Published online Apr 15, 2019. doi: 10.4251/wjgo.v11.i4.322
Peer-review started: November 19, 2018
First decision: December 7, 2018
Revised: January 3, 2019
Accepted: January 8, 2019
Article in press: January 9, 2019
Published online: April 15, 2019
Processing time: 147 Days and 5.8 Hours
Liver transplantation (LT) is regarded as the best treatment for both primary and recurrent hepatocellular carcinoma (HCC). Post-transplant HCC recurrence rate is relatively low but significant, ranging from 10%-30% according to different series. When recurrence happens, it is usually extrahepatic and associated with poor prognosis. A predictive model that allows patient stratification according to recurrence risk can help to individualize post-transplant surveillance protocol and guidance of the use of anti-tumor immunosuppressive agents.
To develop a scoring system to predict HCC recurrence after LT in an Asian population.
Consecutive patients having LT for HCC from 1995 to 2016 at our hospital were recruited. They were randomized into the training set and the validation set in a 60:40 ratio. Multivariable Cox regression model was used to identity factors associated with HCC recurrence. A risk score was assigned to each factor according to the odds ratio. Accuracy of the score was assessed by the area under the receiver operating characteristic curve.
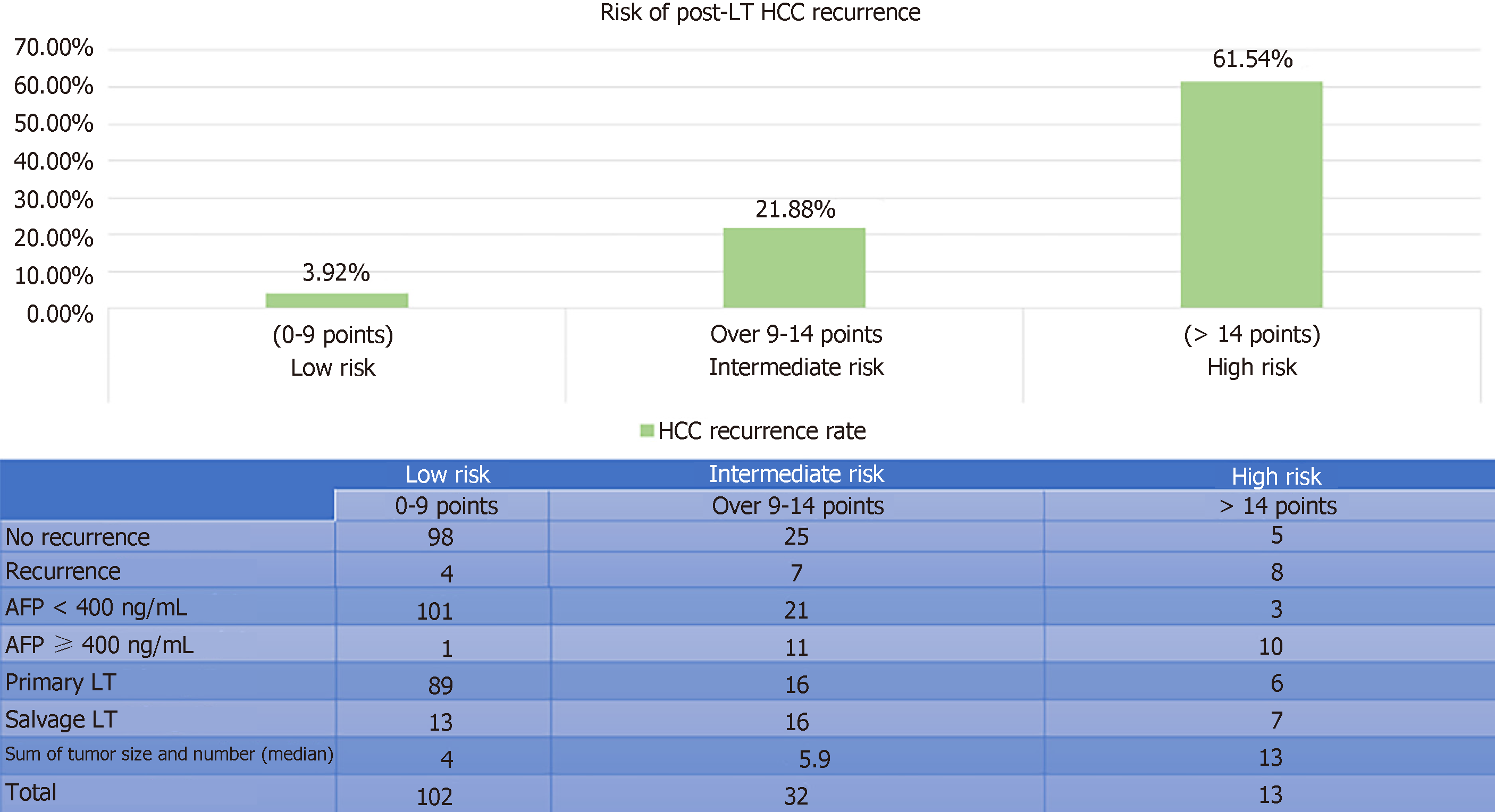
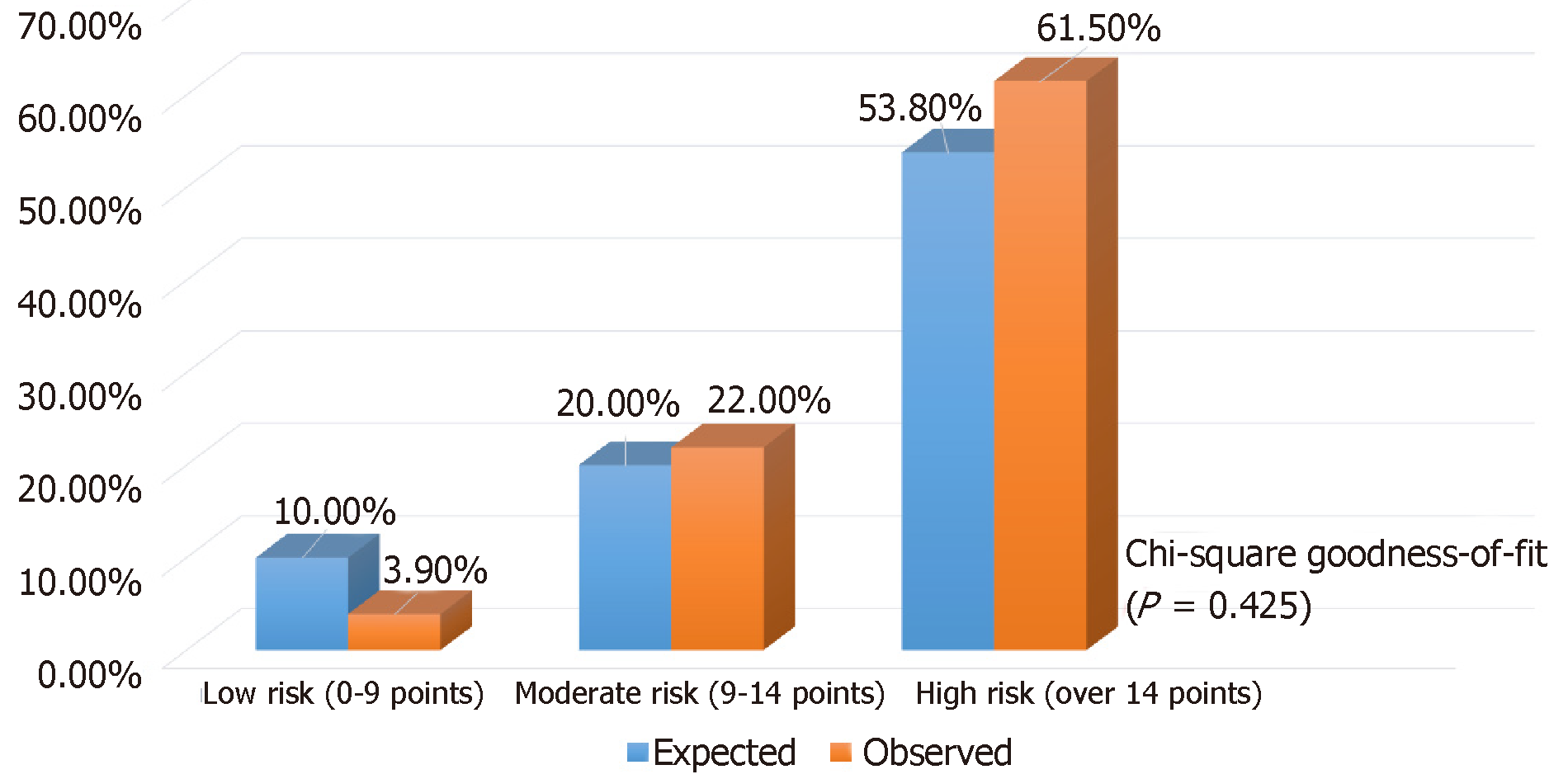
In total, 330 patients were eligible for analysis (183 in training and 147 in validation). Recurrent HCC developed in 14.2% of them. The median follow-up duration was 65.6 mo. The 5-year disease-free and overall survival rates were 78% and 80%, respectively. On multivariate analysis, alpha-fetoprotein > 400 ng/mL [P = 0.012, hazard ratio (HR) 2.92], sum of maximum tumor size and number (P = 0.013, HR 1.15), and salvage LT (P = 0.033, HR 2.08) were found to be independent factors for disease-free survival. A risk score was calculated for each patient with good discriminatory power (c-stat 0.748 and 0.85, respectively, in the training and validation sets). With the derived scores, patients were classified into low- (0–9), moderate- (> 9–14), and high-risk groups (> 14), and the risk of HCC recurrence in the training and validation sets was 10%, 20%, 54% (c-stat 0.67) and 4%, 22%, 62% (c-stat 0.811), accordingly. The risk stratification model was validated with chi-squared goodness-of-fit test (P = 0.425).
A validated predictive model featuring alpha-fetoprotein, salvage LT, and the sum of largest tumor diameter and total number of tumor nodule provides simple and reliable guidance for individualizing postoperative surveillance strategy.
Core tip: Recurrent hepatocellular carcinoma is the leading cause of death after liver transplantation. A validated predictive system for the chance of post-transplant recurrence of hepatocellular carcinoma is indispensable for stratifying patients into different risk groups. This study found that salvage liver transplantation, pre-transplant alpha-fetoprotein level, and the sum of pathological tumor size and number were the three independent factors associated with recurrence. Based on this, a scoring model was derived, validated, and found to have good concordance and is therefore recommendable to be used as a guide for postoperative patient surveillance.
- Citation: Ma KW, She WH, Chan ACY, Cheung TT, Fung JYY, Dai WC, Lo CM, Chok KSH. Validated model for prediction of recurrent hepatocellular carcinoma after liver transplantation in Asian population. World J Gastrointest Oncol 2019; 11(4): 322-334
- URL: https://www.wjgnet.com/1948-5204/full/v11/i4/322.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i4.322
Regardless of which selection criteria are used, post-LT hepatocellular carcinoma (HCC) recurrence happens in around 15%-20% of the cases, and it remains an important cause of death for HCC patients after liver transplantation (LT). A number of factors have been found to be associated with post-LT HCC recurrence, including alpha-fetoprotein (AFP)[1,2], PIVKA-II[3], lympho-vascular permeation[4,5], tumor differentiation[6], response to down-staging or bridging therapies[7-10], etc. However, studies focusing on the development of a risk prediction model for HCC recurrence had been limited, and this set the stage for research of predictive scoring model at different centers[11-14]. One of the scoring systems known as Risk Estimation of Tumor Recurrence After Transplant (RETREAT) score was proposed by a group at the University of California at San Francisco (UCSF)[12]. It is composed of three parameters, namely serum AFP level, presence of microvascular invasion, and the sum of largest tumor diameter and total number of tumor nodules. Such predictive scores were shown to have a high concordance index in relation to HCC recurrence. Nonetheless, characteristics of the population from which the RETREAT score was derived were quite different from those of Asian regions. For example, only 20% of the population from which the RETREAT score derived was hepatitis B virus (HBV) carriers. In contrast, 80% of our population was HBV carriers. There is a need to develop a scoring system that is readily generalizable to Asian HCC patients. In this study, we derive and validate a new scoring system, aiming to delineate better the post-LT HCC recurrence risk so that future surveillance strategies can be more individualized and immunosuppressive therapy with anti-tumor properties can be commenced in a timely manner.
Consecutive patients diagnosed with HCC and admitted to our center for LT from 1995 to 2016 were recruited. Pediatric patients and patients with final pathological diagnosis other than HCC (i.e., mixed hepato-cholangiocarcinoma) were excluded from analysis. Demographic characteristics, preoperative biochemical and imaging data, operative outcomes, pathological findings, and survival data were extracted from our prospectively maintained database. Categorical data and continuous data were compared with chi-squared/Fisher’s exact text and the Mann-Whitney U/t-test whenever appropriate.
All recruited patients were randomly allocated to the training or validation set by a computer software program in a 60:40 proportion. Disease-free survival was defined as the duration between the day of LT and the day of HCC recurrence as detected by imaging or occurrence of death. Independent factors affecting disease-free survival were identified using Cox-regression model with the forward conditional method. A risk score was assigned to each independent factor according to the value of the odds ratio. The total (sum of all risk scores) risk score was used to predict post-LT HCC recurrence. Patients were then stratified into three risk groups, namely low, moderate, and high risk of HCC recurrence. The accuracy of total risk score and risk stratification in predicting HCC recurrence was evaluated by area under the receiver-operating characteristic (ROC) curve.
Total risk scores of patients in the validation set were calculated using the model derived from the training set. Correlations between the total risk score, risk stratification model, and HCC recurrence in the validation population were again assessed by area under ROC curve. The validity of the risk stratification model was assessed by chi-squared goodness-of-fit test. P-value of over 0.05 signified no significant difference between the expected and observed HCC recurrence rates.
HCC patients have to meet the UCSF criteria[1] before they are considered eligible for LT listing. Bonus Model for End Stage Liver Disease (MELD) score of 18 is granted to a patient whose HCC remains in United Network for Organ Sharing stage II for over 6 mo, and an extra two points will be added every subsequent 3 mo[15,16]. Down-staging therapy is not adopted in our center partly due to the severe graft shortage in our locality. Bridging therapy with trans-arterial chemoembolization or stereotactic external beam radiotherapy is given to reduce dropout due to disease progression[17,18]. Bonus MELD score granting is stopped when HCC has progressed beyond United Network for Organ Sharing T2, and patients are delisted if their HCC tumor status has progressed beyond UCSF criteria. A patient with a tumor slightly beyond UCSF criteria might still be given LT if a living donor is available. Techniques of living-donor LT have been described elsewhere[19,20]. In brief, the donor should be a completely healthy individual, and the residual liver volume to estimated standard liver volume must be over 30%. The calculated graft-to-estimated standard liver volume should be over 35% so as to minimize the chance of small-for-size syn-drome[21-23]. The middle hepatic vein is always taken with the liver graft, and venoplasty for hepatic outflow reconstruction is routine for all right lobe liver grafts[24]. In the first 3 mo after LT, mycophenolate mofetil and tacrolimus are given. After 3 mo, tacrolimus monotherapy is used for immunosuppression. AFP level check and contrasted computed tomography/magnetic resonance imaging are performed every 6 mo as surveillance for post-LT HCC recurrence.
Salvage LT in this study referred to LT performed for recurrent HCC. Patients might have received prior curative treatments such as radiofrequency ablation or hepatectomy. To be listed for deceased-donor LT, the patients’ tumor status in the primary or recurrent episode must be within UCSF criteria. However, patients with a tumor beyond UCSF criteria might still be given living-donor LT if keen living donors were available, and this decision would be reached jointly by surgeons and relatives. This study was retrospective, observational in nature. Results were generated by meticulous analysis of the data from the local LT database. Approval from institutional reviewer board for this study was not applicable.
There were 330 eligible patients recruited in the study period. The median age was 56 years, and the majority of them were male. The median follow-up duration of the series was 65.4 mo. The median MELD score was 12.4, the median pre-LT AFP level was 18 ng/mL, and the median explant tumor size was 3 cm. Over half of the patients had a solitary HCC nodule. Living donor graft was the predominant graft type in this series. Micro-vascular invasion and poorly differentiated tumor grading were identified in 29.4% and 15.3% of the patients, respectively. Salvage LT was performed in 71 patients (21.5%), and 33.8% of this group of patients had microvascular invasion while 71.4% had well/moderate tumor differentiation. The median time from last curative treatment to salvage LT was 28 mo. Hospital mortality and serious morbidity (Clavien-Dindo classification IIIa or above) was 1.6% and 23.3%, respectively. Post-LT HCC recurrence was diagnosed in 47 (14.2%) out of 330 patients. The 5-year disease-free and overall survival rates were 78% and 80%, respectively (Table 1).
| Characteristic | Total, n = 330 |
| Age (yr, range) | 56 (30-73) |
| Sex (male) | 269 (81.5%) |
| Hepatitis B carrier (%) | 81.6% |
| Waiting time from listing to transplantation (mo) | 1 (0-89) |
| MELD | 12.4 (6-59) |
| Size of tumor in explant (cm) | 3(0.25-19.5) |
| No. of tumor nodules in explant One Two Three | 163 (51.1%) 85 (26.6%) 28 (8.8%) |
| Within UCSF criteria | 75% |
| Within Milan Criteria | 65.6% |
| Serum AFP level before time of LT (ng/mL) | 18.0 (1-117850) |
| Salvage LT (%) | 71 (21.5%) |
| LDLT (%) | 204 (61.8%) |
| Microvascular invasion (%) | 97 (29.4%) |
| Well/mod differentiation (%) | 271 (84.7%) |
| Operation duration (min) | 671 |
| Blood loss (mL) | 2600 (200-30800) |
| Hospital mortality (%) | 5 (1.6%) |
| Morbidity (ClavienIIIa or above) (%) | 23.3% |
| Length of hospital stay (d) | 16 (7-378) |
| Post-transplant HCC recurrence (%) | 73 (23.9%) |
| Median disease-free survival (mo) | 60.8 (0-263) |
| Median overall survival (mo) | 65.4 (0-263) |
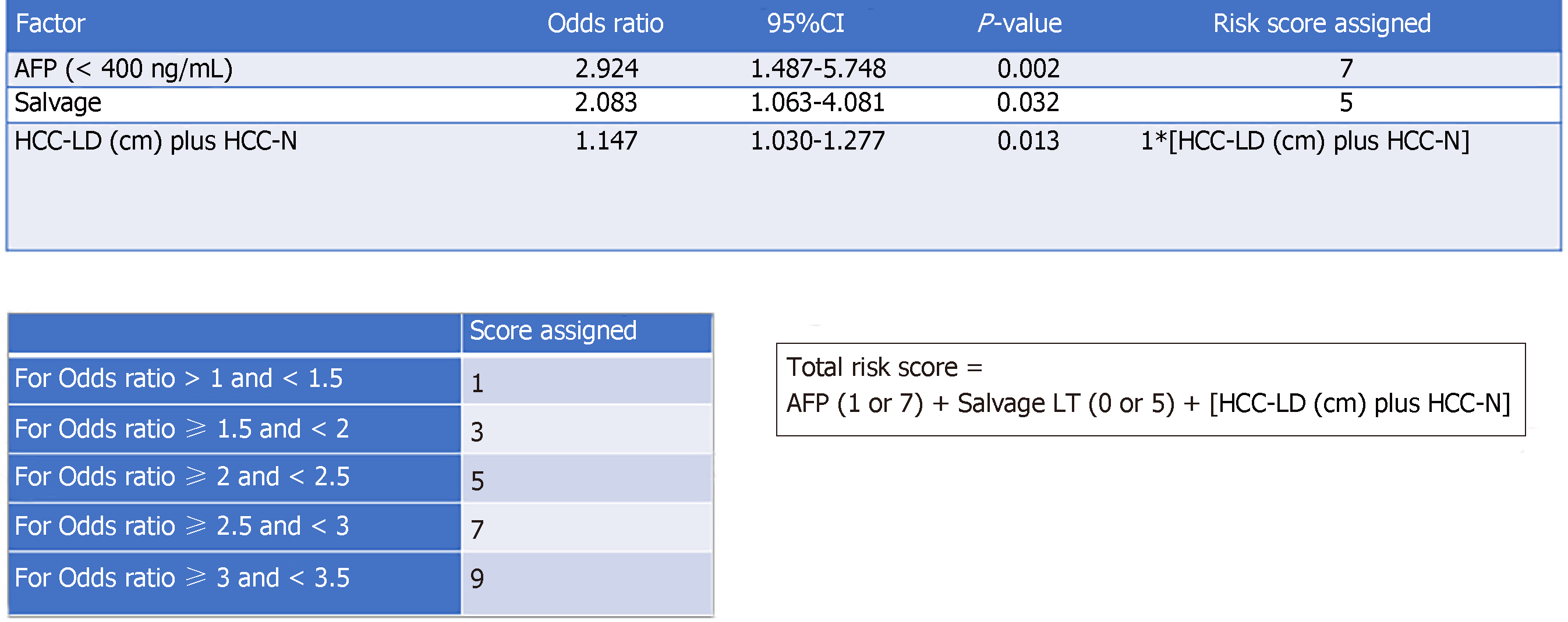
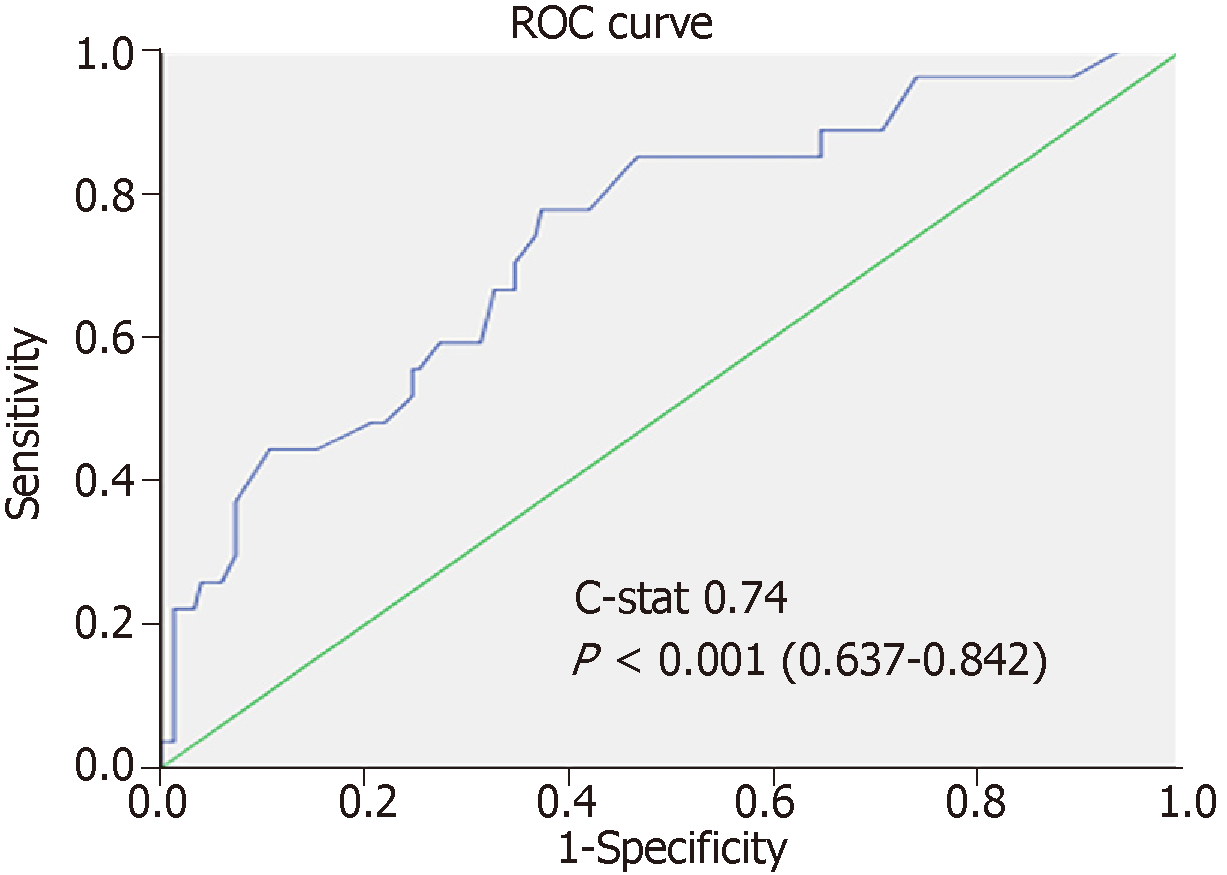
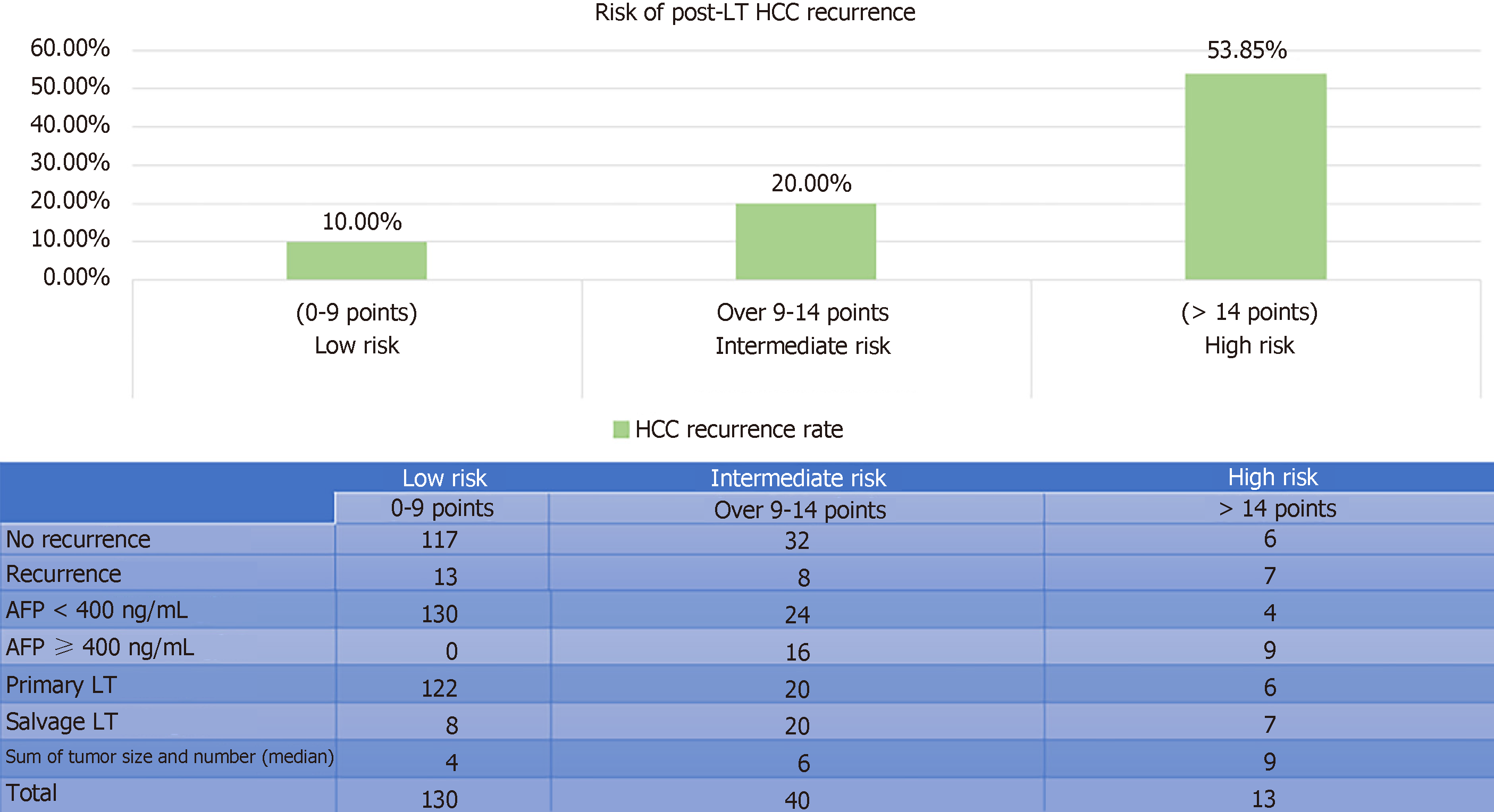
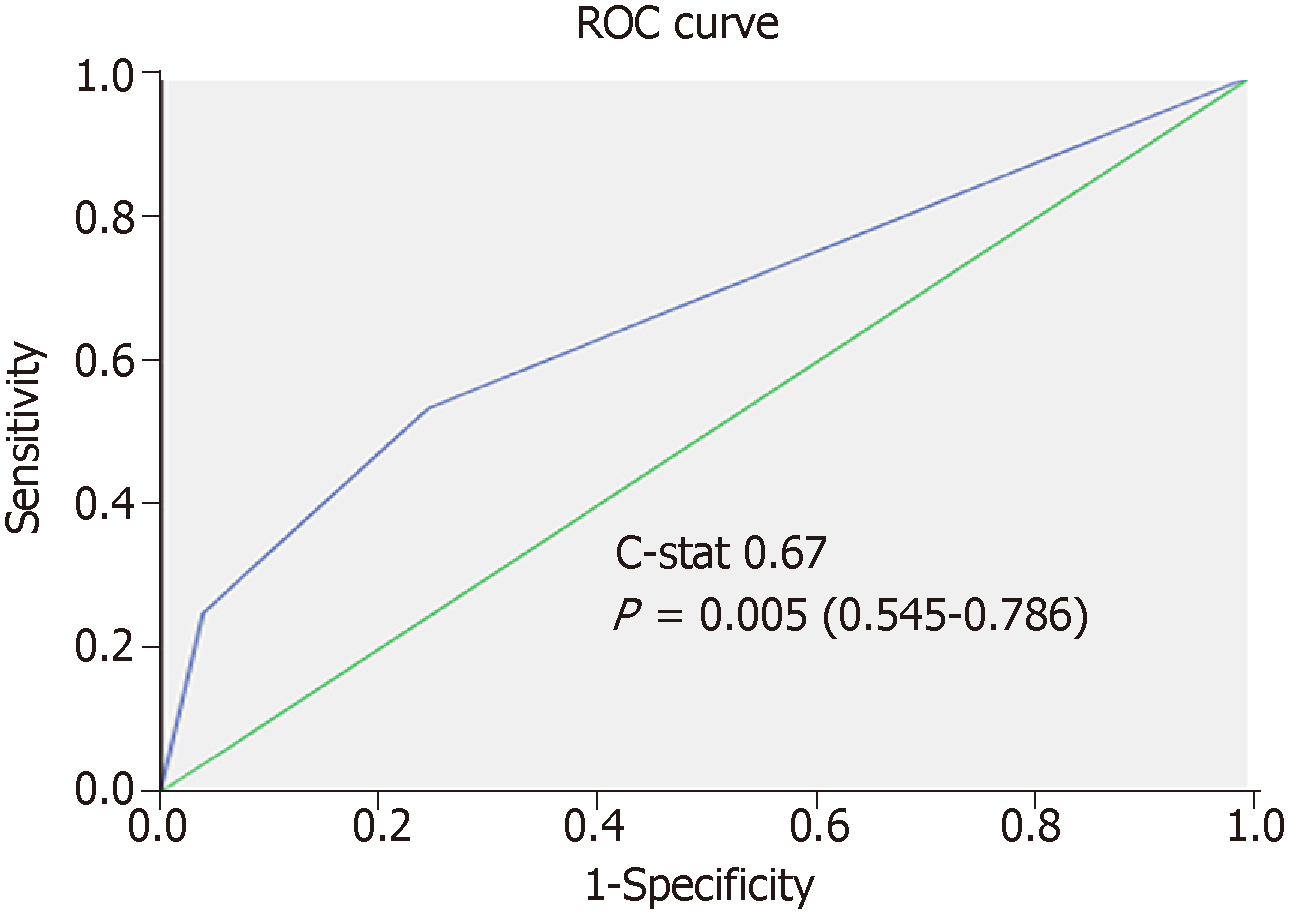
After randomization, 183 and 147 patients were allocated to the training set and the validation set, respectively. There was no statistically significant difference between the groups in terms of demographic characteristics, biomarkers, graft type, tumor characteristics, or operative outcomes (Table 2). Five factors were found to be associated with inferior disease-free survival, namely number of tumor nodule in explant (P = 0.007), serum AFP over 400 ng/mL before LT (P = 0.002), salvage LT (P = 0.019), UCSF criteria (P = 0.009), and the sum of large tumor size and total number of HCC nodule in explant (P = 0.002). After multivariate analysis, only three factors were shown to be independent risk factors: AFP over 400 ng/mL (P = 0.012, OR 2.92), salvage LT (P = 0.033, OR 2.08), and the sum of largest tumor size and total number of HCC nodule in explant (P = 0.013, OR 1.15) (Table 3). According to their respective odds ratios, patients with preoperative serum AFP level over 400 ng/mL were given a score of 7, those who underwent LT for recurrent HCC were given a score of 5, and the sum of largest tumor diameter (cm) and total number of tumor nodule would contribute to the total risk score directly (Figure 1). The accuracy of this scoring system was tested by area under the ROC curve, and the c-index in the training set was 0.74 (P < 0.001, 95%CI: 0.637-0.842) (Figure 2). Patients were stratified into low-, moderate-, and high-risk groups according to their corresponding total risk scores, and the chance of HCC recurrence was 10.6%, 22.7% and 53.9%, respectively. The corresponding numbers of patients exposed to the three independent predictors were tabulated in Figure 3. The concordance index for this risk stratification model was 0.67 (P = 0.005, 95%CI: 0.545-0.786) (Figure 4).
| Characteristic | Training set, n = 183 | Validation set, n = 147 | P-value |
| Age | 55 ( ± 7.1) | 55 ( ± 7.0) | 0.768 |
| Sex (male, %) | 80% | 83% | 0.570 |
| HBsAg positivity (%) | 82% | 80.3% | 0.777 |
| MELD | 14.7 (± 7.5) | 13.8 (± 7.0) | 0.284 |
| Waiting time from listing to transplantation (mo) | 1 (0-89) | 1 (0-81) | 0.051 |
| Size of tumor in explant (cm) | 3.0 (± 1.4) | 3.3 (± 2.1) | 0.116 |
| No. of tumor nodules in explant | 2.1 (± 1.8) | 2.3 (± 2.5) | 0.609 |
| Serum AFP level before time of LT (ng/mL) | 586 ( ± 3169) | 1719 ( ± 10541) | 0.169 |
| Salvage LT (%) | 19.1% | 24.5% | 0.281 |
| LDLT (%) | 112 (62.9%) | 91 (61.9%) | 0.908 |
| Microvascular invasion (%) | 28.8% | 32.2% | 0.713 |
| Well/mod differentiation | 87.5% | 81.1% | 0.237 |
| HCC recurrence | 24% | 23.8% | 1.000 |
| 5 yr disease-free survival (%) | 78.8% | 76.8% | 0.733 |
| 5 yr overall survival (%) | 80.8% | 78.7% | 0.733 |
| Variable | Univariate | Multivariate | HR (95%CI) |
| Sex | 0.525 | - | - |
| Age | 0.062 | - | - |
| Albumin | 0.816 | - | - |
| Platelet count | 0.094 | - | - |
| MELD | 0.874 | - | - |
| Tumor size in explant | 0.059 | - | - |
| Number of tumors in explant | 0.007 | NS | - |
| Microvascular invasion | 0.895 | - | - |
| Well/mod differentiation | 0.811 | - | - |
| Serum AFP level before LT ( ≥ 400 ng/mL) | 0.002 | 0.012 | 2.92 (1.487-5.748) |
| Graft (g)/ESLW | 0.083 | - | - |
| Waiting time from listing to transplantation | 0.77 | - | - |
| Salvage LT | 0.019 | 0.033 | 2.08 (1.063-4.081) |
| Milan criteria | 0.052 | NS | - |
| UCSF criteria | 0.009 | NS | - |
| Sum of tumor size and number | 0.002 | 0.013 | 1.15 (1.03-1.28) |
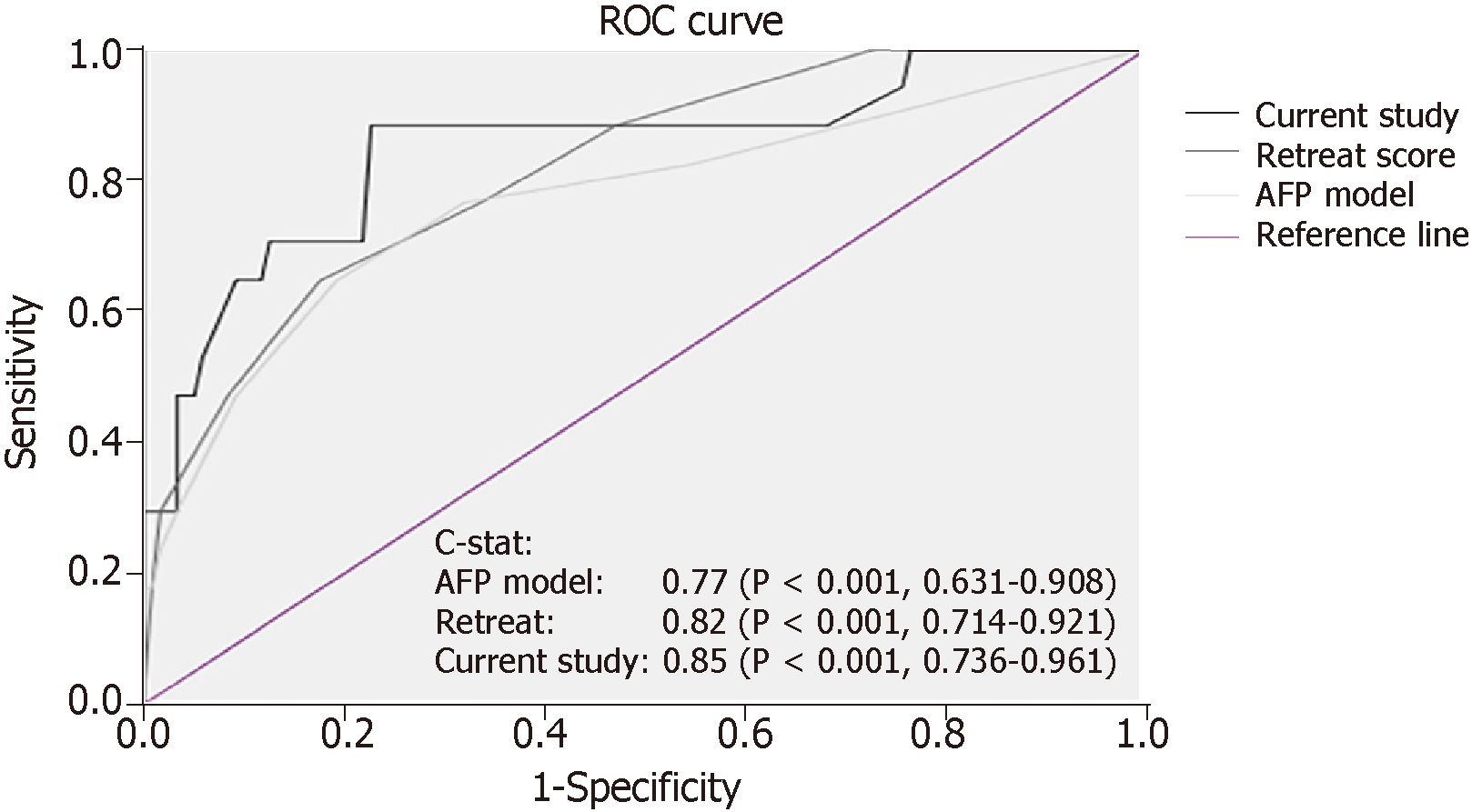
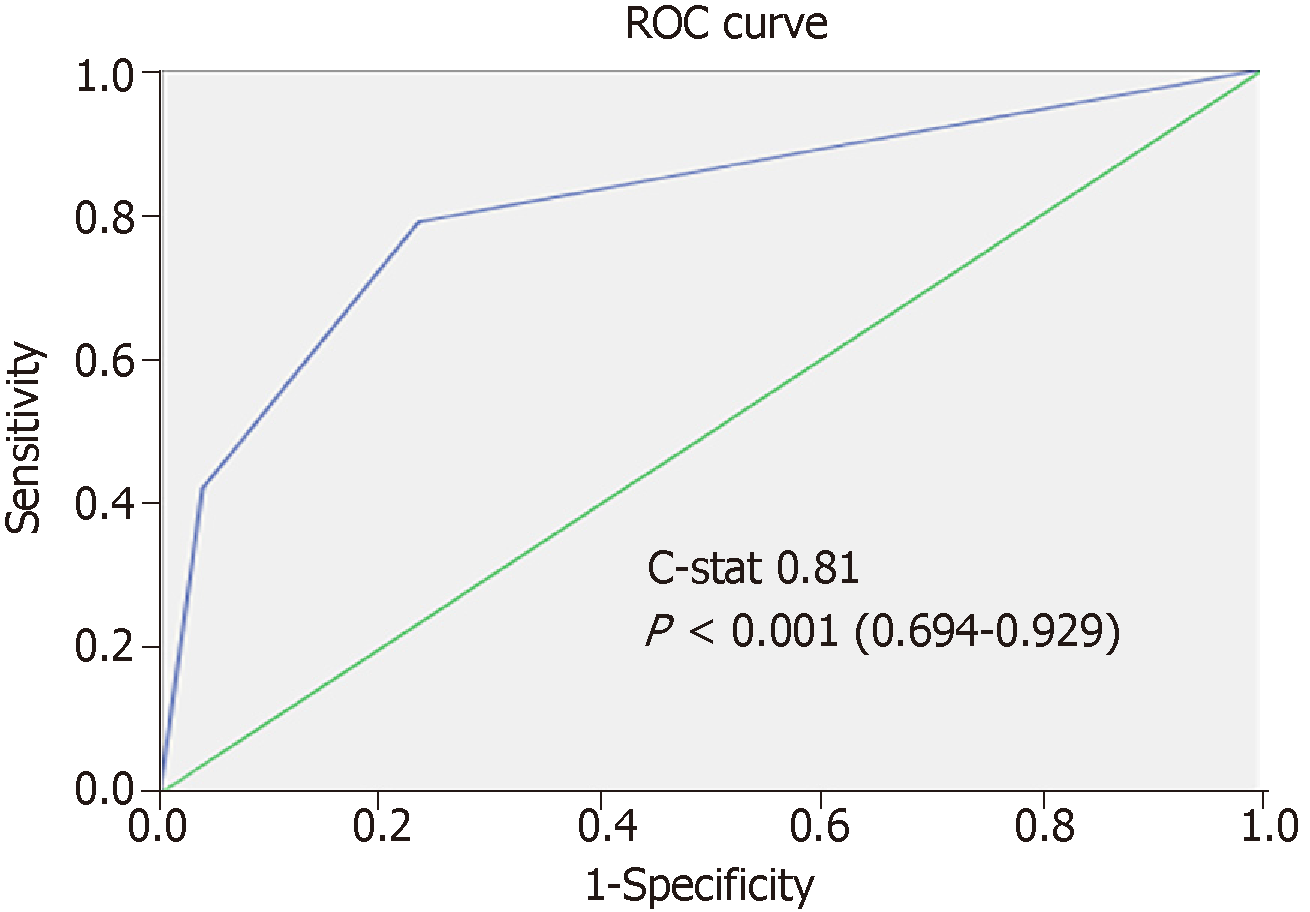
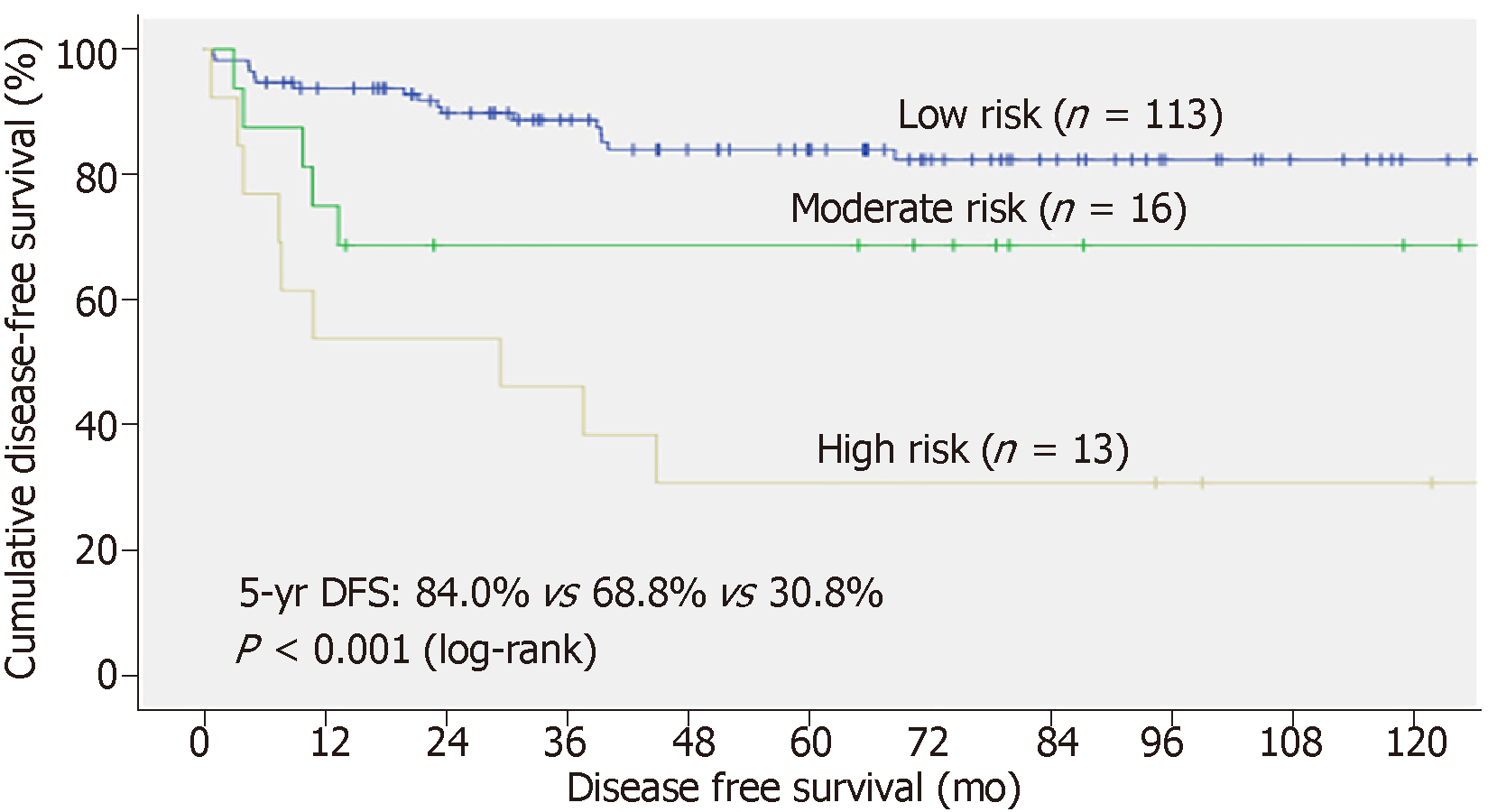
In the validation set, total risk score, AFP model score, and RETREAT score were calculated for each patient. The scoring system in the current study was validated [c-index 0.85 (P < 0.001, 0.736-0.961)] and was shown to have better correlation with post-LT HCC recurrence than the AFP model and the RETREAT scoring system [c-index 0.77 (P < 0.001, 0.631-0.908) and 0.82 (P < 0.001, 0.714-0.921), respectively] (Figure 5). Patients in the validation set were stratified into three risk groups as per the training set (Figure 6), and the concordance index was 0.81 [P < 0.001 (0.645-0.919)] (Figure 7). This risk stratification model was then tested by the chi-squared goodness-of-fit test, and the result showed no statistically significant difference between the expected and observed HCC recurrence rates with this model (P = 0.459) (Figure 8). There was distinct difference in the disease-free survival of the patients in the low, moderate, and high risk groups, i.e., 84.0% vs 68.8% vs 30.8%, respectively (Log-rank P < 0.001) (Figure 9).
This study illustrated the development of a new scoring system featuring pre-LT AFP, salvage LT, and the sum of largest tumor size and number of tumor nodule on explant examination. In the validation process, this scoring system exhibited a high concordance index (c-index 0.85) in relation to HCC recurrence and demonstrated performance at least comparable to the AFP model (c-stat 0.77) and the RETREAT score (c-index 0.82). The proposed risk stratification model showed satisfactory goodness-of-fit between the expected and observed HCC recurrence rates, with high discrimination ability as exemplified by the Kaplan-Meier curve separation for the disease-free survival in patients from three different risk groups.
Since the introduction of the RETREAT score in 2017, it was widely accepted in Europe and North America as one of the most useful tools in predicting HCC recurrence after transplantation. RETREAT score was derived and externally validated using multicenter data of over 1000 patients. The good study design, scientific analyses, and encouraging results accounted for its success and popularity. In contrast, our current model was developed and internally validated based on a single center data with sample size of roughly one third to that of the RETREAT score. These facts do not negate the value of our current model, as the RETREAT score might not be readily generalizable to Asian populations (19.1% were Asian in the RETREAT score population), where prevalence of HBV carrier state is up to 80%. In addition, we are the first to incorporate quantitatively and qualitatively salvage transplantation, which is a common strategy for HCC in many centers, as one of the variables in the predictive model. We believe that the current model should work complementarily with RETREAT score in predicting HCC recurrence in particularly for patients of Asian ethnicity.
In this study, a number of preoperative and pathological factors were put into multivariate analysis. The influence of tumor size and multiplicity on post-LT HCC recurrence had been well recognized since the introduction of Milan criteria and its subsequent variants from other series. The effect of the sum of largest tumor diameter and total tumor number was illustrated by the “Metro-ticket” concept introduced by Mazzaferro et al[25]. The tumor size and number used in the current analyses were from explant measurement rather than preoperative imaging. The authors considered this a more objective and accurate assessment of tumor metrics when compared to preoperative imaging measurement. This is because the discrepancy between different imaging modalities (computed tomography, magnetic resonance imaging, or positron emission tomography), contrast injection protocols, and presence of inter-observer variability during reporting could lead to substantial errors, such as over- or under-reporting of tumor size and number. Using explant measurements should be theoretically more objective and accurate. Pathological tumor size and number should be inferable to pre-LT imaging during patient selection and counselling process.
Pre-LT serum AFP over 400 ng/mL was shown to be the most influential factor associated with disease-free survival. The role of AFP in HCC recurrence after LT has been demonstrated in the literature[26-28]. Moreover, it has been suggested that AFP over 1000 ng/mL should be a contraindication to LT even if Milan criteria are met[29]. The fact that AFP is frequently included as a parameter in other published scoring systems suggests that it is an important recurrence predictor[11,12,30,31]. In addition, there is a growing body of evidence that suggests that AFP level response to bridging loco-regional therapy has a significant prognostic effect in terms of post-LT HCC recurrence[32,33]. Although AFP response to pre-LT therapy was not investigated in our current series, future study should look into the effect of incorporating this variable into the existing risk prediction models.
Since its introduction by Majno et al[34], the approach of salvage LT has been a topic of debate. Theoretically, by delaying (resection first, transplant when recurrence occurs) or negating (curative hepatectomy in some HCC patients) LT, this “salvage” policy should be able to relieve the donor pool burden[35]. However, there are worries about the development of un-transplantable HCC recurrence after initial resection and inferior oncological outcomes[36-38]. In an intention-to-treat analysis by Fuks et al[36], half of the patients with recurrent HCC did not receive salvage LT because of disease beyond Milan criteria and deteriorated physical conditions. Patients who received salvage LT were also shown to have inferior disease-free survival[37,38]. In our current analysis, salvage LT, regardless of the previous treatment modality and number of tumor recurrence, was a significant predisposing factor for HCC recurrence.
Microvascular invasion has been shown to be an important factor in post-LT HCC recurrence in the literature. Failure to demonstrate this relationship in our series could be explained by the low prevalence of HCC recurrence (14.2%) and microvascular invasion (29.4%) in this study population. In our multivariate analysis, AFP over 400 ng/mL, salvage LT, and the sum of tumor size and number in explant were the more influential factors in this group of good risk HCC population. AFP over 400 ng/mL before LT represented a remarkable risk of post-LT HCC recurrence as suggested by the risk score of 7 for this predictor.
There were some limitations of the current study. Firstly, confounders associated with retrospective study, such as missing data and selection bias, could not be totally avoided. Secondly, the fact that some of the HCC patients in this analysis were transplanted with MELD bonus scores might have led to data contamination as patients from pre- and post-MELD-bonus eras had different tumor characteristics and prognosis; the random allocation of patients to the training and validation sets could have alleviated this problem. Thirdly, parameters such as neutrophil/platelet to lymphocyte ratio, total tumor volume, and other newer biomarkers were not investigated. Last but not least, despite good performance of our proposed scoring system demonstrated in the independent, randomly allocated validation population, the relatively small patient number (n = 147) might have limited its validating power, stressing the importance of multicenter patient recruitment in a future external validation study. Nonetheless, this new predictive model provides a simple and reliable way to stratify patients into different recurrence risk groups. For patients who belong to the high HCC recurrence risk group, close surveillance and early commencement of m-TOR inhibitor are strongly recommended, as the predicted recurrence risk is over 50%. To extrapolate further the application of our study, when a patient has a high pre-LT AFP level (i.e., over 400 ng/mL) and tumor metrics unequivocally approaching the “up-to-7” Metro-ticket limit value on pre-LT imaging, his/her baseline risk score is expected to be close to 14. Salvage LT in such a scenario should be rejected or at least deferred until the AFP level and tumor metrics could be brought down to a “safer” level by bridging loco-regional therapy.
In conclusion, a scoring system featuring pre-LT AFP level, salvage LT, and the sum of tumor largest diameter and total number of tumor nodule was developed and validated. This scoring system helps to stratify patients into different HCC recurrence risk groups, and thus an individualized surveillance strategy and immuno-suppressive protocol can be implemented.
Disease recurrence remains the chief reason for post-transplant mortality for hepatocellular carcinoma (HCC) patients. High risk patients should undergo close biochemical and radiological surveillance and start immunosuppressive agent with anti-tumor effect soon after the operation. A risk predictive model helps to implement this strategy selectively; however, the availability of such model is limited in the literature.
A more well-known validated model, the RETREAT score, had been popularized in Western Europe and North America. However, this model had not been validated in Asian populations where the prevalence of hepatitis B virus (HBV) infection is high. Therefore, another validated model that serves a complementary role to the RETREAT score has value.
This study aimed to derive and validate a predictive model using a database from a large transplant center.
All patients were randomly allocated to training and validation sets. Factors that predict HCC recurrence were identified using multivariate analysis. A risk score was assigned to each of these factors according to their corresponding odds ratio and then a scoring model was developed. The accuracy of this model was validated and compared with other scoring models using data in the validation set with receiver-operating characteristic curve.
This is the first scoring model derived and validated in an Asian population. It is also the first time to incorporate salvage liver transplantation (LT) as one of the variables in the predictive system. This new model compared favorably with the RETREAT score, which did not include salvage LT in the multivariate analysis.
We believe that salvage LT should be included in the predictive model for post-LT HCC recurrence. This new model could be an improvement for Asian populations where HBV infection is prevalent.
Patients who were classified as high risk of HCC recurrence should be given close biochemical and radiological surveillance to detect early recurrence. In addition, immunosuppressant with anti-tumor effect (i.e., m-TOR inhibitor) should be commenced after 1 mo together with the minimization of calcineurin inhibitor. Future multi-center external validation should be contemplated to define further its accuracy and role.
The authors would like to thank Ms. Lam Ka Yiu Banny (BSc) and Mr. Yuen Kim [BSc (Hons), Stat and Comp] for data and statistical support.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gad EH, Stanciu C S-Editor: Ji FF L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1695] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 2. | Nörthen A, Asendorf T, Walson PD, Oellerich M. Diagnostic value of alpha-1-fetoprotein (AFP) as a biomarker for hepatocellular carcinoma recurrence after liver transplantation. Clin Biochem. 2018;52:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Furukawa H, Shimamura T, Suzuki T, Taniguchi M, Nakanishi K, Yamashita K, Kamiyama T, Matsushita M, Todo S. Liver transplantation for hepatocellular carcinoma: the Japanese experience. J Hepatobiliary Pancreat Sci. 2010;17:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17 Suppl 2:S72-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD, Grant DR. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 229] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Agopian VG, Harlander-Locke MP, Ruiz RM, Klintmalm GB, Senguttuvan S, Florman SS, Haydel B, Hoteit M, Levine MH, Lee DD, Taner CB, Verna EC, Halazun KJ, Abdelmessih R, Tevar AD, Humar A, Aucejo F, Chapman WC, Vachharajani N, Nguyen MH, Melcher ML, Nydam TL, Mobley C, Ghobrial RM, Amundsen B, Markmann JF, Langnas AN, Carney CA, Berumen J, Hemming AW, Sudan DL, Hong JC, Kim J, Zimmerman MA, Rana A, Kueht ML, Jones CM, Fishbein TM, Busuttil RW. Impact of Pretransplant Bridging Locoregional Therapy for Patients With Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation: Analysis of 3601 Patients From the US Multicenter HCC Transplant Consortium. Ann Surg. 2017;266:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 8. | Lai Q, Nicolini D, Inostroza Nunez M, Iesari S, Goffette P, Agostini A, Giovagnoni A, Vivarelli M, Lerut J. A Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) Score. Ann Surg. 2016;264:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Cucchetti A, Cescon M, Bigonzi E, Piscaglia F, Golfieri R, Ercolani G, Cristina Morelli M, Ravaioli M, Daniele Pinna A. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl. 2011;17:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Otto G, Herber S, Heise M, Lohse AW, Mönch C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A, Pitton M. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 314] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, Dharancy S, Gugenheim J, Bernard PH, Adam R, Radenne S, Muscari F, Conti F, Hardwigsen J, Pageaux GP, Chazouillères O, Salame E, Hilleret MN, Lebray P, Abergel A, Debette-Gratien M, Kluger MD, Mallat A, Azoulay D, Cherqui D; Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-94.e3; quiz e14-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 726] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 12. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 13. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 475] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 14. | Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AM, Kneteman NM. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Chan SC, Sharr WW, Chok KS, Chan AC, Lo CM. Wait and transplant for stage 2 hepatocellular carcinoma with deceased-donor liver grafts. Transplantation. 2013;96:995-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Ma KW, Cheung TT. When to consider liver transplantation in hepatocellular carcinoma patients? Hepat Oncol. 2017;4:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | She WH, Cheung TT. Bridging and downstaging therapy in patients suffering from hepatocellular carcinoma waiting on the list of liver transplantation. Transl Gastroenterol Hepatol. 2016;1:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Katz AW, Chawla S, Qu Z, Kashyap R, Milano MT, Hezel AF. Stereotactic hypofractionated radiation therapy as a bridge to transplantation for hepatocellular carcinoma: clinical outcome and pathologic correlation. Int J Radiat Oncol Biol Phys. 2012;83:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Chan SC, Fan ST, Lo CM, Liu CL, Wei WI, Chik BH, Wong J. A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes. Ann Surg. 2008;248:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Ma KW, Chok KSH, Chan ACY, Tam HSC, Dai WC, Cheung TT, Fung JYY, Lo CM. A new formula for estimation of standard liver volume using computed tomography-measured body thickness. Liver Transpl. 2017;23:1113-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Chan SC, Liu CL, Lo CM, Lam BK, Lee EW, Wong Y, Fan ST. Estimating liver weight of adults by body weight and gender. World J Gastroenterol. 2006;12:2217-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 55] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Chan SC. Section 2. Small-for-size liver graft and hepatocellular carcinoma recurrence. Transplantation. 2014;97 Suppl 8:S7-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Lo CM, Fan ST, Liu CL, Wong J. Hepatic venoplasty in living-donor liver transplantation using right lobe graft with middle hepatic vein. Transplantation. 2003;75:358-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P; Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1572] [Article Influence: 92.5] [Reference Citation Analysis (1)] |
| 26. | Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Mailey B, Artinyan A, Khalili J, Denitz J, Sanchez-Luege N, Sun CL, Bhatia S, Nissen N, Colquhoun SD, Kim J. Evaluation of absolute serum α-fetoprotein levels in liver transplant for hepatocellular cancer. Arch Surg. 2011;146:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Agopian VG, Harlander-Locke MP, Markovic D, Zarrinpar A, Kaldas FM, Cheng EY, Yersiz H, Farmer DG, Hiatt JR, Busuttil RW. Evaluation of Patients With Hepatocellular Carcinomas That Do Not Produce α-Fetoprotein. JAMA Surg. 2017;152:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 228] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 30. | Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, Kato T, Verna EC, Emond JC, Brown RS. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg. 2017;265:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 31. | Yang SH, Suh KS, Lee HW, Cho EH, Cho JY, Cho YB, Kim IH, Yi NJ, Lee KU. A revised scoring system utilizing serum alphafetoprotein levels to expand candidates for living donor transplantation in hepatocellular carcinoma. Surgery. 2007;141:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Lai Q, Avolio AW, Graziadei I, Otto G, Rossi M, Tisone G, Goffette P, Vogel W, Pitton MB, Lerut J; European Hepatocellular Cancer Liver Transplant Study Group. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19:1108-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 33. | Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Majno PE, Sarasin FP, Mentha G, Hadengue A. Primary liver resection and salvage transplantation or primary liver transplantation in patients with single, small hepatocellular carcinoma and preserved liver function: an outcome-oriented decision analysis. Hepatology. 2000;31:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Belghiti J, Cortes A, Abdalla EK, Régimbeau JM, Prakash K, Durand F, Sommacale D, Dondero F, Lesurtel M, Sauvanet A, Farges O, Kianmanesh R. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg. 2003;238:885-892; discussion 892-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 319] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 36. | Fuks D, Dokmak S, Paradis V, Diouf M, Durand F, Belghiti J. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology. 2012;55:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 37. | Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508-18; discussion 518-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 239] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Bhangui P, Allard MA, Vibert E, Cherqui D, Pelletier G, Cunha AS, Guettier C, Vallee JC, Saliba F, Bismuth H, Samuel D, Castaing D, Adam R. Salvage Versus Primary Liver Transplantation for Early Hepatocellular Carcinoma: Do Both Strategies Yield Similar Outcomes? Ann Surg. 2016;264:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |