Published online Apr 15, 2020. doi: 10.4251/wjgo.v12.i4.447
Peer-review started: December 27, 2019
First decision: January 19, 2020
Revised: March 13, 2020
Accepted: March 25, 2020
Article in press: March 25, 2020
Published online: April 15, 2020
Processing time: 110 Days and 5.9 Hours
Lymph node (LN) metastasis is an important prognostic factor in patients with gastric cancer (GC). However, the evaluation of LN metastasis status in the preoperative setting is not accurate. Therefore, precise preoperative prediction of LN metastasis status is crucial for optimal treatment in patients with GC.
To develop a preoperative nomogram for LN metastasis using F-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography/computed tomography (PET/CT) and preoperative laboratory test findings in GC.
In this study, the data of 566 GC patients who underwent preoperative F-18 FDG PET/CT and subsequent surgical resection were analyzed. The LN metastasis prediction model was developed in the training cohort and validated in the internal validation cohort. Routine preoperative laboratory tests, including albumin and carbohydrate antigen (CA) 19-9 were performed in all patients. Univariate and multivariable logistic regression was performed to validate the preoperative predictive indicators for LN metastasis.
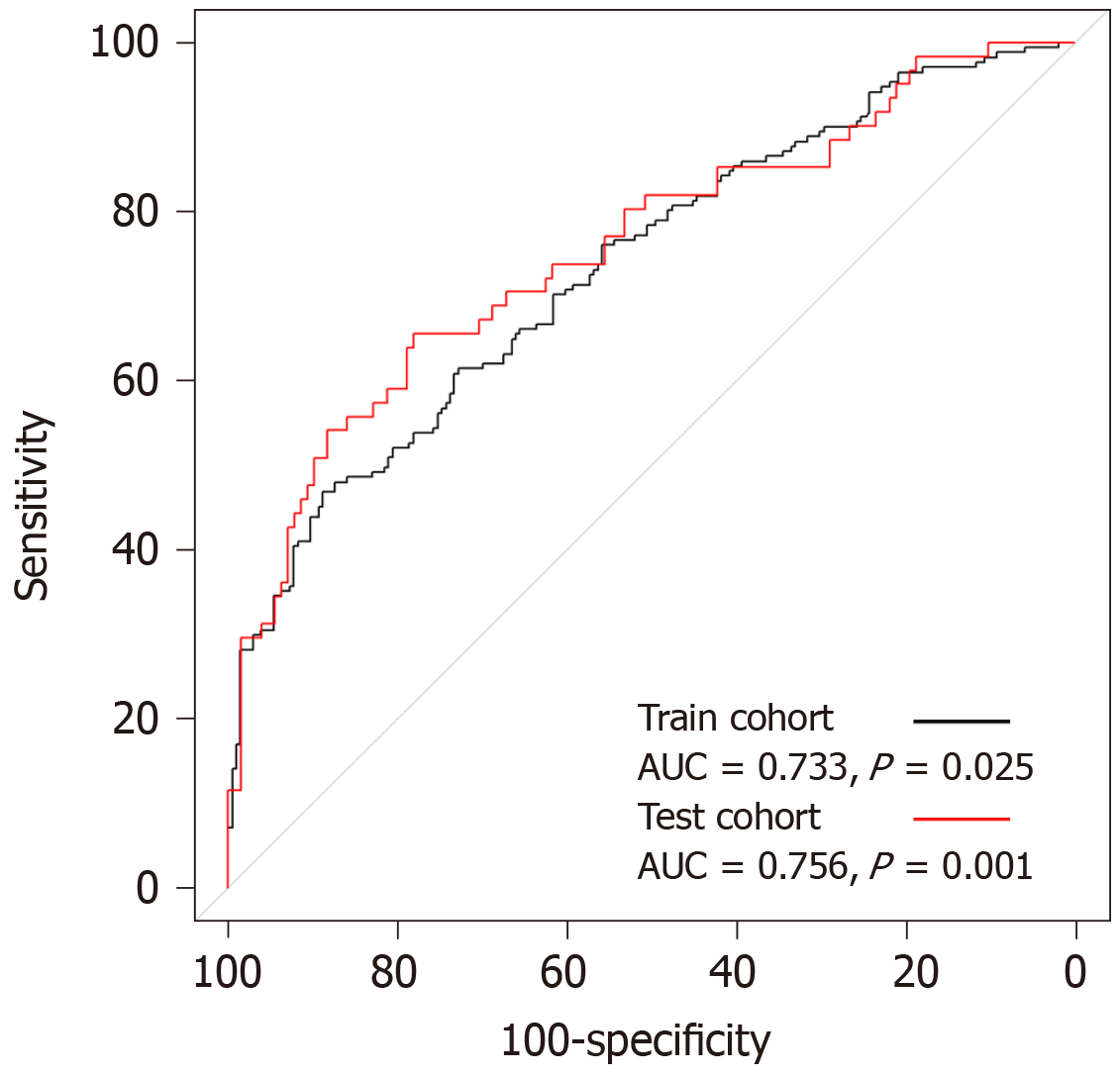
Of the 566 patients, 232 (41%) had confirmed histopathologic LN metastasis. Univariate logistic regression revealed that the tumor location, blood hemoglobin, serum albumin levels, neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, CA 19-9, maximum standardized uptake value (SUVmax) of the primary tumor (T_SUVmax), and SUVmax of LN (N_SUVmax) were significantly associated with LN metastasis. In multivariate analysis, T_SUVmax (OR = 1.08; 95%CI: 1.02–1.15; P = 0.011) and N_SUVmax (OR = 1.49; 95%CI: 1.19–1.97; P = 0.002) were found to be significant predictive factors for LN metastasis. The LN metastasis prediction model using T_SUVmax, N_SUVmax, serum albumin, and CA 19-9 yielded an area under the curve (AUC) of 0.733 (95%CI: 0.683–0.784, P = 0.025) in the training cohort and AUC of 0.756 (95%CI: 0.678–0.833, P < 0.001) in the test cohort.
T_SUVmax and N_SUVmax measured by preoperative F-18 FDG PET/CT are independent predictive factors for LN metastasis in GC.
Core tip: The maximum standardized uptake values of the primary tumor and lymph node (LN), measured by preoperative F-18 fluorodeoxyglucose positron emission tomography/computed tomography, are independent predictive factors for LN metastasis in patients with gastric cancer. Moreover, a nomogram using a combination of these metabolic information and laboratory parameters, such as serum albumin and carbohydrate antigen 19-9, for risk estimation of LN metastasis in gastric cancer was successfully developed in the training cohort and validated in the internal validation cohort.
- Citation: Song BI. Nomogram using F-18 fluorodeoxyglucose positron emission tomography/computed tomography for preoperative prediction of lymph node metastasis in gastric cancer. World J Gastrointest Oncol 2020; 12(4): 447-456
- URL: https://www.wjgnet.com/1948-5204/full/v12/i4/447.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i4.447
Gastric cancer (GC) is one of the most commonly diagnosed malignancies and the second leading cause of cancer-related deaths worldwide[1]. The status of lymph node (LN) metastasis is an important prognostic factor in GC, and complete dissection of the metastatic LNs is the only curative treatment for GC[2]. Although contrast-enhanced computed tomography (CT) and endoscopic ultrasonography (EUS) are used for the diagnosis of LN metastasis in GC, the accuracy of diagnostic performance for LN metastasis is imperfect[3,4].
Positron emission tomography/computed tomography (PET/CT) with 18F-fluorodeoxyglucose (F-18 FDG) has become a useful diagnostic modality for staging, treatment response evaluation, and detection of recurrence in GC[5,6]. However, F-18 FDG PET/CT has shown relatively low sensitivity in the detection of LN metastasis in GC[7,8]. Recently, metabolic information of the primary tumor obtained using F-18 FDG PET/CT has been suggested as a promising predictive marker for LN metastasis[9-11]. Glucose metabolism in the primary tumor reflects not only the total tumor burden, but also the aggressiveness of cancer associated with LN metastasis. Therefore, a combination of the metabolic information of the primary tumor and metastatic LN could be useful in predicting LN metastasis in GC.
Recently, a few studies have been undertaken to develop a nomogram for the prediction of LN metastasis in GC[12,13]. However, these LN metastasis prediction models are based on postoperative parameters. Nevertheless, a preoperative LN metastasis prediction model, based on the tumor metabolic information as measured by F-18 FDG PET/CT, does not exist for GC. This model would be crucial for clinicians to determine the most effective treatment strategy.
The aim of this retrospective study was to determine whether the metabolic information of LN, as well as the primary tumor, could be prognostic factors for the prediction of LN metastasis in GC and to develop a preoperative nomogram for the prediction of LN metastasis in GC.
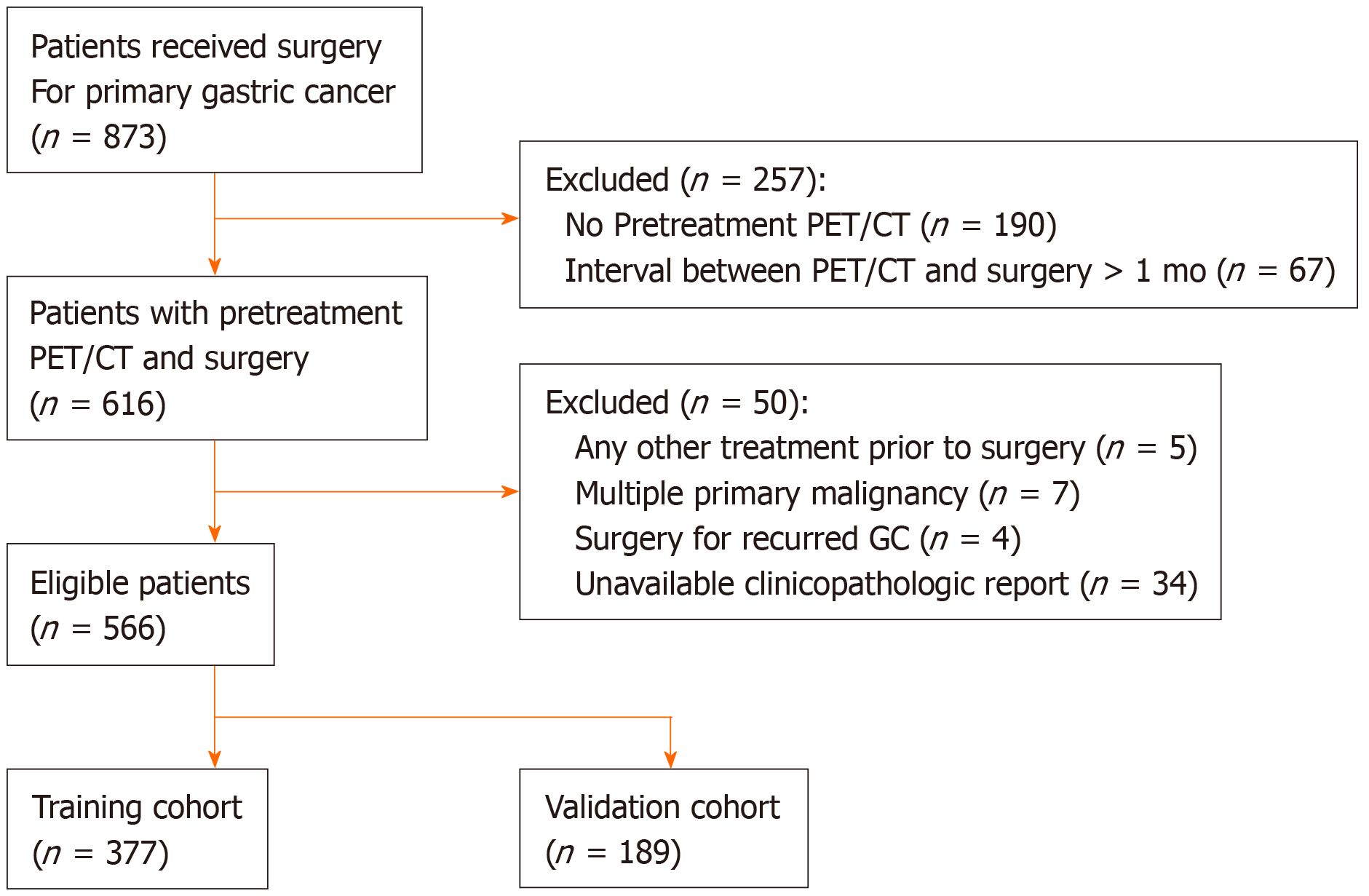
Between January 2008 and December 2010, the medical records of 873 consecutive patients who underwent surgery for primary GC at Keimyung University Dongsan Medical Center (Daegu, South Korea) were retrospectively reviewed. Of these, 566 patients who underwent preoperative F-18 FDG PET/CT and subsequent surgical treatment without any preoperative intervention were enrolled in this study. The exclusion criteria were as follows: any other treatment prior to surgery such as gastric endoscopic submucosal dissection or chemotherapy, multiple primary malignancies, surgery for recurred GC, unavailable clinicopathological report, or an interval over 1 month between F-18 FDG PET/CT and surgery. A total of 566 GC patients were randomly divided into 377 of the training cohort and 189 of the internal validation cohort (2:1) (Figure 1). This retrospective study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center. The need for informed consent was waived, and all data were anonymized prior to the analysis.
All patients underwent subtotal or total gastrectomy along with D1+β or D2 lymphadenectomy in early GC and D2 lymphadenectomy in advanced GC. Clinicopathological data, including age at surgery, sex, location of the tumor, pathologic T (pT) stage, serum white blood cell (WBC) count, blood hemoglobin and serum albumin levels, platelet count, neutrophil count, lymphocyte count, platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR), preoperative carcinoembryonic antigen (CEA), and carbohydrate antigen (CA) 19-9 were retrieved from the patients’ medical records. The pT stage was classified according to the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system.
All the patients fasted for at least 6 h before F-18 FDG injection, and the blood glucose level was managed to be lower than 150 mg/dL. The PET/CT scan was performed 60 min after F-18 FDG was administered. PET/CT scans were performed using a Discovery STE PET/CT scanner (GE Healthcare, Milwaukee, WI, United States). First, a low-dose CT scan (peak voltage of 120 kVp, automated tube current ranging from 60 to 150 mA, and slice thickness of 3.75 mm) for attenuation correction without contrast enhancement was acquired. After CT scan, PET scan was performed immediately with an acquisition time of 3 min per bed position in 3D mode. The PET images were reconstructed using an ordered-subset expectation maximum iterative reconstruction algorithm.
All the F-18 FDG PET/CT images were retrospectively interpreted on an Advantage Workstation 4.3 (GE Healthcare), blinded to the status of LN metastasis. First, all F-18 FDG PET/CT images were visually assessed and classified as positive or negative with respect to F-18 FDG uptake by the primary tumor or LN. A positive uptake was defined as abnormally increased F-18 FDG uptake that exceeded the physiologic uptake by the surrounding stomach wall and corresponding cancer lesions on esophagogastroduodenoscopy. Consequently, the maximum standardized uptake value (SUVmax) of the primary tumor (T_SUVmax) was obtained only in positive F-18 FDG uptake lesions. In case of LNs, SUVmax of LN (N_SUVmax) was acquired in the highest focal F-18 FDG avid LN on PET image regardless of size on CT. A spherical volume of interest was manually drawn over the maximum F-18 FDG uptake lesions on the attenuation-corrected transaxial F-18 FDG PET images for semi-quantitative analysis. The SUVmax was calculated using the following formula: SUVmax = maximum activity in the region of interest (MBq/g)/ [injected dose (MBq)/body weight (g)].
Numeric data were expressed as the mean ± SD. First, all the factors that were significantly associated (P < 0.05) with LN metastasis were identified in univariate analysis, and these significant factors were then evaluated to determine the variables independently associated with LN metastasis using multivariate logistic regression. Second, the LN metastasis prediction model was developed using the multivariate logistic analysis with a stepwise backward elimination method in the training cohort, and validated in the internal validation cohort. All variables with P < 0.05 in the univariate logistic analysis were selected for multivariate logistic analysis in the training cohort, and deleting the variable whose loss gives the most statistically insignificant deterioration of the prediction model fit. Lastly, we developed a nomogram as a graphical tool for calculating the risk of LN metastasis in individual patients. All statistical analyses were performed using R version 3.5.0 software (http://www.r-project.org, R Foundation for Statistical Computing, Vienna, Austria). A P < 0.05 was considered statistically significant.
The characteristics of the enrolled patients and the associations of these characteristics with LN metastasis in the training cohort (n = 377) and internal validation cohort (n = 189) are summarized in Table 1. Of the 566 patients enrolled in the present study, 232 (41.0%) had pathologically confirmed LN metastasis and 334 patients (59.0%) presented with no LN metastasis. The sensitivity, specificity, and accuracy of F-18 FDG PET/CT for the diagnosis of LN metastasis in GC patients were 28.9%, 97.3%, and 69.3%, respectively. Clinicopathological findings; tumor location, pT stage, blood hemoglobin levels, platelet count, lymphocyte count, PLR, NLR, CA 19-9, serum albumin, and metabolic parameters; T_SUVmax, and N_SUVmax were significantly different between the two groups (with or without LV metastasis); however, no significant differences were found with respect to age, sex, WBC count, neutrophil count, and serum CEA in the training cohort.
| Training cohort | Validation cohort | |||||
| Variables | LN metastasis (-) | LN metastasis (+) | P value | LN metastasis (-) | LN metastasis (+) | P value |
| (n = 206) | (n = 171) | (n = 128) | (n = 61) | |||
| Age | 59.2 ± 11.5 | 59.6 ± 12.1 | 0.701 | 59.5 ± 12.1 | 61.8 ± 12.8 | 0.218 |
| Sex | 0.577 | 0.456 | ||||
| Male | 122 (59.2%) | 107 (62.6%) | 73 (57.0%) | 39 (63.9%) | ||
| Female | 84 (40.8%) | 64 (37.4%) | 55 (43.0%) | 22 (36.1%) | ||
| Tumor Location | < 0.001 | 0.034 | ||||
| Upper | 46 (22.3%) | 27 (15.8%) | 25 (19.5%) | 15 (24.6%) | ||
| Middle | 32 (15.5%) | 35 (20.5%) | 24 (18.8%) | 11 (18.0%) | ||
| Low | 122 (59.2%) | 81 (47.4%) | 78 (60.9%) | 30 (49.2%) | ||
| Mixed | 6 (2.9%) | 28 (16.4%) | 1 ( 0.8%) | 5 (8.2%) | ||
| Pathologic T stage | < 0.001 | < 0.001 | ||||
| 1 | 158 (76.7%) | 28 (16.4%) | 100 (78.1%) | 10 (16.4%) | ||
| 2 | 20 (9.7%) | 27 (15.8%) | 11 (8.6%) | 12 (19.7%) | ||
| 3 | 18 (8.7%) | 27 (15.8%) | 8 (6.2%) | 18 (29.5%) | ||
| 4 | 10 (4.9%) | 89 (52.0%) | 9 (7.0%) | 21 (34.4%) | ||
| WBC counts (103 cells/μL) | 6.4 ± 1.8 | 6.2 ± 1.7 | 0.283 | 6.2 ± 1.6 | 6.5 ± 1.8 | 0.295 |
| Blood hemoglobin levels (g/dL) | 12.5 ± 1.6 | 12.0 ± 1.8 | 0.004 | 13.3 ± 9.7 | 12.2 ± 2.1 | 0.211 |
| Platelet counts (103 cells/μL) | 267.2 ± 72.6 | 287.9 ± 91.0 | 0.017 | 267.3 ± 74.4 | 282.0 ± 67.3 | 0.192 |
| Neutrophil counts (cells/μL) | 3777.4 ± 1458.7 | 3810.9 ± 1333.2 | 0.818 | 3686.8 ± 1367.7 | 3902.9 ± 1405.2 | 0.315 |
| Lymphocyte counts (cells/μL) | 1860.3 ± 609.4 | 1661.1 ± 589.8 | 0.001 | 1835.0 ± 521.7 | 1855.1 ± 764.4 | 0.853 |
| PLR | 159.3 ± 72.8 | 196.4 ± 98.8 | < 0.001 | 155.3 ± 60.3 | 184.8 ± 111.5 | 0.057 |
| NLR | 2.3 ± 1.4 | 2.5 ± 1.2 | 0.036 | 2.2 ± 1.2 | 2.6 ± 2.0 | 0.136 |
| CEA (ng/mL) | 4.8 ± 25.9 | 7.9 ± 27.9 | 0.268 | 2.6 ± 3.5 | 3.7 ± 6.1 | 0.195 |
| CA 19-9 (U/mL) | 18.1 ± 62.7 | 96.0 ± 364.9 | 0.006 | 10.0 ± 7.6 | 12.7 ± 8.6 | 0.029 |
| Albumin (g/dL) | 4.1 ± 0.3 | 3.9 ± 0.4 | < 0.001 | 4.0 ± 0.3 | 3.9 ± 0.4 | 0.032 |
| T_SUVmax | 2.9 ± 4.4 | 6.1 ± 5.5 | < 0.001 | 2.2 ± 3.7 | 6.9 ± 6.7 | < 0.001 |
| N_SUVmax | 0.1 ± 0.9 | 1.8 ± 3.7 | < 0.001 | 0.1 ± 0.5 | 1.7 ± 3.9 | 0.002 |
Univariate logistic regression analysis revealed that tumor location, blood hemoglobin levels, platelet count, lymphocyte count, PLR, NLR, CA 19-9, serum albumin, T_SUVmax, and N_SUVmax were significantly associated with LN metastasis in the training cohort. In multivariate analysis, T_SUVmax (OR = 1.08; 95%CI: 1.02–1.15; P = 0.011) and N_SUVmax (OR = 1.49; 95%CI: 1.19–1.97; P = 0.002) were found to be independent predictive factors for LN metastasis in the training set (Table 2). Also, T_SUVmax (OR = 1.17; 95%CI: 1.07–1.29; P < 0.001) and N_SUVmax (OR = 1.60; 95%CI: 1.09–2.69; P = 0.038) were independent predictive factors for LN metastasis in the test set (Table 3).
| Univariate logistic analysis | Multivariate logistic analysis | |||
| Variables | OR (95%CI) | P value | OR (95%CI) | P value |
| Age, yr | 1.00 (0.99–1.02) | 0.700 | ||
| Sex (male vs female) | 0.87 (0.57–1.32) | 0.507 | ||
| Tumor location | 1.31 (1.04–1.65) | 0.022 | 1.19 (0.92–1.55) | 0.178 |
| WBC counts | 0.94 (0.83–1.05) | 0.283 | ||
| Blood hemoglobin levels | 0.84 (0.74–0.94) | 0.005 | 1.07 (0.91–1.26) | 0.433 |
| Platelet counts | 1.00 (1.00–1.01) | 0.016 | 1.00 (1.00–1.01) | 0.625 |
| Neutrophil counts | 1.00 (1.00–1.00) | 0.817 | ||
| Lymphocyte counts | 1.00 (1.00–1.00) | 0.002 | 1.00 (1.00–1.00) | 0.342 |
| PLR | 1.01 (1.00–1.01) | < 0.001 | 1.00 (0.99–1.01) | 0.816 |
| NLR | 1.19 (1.01–1.41) | 0.041 | 0.85 (0.65–1.10) | 0.234 |
| CEA | 1.00 (1.00–1.01) | 0.290 | ||
| CA 19-9 | 1.00 (1.00–1.01) | 0.018 | 1.00 (1.00–1.01) | 0.133 |
| Albumin | 0.26 (0.13–0.49) | < 0.001 | 0.52 (0.23–1.15) | 0.110 |
| T_SUVmax | 1.17 (1.11–1.24) | < 0.001 | 1.08 (1.02–1.15) | 0.011 |
| N_SUVmax | 1.81 (1.45–2.40) | < 0.001 | 1.49 (1.19–1.97) | 0.002 |
| Univariate Logistic Analysis | Multivariate Logistic Analysis | |||
| Variables | OR (95%CI) | P value | OR (95%CI) | P value |
| Age, yr | 1.02 (0.99–1.04) | 0.218 | ||
| Sex (male vs female) | 0.75 (0.40–1.40) | 0.367 | ||
| Tumor location | 0.97 (0.68–1.40) | 0.881 | ||
| WBC counts | 1.10 (0.92–1.33) | 0.295 | ||
| Blood hemoglobin levels | 0.95 (0.82–1.02) | 0.451 | ||
| Platelet counts | 1.00 (1.00–1.01) | 0.194 | ||
| Neutrophil counts | 1.00 (1.00–1.00) | 0.315 | ||
| Lymphocyte counts | 1.00 (1.00–1.00) | 0.832 | ||
| PLR | 1.00 (1.00–1.01) | 0.027 | 1.00 (0.99–1.00) | 0.632 |
| NLR | 1.19 (0.98–1.46) | 0.087 | ||
| CEA | 1.05 (0.99–1.13) | 0.138 | ||
| CA 19-9 | 1.04 (1.00–1.08) | 0.032 | 1.03 (0.99–1.07) | 0.185 |
| Albumin | 0.31 (0.12–0.80) | 0.017 | 1.00 (0.30–3.32) | 0.994 |
| T_SUVmax | 1.23 (1.14–1.34) | < 0.001 | 1.17 (1.07–1.29) | < 0.001 |
| N_SUVmax | 2.02 (1.43–3.29) | < 0.001 | 1.60 (1.09–2.69) | 0.038 |
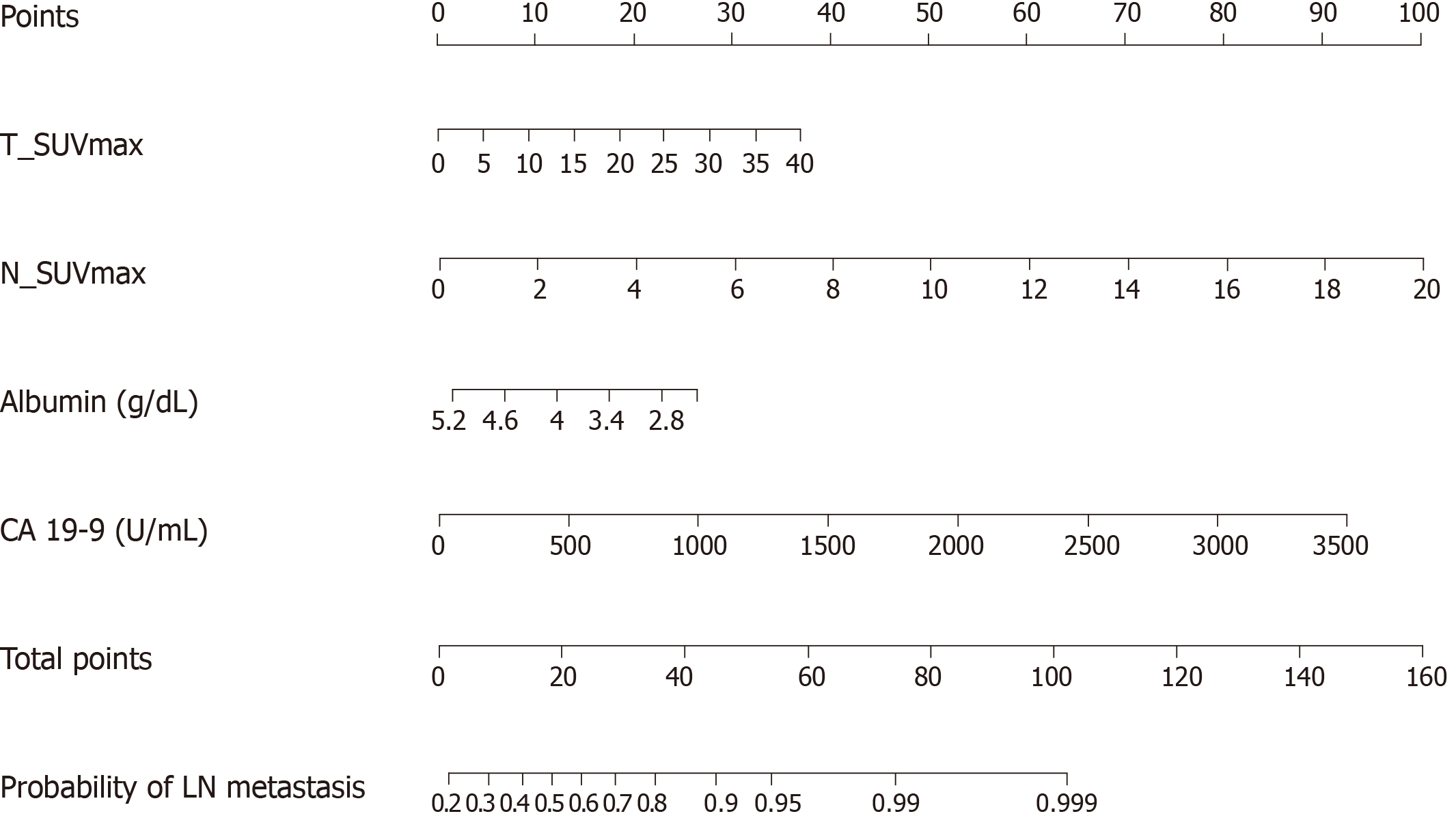
The result of the stepwise backward regression showed that a prediction model that combines T_SUVmax, N_SUVmax, serum albumin, and CA 19-9 was the best model to predict the risk of LN metastasis in the training cohort. The Hosmer and Lemeshow test generated a P value of 0.484, indicating that this predictive model fits well. A nomogram for predicting the probability of LN metastasis using pretreatment F-18 FDG PET/CT parameters and laboratory findings was successfully developed (Figure 2). The performance of this LN metastasis prediction model was good with the area under the receiver operating characteristic curve (AUC) of 0.733 (95%CI: 0.683–0.784, P = 0.025) in the training cohort and AUC of 0.756 (95%CI: 0.678–0.833, P < 0.001) in the test cohort (Figure 3).
In the present study, the incidence of LN metastasis in GC patients was 41% and the diagnostic performance of F-18 FDG PET/CT was highly specific for LN metastasis status; however, it had a limitation due to its relatively low sensitivity. The present study revealed that T_SUVmax and N_SUVmax measured by preoperative F-18 FDG PET/CT are independent predictive factors for LN metastasis in patients with GC. Moreover, the combination of these metabolic parameters with clinical laboratory findings (albumin and CA 19-9) significantly improved prediction of LN metastasis, compared with each parameter alone.
Several previous studies demonstrated that F-18 FDG PET/CT had relatively low sensitivity in detecting LN metastasis in GC patients[7,8,14]. In agreement with those studies, the results of the present study showed relatively low sensitivity. Despite the high specificity in the detection of LN metastasis, routine use of F-18 FDG PET/CT for GC stating is still controversial due to its low sensitivity[14-16]. A few studies have found that F-18 FDG uptake by the primary gastric tumor may predict LN metastasis status. Oh et al[17] reported that the peak-SUV of the primary gastric tumor is a useful indicator for LN metastasis. Kim et al[18] demonstrated that the T_SUVmax was the only independent factor to be significantly related to sensitivity for LN metastasis. However, no study has evaluated the predictive value of the combination of T_SUVmax and N_SUVmax for LN metastasis in GC. Notably, the present study showed that T_SUVmax and N_SUVmax were independent predictive factors for LN metastasis in the validation cohort as well as the training cohort.
Several studies have found that F-18 FDG uptake by the primary tumor is positively correlated with the status of LN metastasis in various cancers[9,19,20]. The present study also suggested that T_SUVmax was an independent prognostic factor for LN metastasis in patients with GC. This result could be explained by the fact that T_SUVmax reflects not only the tumor’s metabolic information, but also the tumor aggressiveness[21,22]. In this regard, T_SUVmax could have an additional value in predicting LN metastasis by reducing the high false-negative rate of F-18 FDG PET/CT for LN metastasis in patients with GC.
Some studies have found that pretreatment serum albumin[23,24] and CA 19-9[25-27] levels are correlated with LN metastasis. The chronic systematic inflammatory state increases the vascular permeability and loss of serum protein. Hypoalbuminemia, therefore, results from and reflects the systematic inflammatory condition. The inflammatory component contributes to tumor proliferation, angiogenesis, and metastasis[28]. For this reason, the serum albumin level is associated with LN metastasis. Meanwhile, CA 19-9 is a tumor-associated antigen and has recently been demonstrated to be a marker of digestive tract malignancies, especially pancreatic cancer[29]. Accordingly, the pretreatment serum albumin and CA 19-9 levels could be promising predictive factors for LN metastasis in patients with GC.
Meanwhile, the positive rate of LN differs by T stage, and the clinical significance of preoperative prediction of LN also depends on T stage. However, the endpoint of this study was the development of the preoperative LN metastasis prediction model. Therefore, despite the positive rate of LN differing by T stage, T stage could not be considered as a predictive parameter in this study. Although, there are several studies for the precise diagnosis of T stage using endoscopic ultrasonography (EUS), the accuracy of EUS for T stage ranged between < 50% and > 90%[30-33]. In GC, accurate preoperative prediction of LN status according to the specific T stage could provide more detailed pretreatment decision making. Since T stage is one of the most important factors for not only LN status prediction but also treatment decision making, establishment of reliable and objective method for the accurate T stage could be a useful co-consideration parameter for the prediction of LN metastasis and GC treatment.
Recently, validation of nomograms for calculating the risk of LN metastasis in GC has been reported[12,13]. However, no study has yet established a nomogram for prediction of LN metastasis using preoperative clinical parameters. The present study successfully developed an effective nomogram to predict LN metastasis in GC using T_SUVmax, N_SUVmax, serum albumin, and CA 19-9. Considering the feasibility of F-18 FDG PET/CT in the preoperative setting of GC, F-18 FDG PET/CT could be used as a non-invasive diagnostic tool for assessment of LN metastasis status in patients with GC and can be used to optimize current treatment strategy for patients with GC patients. The accurate preoperative prediction of LN can support clinicians in classifying patients who could receive minimal surgery or may derive greater clinical benefit from more extensive treatment.
This study had a few limitations. First, this study was a single-institution, retrospective study that might have been subject to selection bias. External validation is necessary to assess transferability of the LN prediction model. Second, the SUV of the small-sized primary tumor and LNs could be underestimated due to partial-volume effects. Lastly, since F-18 FDG uptake can be elevated by not only the malignant cell, but also the inflammatory lesion, SUVmax might be overestimated in some patients.
In conclusion, T_SUVmax and N_SUVmax were independent prognostic factors for the prediction LN metastasis in GC patients. Further, a prediction model using metabolic parameters (T_SUVmax and N_SUVmax) and laboratory findings (albumin and CA 19-9) could provide a more precise prediction of LN metastasis in the preoperative setting. The use of preoperative F-18 FDG PET/CT could be a useful tool for LN metastasis evaluation and treatment planning in patients with GC.
Gastric cancer (GC) is one of the most commonly diagnosed malignancies and the second leading cause of cancer-related deaths worldwide. The status of lymph node (LN) metastasis is an important prognostic factor in patients with GC. However, the evaluation of LN metastasis status in the preoperative setting is not accurate.
A few studies have been conducted to develop a nomogram for the prediction of LN metastasis in GC. However, a preoperative LN metastasis prediction model, based on the tumor metabolic information as measured by F-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography/computed tomography (PET/CT) and laboratory findings, does not exist for GC. The purpose of this study was to develop a preoperative nomogram for LN metastasis in patients with GC.
This study aims to identify predictive factors and to develop a preoperative nomogram for the prediction of LN metastasis using F-18 FDG PET/CT and preoperative laboratory findings in patients with GC.
Between 2008 and 2010, a total of 566 GC patients who underwent preoperative F-18 FDG PET/CT and subsequent surgical treatment without any preoperative intervention were analyzed. The LN metastasis prediction model was developed in the training cohort (n = 377) and validated in the internal validation cohort (n = 189). Clinicopathological data were retrieved from the patients’ medical records and the F-18 FDG PET/CT images were retrospectively interpreted. Univariate and multivariable logistic regression was performed to validate the preoperative predictive factors for LN metastasis.
The multivariate logistic analysis showed that the combination of maximum standardized uptake value (SUVmax) of the primary tumor (T_SUVmax) and SUVmax of LN (N_SUVmax), serum albumin, and carbohydrate antigen (CA) 19-9 was the best model to predict the risk of LN metastasis. The preoperative nomogram for the prediction of LN metastasis using T_SUVmax, N_SUVmax, serum albumin, and CA 19-9 showed good performance in the validation cohort as well as the training cohort.
The combination of preoperative F-18 FDG PET/CT metabolic parameters (T_SUVmax and N_SUVmax) and laboratory findings (albumin and CA 19-9) could be a useful tool for LN metastasis assessment and treatment planning in patients with GC.
The preoperative nomogram for the prediction of LN should be verified on a larger and external validation cohort for widespread acceptance.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aurello P, Ichimasa K, Tanabe S S-Editor: Ma YJ L-Editor: A E-Editor: Qi LL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13158] [Article Influence: 1879.7] [Reference Citation Analysis (4)] |
| 2. | Deng JY, Liang H. Clinical significance of lymph node metastasis in gastric cancer. World J Gastroenterol. 2014;20:3967-3975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 142] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Kim SH, Kim JJ, Lee JS, Kim SH, Kim BS, Maeng YH, Hyun CL, Kim MJ, Jeong IH. Preoperative N staging of gastric cancer by stomach protocol computed tomography. J Gastric Cancer. 2013;13:149-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Rösch T. Endosonographic staging of gastric cancer: a review of literature results. Gastrointest Endosc Clin N Am. 1995;5:549-557. [PubMed] |
| 5. | Park S, Ha S, Kwon HW, Kim WH, Kim TY, Oh DY, Cheon GJ, Bang YJ. Prospective Evaluation of Changes in Tumor Size and Tumor Metabolism in Patients with Advanced Gastric Cancer Undergoing Chemotherapy: Association and Clinical Implication. J Nucl Med. 2017;58:899-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Malibari N, Hickeson M, Lisbona R. PET/Computed Tomography in the Diagnosis and Staging of Gastric Cancers. PET Clin. 2015;10:311-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, Cho A, Lee JD. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582-1588. [PubMed] |
| 8. | Song BI, Kim HW, Won KS, Ryu SW, Sohn SS, Kang YN. Preoperative Standardized Uptake Value of Metastatic Lymph Nodes Measured by 18F-FDG PET/CT Improves the Prediction of Prognosis in Gastric Cancer. Medicine (Baltimore). 2015;94:e1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Song BI, Kim HW, Won KS. Predictive Value of 18F-FDG PET/CT for Axillary Lymph Node Metastasis in Invasive Ductal Breast Cancer. Ann Surg Oncol. 2017;24:2174-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Kim DH, Song BI, Hong CM, Jeong SY, Lee SW, Lee J, Ahn BC. Metabolic parameters using ¹⁸F-FDG PET/CT correlate with occult lymph node metastasis in squamous cell lung carcinoma. Eur J Nucl Med Mol Imaging. 2014;41:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Kim SH, Song BI, Kim BW, Kim HW, Won KS, Bae SU, Jeong WK, Baek SK. Predictive Value of [18F]FDG PET/CT for Lymph Node Metastasis in Rectal Cancer. Sci Rep. 2019;9:4979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Zheng Z, Zhang Y, Zhang L, Li Z, Wu X, Liu Y, Bu Z, Ji J. A nomogram for predicting the likelihood of lymph node metastasis in early gastric patients. BMC Cancer. 2016;16:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Guo CG, Zhao DB, Liu Q, Zhou ZX, Zhao P, Wang GQ, Cai JQ. A nomogram to predict lymph node metastasis in patients with early gastric cancer. Oncotarget. 2017;8:12203-12210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Wu CX, Zhu ZH. Diagnosis and evaluation of gastric cancer by positron emission tomography. World J Gastroenterol. 2014;20:4574-4585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Serrano OK, Love C, Goldman I, Huang K, Ng N, Abraham T, Da Silva R, Friedmann P, Libutti SK, Kennedy TJ. The value of FDG-PET in the staging of gastric adenocarcinoma: A single institution retrospective review. J Surg Oncol. 2016;113:640-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | De Raffele E, Mirarchi M, Cuicchi D, Lecce F, Cola B. Evolving role of FDG-PET/CT in prognostic evaluation of resectable gastric cancer. World J Gastroenterol. 2017;23:6923-6926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Oh HH, Lee SE, Choi IS, Choi WJ, Yoon DS, Min HS, Ra YM, Moon JI, Kang YH. The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol. 2011;104:530-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, Lee JH, Ryu KW, Kim YW, Bae JM. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Jung JH, Kim CY, Son SH, Kim DH, Jeong SY, Lee SW, Lee J, Ahn BC. Preoperative Prediction of Cervical Lymph Node Metastasis Using Primary Tumor SUVmax on 18F-FDG PET/CT in Patients with Papillary Thyroid Carcinoma. PLoS One. 2015;10:e0144152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Moon SH, Kim HS, Hyun SH, Choi YS, Zo JI, Shim YM, Lee KH, Kim BT, Choi JY. Prediction of occult lymph node metastasis by metabolic parameters in patients with clinically N0 esophageal squamous cell carcinoma. J Nucl Med. 2014;55:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, Shishikura T, Imazeki K, Nakajima N. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer. 1998;82:2227-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Song BI, Hong CM, Lee HJ, Kang S, Jeong SY, Kim HW, Chae YS, Park JY, Lee SW, Ahn BC, Lee J. Prognostic Value of Primary Tumor Uptake on F-18 FDG PET/CT in Patients with Invasive Ductal Breast Cancer. Nucl Med Mol Imaging. 2011;45:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | González-Trejo S, Carrillo JF, Carmona-Herrera DD, Baz-Gutiérrez P, Herrera-Goepfert R, Núñez G, Ochoa-Carrillo FJ, Gallardo-Rincón D, Aiello-Crocifoglio V, Oñate-Ocaña LF. Baseline serum albumin and other common clinical markers are prognostic factors in colorectal carcinoma: A retrospective cohort study. Medicine (Baltimore). 2017;96:e6610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Zhao F, Zhen FX, Zhou Y, Huang CJ, Yu Y, Li J, Li QF, Zhu CX, Yang XY, You SH, Wu QG, Qin XY, Liu Y, Chen L, Wang W. Clinicopathologic predictors of metastasis of different regional lymph nodes in patients intraoperatively diagnosed with stage-I non-small cell lung cancer. BMC Cancer. 2019;19:444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Stojkovic Lalosevic M, Stankovic S, Stojkovic M, Markovic V, Dimitrijevic I, Lalosevic J, Petrovic J, Brankovic M, Pavlovic Markovic A, Krivokapic Z. Can preoperative CEA and CA19-9 serum concentrations suggest metastatic disease in colorectal cancer patients? Hell J Nucl Med. 2017;20:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 26. | Kaneko M, Ishihara S, Murono K, Sasaki K, Otani K, Yasuda K, Nishikawa T, Tanaka T, Kiyomatsu T, Hata K, Kawai K, Nozawa H, Nakayama H, Watanabe T, Sasaki S, Watanabe T. Carbohydrate Antigen 19-9 Predicts Synchronous Peritoneal Carcinomatosis in Patients with Colorectal Cancer. Anticancer Res. 2017;37:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Wentz SC, Zhao ZG, Shyr Y, Shi CJ, Merchant NB, Washington K, Xia F, Chakravarthy AB. Lymph node ratio and preoperative CA 19-9 levels predict overall survival and recurrence-free survival in patients with resected pancreatic adenocarcinoma. World J Gastrointest Oncol. 2012;4:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Wu Y, Zhou BP. Inflammation: a driving force speeds cancer metastasis. Cell Cycle. 2009;8:3267-3273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 248] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 29. | Magnani JL, Nilsson B, Brockhaus M, Zopf D, Steplewski Z, Koprowski H, Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982;257:14365-14369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Yoshinaga S, Oda I, Nonaka S, Kushima R, Saito Y. Endoscopic ultrasound using ultrasound probes for the diagnosis of early esophageal and gastric cancers. World J Gastrointest Endosc. 2012;4:218-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Lee HH, Lim CH, Park JM, Cho YK, Song KY, Jeon HM, Park CH. Low accuracy of endoscopic ultrasonography for detailed T staging in gastric cancer. World J Surg Oncol. 2012;10:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Kutup A, Vashist YK, Groth S, Vettorazzi E, Yekebas EF, Soehendra N, Izbicki JR. Endoscopic ultrasound staging in gastric cancer: Does it help management decisions in the era of neoadjuvant treatment? Endoscopy. 2012;44:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Jürgensen C, Brand J, Nothnagel M, Arlt A, Neser F, Habeck JO, Schreiber S, Stölzel U, Zeitz M, Hampe J. Prognostic relevance of gastric cancer staging by endoscopic ultrasound. Surg Endosc. 2013;27:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |