which might occur within days from being on immunotherapy and to open the way for future pathological and clinical research to further understand the pathophysiology behind it, and its possible relationship to IBD which might help in developing methods to avoid such severe complication and aid in shedding the light on some poorly understood concepts of IBD.
Thank you to Nitya Prabhakaran, the pathology chief resident for helping out in getting the pathology slides.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aykan NF, Cao ZF, Ebrahimifar M, Rassy EE S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Herzberg B, Fisher DE. Metastatic melanoma and immunotherapy. Clin Immunol. 2016;172:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Bertha M, Bellaguara E, Kuzel T, Hanauer S. Checkpoint Inhibitor-Induced Colitis: A New Type of Inflammatory Bowel Disease? ACG Case Rep J. 2017;4:e112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists' perspective. J Clin Pathol. 2018;71:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Matthew C, Abraham S, Cotton I, Zuazua R. A Unique Case of Immunotherapy-Induced Colitis in a 50-year-old Female With Bladder Cancer. Am J Gastroenterol. 2017;112:S798-S800. |
| 5. | US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. [published 28 May 2009]. v4.03: June 14, 2010. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. |
| 6. | Parakh S, Randhawa M, Nguyen B, Warburton L, Hussain MA, Cebon J, Millward M, Yip D, Ali S. Real-world efficacy and toxicity of combined nivolumab and ipilimumab in patients with metastatic melanoma. Asia Pac J Clin Oncol. 2019;15:26-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Wei W, Luo Z. Risk of gastrointestinal toxicities with PD-1 inhibitors in cancer patients: A meta-analysis of randomized clinical trials. Medicine (Baltimore). 2017;96:e8931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Yamauchi R, Araki T, Mitsuyama K, Tokito T, Ishii H, Yoshioka S, Kuwaki K, Mori A, Yoshimura T, Tsuruta O, Torimura T. The characteristics of nivolumab-induced colitis: an evaluation of three cases and a literature review. BMC Gastroenterol. 2018;18:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Gupta A, Khanna S. Ipilimumab-associated colitis or refractory Clostridium difficile infection? BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Mir R, Shaw HM, Nathan PD. Mycophenolate mofetil alongside high-dose corticosteroids: optimizing the management of combination immune checkpoint inhibitor-induced colitis. Melanoma Res. 2019;29:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Hsieh AH, Ferman M, Brown MP, Andrews JM. Vedolizumab: a novel treatment for ipilimumab-induced colitis. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Kohoutova D, Drahosova M, Moravkova P, Rejchrt S, Bures J. Anti-Outer membrane protein C and anti-glycoprotein 2 antibodies in inflammatory bowel disease and their association with complicated forms of Crohn's disease. BMC Gastroenterol. 2014;14:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |