Published online Mar 15, 2020. doi: 10.4251/wjgo.v12.i3.323
Peer-review started: September 27, 2019
First decision: November 18, 2019
Revised: November 27, 2019
Accepted: January 14, 2020
Article in press: January 14, 2020
Published online: March 15, 2020
Processing time: 167 Days and 4.1 Hours
Malignant bowel obstruction (MBO) is a common event for end-stage gastrointestinal cancer patients. Previous studies had demonstrated manifestations and clinical management of MBO with mixed malignancies. There still lack reports of the surgical treatment of MBO.
To analyze the short-term outcomes and prognosis of palliative surgery for MBO caused by gastrointestinal cancer.
A retrospective chart review of 61 patients received palliative surgery between January 2016 to October 2018 was performed, of which 31 patients underwent massive debulking surgery (MDS) and 30 underwent ostomy/by-pass surgery (OBS). The 60-d symptom palliation rate, 30-d morbidity and mortality, and overall survival rates were compared between the two groups.
The overall symptom palliation rate was 75.4% (46/61); patients in the MDS group had significantly higher symptom palliation rate than OBS group (90% vs 61.2%, P = 0.016). Patients with colorectal cancer who were in the MDS group showed significantly higher symptom improvement rates compared to the OBS group (overall, 76.4%; MDS, 61.5%; OBS, 92%; P = 0.019). However, patients with gastric cancer did not show a significant difference in symptom palliation rate between the MDS and OBS groups (OBS, 60%; MDS, 80%; P = 1.0). The median survival time in the MDS group was significantly longer than in the OBS group (10.9 mo vs 5.3 mo, P = 0.05).
For patients with MBO caused by peritoneal metastatic colorectal cancer, MDS can improve symptom palliation rates and prolong survival, without increasing mortality and morbidity rates.
Core tip: Malignant bowel obstruction (MBO) is a frequent event for patients with end-stage gastrointestinal cancer. There is no consensus on the optimal treatment strategy for improving quality of life and prolonging survival. We performed a retrospective study at a single institution to determine the effects of palliative surgery for MBO in patients with gastrointestinal cancers. In this cohort, we observed higher symptom relief rates and prolonged survival after massive debulking surgery compared with ostomy/by-pass surgery in MBO patients. For select patients with MBO caused by metastatic colorectal cancer, massive debulking surgery can result in higher symptom palliation rates and prolonged survival without increasing mortality and morbidity rates compared with ostomy/by-pass surgery.
- Citation: Chen PJ, Wang L, Peng YF, Chen N, Wu AW. Surgical intervention for malignant bowel obstruction caused by gastrointestinal malignancies. World J Gastrointest Oncol 2020; 12(3): 323-331
- URL: https://www.wjgnet.com/1948-5204/full/v12/i3/323.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i3.323
Malignant bowel obstruction (MBO) is a frequent event for patients with end-stage cancer, especially in gastrointestinal (GI) and ovarian cancer[1-3]. It had been reported that 10%-50% of patients with cancer will develop MBO during the preterminal stage[4]. Patients with MBO suffered from an inability to eat, abdominal pain, distention, nausea, and vomiting, resulting in a poor quality of life (QOL), stress, and emotional problems.
The clinical management of MBO requires a specific and individualized approach based on the expected rest time of the disease, objectives of care, and the patient's preferences. The primary treatment objective in patients with MBO is to relieve symptoms, restore food intake, and improve nutrition[5]. The most common treatment options for patients with MBO included nasogastric drainage, total parenteral nutrition, pain control, somatostatin, endoscopically placed stents, and palliative surgery[4]. Due to the heterogeneity of MBO patients, there is no consensus on the optimal treatment strategy for improving QOL and prolonging survival. The decision making of the management of MBO should be individualized[6].
Surgical intervention is usually considered to be the last treatment option that may relieve the symptoms of MBO. However, the decision to proceed with palliative surgery is usually difficult, especially for patients who may only have a few weeks to live. Additionally, MBO patients suffering from malnutrition are not good candidates for surgical intervention because of the high postoperative mortality and morbidity rates. Previous studies included several types of malignancies in their analysis, and most focused on the treatment of ovarian cancer. At present, there is limited evidence on the effectiveness of surgical intervention for MBO[7].
The surgical treatments for MBO include percutaneous venting gastrostomy, stoma diversion and cytoreductive surgery, etc.[8-10]. However, some patients with extensive peritoneal metastasis may have multiple obstruction sites, and an ostomy or by-pass surgery may not be feasible. Previous studies have shown that some selected patients with recurrent, unresectable colorectal cancer may benefit from cytoreductive surgery, massive tumor debulking surgery and hyperthermic intraperitoneal chemotherapy[11-14].
We performed a retrospective study at a single institution to determine the effects of palliative surgery for MBO in patients with GI cancers and analyzed symptom palliation rates, postoperative mortality, complications after surgery, and survival rates.
Between January 2016 to October 2018, we enrolled 61 patients with MBO caused by peritoneal metastasis of gastric and colorectal cancer underwent surgical treatment at the Peking University Cancer Hospital.
Including criteria for patients are: (1) Clinical evidence of bowel obstruction; (2) Obstruction distal to the Treitz ligament; (3) The presence of primary gastric and colorectal cancer; (4) The absence of curable possibilities; (5) Ineffective conservative treatment for obstruction; (6) Expected survival of more than 2 mo; and (7) Patients and their families willing to undergo surgery[4].
All patients discussed their options with the Multiple Disciplinary Team before the operation, and a treatment plan was developed based on the patients' preferences. The surgical options included ostomy/by-pass surgery (OBS) and massive debulking surgery (MDS).
Indications for OBS: (1) Localized or single-site tumor obstruction; (2) Patients may benefit from further chemotherapy; and (3) Patients and their families do not receive aggressive tumor reductive surgery.
Indications for MDS: (1) Perioperative risk evaluation indicates that patients can tolerate surgery; (2) After imaging and physical examination, it is expected that the obstruction and symptoms can be effectively relieved after operation; (3) Patients can endure at least 2 months of oral feeding and non-obstructive survival; (4) It is expected that at least 2 meters of small intestine will remain after the operation; and (5) Patients and their families had a strong preference for surgery.
Evaluation of surgical effectiveness: (1) Solid food intake and symptom relief rates 60 d after surgery[15]; (2) Postoperative complications and mortality rates within 30 d; and (3) Postoperative survival time.
Statistical analysis was performed by using the IBM-SPSS19 software package. The categorical variables were compared by the Pearson’s chi-square test. Overall survival rates were analyzed by the Kaplan-Meier method and the difference between the groups were evaluated by a log-rank test. P < 0.05 was considered as statistically significant. Significant prognostic factors were analyzed using the Cox proportional hazards regression model to determine the independent prognostic factors of MBO caused by colorectal cancer.
A total of 61 patients with GI cancer were enrolled in this study. Of the 61 patients, 38 were male and 23 were female. Patient ages ranged from 19 to 88 years, with an average age of 59.6 ± 11.5 years and a median age of 61 years. The clinical and pathological data of all the patients in this study are shown in Table 1.
| Clinicopathological features | n (%) | 6-mo OS | Median survival time (mo) | P value |
| Gender | 0.923 | |||
| Male | 38 (65.2) | 54.7% | 6.6 | |
| Female | 23 (34.8) | 59.2% | 6.4 | |
| Age (yr) | 0.516 | |||
| ≥ 60 | 33 (54.1) | 50.4% | 6.5 | |
| < 60 | 28 (45.9) | 61.5% | 10.6 | |
| Primary tumor | 0.015 | |||
| Colorectal | 51 (69.6) | 83.6% | 10.6 | |
| Gastric | 10 (30.4) | 16.4% | 1.8 | |
| Differentiation | 0.005 | |||
| Well + Moderate | 41 (67.2) | 71.5% | 10.9 | |
| Poor | 20 (32.8) | 26.5% | 4.1 | |
| ECOG | 0.031 | |||
| 0-1 | 40 (65.6) | 64.4% | 10.6 | |
| > 1 | 21 (34.4) | 42.9% | 5.1 | |
| Surgical approach | 0.053 | |||
| OBS | 31 (50.8) | 44.6% | 5.3 | |
| MDS | 30 (49.2) | 68.6% | 10.9 | |
| Obstruction | 0.25 | |||
| Single | 41 (67.2) | 57% | 6.6 | |
| Multiple | 20 (32.8) | 55% | 6.1 | |
| Intestinal obstruction | 0.084 | |||
| Yes | 31 (50.8) | 53.2% | 6.1 | |
| No | 30 (49.2) | 60.1% | 10.9 | |
| Colon/rectum obstruction | 0.389 | |||
| Yes | 37 (60.7) | 57.4% | 10.9 | |
| No | 24 (39.3) | 54.8% | 6.5 | |
| Ascites | 0.691 | |||
| Yes | 15 (24.6) | 41.1% | 5.1 | |
| No | 46 (75.4) | 61.1% | 6.6 | |
| Greater omentum metastasis | 0.044 | |||
| Yes | 42 (68.9) | 51.2% | 6.1 | |
| No | 19 (31.1) | 67.5% | 17.3 | |
| Distant metastasis | 0.13 | |||
| Yes | 24 (39.3) | 50% | 5.2 | |
| No | 37 (60.7) | 60.4% | 10.9 | |
| Symptom relief | 0.007 | |||
| Yes | 46 (75.4) | 63.7% | 10.9 | |
| No | 15 (24.6) | 31.5% | 3.9 |
In the MDS group, 3 patients showed no evidence of disease. The overall symptom improvement rate was 75.4% (46/51). The overall symptom improvement rate in the MDS group was significantly higher than in OBS group (90% vs 61.3%, respectively, P = 0.016). Re-obstruction occurred in 3 patients after the operation, including 2 in the OBS group and 1 in the MDS group.
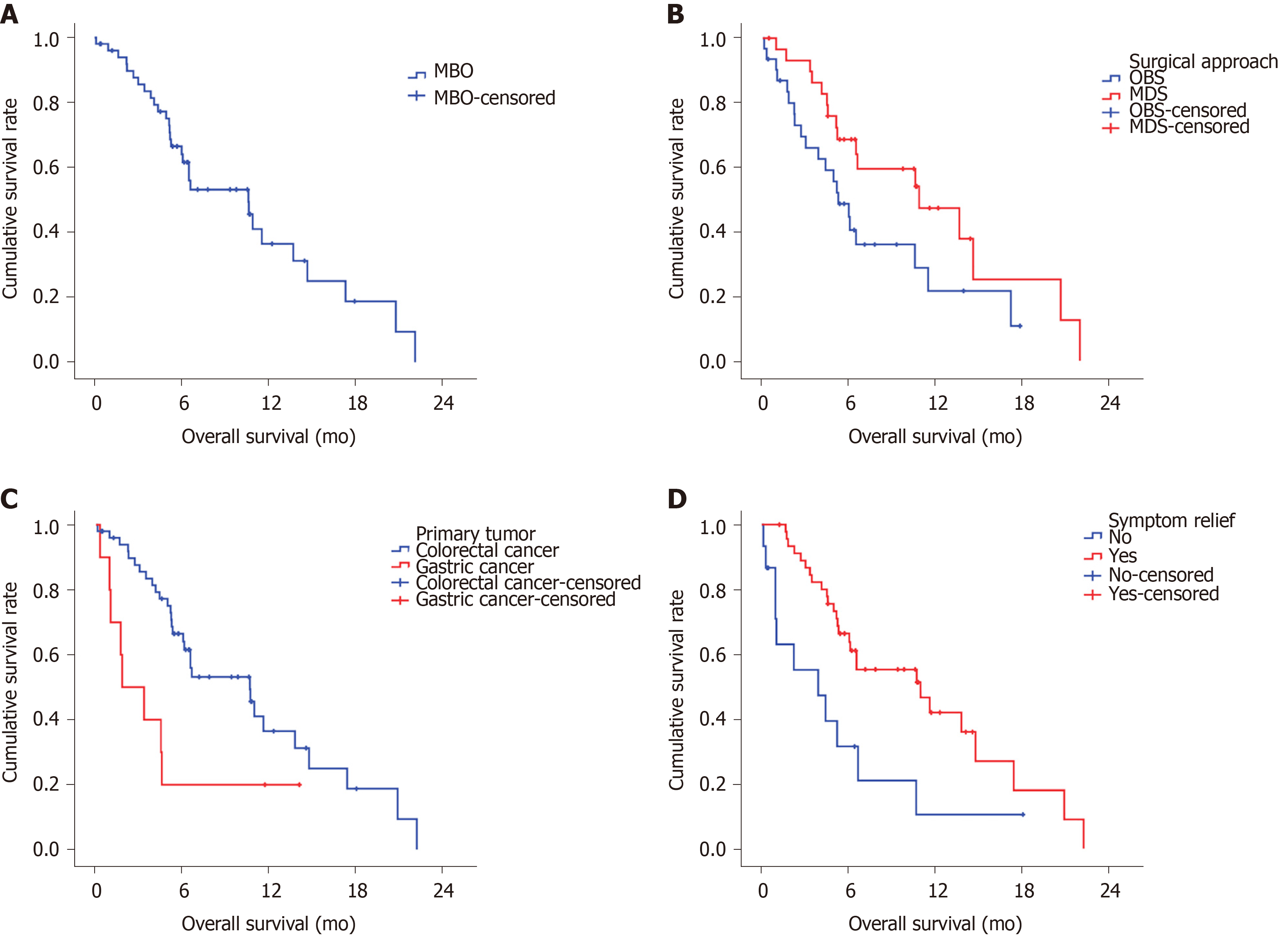
Ten patients, including 4 patients in OBS group and 6 patients in the MDS group, continued medical treatment and radiotherapy after the operation. By the final follow-up in June 2019, 38 of the 61 patients had died. The median survival time of the whole group was 6.5 mo, and the 6-mo survival rate was 56.5% (Figure 1A). Patients with colorectal cancer had significant longer median survival than gastric cancer (10.6 mo vs 1.8 mo, P = 0.015, Figure 1B). Patients in MDS group had significant longer median survival than in OBS group (10.9 mo vs 5.3 mo, P = 0.05, Figure 1C). Patients with improvement of symptoms after operation had significant longer median survival than those without (10.9 mo vs 3.9 mo, P = 0.007, Figure 1D).
In this cohort, 32 patients had complications within 30-d of surgery. In the MDS group, 1 patient had secondary surgery due to wound infection.
The overall complication rates in the MDS and OBS groups were 48.4% (15/31) and 56.7% (17/30), respectively. There was no significant difference between the two groups (P = 0.611). The mortality rates within 30 days of the operation in the MDS and OBS groups were 19.4% (6/31) and 6.7% (2/30), respectively. There was no significant difference between the two groups (P = 0.255, Table 2).
| Complications | OBS group (n = 31) | MDS group (n = 30) |
| Pulmonary infection | 6 (19.4) | 4 (13.3) |
| Abdominal infection | 2 (6.5) | 4 (13.3) |
| Incision infection | 3 (9.7) | 4 (13.3) |
| Urinary tract infection | 1 (3.2) | - |
| Diarrhea | - | 1 (3.3) |
| Arrhythmia | 1 (3.2) | - |
| Hemorrhage | 1 (3.2) | 4 (13.3) |
| Cerebral infarction | - | 2 (6.7) |
| Short intestine | - | 2 (6.7) |
| Necrosis of ostomy mucosa | 2 (6.5) | - |
| Clavien-Dindo classification[16] | ||
| I | - | 1(3.3) |
| II | 9 (29) | 13 (43.3) |
| III | - | - |
| IV | - | 1 (2.3) |
| V | 6 (19.4) | 2 (6.7) |
Because of the short survival time of patients with gastric cancer, we conducted a univariate analysis of MBO in patients with colorectal cancer, and found that surgical approach [hazard ratio (HR) = 2.301, 95%CI: 0.999-5.299, P = 0.05], differentiation (HR = 8.509, 95%CI: 2.455-26.448, P= 0.001), greater omentum metastasis (HR = 7.718, 95%CI: 2.224-26.782, P = 0.001) and distant organ metastasis (HR = 2.375, 95%CI: 1.022-5.253, P = 0.044, Table 3) were independent prognostic factors for MBO caused by metastatic colorectal cancer (mCRC).
| Variables | HR | 95%CI | P value |
| Surgical approach (OBS vs MDS) | 2.301 | 0.999-5.299 | 0.05 |
| Greater omentum metastasis (Yes vs No) | 7.718 | 2.224-26.782 | 0.001 |
| Differentiation (Poor vs Well+ Moderate) | 8.059 | 2.455-26.448 | 0.001 |
| Distant organ metastasis (Yes vs No) | 2.375 | 1.022-5.253 | 0.044 |
MBO patients represent a complex and heterogeneous population, and there are no evidence-based guidelines to help with the decision-making process for clinical management of the disease. Previous studies have reported on the effectiveness of palliative surgery for MBO, most of which focused on gynecologic and GI cancer populations[17-19]. However, there is little data on palliative surgery for MBO in colorectal cancer patients and most of the literature that included colorectal cancer combined several types of malignancies.
In this cohort study focused on GI cancer, we observed higher symptom relief rates and prolonged survival after MDS compared with OBS in MBO patients. For select patients with MBO caused by peritoneal mCRC, MDS can result in higher symptom palliation rates and prolonged survival without increasing mortality and morbidity rates compared with OBS. However, because of the short survival time of GI cancer patients, no surgical intervention which may cause a patient to spend the remainder of their life in the hospital should be seriously considered.
The priority of care for inoperable and conservative MBO patients is to control their symptoms and improve their QOL. Medical treatment combining total parenteral nutrition, opioids, antiemetics, and somatostatin drugs is usually considered to be the preferred treatment[20]. The primary goals in palliative surgery are symptom relief and the restoration of oral feeding. Single site obstructions can be resolved by endoscopic treatment and ostomy[21,22]. However, some patients with complete obstructions may have multiple sites that are occlusive, and therefore, tumor reduction surgery becomes a necessity.
The overall symptom relief rate of all the patients included in this study was 76.1%. The MDS group achieved a higher symptom relief rate than the OBS group (91.3% vs 60.9%, respectively), which was higher than that reported in the literature[15]. The re-obstruction rate was 6.5% in this group, which was lower than the 9% reported in the literature[23]. In this group, although the obstructions were removed, the symptom improvement rate in the OBS group was not high, and 40% of these patients still needed parenteral nutritional support.
Previous studies reported that the median survival for MBO patients treated with conservative care was no longer than 4–5 wk[24]. For some MBO patients, surgical intervention can prolong survival[1,4,25-27]. In this study, the overall median survival time was 6.5 mo. The patients who underwent MDS had a longer survival time than those who underwent OBS (median survival time was 11.5 mo vs 5.1 mo, respectively), and a longer survival time than those reported in previous literature (4-9 mo)[28,29].
In this cohort, the patients that showed significant symptom improvement and good oral food intake had significantly longer survival times than those who did not. This indicates that palliative surgery, although aimed at relieving symptoms, has also prolonged survival, likely due to the improvement in nutrition and subsequent systemic treatment. These results were in accordance with the literature[26]. However, due to the high heterogeneity of patients with malignant intestinal obstructions, it is usually difficult for surgeons to determine the best surgical approach before the operation is performed[5].
Although MDS and OBS can improve the QOL and potentially the survival time, these benefits are accompanied by high mortality and complication rates. In this study, the 30-d postoperative complication rates were as high as 54.3% and 13%, respectively, which are similar to those reported in previous literature (6%-40%) and 5%-15%, respectively[5,18,28,30]. Notably, patients undergoing OBS had comparable complication rate and mortality after surgery as patients in the MDS group, suggesting that the short-term prognosis of patients cannot be overlooked.
Much of the literature defines surgical benefit as at least 60 d of survival after the operation. In this cohort, the median survival of gastric cancer patients was only 1.8 mo, suggesting surgery should not be routinely undertaken in patients with end-stage gastric cancer. However, MBO patients with colorectal cancer may benefit from surgical intervention especially for select patients that can withstand treatment with MDS. Previous studies have suggested candidate prognostic indicators that a patient has a low likelihood of benefiting from surgery for MBO. Age, surgical treatment, ascites, low albumin, hypoproteinemia, and primary tumors are reported to be the main prognostic factors. The number of combined risk factors determines survival rates and the incidence rates of short-term complications after surgery[6,18,27,28,31]. Before any palliative surgical intervention is performed, the feasibility of surgery and the probability that a patient will benefit not only from an improvement in QOL but in terms of survival should be taken into consideration[32]. In this cohort, survival analysis showed that the primary tumor site, differentiation, the surgical approach, the ECOG score and greater omentum metastasis were the prognostic factors for MBO patients with GI cancer. Whereas in mCRC, differentiation, surgical approach, greater omentum metastasis and distant organ metastasis were the most important independent prognostic factors.
The limitations of this study were the small sample size and the high heterogeneity of the patients. We lacked the data on the use of medical antineoplastic drugs and the patient KARS/BRAF status. There may also be selection bias that affects the conclusions. Although symptom relief and survival benefit were observed in this group, the risk of serious complications and mortality cannot be ignored. Surgeons should fully weigh the life-expectancy, potential benefits and risks, and the QOL of patients after the operation. The aim of surgical and other approaches must be considered and prioritized based on each patient’s and family’s goals and preferences. Although surgeons usually actively treat postoperative complications, it is may be unacceptable for some patients with end-stage diseases spending the rest life in hospital.
Because of the patient heterogeneity, it seems impossible to conduct a random study of malignant intestinal obstruction. In the future, registry studies may be needed to provide high-level evidence about the treatment of MBO and the QOL of patients should be taken into account.
In conclusion, because of the short expected-survival time for patients with gastric cancer and MBO, the high perioperative complication and mortality rates should be seriously considered before any surgical intervention is performed even for an ostomy. For select patients with MBO caused by peritoneal mCRC, MDS may result in high symptom palliation rates and prolonged survival compared with OBS, without increasing mortality and morbidity rates.
Malignant bowel obstruction (MBO) is a frequent event for end-stage malignant cancers. There is no consensus on the optimal treatment strategy for improving quality of life and prolonging survival. There were fewer studies focused on the surgical intervention of MBO with gastrointestinal (GI) cancers.
We wanted to investigate the effects of palliative surgery for MBO in patients with GI cancers in order to guide treatment.
To define the surgical outcome difference between massive debulking surgery (MDS) and ostomy/by-pass surgery (OBS) for MBO patients with GI cancer.
MBO patients with GI cancer receive palliative surgery were included MDS group and OBS group. This study mainly investigated the difference of short outcome and survival between the two groups.
This study reported that patients in the MDS group had significantly higher symptom palliation rate than OBS group, and the median survival time in the MDS group was significantly longer than in the OBS group.
Massive debulking surgery can significantly improve symptom and prolong survival for MBO patients with colorectal cancer, without increasing mortality and morbidity rates compared with ostomy/by-pass surgery. However, MDS had no such advantage in gastric cancer.
The treatment of MBO remained controversial and no well-evidenced. This small sample study demonstrates the effectiveness, safety and survival benefit of massive debulking surgery in colorectal cancer patients with MBO. It is difficult to carry out large sample randomized controlled study. In the future, it is necessary to establish a large sample registration study.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tokunaga Y, Majewski M, Nair KS S-Editor: Wang JL L-Editor: A E-Editor: Ma YJ
| 1. | Anthony T, Baron T, Mercadante S, Green S, Chi D, Cunningham J, Herbst A, Smart E, Krouse RS. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manage. 2007;34:S49-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Miller G, Boman J, Shrier I, Gordon PH. Small-bowel obstruction secondary to malignant disease: an 11-year audit. Can J Surg. 2000;43:353-358. [PubMed] |
| 3. | Nelen SD, van Putten M, Lemmens VEPP, Bosscha K, de Wilt JHW, Verhoeven RHA. Effect of age on rates of palliative surgery and chemotherapy use in patients with locally advanced or metastatic gastric cancer. Br J Surg. 2017;104:1837-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res. 2012;4:159-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Paul Olson TJ, Pinkerton C, Brasel KJ, Schwarze ML. Palliative surgery for malignant bowel obstruction from carcinomatosis: a systematic review. JAMA Surg. 2014;149:383-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Bento JH, Bianchi ET, Tustumi F, Leonardi PC, Junior UR, Ceconello I. Surgical Management of Malignant Intestinal Obstruction: Outcome and Prognostic Factors. Chirurgia (Bucur). 2019;114:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Lilley EJ, Cooper Z, Schwarze ML, Mosenthal AC. Palliative Care in Surgery: Defining the Research Priorities. Ann Surg. 2018;267:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Dolan EA. Malignant bowel obstruction: a review of current treatment strategies. Am J Hosp Palliat Care. 2011;28:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol. 2008;12:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Thampy S, Najran P, Mullan D, Laasch HU. Safety and Efficacy of Venting Gastrostomy in Malignant Bowel Obstruction: A Systematic Review. J Palliat Care. 2019;825859719864915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Verwaal VJ, Boot H, Aleman BM, van Tinteren H, Zoetmulder FA. Recurrences after peritoneal carcinomatosis of colorectal origin treated by cytoreduction and hyperthermic intraperitoneal chemotherapy: location, treatment, and outcome. Ann Surg Oncol. 2004;11:375-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Kecmanovic DM, Pavlov MJ, Ceranic MS, Sepetkovski AV, Kovacevic PA, Stamenkovic AB. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Anwar S, Peter MB, Dent J, Scott NA. Palliative excisional surgery for primary colorectal cancer in patients with incurable metastatic disease. Is there a survival benefit? A systematic review. Colorectal Dis. 2012;14:920-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Gootjes EC, Bakkerus L, Ten Tije AJ, Witteveen PO, Buffart TE, Bridgewater JA, Primrose JN, Verhoef C, Verheul HMW. The value of tumour debulking for patients with extensive multi-organ metastatic colorectal cancer. Eur J Cancer. 2018;103:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Blair SL, Chu DZ, Schwarz RE. Outcome of palliative operations for malignant bowel obstruction in patients with peritoneal carcinomatosis from nongynecological cancer. Ann Surg Oncol. 2001;8:632-637. [PubMed] |
| 16. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24805] [Article Influence: 1181.2] [Reference Citation Analysis (0)] |
| 17. | Tran E, Spiceland C, Sandhu NP, Jatoi A. Malignant Bowel Obstruction in Patients With Recurrent Ovarian Cancer. Am J Hosp Palliat Care. 2016;33:272-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Alese OB, Kim S, Chen Z, Owonikoko TK, El-Rayes BF. Management patterns and predictors of mortality among US patients with cancer hospitalized for malignant bowel obstruction. Cancer. 2015;121:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Chen JH, Huang TC, Chang PY, Dai MS, Ho CL, Chen YC, Chao TY, Kao WY. Malignant bowel obstruction: A retrospective clinical analysis. Mol Clin Oncol. 2014;2:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Currow DC, Quinn S, Agar M, Fazekas B, Hardy J, McCaffrey N, Eckermann S, Abernethy AP, Clark K. Double-blind, placebo-controlled, randomized trial of octreotide in malignant bowel obstruction. J Pain Symptom Manage. 2015;49:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Jang SH, Lee H, Min BH, Kim SM, Kim HS, Carriere KC, Min YW, Lee JH, Kim JJ. Palliative gastrojejunostomy versus endoscopic stent placement for gastric outlet obstruction in patients with unresectable gastric cancer: a propensity score-matched analysis. Surg Endosc. 2017;31:4217-4223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Vashi PG, Dahlk S, Vashi RP, Gupta D. Percutaneous endoscopic gastrostomy tube occlusion in malignant peritoneal carcinomatosis-induced bowel obstruction. Eur J Gastroenterol Hepatol. 2011;23:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Fiori E, Lamazza A, Schillaci A, Femia S, Demasi E, Decesare A, Sterpetti AV. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy versus endoscopic stenting: final results of a prospective randomized trial. Am J Surg. 2012;204:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Santangelo ML, Grifasi C, Criscitiello C, Giuliano M, Calogero A, Dodaro C, Incollingo P, Rupealta N, Candida M, Chiacchio G, Riccio E, Pisani A, Tammaro V, Carlomagno N. Bowel obstruction and peritoneal carcinomatosis in the elderly. A systematic review. Aging Clin Exp Res. 2017;29:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Jiménez-Pérez J, Casellas J, García-Cano J, Vandervoort J, García-Escribano OR, Barcenilla J, Delgado AA, Goldberg P, Gonzalez-Huix F, Vázquez-Astray E, Meisner S. Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol. 2011;106:2174-2180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Goto T, Takano M, Aoyama T, Miyamoto M, Watanabe A, Kato M, Sasaki N, Hirata J, Sasa H, Furuya K. Outcomes of palliative bowel surgery for malignant bowel obstruction in patients with gynecological malignancy. Oncol Lett. 2012;4:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Henry JC, Pouly S, Sullivan R, Sharif S, Klemanski D, Abdel-Misih S, Arradaza N, Jarjoura D, Schmidt C, Bloomston M. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery. 2012;152:747-56; discussion 756-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Abbas SM, Merrie AE. Resection of peritoneal metastases causing malignant small bowel obstruction. World J Surg Oncol. 2007;5:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Krouse RS. Surgical management of malignant bowel obstruction. Surg Oncol Clin N Am. 2004;13:479-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Miner TJ, Brennan MF, Jaques DP. A prospective, symptom related, outcomes analysis of 1022 palliative procedures for advanced cancer. Ann Surg. 2004;240:719-26; discussion 726-7. [PubMed] |
| 31. | Cousins SE, Tempest E, Feuer DJ. Surgery for the resolution of symptoms in malignant bowel obstruction in advanced gynaecological and gastrointestinal cancer. Cochrane Database Syst Rev. 2016;CD002764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Ripamonti C, Bruera E. Palliative management of malignant bowel obstruction. Int J Gynecol Cancer. 2002;12:135-143. [PubMed] |