Published online Feb 15, 2019. doi: 10.4251/wjgo.v11.i2.71
Peer-review started: November 10, 2018
First decision: November 28, 2018
Revised: December 11, 2018
Accepted: January 1, 2019
Article in press: January 1, 2019
Published online: February 15, 2019
Processing time: 101 Days and 18.8 Hours
Trans-acting factors controlling mRNA fate are critical for the post-transcriptional regulation of inflammation-related genes, as well as for oncogene and tumor suppressor expression in human cancers. Among them, a group of RNA-binding proteins called “Adenylate-Uridylate-rich elements binding proteins” (AUBPs) control mRNA stability or translation through their binding to AU-rich elements enriched in the 3’UTRs of inflammation- and cancer-associated mRNA transcripts. AUBPs play a central role in the recruitment of target mRNAs into small cytoplasmic foci called Processing-bodies and stress granules (also known as P-body/SG). Alterations in the expression and activities of AUBPs and P-body/SG assembly have been observed to occur with colorectal cancer (CRC) progression, indicating the significant role AUBP-dependent post-transcriptional regulation plays in controlling gene expression during CRC tumorigenesis. Accordingly, these alterations contribute to the pathological expression of many early-response genes involved in prostaglandin biosynthesis and inflammation, along with key oncogenic pathways. In this review, we summarize the current role of these proteins in CRC development. CRC remains a major cause of cancer mortality worldwide and, therefore, targeting these AUBPs to restore efficient post-transcriptional regulation of gene expression may represent an appealing therapeutic strategy.
Core tip: Colorectal cancer (CRC) is a deadly cancer associated with the deregulation of multiple genetic and epigenetic mechanisms, leading to the silencing of tumor suppressors and the induction of both oncogenes and inflammation-related genes. Among them, a novel class of RNA-binding proteins called Adenylate-Uridylate-rich element-binding proteins have been involved in the post-transcriptional regulation of genes linked to CRC tumorigenesis. Current findings indicate the major regulatory roles these RNA-binding proteins have on deregulated pathways associated with CRC. Therefore, targeting these proteins may represent a novel and efficient therapeutic approach.
- Citation: Legrand N, Dixon DA, Sobolewski C. AU-rich element-binding proteins in colorectal cancer. World J Gastrointest Oncol 2019; 11(2): 71-90
- URL: https://www.wjgnet.com/1948-5204/full/v11/i2/71.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i2.71
Colorectal cancers (CRCs) represent the third most frequent cancer and a leading cause of cancer mortality worldwide[1,2]. The progression model of CRC tumorigenesis results from a long deregulated process that initiates with the development of small adenomas, large adenomas and finally CRC[3]. In the majority of cases, CRCs develop sporadically (70% of cases) and rarely are due to inherited mutations underlying familial adenomatous polyposis (FAP) and the Lynch syndromes[4]. The causes of sporadic CRC development are still unclear, but several risk factors have been identified, including older age, obesity, diabetes, sedentary lifestyle, alcohol consumption or chronic inflammatory diseases (i.e., inflammatory bowel diseases, Crohn’s disease and ulcerative colitis)[2,5,6]. Considering the pandemic of obesity and type-2 diabetes in developed countries[7], the incidence of CRC is expected to increase in the future, making it a major public health concern and economic burden. CRC is primarily treated by surgery, but also by chemotherapy (e.g., FOLFOX: Folinic acid, 5-fluorouracil, oxaliplatin) and targeted therapy[4]. However, despite this myriad of therapeutic approaches, CRC remains one of the most deadly cancers[2]. Several causes contribute to the development of aggressive metastatic tumors, which include the development of chemoresistance and late diagnosis due to the lack of symptoms at early stages. Therefore, deciphering the molecular features of CRC is still a major research effort to identify novel/early biomarkers and therapeutic approaches.
At the molecular level, CRC has been associated with chromosomal instability and microsatellite instability that can affect tumor suppressor (TS) and oncogene (ONC) expression[4]. These mutations trigger various signaling pathways (i.e., Wnt/β-catenin, TP53, KRAS) involved in most cancer hallmarks (i.e., proliferation, migration, survival)[4]. Unfortunately, many of these mutated genes have proven to be difficult to target therapeutically, considerably limiting the amount of therapeutic options. Nevertheless, ONC/TS and inflammation-associated gene expression are also deregulated through mutational-independent mechanisms or aberrant signaling within the tumor microenvironment. These alterations can be mediated by the metabolic status of the intestinal epithelium, the gut microbiota, epigenetic changes (i.e., DNA methylation, histones acetylation)[8] or the pro-inflammatory environment. Among them, increasing evidence indicates that post-transcriptional mechanisms controlling mRNA stability and translation contribute to CRC tumor progression. Over the last decade, extensive efforts have been devoted to deciphering the impact of non-coding RNAs (i.e., long-non-coding RNAs, microRNAs) during CRC development. More recently, the role of a family of RNA-binding proteins (RBP) called “Adenylate and Uridylate-rich elements-binding proteins” (AUBPs) regulating mRNA stability and translation have been highlighted. Alterations in AUBPs expression/activity have been associated with the development of several inflammatory, metabolic disorders (i.e., osteoarthritis, diabetes) and cancers[9]. During carcinogenesis, these proteins contribute to the activation of various ONC and the silencing of TS, thereby triggering critical pathways involved in CRC development. In this review, we discuss the specific role of these proteins in the onset and progression of CRC, with particular emphasis on their ability to regulate the expression of key ONC, TS and inflammation-related genes. Finally, we discuss the potential of targeting these proteins for therapeutic purposes.
Post-transcriptional regulation of gene expression encompasses various mechanisms that control mRNA processing, splicing, stability and translation. In this regard, the 3’UTR is a determinant region of mRNA transcripts that can be targeted by various trans-acting factors, such as microRNAs, long-non-coding RNA or RBP. In particular, the AU-rich element present in the 3’UTR of various mRNAs plays a critical role in the control of mRNA stability and translation. The ARE is usually defined as a core AUUUA sequence, and is most often composed of multiple copies of the AUUUA motif. Several ARE classes and clusters have been defined based on the number and context of the AUUUA pentamers[10,11]. The presence of one or more ARE in the 3’UTR of mRNAs is frequently observed in immediate-early response genes (e.g., pro-inflammatory cytokines, ONC), which make ARE-dependent regulation critical for several processes like inflammation[12]. Currently, it is estimated that 5-8% of human genes contain an ARE in their 3’UTR[11], thus highlighting the importance of ARE-dependent regulation. The development of several ARE databases, such as AREsite (http://rna.tbi.univie.ac.at/cgi-bin/AREsite.cgi) or ARED (http://brp.kfshrc.edu.sa/ARED/), has provided researchers with potent bioinformatic tools to identify the presence of AREs in eukaryotic mRNAs[13,14]. Moreover, high-throughput gene expression analyses have allowed the identification of several ARE-containing genes deregulated in as early as stage I CRC[15].
Aberrant ARE-dependent post-transcriptional regulation has been observed in all cancer types, and contributes to the overexpression of pro-inflammatory mediators [e.g., Cyclooxygenase-2 (COX-2)], ONC (e.g., c-myc) and to the silencing of TS [e.g., Insulin-like growth factor-binding protein 3 (IGFBP3), Programmed cell death protein 4 (PDCD4)], thereby triggering key oncogenic pathways involved in the establishment of neoplastic phenotypes[10]. Over the last decade, efforts have been devoted to fully characterize the underlying mechanisms associated with this aberrant ARE-dependent regulation in CRC. Among them, several non-coding RNAs and RBPs have been identified, which may represent novel biomarkers and/or therapeutic targets.
The ARE can be targeted by RBPs called “AU-rich Element-Binding Proteins”, which display high affinity for binding to adenine and uridine-rich elements present in the 3’UTR of several immediate-early response genes, such as pro-inflammatory cytokines, growth factors and ONC. To date, more than 20 different AUBPs involved in mRNA stability and translation regulation have been described[11]. Importantly, the expression and role of these proteins may differ depending on the tissue type or the cellular context (e.g., inflammation, hypoxia)[16,17]. AUBPs can promote mRNA decay or regulate mRNA translation by directing ARE-containing mRNAs to P-bodies and stress granules, respectively[18,19]. Therefore, the identification of target mRNAs through transcriptomic approaches is not always suitable, particularly for those involved in translation regulation [i.e., T-Cell-Restricted Intracellular Antigen-1 (TIA1)]. A primary method to identify direct mRNA targets consists of crosslinking ribonucleoprotein (RNP) complexes, followed by the immunoprecipitation of AUBP and RNA sequencing (CLIP-seq, RNP-IP)[6]. Many published studies investigating AUBPs have used in vitro cell models. More recently, however, the development of several in vivo transgenic models have allowed researchers to better characterize the physiological and pathological functions of several AUBPs in the context of tissue-specific expression.
Most AUBPs are regulated by post-translational modifications (e.g., phosphorylation) that can impact their subcellular localization and activity[20,21]. Therefore, not only the expression but also the activity/localization of AUBPs should be considered in research projects. Moreover, AUBPs can regulate the fate of their own mRNA transcript, as demonstrated for tristetraprolin (TTP)[22]. This may result in the transient modulation of their expression, as previously demonstrated for TTP with insulin stimulation[23]. This aspect should also be considered in the design of experimental settings aimed at measuring the level of AUBPs in physiological or pathological conditions.
The complexity of ARE-dependent regulation is further enhanced by the fact that one particular AUBP can regulate several mRNAs and, conversely, one mRNA can also be targeted by distinct AUBPs (e.g., COX-2)[24]. This derives from the fact that AUBPs can bind to different classes of AREs or share the same binding sites (e.g., HuR and TTP for COX-2 regulation). Therefore, it is likely that the phenotype observed with the manipulation of one given AUBP’s expression may contribute to the concerted deregulation of several mRNA transcripts.
Another layer of complexity is provided by the competition of AUBPs and miRNAs for the same binding site on a given target (e.g., HuR and miR-16)[25]. Moreover, AUBPs can also directly sequester miRNAs and prevent their binding to their mRNA targets (e.g., HuR and miR-21)[26]. Such competitions have also been reported between long-non-coding RNAs, miRNAs or AUBPs[27], however it is still unclear whether this interplay exists in CRC. Most studies focus on the role of one given miRNA, AUBP or lncRNA, so this dynamic equilibrium between AUBPs and non-coding RNAs is thus poorly considered.
ARE-dependent post-transcriptional regulation plays an important role in the development and progression of CRC. This importance derives from an ability of these proteins to directly regulate the mRNA decay and/or translation of several ONC, TS and/or inflammatory mediators.
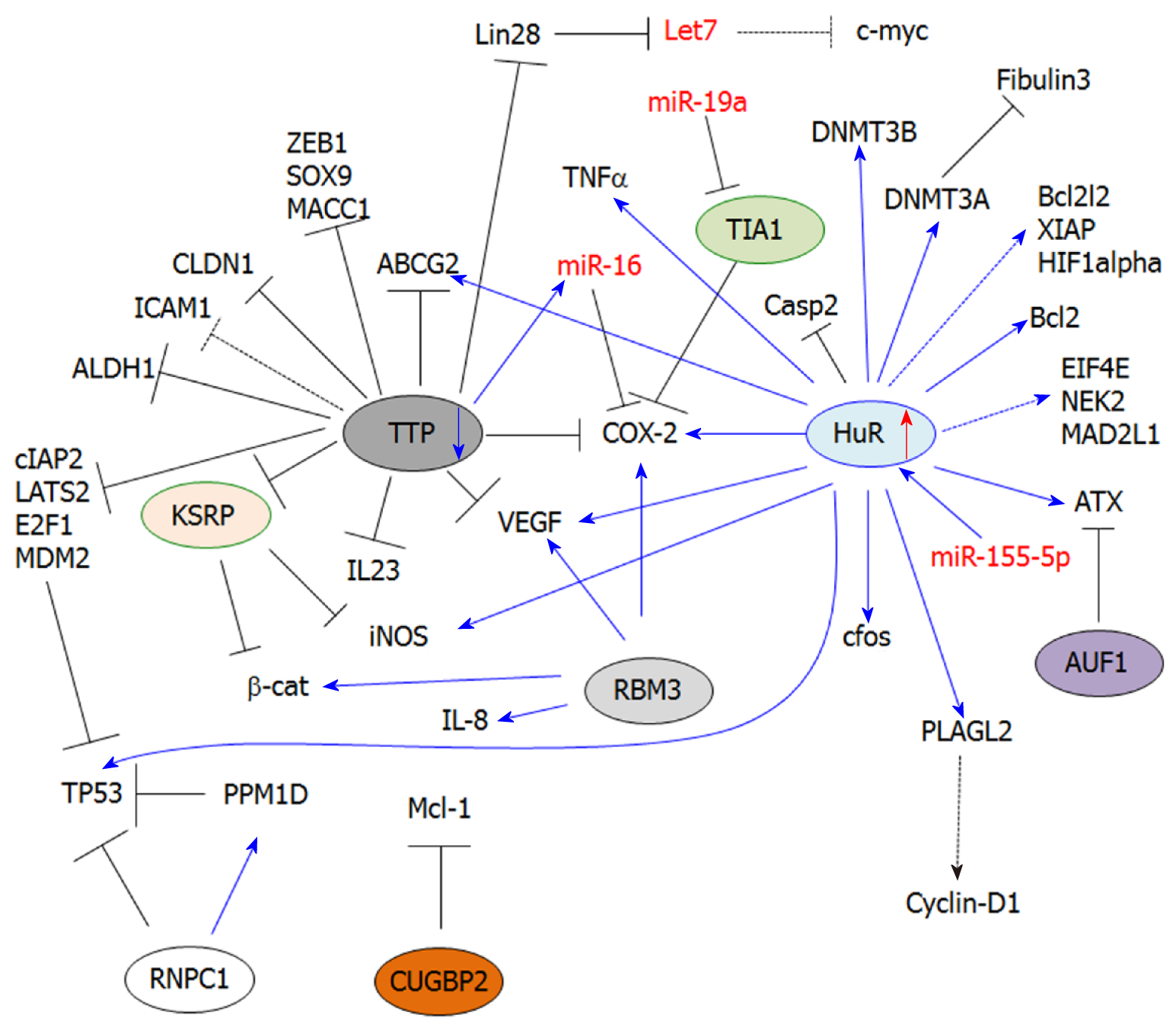
HuR (ELAVL1) belongs to the Embryonic-Lethal Abnormal Vision in Drosophila (ELAV) family of RBPs[28]. This protein is ubiquitously expressed and primarily localized in the nucleus, where it contributes to nucleo-cytoplasmic export[20,29]. The protein displays two tandem RNA-recognition motifs (RRM), followed by a hinge region and a third RRM. The hinge region contains a HuR nucleocytoplasmic shuttling (HNS) domain that can be phosphorylated by various kinases, and is involved in nucleo-cytoplasmic shuttling of the protein. In the cytosol, HuR stabilizes ARE-containing mRNA transcripts (Class I and II mostly) by competing or displacing destabilizing factors, such as microRNAs or other AUBPs (i.e., TTP), that share the same ARE binding sites. Moreover, HuR can directly bind to miRNAs (e.g., miR-21) and thus prevent the downregulation of their targets[26]. HuR is frequently upregulated in most human cancers and exerts oncogenic functions.
HuR is one of the most studied AUBPs in CRC. HuR is overexpressed in CRC when compared to normal colon epithelium[30]. It contributes to the stabilization of inflammation-related transcripts, as well as various oncogenic transcripts involved in cancer cell proliferation, migration, invasion and angiogenesis. Interestingly, HuR was also found overexpressed in colonic epithelial cells from patients with inflammatory bowel disease[31], thus suggesting that HuR induction may represent an early event that promotes CRC development.
The role of HuR in CRC has been extensively studied in various in vitro and in vivo models. HuR silencing in CRC cells (i.e., HCT116) is associated with decreased tumor growth in xenograft models[32]. Conversely, HuR-overexpressing RKO cells display larger tumors in nude mice[33]. Finally, intestinal-specific HuR KO mice (HuRiKO) display reduced tumor burden in a model of FAP (APCmin/+ mice), and increased acute intestinal injury following doxorubicin treatment[34].
Efforts have been devoted to identify all HuR targets involved in colorectal carcinogenesis. In this regard, transcriptomic analyses have been performed in various in vitro and in vivo models with varying levels of HuR. Furthermore, immunoprecipitation of HuR/mRNA complexes has allowed the identification of several HuR targets with significantly more specificity[35]. However, depending on the colon cancer cell lines used for analysis, different targets can be identified. Considering the heterogeneity that exists between CRC tumors, different cellular models should be considered.
Prostaglandin (PG) biosynthesis and inflammation: PGs are bioactive lipid mediators derived from arachidonic acid metabolism. PGs play important functions in the regulation of physiological processes[36]. Thus, the alteration of PG homeostasis is often associated with the development of inflammatory diseases and cancer[37,38]. Following their synthesis, PGs are secreted and act in a paracrine or autocrine manner by binding to nuclear receptors or G-coupled receptors localized at the cellular surface (e.g., EP receptors)[39]. Prostanoid biosynthesis requires several enzymes, including phospholipases, COXs and PGs synthases. In particular, the inducible isoform of COXs, COX-2, is frequently overexpressed in CRC[30,40], thus leading to aberrant PG synthesis while promoting inflammation, immune escape of cancer cells[41], tumor growth and metastasis. In CRC, several PGs were found to be aberrantly expressed (e.g., PGE2), and their secretion in the tumor microenvironment contributes to both the development and progression of CRC[42]. HuR is a positive regulator of COX-2 expression through its ability to bind the COX-2 3’UTR and mediate its stabilization[24,25,30,43]. Therefore, both HuR and COX-2 are not only frequently overexpressed in colorectal tumors, but also in early adenomas and FAP[44,45]. Moreover, the binding of HuR to the 3’UTR of COX-2 prevents miR-16-dependent COX-2 downregulation[25]. Importantly, HuR indirectly induces COX-2 expression by stabilizing the mRNA transcripts of pro-inflammatory cytokines involved in the transcriptional induction of COX-2 expression (e.g., TNFα)[46,47]. Finally, HuR stabilizes Inducible Nitric Oxide Synthase (iNOS) mRNA transcripts, thereby fostering nitric oxide synthesis, which is a major inflammatory mediator[48].
Cancer cell proliferation: The ability of HuR to regulate cancer cell proliferation is tightly linked to its ability to stabilize COX-2 mRNA. Indeed, PG-related signaling[40,49] can trigger various pathways that promote cancer cell proliferation, such as JAK/STAT, PI3K, MAPKs, Wnt/β-cat signaling and mTORC1 (see[50-52] for more detailed reviews). These all control the transcription of cell cycle-related genes (e.g., cyclin D1, c-myc). In particular, PGE2 levels are increased in CRC, and has been associated with strong oncogenic properties[53].
HuR promotes the overexpression of several proliferation-associated genes. In particular, gene expression analysis of RKO cells (colon carcinoma cell line) displaying different levels of HuR expression revealed 26 upregulated genes when HuR is induced, including cell cycle-related genes (e.g., cyclin D1, cyclin A)[54]. However, only a few of them, including TNFα, c-fos and β-catenin, were identified to be direct HuR targets. It is therefore likely that HuR controls gene expression indirectly by affecting the mRNA stability of key transcription factors. This idea is supported by the fact that HuR can stabilize PLAGL2 (Pleomorphic Adenoma Gene Like-2)[55], a transcription factor frequently overexpressed in CRC and involved in the regulation of several genes, including cyclin-D1[56]. Paradoxically, despite the numerous studies attributing a tumor-promoting function to HuR, another study has reported that HuR can bind to the 3’UTR of p53 and enhance its translation in RKO cells under stress conditions (ultraviolet light irradiation)[57].
Cell death: HuR is an important regulator of apoptosis, which stabilizes the mRNA of anti-apoptotic genes such as Bcl-2[58]. However, the role of HuR in cell death-related processes in CRC remains poorly understood. Only a few studies have shown that HuR is involved in the intrinsic apoptotic pathway by directly regulating Bcl-2 mRNA stability[56]. This effect has been associated with chemoresistance (epirubicin). Similarly, several previously reported HuR targets (in other models) involved in apoptosis (BCL2L2, XIAP, HIF1α) were found downregulated in normal/tumor tissues from intestinal-specific HuRiKO mice as compared to their respective controls[34]. These in vivo studies suggest that these apoptosis-associated transcripts are direct HuR targets, consistent with previously reported HuR targets in other models. Moreover, HuRiKO mice display decreased β-catenin expression, leading to the downregulation of target genes, including survivin[34]. This thus indicates that HuR can also inhibit apoptosis indirectly. Furthermore, HuR can also indirectly prevent apoptosis through COX-2/PG pathways (e.g., PGE2), which can trigger the transcription of anti-apoptotic genes (e.g., Bcl-2)[59].
In addition to its regulation of anti-apoptotic genes, HuR can also impair the expression of pro-apoptotic factors like caspases. In particular, HuR blocks IRES-dependent translation of caspase-2 by binding to its 5’UTR[60,61]. This effect confers resistance to radiotherapy in colon cancer cells (i.e., DLD-1 and HCT-15 cells)[62]. Finally, HuR can also mediate chemoresistance by favoring Multidrug Resistance genes, such as ABCG2, in CRC cells[63].
Cancer cell migration/invasion: The development of CRC-derived metastasis is one of the leading causes of CRC mortality[64,65]. HuR overexpression contributes to the stabilization of various mRNAs involved in this process. For instance, HuR contributes to the regulation of lysophosphatidic acid (LPA) by controlling the regulation of a key enzyme involved in its biosynthesis, Autotaxin (ATX). LPA exerts pleiotropic functions by activating G-coupled receptors (LPA1-6)[66] and triggering intracellular signaling cascades that inhibit cell death and promote cell proliferation, angiogenesis[66] and cancer cell migration[67,68].
In another study, HuR was found to control HCT116 colon cancer cell migration/invasion by downregulating fibulin 3 expression[69]. The loss of fibulin 3 expression was previously reported in CRC patients, which involved the methylation of its promoter[70]. Moreover, fibulin 3 downregulation correlates with higher tumor stages and lymph node metastasis. HuR plays a critical function in fibulin 3 silencing by promoting the methylation of its promoter. This effect is mediated via DNMT3A mRNA stabilization by HuR, following HuR phosphorylation by p38MAPK. Interestingly, HuR was previously reported to also stabilize DNMT3B in RKO cells[71]. Together, these findings indicate that HuR can function on an epigenetic level by regulating key genes that methylate target genes commonly repressed in CRC[72,73].
The intestinal-specific HuR KO mice (HuRiKO) were also useful to identify potential HuR targets. In this regard, the expression of olfactomedin4 (Olfm4) was found highly upregulated in the small intestine and colon of HuRiKO[34]. Olfm4 is frequently upregulated in human CRC tumors, and is mostly considered to be a stem cell marker involved in cancer cell proliferation and migration[74].
Other specific mechanisms have been associated with the migration-promoting effect of HuR. Claudin-1 overexpression has been tightly associated with CRC progression, invasion and metastasis[75], and HuR stabilizes the claudin-1 transcript[76]. Finally, increased PGE2 synthesis associated with COX-2 mRNA stabilization by HuR can also increase cancer cell migration/invasion through the activation of membrane receptors that promote the expansion of cancer stem cells. Furthermore, PGE2 synthesis can also inducing key regulators of migration/invasion, such as urokinase-type plasminogen activator receptor (uPAR)[42], MMP-2/9[77,78], VEGFR1[79] and VEGF[52].
The mechanisms involved in HuR overexpression in CRC are still unclear, but increasing evidence indicates that non-coding RNAs are involved in HuR induction. For instance, the long non-coding RNA Overexpressed in Colon Carcinoma-1 (OCC1)[80] has been involved in the regulation of HuR overexpression. OCC1 expression is decreased in CRC patients and in colon cancer cell lines, indicating it to be a negative regulator of HuR expression. In work by Lan et al[80], OCC1 was shown to promote HuR protein degradation by enhancing the binding of ubiquitin E3 ligase β-TrCP1 to HuR. In agreement with the role of HuR in the regulation of cell cycle-related genes, OCC1-dependent HuR downregulation leads to an arrest of cancer cells in the G0/G1 phase of the cell cycle, as well as to decreased expression of direct HuR target genes (i.e., eIF4E, NEK2, MAD2L1, HNRNPA1, HNRNPK).
Deregulation in microRNA expression is also associated with HuR upregulation in human cancers. Interestingly, based on the miRwalk database, more than 3000 miRs (predicted by at least three different algorithms) are predicted to target HuR mRNA in human, but only a few of them have been experimentally validated[58]. In CRC, miR-519c has been reported to downregulate HuR expression[81], leading to an overexpression of HuR targets, including the multidrug resistance gene ABCG2, and thus chemoresistance[63].
Interestingly, although microRNAs have been mostly associated with mRNA decay or translation inhibition, miR-155-5p seems to be a positive regulator of HuR expression[82]. The underlying mechanism is still unclear, but involves the binding of miR-155-5P to ARE (AUUA and AUUU) within the HuR 3’UTR. This study suggests that some miRNAs can inhibit gene expression, while others may stabilize some transcripts similar to AUBPs. This effect may depend on the binding site and/or may also result from interplay between miRNAs and stabilizing RBPs, as previously demonstrated[83].
Several post-translational modifications (e.g., phosphorylation) have been involved in the subcellular localization and activity of HuR (see[20] for more detailed reviews). For instance, the cytoplasmic localization of HuR is affected by kinases such as p38, cdK1, PKC and AMPK, which phosphorylate HuR at different residues[29]. In CRC, an increase in cytosolic HuR has been observed in inflamed tissue from patients with inflammatory bowel disease, early adenomas and CRC, indicating that the nuclear-cytosolic shuttling mechanisms are potentially deregulated in both CRC and preneoplastic conditions[31]. While these and other mechanisms have been well-characterized in various cancers, connecting these CRC-related post-translational alterations to HuR is under current investigation. One study has shown that the neddylation of HuR by Mdm2 contributes to its protein stabilization in hepatocellular carcinoma and CRC[84,85]. The phosphorylation of HuR at Ser318 by PKC (delta), and its cytoplasmic localization in DLD-1 colon cancer cells[86], is also involved in HuR-dependent stabilization of COX-2 mRNA. Furthermore, phosphorylation of nuclear HuR by Chk2 and p38MAPK at Ser88 and Thr118, respectively, in oxidative stress conditions is critical for the regulation of the splicing of TRA2β4, particularly by favoring exon 2 incorporation[87]. Interestingly, the silencing of p38α MAPK was also associated with decreased expression of HuR in HCT116 cells[69]. In addition, several studies have associated p38α MAPK with oncogenic properties, including the promotion of cell proliferation, migration, invasion and angiogenesis[88].
Altogether, these data indicate that increased expression/activity of HuR in CRC is most likely not the consequence of a single mechanism, but rather the concerted deregulation of several factors during transcription, post-transcription and post-translation.
HuR overexpression can modulate a whole network of ONC and TS involved in the various hallmarks of cancer[89]. Therefore, targeting HuR in CRC may represent an appealing therapeutic strategy alone or in combination with existing therapeutic approaches (i.e., chemotherapy, radiotherapy). Several small molecules compounds that have the capacity to block HuR/ARE interactions have been identified by high-throughput screenings[90-93], with a few of them further characterized for HuR specificity. In particular, polyketides purified from plants or microbial extracts (i.e., MS-444, okicenone, dehydromutactin) have shown HuR inhibitory properties[91]. Among them, MS-444 has been further studied and displays several anti-tumor properties[94]. MS-444 is a potent inhibitor of HuR homodimerization, preventing its cytoplasmic export and the stabilization of several mRNA transcripts[94]. The anti-tumor effect of MS-444 was further observed in vivo in a model of inflammatory bowel disease and also in a genetic model of FAP (i.e., APCMin mice), but not in inflammatory colon cancer (AOM/DSS mice)[31].
Other compounds have demonstrated an ability to impede HuR expression or activity. For instance, ar-turmerone from Curcuma longa prevents LPS-induced translocation of HuR[95]. However, the effects observed with these molecules are not restricted to HuR and, thus, it is likely that the beneficial effects observed may also result from several HuR-independent mechanisms.
TTP belongs to a small family of Cys-Cys-Cys-His zinc finger proteins comprised of TTP, BRF1 and BRF-2[96,97]. Zfp36 is an immediate-early response gene[98] whose expression can be induced by diverse stimuli like insulin[98,99], TGF-β[100,101], LPS[102] and TNFα[103]. TTP is one of the best-characterized AUBPs that promote ARE-dependent mRNA decay[96,97,104]. This process is mediated by the nucleation of small cytoplasmic foci called Processing-bodies (P-bodies)[100,105,106], where targeted mRNA transcripts are bound by mRNA deadenylases, translational repressors and decapping enzymes[107-109]. Alternatively, TTP can recruit the exosome complex to degrade ARE-containing transcripts[110,111]. Finally, TTP is also involved in miRNA-dependent post-transcriptional regulation (e.g., miR-16) through its binding to argonaute proteins (Ago/eiF2C). This interaction promotes complex formation with miRNAs, which allow their binding to ARE sequences[112,113].
The physiological significance of TTP is highlighted by Zfp36 knockout mice, which develop severe inflammatory syndromes and growth retardation[114]. Moreover, TTP expression is frequently lost in human cancers, and this loss is often associated with poor clinical outcomes[115,116].
Several studies have demonstrated that TTP expression is downregulated in early adenomas and adenocarcinomas[24,117,118]. TTP is considered as a TS, whose downregulation in CRC contributes to the enhanced expression of pro-inflammatory cytokines. However, TTP is also critical to ONC and TS gene regulation. Interestingly, no significant difference was observed in the survival rate of patients with colon adenocarcinoma in TTP low-expressing versus TTP high-expressing individuals[119]. Moreover, there were no differences noted in the stage or aggressiveness of the tumors in TTP low and TTP high patients compared to other cancer types (i.e., breast cancer, lung adenocarcinoma)[119]. However, it should be noted that the loss of TTP is frequently associated with an overexpression of HuR during CRC development[24], thus favoring the overexpression of their common targets. Accordingly, not only the loss of TTP, but also the concomitant induction of HuR expression should be considered in these analyses.
PG biosynthesis and inflammation: TTP plays a critical role in inflammation, since most of these targets are inflammatory mediators like TNFα[103,120,121], GM-CSF[122] and COX-2[24,123]. All of them contain AREs in their 3’UTR mRNA. Furthermore, TTP knockout mice develop a severe inflammatory syndrome characterized by cachexia, arthritis, dermatitis, inflammation and autoimmunity[114,124].
In colon epithelium, TTP is a potent inducer of COX-2 mRNA decay through its direct binding to ARE within the 3’UTR[125]. Several studies have reported the downregulation of COX-2 mRNA by TTP in various in vitro and in vivo models, as well as in human tissues[24,123]. TTP expression is silenced in CRC, and together with HuR overexpression, stabilization of the COX-2 mRNA occurs. These combined AUBP effects allow for pathological protein and PGE2 production, with the subsequent activation of downstream signaling pathways involved in CRC development[24].
TTP is well-known for its ability to mediate the mRNA decay of pro-inflammatory cytokines, including IL-6 and TNFα[12]. However, the regulation of these cytokines by TTP in CRC is not known. Nevertheless, other pro-inflammatory cytokines seem to be regulated by TTP, such as IL-23, which plays a key role in colon cancer promotion[126]. A study performed in CT26 cells (murine colon cancer cell line) has shown that TTP can induce IL-23 mRNA decay by directly binding to the ARE present within its 3’UTR[127].
Cancer cell proliferation and cell death: The ability of TTP to promote the mRNA decay of several cell cycle-related genes, including cyclin-D1, c-myc or c-fos, is well established in various cancers[128,129]. However, the regulation of all these targets by TTP in CRC remains to be established[117]. Nonetheless, TTP can negatively control cell proliferation by promoting COX-2 mRNA decay, thus leading to decreased PG signaling (e.g., PGE2)[130].
TTP also exerts negative control on tumor growth by mediating mRNA decay of the RBP Lin28[131]. Importantly, Lin28 overexpression fosters adenocarcinoma development[132] through several mechanisms, including the downregulation of let-7 miRNA[132,133]. Let-7 is a critical regulator of cell cycle-related genes (e.g., c-myc)[134], and it is thus likely that the loss of TTP in CRC may indirectly contribute to the overexpression of Let-7 targets involved in cell proliferation. The role of TTP in cell proliferation and cell death was further highlighted by its induction by resveratrol in HCT116 and SNU81 cells. In this study, the induction of TTP expression was associated with a direct binding to several genes involved in apoptosis and cell cycle progression, including cIAP2, LATS2, MDM2 and E2F1[135].
Cancer cell migration, invasion: The loss of TTP in cancer is often associated with an increase in cancer cell migration and invasion. This effect has been attributed to an ability of TTP to destabilize the mRNAs of several migration-related genes such as MMP9 and uPAR[9,136]. In CRC, the relationship between these genes and TTP is not known. However, several genes promoting epithelial-to-mesenchymal transition (EMT) were reported as direct TTP targets, including ZEB1, SOX-9 and MACC1 as evidenced in colon cancer cell lines[118].
Furthermore, the loss of TTP in CRC (e.g., HCT116 and SW480 cells) correlates with an increase in stemness markers (i.e., Bmi-1, ALDH-1 and ABCG2)[137], thus indicating that TTP is a critical regulator of colon cancer cell differentiation. Some cell adhesion molecules (CAMs) are negatively regulated by TTP[138], and thus their overexpression following TTP loss will contribute to the establishment of a metastatic phenotype. TTP promotes the decay of Claudin-1 mRNA in human colon cancer cells through direct binding to its 3’UTR[76]. Claudin-1 overexpression has been associated with TNFα-induced EMT and cancer cell migration[139].
Paradoxically, TTP induction in colon cancer cell lines (e.g., HT-29) has been involved in the inhibition of anti-tumor immunity, thereby fostering tumor growth and metastasis formation. In this study, TTP is induced by Heme Oxygenase-1 (HO-1) and mediates Intercellular Adhesion Molecule-1 (ICAM-1) mRNA decay, thus impairing leucocyte recruitment/adhesion[138,140,141]. However, these effects were observed in vitro and, therefore, it is still unclear whether TTP plays the same function in vivo. Moreover, it is not clear whether TTP binds directly to ICAM-1 mRNA.
Finally, TTP is a negative regulator of angiogenesis, as evidenced by the increased VEGF mRNA decay in TTP-overexpressing CRC cells (i.e., KM12C, HT-29, SW620 and Colo320 cell lines)[117]. This effect involves the dephosphorylation of p38 MAPK by MAPK phosphatase-1, which is activated by casein kinase 2 (CK2). The dephosphorylation of p38 MAPK prevents the inhibition of TTP activity and thus promotes VEGF mRNA decay[142]. Moreover, TGFβ increases CK2 (Casein Kinase 2) activity and, consequently, increased TTP mRNA decay activity in Colo320 cells[142].
Another important role of TTP in angiogenesis has been associated with an ability to prevent K-homology splicing regulator protein (KSRP)-induced iNOS mRNA decay[48]. iNOS is a critical enzyme involved in NO synthesis, which promotes tumor angiogenesis in CRC[143]. KSRP is another AUBP that promotes iNOS mRNA decay. However, TTP interacts directly with the KSRP protein and prevents its binding to the iNOS 3’UTR in colorectal adenocarcinoma cells[48].
Several mechanisms contribute to the silencing of TTP expression in CRC. Among them, an epigenetic mechanism involving the silencing of the transcription factor EGR1 by histone deacetylases (HDAC) has been described[144]. Accordingly, EGR1 and TTP expression could be restored in various colon cancer cell lines by HDAC inhibitors (i.e., SAHA, Trichostatin-A, sodium butyrate)[144]. In line with these findings, a study by Krishnan et al[145] has reported that HDAC inhibitors increase the binding of TTP to claudin-1 3’UTR in SW480 and SW620 cells. Therefore, it appeared from these studies that HDAC inhibitors represent an efficient approach to restore TTP expression. Several HDACs inhibitors have been shown to decrease cell proliferation and promote cell apoptosis in CRC[146], and are currently being used in clinical trials.
Some pathways deregulated in colon cancer, such as Wnt/β-catenin, may also contribute to TTP loss. Indeed, an inverse relationship between TTP and the TCF/β-catenin pathway has been reported in colon cancer cell lines (i.e., SW480, HCT116 and SW620). Furthermore, the treatment of these cell lines with an inhibitor of this pathway (i.e., FH535) was associated with an increase in TTP expression, suggesting a role for this pathway in regulating TTP expression[118].
Other mechanisms leading to the silencing of TTP expression in CRC have been described. Of note, one study demonstrated that p53 can directly activate TTP transcription in CRC cell lines following doxorubicin (DOX) treatment. Accordingly, the decreased TTP expression observed in human colonic adenocarcinoma tissues is partially linked to the loss of p53[131]
Finally, EGFR/ERK signaling has been implicated in TTP loss, since Gambogic acid from the Indian Gambodge tree induces TTP expression through the downregulation of EGFR/ERK pathway signaling[137]. Other compounds of natural origin have also been reported to induce TTP expression in colon cancer cells. In this regard, resveratrol from red grapes promotes apoptosis and inhibits both cell proliferation and metastasis by increasing TTP expression in HCT116 and SNU81 cells[135]
TIA1 was originally identified in activated T lymphocytes, where it plays a nucleolytic role against T cell targets. TIA1 is comprised of three RRMs involved in binding to ARE in the 3’UTR of target mRNA transcripts[147,148]. TIA1 acts as a translational repressor and, during stress conditions (i.e., hypoxia, oxidative stress), interacts with various co-factors (e.g., TIAR) to promote the sequestration of target mRNAs into non-membranous cytoplasmic SG[149]. Therefore, target mRNAs are held translationally silenced and can re-enter translation or proceed to mRNA decay in P-bodies. This mechanism allows cancer cells to survive in stressful conditions (e.g., hypoxia, oxidative stress, chemotherapy). Therefore, SG formation represents a post-cellular stress response that allows cancer cells to re-launch mRNA translation without the high-energy demand of de novo transcription[150]. Stress granule formation has been proposed to be a survival mechanism for cancer cells, thus suggesting that targeting SG components may represent an appealing therapeutic approach in combination with chemotherapy/targeted therapy[151,152].
The role of TIA1 in cancer is currently unclear. Depending on the cancer type, TIA1 behaves either as a TS or an ONC[153,154]. TIA1 possesses pleiotropic functions and, in addition to its ability to regulate mRNA translation, also contributes to the alternative mRNA splicing of various cancer factors (e.g., SIRT1, CD95)[155,156]. While limited studies have been conducted to decipher the role of TIA1, TIAR and SGs in CRC, COX-2 overexpression in CRC has been associated with a deficiency of TIA1 binding to the COX-2 3’UTR[16]. This study suggests a tumor suppressive function of TIA1 in CRC, and provides further evidence that COX-2 overexpression in CRC is mediated through the deregulation of several AUBPs (i.e., TTP loss, HuR overexpression). The idea of a tumor suppressive role of TIA1 is further supported by survival analysis of CRC patients, showing that high-expressing TIA1 patients display a better prognosis (TCGA/human protein atlas database). Paradoxically, TIA1 has been associated with SG assembly[157], while SG formation has been associated with chemoresistance to 5-FU in CRC cells (HT-29 and HCT-116 cells)[158]. Together, the data currently available are more in favor of a tumor suppressive role of TIA1, yet are still insufficient to fully understand the role of TIA1/TIAR/SGs function in CRC development. Thus, further studies are required to identify TIA1-regulated mRNA targets in CRC.
The mechanisms involved in TIA1 silencing are still poorly known. To date, only one study has involved the overexpression of miR-19a, which directly targets the TIA1 3’UTR in CRC tissues and cell lines[159]. Nevertheless, other miRNAs have been involved in TIA1 silencing in other cancers, such as miR-487a in gastric cancer[160]. The role of these miRNAs in TIA1 silencing remains to be investigated in the context of CRC. Moreover, many other miRNAs are potential regulators of TIA1 expression (TargetsScan analysis: http://www.targetscan.org/vert_72/), such as miR-199-3p, which is upregulated in CRC[161].
The butyrate response factor 1 (BRF1) encoded by the ZFP36L1 gene, also known as TIS11B, belongs to the ZFP36 zinc finger protein family[96,97]. The BRF1 gene is localized on chromosome 14q22-q24[162]. Similarly to TTP, BRF1 contains a tandem zinc finger domain bearing a double zinc finger motif (Cys-Cys-Cys-His) and can target mRNAs bearing AREs to P-bodies[106]. BRF1 has mostly been associated with tumor suppressive functions[96] due to its ability to target and promote the mRNA decay of key mediators of angiogenesis (i.e., VEGF)[163] or apoptosis (i.e., cIAP2)[164]. However, information addressing its expression and role in CRC remains limited. To date, only one study has suggested that 17beta-oestradiol induces an ensemble of genes involved in apoptosis, including BRF1 in COLO205 colon cancer cells[165].
ARE/poly(U)-binding/degradation factor-1 (AUF1) is an RBP implicated in promoting mRNA decay[66]. Moreover, AUF1 can antagonize HuR function[166], and may thus also indirectly regulate the expression of HuR targets. Cancer studies have revealed differential expression and functions of AUF1 that is dependent on the cancer type[167-169]. In CRC, one study has reported the interplay between HuR and AUF1 in the regulation of ATX expression[66]. ATX is a key enzyme involved in the regulation of lysophosphatitic acid (LPA) synthesis, which converts lysophosphatidylcholine (LPC) into LPA. Importantly ATX is involved in cancer cell migration[170]. AUF1 promotes the decay of ATX mRNA in Colo320 cells[66], while HuR mediates its stabilization by preventing AUF1 binding.
KSRP is a RNA-binding protein involved in mRNA stability regulation and miRNA-mediated regulation. KSRP displays pleiotropic functions, such as regulation of pre-mRNA splicing, transcription, and miRNA biogenesis/maturation[171-173]. Current studies in cancer suggest that this protein may exert a tumor suppressive function, such as in lung cancer[174]. In colon cancer, one study has shown that KSRP impairs Wnt/β-catenin signaling by directly binding to the CTNNB1 3’UTR and mediating its degradation[175]. KSRP is also a negative regulator of NO synthesis by promoting the decay of iNOS[48]. Increased NO synthesis has been associated with several oncogenic properties (e.g., inflammation, proliferation, migration, angiogenesis) in CRC, and the inhibitory effect of KSRP on iNOS reinforces the idea of a tumor suppressive function of KSRP. Importantly, KSRP can compete with HuR for binding to the iNOS 3’UTR in colorectal adenocarcinoma cells[176]. Moreover, the binding of KSRP to iNOS mRNA can be impaired by a direct protein-protein interaction with TTP[48]. Therefore, considering the importance of HuR and TTP in the pathophysiology of CRC, the deregulation of many ARE-containing genes may result from a complex interplay between KSRP, TTP and HuR.
CUGBP2 is a member of the CUGBP-ETR-3-like factors family, which is ubiquitously expressed. This protein is comprised of two N-terminal RRMs and one C-terminal RRM[177]. CUGBP2 is involved in mRNA alternative splicing, RNA editing and translation inhibition[178]. CUGBP2 is mostly considered as a TS and its expression is lost in various cancers (e.g., breast cancer)[179,180]. In CRC, the loss of CUGBP2 expression is mediated by PGE2 and contributes to the radiation-induced mitotic catastrophe in CRC cells[181]. Moreover, HCT-116 cells stably overexpressing CUGBP2[178] display a cell cycle arrest in G2/M and an induction of apoptosis. This effect was notably associated with the downregulation of the anti-apoptotic protein Mcl-1 through the direct binding of CUGBP2 to the 3’UTR of Mcl-1 mRNA and a blockade of its translation[178].
RBM3 belongs to a family of glycine-rich RBP and is comprised of a single RRM[182]. RBM3 is a cold shock protein induced by both hypothermia as well as other cellular stresses like hypoxia[183]. RBM3 plays an important role in various cellular processes, including neural differentiation[184], cell cycle progression[185] and DNA-induced innate immune response, as evidenced in RBM3 KO mice[186]. In cancer, RBM3 is primarily considered as a proto-oncogene[187], but studies have documented the role of RBM3 in CRC. RBM3 is overexpressed in CRC and displays potent oncogenic activities, specifically by stabilizing several mRNA transcripts such as COX-2[187], IL-8 or VEGFα[185]. Accordingly, silencing of RBM3 in colon cancer cells triggers caspase-dependent apoptosis and mitotic catastrophe[185], indicating that RBM3 is essential for cancer cell growth. Moreover, overexpression of RBM3 in CRC cells is associated with stem cell characteristics through increased β-catenin signaling[188]. Paradoxically, RBM3 expression in patients with colon cancer is associated with a better prognosis[189,190], thus suggesting a potential tumor suppressive function. These discrepancies might be explained by the fact that oncogenic functions of RBM3 were mostly demonstrated in vitro in colon cancer cells and thus outside of their physiological context. Moreover, the localization of the protein might be associated with its different functions, as demonstrated for HuR or TTP. However, the molecular mechanisms involved in RBM3 activity/localization are currently unknown in CRC. Taken together, these data indicate that although RBM3 has been associated with oncogenic functions in CRC, some clarifications are still required to fully establish the role of this protein in vivo, as well as its clinical relevance.
RNPC1 (encoded by the RBM38 gene) belongs to the RRM-containing RBP family, which includes HuR and Musashi proteins[191]. RBM38 is located on chromosome 20q13[192] and is expressed in several tissues (including breast, colorectal, lung, skin and ovarian tissue)[193]. RNPC1 plays an important role in the regulation of various biological processes, including cell proliferation, cell cycle and myogenic differentiation[193]. Deregulation of RNPC1 expression/activity was reported in a variety of malignancies, such as prostate[193], ovarian[194], esophageal adenocarcinoma[195] and breast cancer[196]. Mice deficient for RNPC1 display accelerated aging and spontaneous tumor development[197]. However, depending on the cancer type, this protein may perform oncogenic or tumor suppressive functions[192,198-200]. In CRC, this protein is a potent inhibitor of p53 translation through its binding to TP53 3’UTR[201], thus suggesting an oncogenic function. This effect can be inhibited by phosphorylation of RNPC1 by GSK3 at serine 195. Moreover, RNPC1 promotes the translation of the p53-inactivating phosphatase Protein Phosphatase, Mg2+/Mn2+ Dependent 1D (PPM1D), which in turn dephosphorylates RNPC1 at serine 195, thus creating a positive feedback loop that impairs p53 translation.
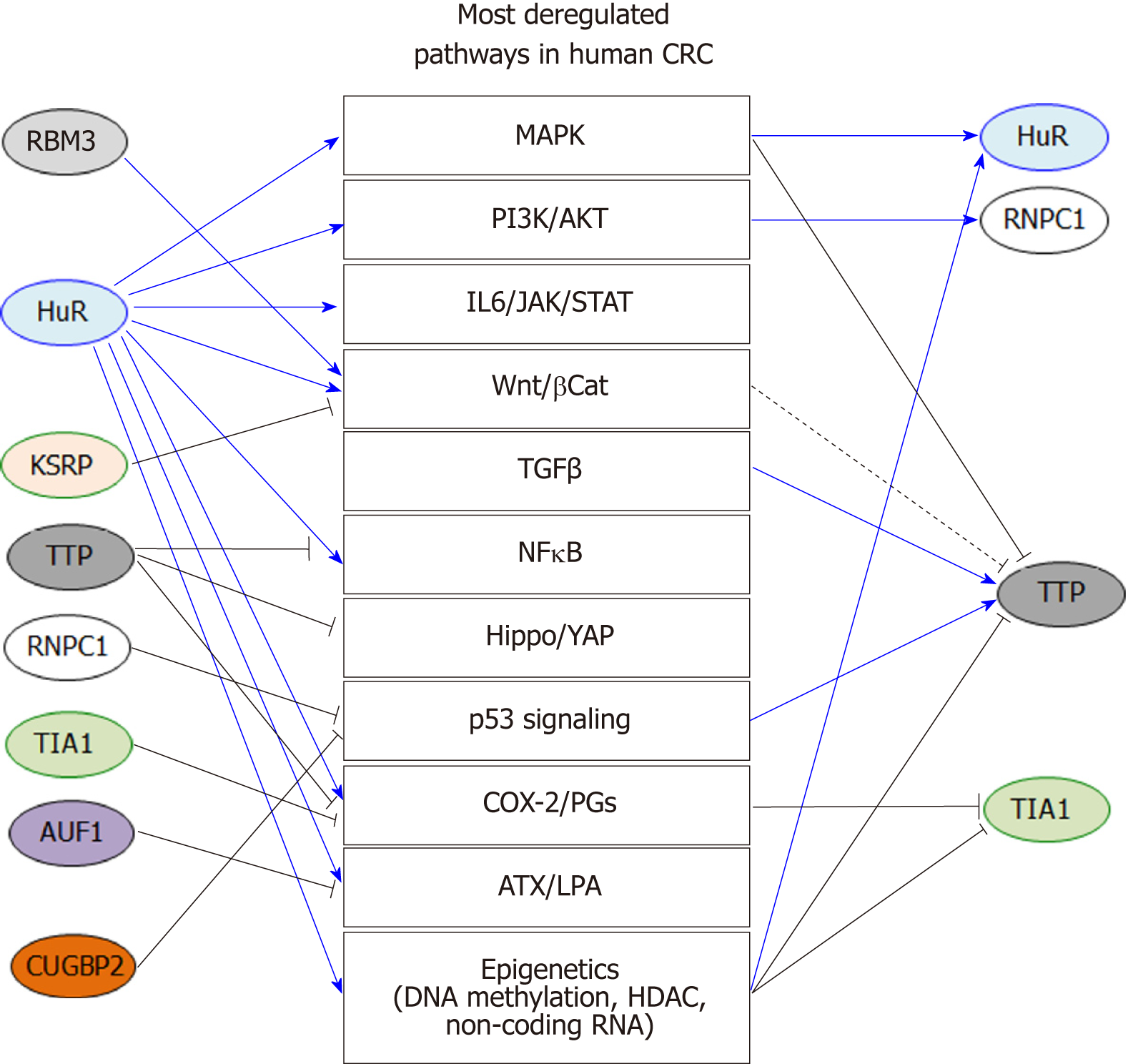
In this review, we summarized the current knowledge related to AUBPs in CRC development and progression. Current studies indicate that these proteins are critical not only for the post-transcriptional control of key inflammatory genes, but also for ONC and TS genes (Figure 1). Importantly, these findings highlight the role post-transcriptional regulation of these genes plays in influencing major oncogenic pathways associated with CRC (Figure 2). Therefore, early alterations of AUBP expression/activity observed in preneoplastic conditions may provide some clues to better understand the development of neoplastic phenotypes and ultimately serve as biomarkers of early-stage CRC. While most studies have focused on the roles of HuR and TTP in CRC, further research will expand our knowledge of the roles of other AUBPs in CRC etiology and in many other cancers. The development of suitable in vivo models will be an indispensable tool to understand the role of these factors in tumor progression. Finally, these proteins may represent appealing therapeutic targets for the treatment of CRC due to their pleiotropic functions influencing the various hallmarks of cancer, as evidenced by small molecule targeting of HuR (Figure 2).
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Switzerland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cao ZF, Ito H, Plaza MA, Salati M S- Editor: Yan JP L- Editor: Filipodia E- Editor: Bian YN
| 1. | Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1625] [Cited by in RCA: 1659] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 2. | Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7:105-114. [PubMed] |
| 3. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8004] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 4. | De Rosa M, Pace U, Rega D, Costabile V, Duraturo F, Izzo P, Delrio P. Genetics, diagnosis and management of colorectal cancer (Review). Oncol Rep. 2015;34:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 5. | Øines M, Helsingen LM, Bretthauer M, Emilsson L. Epidemiology and risk factors of colorectal polyps. Best Pract Res Clin Gastroenterol. 2017;31:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 341] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Ali MK, Siegel KR, Chandrasekar E, Tandon R, Montoya PA, Mbanya JC, Chan J, Zhang P, Narayan KM. Diabetes: An Update on the Pandemic and Potential Solutions. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, editors. Cardiovascular, Respiratory, and Related Disorders. Washington (DC): The International Bank for Reconstruction and Development/The World Bank; 2017. . [PubMed] |
| 8. | Hong SN. Genetic and epigenetic alterations of colorectal cancer. Intest Res. 2018;16:327-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 9. | Wang H, Ding N, Guo J, Xia J, Ruan Y. Dysregulation of TTP and HuR plays an important role in cancers. Tumour Biol. 2016;37:14451-14461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Khabar KS. Hallmarks of cancer and AU-rich elements. Wiley Interdiscip Rev RNA. 2017;8:e1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 11. | Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: Are there unifying principles? Nucleic Acids Res. 2006;33:7138-7150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 784] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 12. | Sanduja S, Blanco FF, Young LE, Kaza V, Dixon DA. The role of tristetraprolin in cancer and inflammation. Front Biosci (Landmark Ed). 2012;17:174-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res. 2001;29:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 311] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Bakheet T, Williams BR, Khabar KS. ARED 2.0: an update of AU-rich element mRNA database. Nucleic Acids Res. 2003;31:421-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Kanies CL, Smith JJ, Kis C, Schmidt C, Levy S, Khabar KS, Morrow J, Deane N, Dixon DA, Beauchamp RD. Oncogenic Ras and transforming growth factor-beta synergistically regulate AU-rich element-containing mRNAs during epithelial to mesenchymal transition. Mol Cancer Res. 2008;6:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Dixon DA, Balch GC, Kedersha N, Anderson P, Zimmerman GA, Beauchamp RD, Prescott SM. Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J Exp Med. 2003;198:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Subramaniam K, Ooi LL, Hui KM. Transcriptional down-regulation of IGFBP-3 in human hepatocellular carcinoma cells is mediated by the binding of TIA-1 to its AT-rich element in the 3'-untranslated region. Cancer Lett. 2010;297:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Beisang D, Bohjanen PR. Perspectives on the ARE as it turns 25 years old. Wiley Interdiscip Rev RNA. 2012;3:719-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat Rev Mol Cell Biol. 2007;8:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 990] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 20. | Grammatikakis I, Abdelmohsen K, Gorospe M. Posttranslational control of HuR function. Wiley Interdiscip Rev RNA. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 188] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 21. | Sandler H, Stoecklin G. Control of mRNA decay by phosphorylation of tristetraprolin. Biochem Soc Trans. 2008;36:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 22. | Tchen CR, Brook M, Saklatvala J, Clark AR. The stability of tristetraprolin mRNA is regulated by mitogen-activated protein kinase p38 and by tristetraprolin itself. J Biol Chem. 2004;279:32393-32400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Lin NY, Lin CT, Chen YL, Chang CJ. Regulation of tristetraprolin during differentiation of 3T3-L1 preadipocytes. FEBS J. 2007;274:867-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Young LE, Sanduja S, Bemis-Standoli K, Pena EA, Price RL, Dixon DA. The mRNA binding proteins HuR and tristetraprolin regulate cyclooxygenase 2 expression during colon carcinogenesis. Gastroenterology. 2009;136:1669-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 188] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Young LE, Moore AE, Sokol L, Meisner-Kober N, Dixon DA. The mRNA stability factor HuR inhibits microRNA-16 targeting of COX-2. Mol Cancer Res. 2012;10:167-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 26. | Poria DK, Guha A, Nandi I, Ray PS. RNA-binding protein HuR sequesters microRNA-21 to prevent translation repression of proinflammatory tumor suppressor gene programmed cell death 4. Oncogene. 2016;35:1703-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Yu D, Zhang C, Gui J. RNA-binding protein HuR promotes bladder cancer progression by competitively binding to the long noncoding HOTAIR with miR-1. Onco Targets Ther. 2017;10:2609-2619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001;58:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 842] [Cited by in RCA: 872] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 29. | Srikantan S, Gorospe M. HuR function in disease. Front Biosci (Landmark Ed). 2012;17:189-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 30. | Dixon DA, Tolley ND, King PH, Nabors LB, McIntyre TM, Zimmerman GA, Prescott SM. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J Clin Invest. 2001;108:1657-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 349] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 31. | Lang M, Berry D, Passecker K, Mesteri I, Bhuju S, Ebner F, Sedlyarov V, Evstatiev R, Dammann K, Loy A, Kuzyk O, Kovarik P, Khare V, Beibel M, Roma G, Meisner-Kober N, Gasche C. HuR Small-Molecule Inhibitor Elicits Differential Effects in Adenomatosis Polyposis and Colorectal Carcinogenesis. Cancer Res. 2017;77:2424-2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Lal S, Cheung EC, Zarei M, Preet R, Chand SN, Mambelli-Lisboa NC, Romeo C, Stout MC, Londin E, Goetz A, Lowder CY, Nevler A, Yeo CJ, Campbell PM, Winter JM, Dixon DA, Brody JR. CRISPR Knockout of the HuR Gene Causes a Xenograft Lethal Phenotype. Mol Cancer Res. 2017;15:696-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | López de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, Gorospe M. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. 2003;22:7146-7154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 235] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Giammanco A, Blanc V, Montenegro G, Klos C, Xie Y, Kennedy S, Luo J, Chang SH, Hla T, Nalbantoglu I, Dharmarajan S, Davidson NO. Intestinal epithelial HuR modulates distinct pathways of proliferation and apoptosis and attenuates small intestinal and colonic tumor development. Cancer Res. 2014;74:5322-5335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | López de Silanes I, Fan J, Galbán CJ, Spencer RG, Becker KG, Gorospe M. Global analysis of HuR-regulated gene expression in colon cancer systems of reducing complexity. Gene Expr. 2004;12:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Salleh N. Diverse roles of prostaglandins in blastocyst implantation. ScientificWorldJournal. 2014;2014:968141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2847] [Cited by in RCA: 2718] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 38. | Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1494] [Cited by in RCA: 1405] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 39. | Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Wu WK, Sung JJ, Lee CW, Yu J, Cho CH. Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett. 2010;295:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Wang D, DuBois RN. An inflammatory mediator, prostaglandin E2, in colorectal cancer. Cancer J. 2013;19:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 42. | Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. FASEB J. 2003;17:1640-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Lim SJ, Lee SH, Joo SH, Song JY, Choi SI. Cytoplasmic expression of HuR is related to cyclooxygenase-2 expression in colon cancer. Cancer Res Treat. 2009;41:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Brosens LA, Keller JJ, Pohjola L, Haglund C, Morsink FH, Iacobuzio-Donahue C, Goggins M, Giardiello FM, Ristimäki A, Offerhaus GJ. Increased expression of cytoplasmic HuR in familial adenomatous polyposis. Cancer Biol Ther. 2008;7:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Denkert C, Koch I, von Keyserlingk N, Noske A, Niesporek S, Dietel M, Weichert W. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol. 2006;19:1261-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 326] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 47. | Dean JL, Wait R, Mahtani KR, Sully G, Clark AR, Saklatvala J. The 3' untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol Cell Biol. 2001;21:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 48. | Fechir M, Linker K, Pautz A, Hubrich T, Förstermann U, Rodriguez-Pascual F, Kleinert H. Tristetraprolin regulates the expression of the human inducible nitric-oxide synthase gene. Mol Pharmacol. 2005;67:2148-2161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 49. | Müller-Decker K, Fürstenberger G. The cyclooxygenase-2-mediated prostaglandin signaling is causally related to epithelial carcinogenesis. Mol Carcinog. 2007;46:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Wang D, Dubois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 657] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 51. | Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 679] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 52. | Dufour M, Faes S, Dormond-Meuwly A, Demartines N, Dormond O. PGE2-induced colon cancer growth is mediated by mTORC1. Biochem Biophys Res Commun. 2014;451:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Mutoh M, Takahashi M, Wakabayashi K. Roles of prostanoids in colon carcinogenesis and their potential targeting for cancer chemoprevention. Curr Pharm Des. 2006;12:2375-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Xia M, Chen Y, Wang LC, Zandi E, Yang H, Bemanian S, Martínez-Chantar ML, Mato JM, Lu SC. Novel function and intracellular localization of methionine adenosyltransferase 2beta splicing variants. J Biol Chem. 2010;285:20015-20021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Su C, Li D, Li N, Du Y, Yang C, Bai Y, Lin C, Li X, Zhang Y. Studying the mechanism of PLAGL2 overexpression and its carcinogenic characteristics based on 3'-untranslated region in colorectal cancer. Int J Oncol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Lin GL, Ting HJ, Tseng TC, Juang V, Lo YL. Modulation of the mRNA-binding protein HuR as a novel reversal mechanism of epirubicin-triggered multidrug resistance in colorectal cancer cells. PLoS One. 2017;12:e0185625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Mazan-Mamczarz K, Galbán S, López de Silanes I, Martindale JL, Atasoy U, Keene JD, Gorospe M. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc Natl Acad Sci U S A. 2003;100:8354-8359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 388] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 58. | Wang J, Guo Y, Chu H, Guan Y, Bi J, Wang B. Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int J Mol Sci. 2013;14:10015-10041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 59. | Singh B, Cook KR, Vincent L, Hall CS, Berry JA, Multani AS, Lucci A. Cyclooxygenase-2 induces genomic instability, BCL2 expression, doxorubicin resistance, and altered cancer-initiating cell phenotype in MCF7 breast cancer cells. J Surg Res. 2008;147:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Badawi A, Biyanee A, Nasrullah U, Winslow S, Schmid T, Pfeilschifter J, Eberhardt W. Inhibition of IRES-dependent translation of caspase-2 by HuR confers chemotherapeutic drug resistance in colon carcinoma cells. Oncotarget. 2018;9:18367-18385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 61. | Winkler C, Doller A, Imre G, Badawi A, Schmid T, Schulz S, Steinmeyer N, Pfeilschifter J, Rajalingam K, Eberhardt W. Attenuation of the ELAV1-like protein HuR sensitizes adenocarcinoma cells to the intrinsic apoptotic pathway by increasing the translation of caspase-2L. Cell Death Dis. 2014;5:e1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 62. | Badawi A, Hehlgans S, Pfeilschifter J, Rödel F, Eberhardt W. Silencing of the mRNA-binding protein HuR increases the sensitivity of colorectal cancer cells to ionizing radiation through upregulation of caspase-2. Cancer Lett. 2017;393:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | To KK, Leung WW, Ng SS. Exploiting a novel miR-519c-HuR-ABCG2 regulatory pathway to overcome chemoresistance in colorectal cancer. Exp Cell Res. 2015;338:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 64. | Duan H, He ZQ, Guo CC, Li JH, Wang J, Zhu Z, Sai K, Chen ZP, Jiang XB, Mou YG. Bone metastasis predicts poor prognosis of patients with brain metastases from colorectal carcinoma post aggressive treatment. Cancer Manag Res. 2018;10:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Luo D, Liu Q, Yu W, Ma Y, Zhu J, Lian P, Cai S, Li Q, Li X. Prognostic value of distant metastasis sites and surgery in stage IV colorectal cancer: a population-based study. Int J Colorectal Dis. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Sun S, Zhang X, Lyu L, Li X, Yao S, Zhang J. Autotaxin Expression Is Regulated at the Post-transcriptional Level by the RNA-binding Proteins HuR and AUF1. J Biol Chem. 2016;291:25823-25836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Yun CC, Kumar A. Diverse roles of LPA signaling in the intestinal epithelium. Exp Cell Res. 2015;333:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Takahashi K, Fukushima K, Onishi Y, Inui K, Node Y, Fukushima N, Honoki K, Tsujiuchi T. Lysophosphatidic acid (LPA) signaling via LPA4 and LPA6 negatively regulates cell motile activities of colon cancer cells. Biochem Biophys Res Commun. 2017;483:652-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Arechederra M, Priego N, Vázquez-Carballo A, Sequera C, Gutiérrez-Uzquiza Á, Cerezo-Guisado MI, Ortiz-Rivero S, Roncero C, Cuenda A, Guerrero C, Porras A. p38 MAPK down-regulates fibulin 3 expression through methylation of gene regulatory sequences: role in migration and invasion. J Biol Chem. 2015;290:4383-4397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Tong JD, Jiao NL, Wang YX, Zhang YW, Han F. Downregulation of fibulin-3 gene by promoter methylation in colorectal cancer predicts adverse prognosis. Neoplasma. 2011;58:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 71. | López de Silanes I, Gorospe M, Taniguchi H, Abdelmohsen K, Srikantan S, Alaminos M, Berdasco M, Urdinguio RG, Fraga MF, Jacinto FV, Esteller M. The RNA-binding protein HuR regulates DNA methylation through stabilization of DNMT3b mRNA. Nucleic Acids Res. 2009;37:2658-2671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Weisenberger DJ, Liang G, Lenz HJ. DNA methylation aberrancies delineate clinically distinct subsets of colorectal cancer and provide novel targets for epigenetic therapies. Oncogene. 2018;37:566-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 73. | Tse JWT, Jenkins LJ, Chionh F, Mariadason JM. Aberrant DNA Methylation in Colorectal Cancer: What Should We Target? Trends Cancer. 2017;3:698-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 74. | Grover PK, Hardingham JE, Cummins AG. Stem cell marker olfactomedin 4: critical appraisal of its characteristics and role in tumorigenesis. Cancer Metastasis Rev. 2010;29:761-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Dhawan P, Singh AB, Deane NG, No Y, Shiou SR, Schmidt C, Neff J, Washington MK, Beauchamp RD. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115:1765-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 438] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 76. | Sharma A, Bhat AA, Krishnan M, Singh AB, Dhawan P. Trichostatin-A modulates claudin-1 mRNA stability through the modulation of Hu antigen R and tristetraprolin in colon cancer cells. Carcinogenesis. 2013;34:2610-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Wang D, Fu L, Sun H, Guo L, DuBois RN. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology. 2015;149:1884-1895.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 78. | Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H, Fan Z, Cai J, Li Q. Berberine Inhibits Invasion and Metastasis of Colorectal Cancer Cells via COX-2/PGE2 Mediated JAK2/STAT3 Signaling Pathway. PLoS One. 2015;10:e0123478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 79. | Fujino H, Toyomura K, Chen XB, Regan JW, Murayama T. Prostaglandin E₂ regulates cellular migration via induction of vascular endothelial growth factor receptor-1 in HCA-7 human colon cancer cells. Biochem Pharmacol. 2011;81:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Lan Y, Xiao X, He Z, Luo Y, Wu C, Li L, Song X. Long noncoding RNA OCC-1 suppresses cell growth through destabilizing HuR protein in colorectal cancer. Nucleic Acids Res. 2018;46:5809-5821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 151] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 81. | Abdelmohsen K, Kim MM, Srikantan S, Mercken EM, Brennan SE, Wilson GM, Cabo Rd, Gorospe M. miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle. 2010;9:1354-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 82. | Al-Haidari A, Algaber A, Madhi R, Syk I, Thorlacius H. MiR-155-5p controls colon cancer cell migration via post-transcriptional regulation of Human Antigen R (HuR). Cancer Lett. 2018;421:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 83. | Orlando RG, Davidorf FH. Spontaneous recovery phenomenon in the presumed ocular histoplasmosis syndrome. Int Ophthalmol Clin. 1983;23:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 84. | McLarnon A. Cancer: Mdm2-regulated stabilization of HuR by neddylation in HCC and colon cancer--a possible target for therapy. Nat Rev Gastroenterol Hepatol. 2011;9:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Embade N, Fernández-Ramos D, Varela-Rey M, Beraza N, Sini M, Gutiérrez de Juan V, Woodhoo A, Martínez-López N, Rodríguez-Iruretagoyena B, Bustamante FJ, de la Hoz AB, Carracedo A, Xirodimas DP, Rodríguez MS, Lu SC, Mato JM, Martínez-Chantar ML. Murine double minute 2 regulates Hu antigen R stability in human liver and colon cancer through NEDDylation. Hepatology. 2012;55:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 86. | Doller A, Winkler C, Azrilian I, Schulz S, Hartmann S, Pfeilschifter J, Eberhardt W. High-constitutive HuR phosphorylation at Ser 318 by PKC{delta} propagates tumor relevant functions in colon carcinoma cells. Carcinogenesis. 2011;32:676-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Akaike Y, Masuda K, Kuwano Y, Nishida K, Kajita K, Kurokawa K, Satake Y, Shoda K, Imoto I, Rokutan K. HuR regulates alternative splicing of the TRA2β gene in human colon cancer cells under oxidative stress. Mol Cell Biol. 2014;34:2857-2873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Grossi V, Peserico A, Tezil T, Simone C. p38α MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol. 2014;20:9744-9758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 179] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 89. | Abdelmohsen K, Gorospe M. Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev RNA. 2010;1:214-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 339] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 90. | Meisner NC, Filipowicz W. Properties of the regulatory RNA-binding protein HuR and its role in controlling miRNA repression. Adv Exp Med Biol. 2010;700:106-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Meisner NC, Hintersteiner M, Mueller K, Bauer R, Seifert JM, Naegeli HU, Ottl J, Oberer L, Guenat C, Moss S, Harrer N, Woisetschlaeger M, Buehler C, Uhl V, Auer M. Identification and mechanistic characterization of low-molecular-weight inhibitors for HuR. Nat Chem Biol. 2007;3:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 92. | Chae MJ, Sung HY, Kim EH, Lee M, Kwak H, Chae CH, Kim S, Park WY. Chemical inhibitors destabilize HuR binding to the AU-rich element of TNF-alpha mRNA. Exp Mol Med. 2009;41:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 93. | D'Agostino VG, Adami V, Provenzani A. A novel high throughput biochemical assay to evaluate the HuR protein-RNA complex formation. PLoS One. 2013;8:e72426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 94. | Blanco FF, Preet R, Aguado A, Vishwakarma V, Stevens LE, Vyas A, Padhye S, Xu L, Weir SJ, Anant S, Meisner-Kober N, Brody JR, Dixon DA. Impact of HuR inhibition by the small molecule MS-444 on colorectal cancer cell tumorigenesis. Oncotarget. 2016;7:74043-74058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 95. | Murakami A, Furukawa I, Miyamoto S, Tanaka T, Ohigashi H. Curcumin combined with turmerones, essential oil components of turmeric, abolishes inflammation-associated mouse colon carcinogenesis. Biofactors. 2013;39:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Sanduja S, Blanco FF, Dixon DA. The roles of TTP and BRF proteins in regulated mRNA decay. Wiley Interdiscip Rev RNA. 2011;2:42-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 97. | Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem Soc Trans. 2002;30:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 98. | Lai WS, Stumpo DJ, Blackshear PJ. Rapid insulin-stimulated accumulation of an mRNA encoding a proline-rich protein. J Biol Chem. 1990;265:16556-16563. [PubMed] |
| 99. | Cao H, Urban JF, Anderson RA. Insulin increases tristetraprolin and decreases VEGF gene expression in mouse 3T3-L1 adipocytes. Obesity (Silver Spring). 2008;16:1208-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Blanco FF, Sanduja S, Deane NG, Blackshear PJ, Dixon DA. Transforming growth factor β regulates P-body formation through induction of the mRNA decay factor tristetraprolin. Mol Cell Biol. 2014;34:180-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Ogawa K, Chen F, Kim YJ, Chen Y. Transcriptional regulation of tristetraprolin by transforming growth factor-beta in human T cells. J Biol Chem. 2003;278:30373-30381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 102. | Chen YL, Jiang YW, Su YL, Lee SC, Chang MS, Chang CJ. Transcriptional regulation of tristetraprolin by NF-κB signaling in LPS-stimulated macrophages. Mol Biol Rep. 2013;40:2867-2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 103. | Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 965] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 104. | Ciais D, Cherradi N, Feige JJ. Multiple functions of tristetraprolin/TIS11 RNA-binding proteins in the regulation of mRNA biogenesis and degradation. Cell Mol Life Sci. 2013;70:2031-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 105. | Kedersha N, Stoecklin G, Ayodele M, Yacono P, Lykke-Andersen J, Fritzler MJ, Scheuner D, Kaufman RJ, Golan DE, Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J Cell Biol. 2005;169:871-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1043] [Cited by in RCA: 1141] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 106. | Franks TM, Lykke-Andersen J. TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU-rich elements. Genes Dev. 2007;21:719-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 107. | Sandler H, Kreth J, Timmers HT, Stoecklin G. Not1 mediates recruitment of the deadenylase Caf1 to mRNAs targeted for degradation by tristetraprolin. Nucleic Acids Res. 2011;39:4373-4386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 108. | Fenger-Grøn M, Fillman C, Norrild B, Lykke-Andersen J. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol Cell. 2005;20:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 380] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 109. | Lykke-Andersen J, Wagner E. Recruitment and activation of mRNA decay enzymes by two ARE-mediated decay activation domains in the proteins TTP and BRF-1. Genes Dev. 2005;19:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 398] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 110. | Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 688] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 111. | Hau HH, Walsh RJ, Ogilvie RL, Williams DA, Reilly CS, Bohjanen PR. Tristetraprolin recruits functional mRNA decay complexes to ARE sequences. J Cell Biochem. 2007;100:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 112. | Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 667] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 113. | Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 723] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 114. | Taylor GA, Carballo E, Lee DM, Lai WS, Thompson MJ, Patel DD, Schenkman DI, Gilkeson GS, Broxmeyer HE, Haynes BF, Blackshear PJ. A pathogenetic role for TNF alpha in the syndrome of cachexia, arthritis, and autoimmunity resulting from tristetraprolin (TTP) deficiency. Immunity. 1996;4:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 652] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 115. | Brennan SE, Kuwano Y, Alkharouf N, Blackshear PJ, Gorospe M, Wilson GM. The mRNA-destabilizing protein tristetraprolin is suppressed in many cancers, altering tumorigenic phenotypes and patient prognosis. Cancer Res. 2009;69:5168-5176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 116. | Rounbehler RJ, Berglund AE, Gerke T, Takhar MM, Awasthi S, Li W, Davicioni E, Erho NG, Ross AE, Schaeffer EM, Klein EA, Karnes RJ, Jenkins RB, Cleveland JL, Park JY, Yamoah K. Tristetraprolin Is a Prognostic Biomarker for Poor Outcomes among Patients with Low-Grade Prostate Cancer. Cancer Epidemiol Biomarkers Prev. 2018;27:1376-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | Lee HH, Son YJ, Lee WH, Park YW, Chae SW, Cho WJ, Kim YM, Choi HJ, Choi DH, Jung SW, Min YJ, Park SE, Lee BJ, Cha HJ, Park JW. Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer. Int J Cancer. 2010;126:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 118. | Montorsi L, Guizzetti F, Alecci C, Caporali A, Martello A, Atene CG, Parenti S, Pizzini S, Zanovello P, Bortoluzzi S, Ferrari S, Grande A, Zanocco-Marani T. Loss of ZFP36 expression in colorectal cancer correlates to wnt/ ß-catenin activity and enhances epithelial-to-mesenchymal transition through upregulation of ZEB1, SOX9 and MACC1. Oncotarget. 2016;7:59144-59157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 119. | Fallahi M, Amelio AL, Cleveland JL, Rounbehler RJ. CREB targets define the gene expression signature of malignancies having reduced levels of the tumor suppressor tristetraprolin. PLoS One. 2014;9:e115517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 120. | Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311-4323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 623] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 121. | Deleault KM, Skinner SJ, Brooks SA. Tristetraprolin regulates TNF TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol. 2008;45:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Carballo E, Lai WS, Blackshear PJ. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood. 2000;95:1891-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 123. | Sawaoka H, Dixon DA, Oates JA, Boutaud O. Tristetraprolin binds to the 3'-untranslated region of cyclooxygenase-2 mRNA. A polyadenylation variant in a cancer cell line lacks the binding site. J Biol Chem. 2003;278:13928-13935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 124. | Moore AE, Young LE, Dixon DA. MicroRNA and AU-rich element regulation of prostaglandin synthesis. Cancer Metastasis Rev. 2011;30:419-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Young LE, Dixon DA. Posttranscriptional Regulation of Cyclooxygenase 2 Expression in Colorectal Cancer. Curr Colorectal Cancer Rep. 2010;6:60-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 126. | Suzuki H, Ogawa H, Miura K, Haneda S, Watanabe K, Ohnuma S, Sasaki H, Sase T, Kimura S, Kajiwara T, Komura T, Toshima M, Matsuda Y, Shibata C, Sasaki I. IL-23 directly enhances the proliferative and invasive activities of colorectal carcinoma. Oncol Lett. 2012;4:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 127. | Lee HH, Yang SS, Vo MT, Cho WJ, Lee BJ, Leem SH, Lee SH, Cha HJ, Park JW. Tristetraprolin down-regulates IL-23 expression in colon cancer cells. Mol Cells. 2013;36:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Marderosian M, Sharma A, Funk AP, Vartanian R, Masri J, Jo OD, Gera JF. Tristetraprolin regulates Cyclin D1 and c-Myc mRNA stability in response to rapamycin in an Akt-dependent manner via p38 MAPK signaling. Oncogene. 2006;25:6277-6290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 129. | Shyu AB, Belasco JG, Greenberg ME. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991;5:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 420] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 130. | Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 937] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 131. | Lee JY, Kim HJ, Yoon NA, Lee WH, Min YJ, Ko BK, Lee BJ, Lee A, Cha HJ, Cho WJ, Park JW. Tumor suppressor p53 plays a key role in induction of both tristetraprolin and let-7 in human cancer cells. Nucleic Acids Res. 2013;41:5614-5625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 132. | Madison BB, Liu Q, Zhong X, Hahn CM, Lin N, Emmett MJ, Stanger BZ, Lee JS, Rustgi AK. LIN28B promotes growth and tumorigenesis of the intestinal epithelium via Let-7. Genes Dev. 2013;27:2233-2245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 133. | Piskounova E, Polytarchou C, Thornton JE, LaPierre RJ, Pothoulakis C, Hagan JP, Iliopoulos D, Gregory RI. Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell. 2011;147:1066-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 134. | Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762-9770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 590] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 135. | Lee SR, Jin H, Kim WT, Kim WJ, Kim SZ, Leem SH, Kim SM. Tristetraprolin activation by resveratrol inhibits the proliferation and metastasis of colorectal cancer cells. Int J Oncol. 2018;53:1269-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 136. | Al-Ahmadi W, Al-Ghamdi M, Al-Souhibani N, Khabar KS. miR-29a inhibition normalizes HuR over-expression and aberrant AU-rich mRNA stability in invasive cancer. J Pathol. 2013;230:28-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 137. | Wei F, Zhang T, Yang Z, Wei JC, Shen HF, Xiao D, Wang Q, Yang P, Chen HC, Hu H, Chen ZP, Huang Q, Li WL, Cao J. Gambogic Acid Efficiently Kills Stem-Like Colorectal Cancer Cells by Upregulating ZFP36 Expression. Cell Physiol Biochem. 2018;46:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 138. | Seo GS, Jiang WY, Chi JH, Jin H, Park WC, Sohn DH, Park PH, Lee SH. Heme oxygenase-1 promotes tumor progression and metastasis of colorectal carcinoma cells by inhibiting antitumor immunity. Oncotarget. 2015;6:19792-19806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 139. | Bhat AA, Ahmad R, Uppada SB, Singh AB, Dhawan P. Claudin-1 promotes TNF-α-induced epithelial-mesenchymal transition and migration in colorectal adenocarcinoma cells. Exp Cell Res. 2016;349:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 140. | Benedicto A, Romayor I, Arteta B. Role of liver ICAM-1 in metastasis. Oncol Lett. 2017;14:3883-3892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 141. | Kelly CP, O'Keane JC, Orellana J, Schroy PC, Yang S, LaMont JT, Brady HR. Human colon cancer cells express ICAM-1 in vivo and support LFA-1-dependent lymphocyte adhesion in vitro. Am J Physiol. 1992;263:G864-G870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 142. | Lee WH, Lee HH, Vo MT, Kim HJ, Ko MS, Im YC, Min YJ, Lee BJ, Cho WJ, Park JW. Casein kinase 2 regulates the mRNA-destabilizing activity of tristetraprolin. J Biol Chem. 2011;286:21577-21587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Cianchi F, Cortesini C, Fantappiè O, Messerini L, Schiavone N, Vannacci A, Nistri S, Sardi I, Baroni G, Marzocca C, Perna F, Mazzanti R, Bechi P, Masini E. Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis. Am J Pathol. 2003;162:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 144. | Sobolewski C, Sanduja S, Blanco FF, Hu L, Dixon DA. Histone Deacetylase Inhibitors Activate Tristetraprolin Expression through Induction of Early Growth Response Protein 1 (EGR1) in Colorectal Cancer Cells. Biomolecules. 2015;5:2035-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 145. | Krishnan M, Singh AB, Smith JJ, Sharma A, Chen X, Eschrich S, Yeatman TJ, Beauchamp RD, Dhawan P. HDAC inhibitors regulate claudin-1 expression in colon cancer cells through modulation of mRNA stability. Oncogene. 2010;29:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 146. | Mariadason JM. HDACs and HDAC inhibitors in colon cancer. Epigenetics. 2008;3:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 147. | Cruz-Gallardo I, Aroca Á, Persson C, Karlsson BG, Díaz-Moreno I. RNA binding of T-cell intracellular antigen-1 (TIA-1) C-terminal RNA recognition motif is modified by pH conditions. J Biol Chem. 2013;288:25986-25994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Bauer WJ, Heath J, Jenkins JL, Kielkopf CL. Three RNA recognition motifs participate in RNA recognition and structural organization by the pro-apoptotic factor TIA-1. J Mol Biol. 2012;415:727-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 149. | Waris S, Wilce MC, Wilce JA. RNA recognition and stress granule formation by TIA proteins. Int J Mol Sci. 2014;15:23377-23388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 150. | Anderson P, Kedersha N, Ivanov P. Stress granules, P-bodies and cancer. Biochim Biophys Acta. 2015;1849:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 151. | Adjibade P, St-Sauveur VG, Quevillon Huberdeau M, Fournier MJ, Savard A, Coudert L, Khandjian EW, Mazroui R. Sorafenib, a multikinase inhibitor, induces formation of stress granules in hepatocarcinoma cells. Oncotarget. 2015;6:43927-43943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 152. | Grabocka E, Bar-Sagi D. Mutant KRAS Enhances Tumor Cell Fitness by Upregulating Stress Granules. Cell. 2016;167:1803-1813.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 153. | Sánchez-Jiménez C, Ludeña MD, Izquierdo JM. T-cell intracellular antigens function as tumor suppressor genes. Cell Death Dis. 2015;6:e1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 154. | Hamada J, Shoda K, Masuda K, Fujita Y, Naruto T, Kohmoto T, Miyakami Y, Watanabe M, Kudo Y, Fujiwara H, Ichikawa D, Otsuji E, Imoto I. Tumor-promoting function and prognostic significance of the RNA-binding protein T-cell intracellular antigen-1 in esophageal squamous cell carcinoma. Oncotarget. 2016;7:17111-17128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 155. | Zhao W, Zhao J, Hou M, Wang Y, Zhang Y, Zhao X, Zhang C, Guo D. HuR and TIA1/TIAL1 are involved in regulation of alternative splicing of SIRT1 pre-mRNA. Int J Mol Sci. 2014;15:2946-2958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 156. | Izquierdo JM, Majós N, Bonnal S, Martínez C, Castelo R, Guigó R, Bilbao D, Valcárcel J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell. 2005;19:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 157. | Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383-5398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 797] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 158. | Chiou GY, Yang TW, Huang CC, Tang CY, Yen JY, Tsai MC, Chen HY, Fadhilah N, Lin CC, Jong YJ. Musashi-1 promotes a cancer stem cell lineage and chemoresistance in colorectal cancer cells. Sci Rep. 2017;7:2172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 159. | Liu Y, Liu R, Yang F, Cheng R, Chen X, Cui S, Gu Y, Sun W, You C, Liu Z, Sun F, Wang Y, Fu Z, Ye C, Zhang C, Li J, Chen X. miR-19a promotes colorectal cancer proliferation and migration by targeting TIA1. Mol Cancer. 2017;16:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 160. | Yang X, Wang M, Lin B, Yao D, Li J, Tang X, Li S, Liu Y, Xie R, Yu S. miR-487a promotes progression of gastric cancer by targeting TIA1. Biochimie. 2018;154:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 161. | Han J, Li J, Tang K, Zhang H, Guo B, Hou N, Huang C. miR-338-3p confers 5-fluorouracil resistance in p53 mutant colon cancer cells by targeting the mammalian target of rapamycin. Exp Cell Res. 2017;360:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 162. | Maclean KN, See CG, McKay IA, Bustin SA. The human immediate early gene BRF1 maps to chromosome 14q22-q24. Genomics. 1995;30:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 163. | Rataj F, Planel S, Desroches-Castan A, Le Douce J, Lamribet K, Denis J, Feige JJ, Cherradi N. The cAMP pathway regulates mRNA decay through phosphorylation of the RNA-binding protein TIS11b/BRF1. Mol Biol Cell. 2016;27:3841-3854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 164. | Lee SK, Kim SB, Kim JS, Moon CH, Han MS, Lee BJ, Chung DK, Min YJ, Park JH, Choi DH, Cho HR, Park SK, Park JW. Butyrate response factor 1 enhances cisplatin sensitivity in human head and neck squamous cell carcinoma cell lines. Int J Cancer. 2005;117:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 165. | Qiu Y, Langman MJ, Eggo MC. Targets of 17beta-oestradiol-induced apoptosis in colon cancer cells: a mechanism for the protective effects of hormone replacement therapy? J Endocrinol. 2004;181:327-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 166. | Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J. 2004;23:3092-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 389] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 167. | Trojanowicz B, Brodauf L, Sekulla C, Lorenz K, Finke R, Dralle H, Hoang-Vu C. The role of AUF1 in thyroid carcinoma progression. Endocr Relat Cancer. 2009;16:857-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 168. | Gouble A, Grazide S, Meggetto F, Mercier P, Delsol G, Morello D. A new player in oncogenesis: AUF1/hnRNPD overexpression leads to tumorigenesis in transgenic mice. Cancer Res. 2002;62:1489-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 169. | Gao Y, Wang W, Cao J, Wang F, Geng Y, Cao J, Xu X, Zhou J, Liu P, Zhang S. Upregulation of AUF1 is involved in the proliferation of esophageal squamous cell carcinoma through GCH1. Int J Oncol. 2016;49:2001-2010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 170. | Leblanc R, Peyruchaud O. New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp Cell Res. 2015;333:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 171. | Baou M, Norton JD, Murphy JJ. AU-rich RNA binding proteins in hematopoiesis and leukemogenesis. Blood. 2011;118:5732-5740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 172. | Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, Filipowicz W, Ramos A, Gherzi R, Rosenfeld MG. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 531] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 173. | Trabucchi M, Briata P, Filipowicz W, Ramos A, Gherzi R, Rosenfeld MG. KSRP promotes the maturation of a group of miRNA precursors. Adv Exp Med Biol. 2010;700:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 174. | Chien MH, Lee WJ, Yang YC, Li YL, Chen BR, Cheng TY, Yang PW, Wang MY, Jan YH, Lin YK, Lee JM, Hsiao M, Chen JS, Hua KT. KSRP suppresses cell invasion and metastasis through miR-23a-mediated EGR3 mRNA degradation in non-small cell lung cancer. Biochim Biophys Acta Gene Regul Mech. 2017;1860:1013-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 175. | Bikkavilli RK, Malbon CC. Dishevelled-KSRP complex regulates Wnt signaling through post-transcriptional stabilization of beta-catenin mRNA. J Cell Sci. 2010;123:1352-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 176. | Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005;33:4813-4827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 177. | Suzuki H, Takeuchi M, Sugiyama A, Alam AK, Vu LT, Sekiyama Y, Dam HC, Ohki SY, Tsukahara T. Alternative splicing produces structural and functional changes in CUGBP2. BMC Biochem. 2012;13:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 178. | Subramaniam D, Natarajan G, Ramalingam S, Ramachandran I, May R, Queimado L, Houchen CW, Anant S. Translation inhibition during cell cycle arrest and apoptosis: Mcl-1 is a novel target for RNA binding protein CUGBP2. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1025-G1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 179. | Mukhopadhyay D, Jung J, Murmu N, Houchen CW, Dieckgraefe BK, Anant S. CUGBP2 plays a critical role in apoptosis of breast cancer cells in response to genotoxic injury. Ann N Y Acad Sci. 2003;1010:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 180. | Jakstaite A, Maziukiene A, Silkuniene G, Kmieliute K, Dauksa A, Paskauskas S, Gulbinas A, Dambrauskas Z. Upregulation of cugbp2 increases response of pancreatic cancer cells to chemotherapy. Langenbecks Arch Surg. 2016;401:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 181. | Natarajan G, Ramalingam S, Ramachandran I, May R, Queimado L, Houchen CW, Anant S. CUGBP2 downregulation by prostaglandin E2 protects colon cancer cells from radiation-induced mitotic catastrophe. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1235-G1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 182. | Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137:899-908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 298] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 183. | Chip S, Zelmer A, Ogunshola OO, Felderhoff-Mueser U, Nitsch C, Bührer C, Wellmann S. The RNA-binding protein RBM3 is involved in hypothermia induced neuroprotection. Neurobiol Dis. 2011;43:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 184. | Zhou RB, Lu XL, Zhang CY, Yin DC. RNA binding motif protein 3: a potential biomarker in cancer and therapeutic target in neuroprotection. Oncotarget. 2017;8:22235-22250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 185. | Sureban SM, Ramalingam S, Natarajan G, May R, Subramaniam D, Bishnupuri KS, Morrison AR, Dieckgraefe BK, Brackett DJ, Postier RG, Houchen CW, Anant S. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene. 2008;27:4544-4556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 186. | Matsuda A, Ogawa M, Yanai H, Naka D, Goto A, Ao T, Tanno Y, Takeda K, Watanabe Y, Honda K, Taniguchi T. Generation of mice deficient in RNA-binding motif protein 3 (RBM3) and characterization of its role in innate immune responses and cell growth. Biochem Biophys Res Commun. 2011;411:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 187. | Wellmann S, Truss M, Bruder E, Tornillo L, Zelmer A, Seeger K, Bührer C. The RNA-binding protein RBM3 is required for cell proliferation and protects against serum deprivation-induced cell death. Pediatr Res. 2010;67:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 188. | Venugopal A, Subramaniam D, Balmaceda J, Roy B, Dixon DA, Umar S, Weir SJ, Anant S. RNA binding protein RBM3 increases β-catenin signaling to increase stem cell characteristics in colorectal cancer cells. Mol Carcinog. 2016;55:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 189. | Jang HH, Lee HN, Kim SY, Hong S, Lee WS. Expression of RNA-binding Motif Protein 3 (RBM3) and Cold-inducible RNA-binding protein (CIRP) Is Associated with Improved Clinical Outcome in Patients with Colon Cancer. Anticancer Res. 2017;37:1779-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 190. | Ye F, Jin P, Cai X, Cai P, Cai H. High RNA-Binding Motif Protein 3 (RBM3) Expression is Independently Associated with Prolonged Overall Survival in Intestinal-Type Gastric Cancer. Med Sci Monit. 2017;23:6033-6041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 191. | Shu L, Yan W, Chen X. RNPC1, an RNA-binding protein and a target of the p53 family, is required for maintaining the stability of the basal and stress-induced p21 transcript. Genes Dev. 2006;20:2961-2972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 192. | Xue JQ, Xia TS, Liang XQ, Zhou W, Cheng L, Shi L, Wang Y, Ding Q. RNA-binding protein RNPC1: acting as a tumor suppressor in breast cancer. BMC Cancer. 2014;14:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 193. | Ding Z, Yang HW, Xia TS, Wang B, Ding Q. Integrative genomic analyses of the RNA-binding protein, RNPC1, and its potential role in cancer prediction. Int J Mol Med. 2015;36:473-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 194. | Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, Borg A, Isola JJ. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin Cancer Res. 2000;6:1833-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 195. | Hötte GJ, Linam-Lennon N, Reynolds JV, Maher SG. Radiation sensitivity of esophageal adenocarcinoma: the contribution of the RNA-binding protein RNPC1 and p21-mediated cell cycle arrest to radioresistance. Radiat Res. 2012;177:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 196. | Lou P, Li C, Shi L, Xia TS, Zhou W, Wu J, Zhou X, Li X, Wang Y, Wei JF, Ding Q. RNPC1 enhances progesterone receptor functions by regulating its mRNA stability in breast cancer. Oncotarget. 2017;8:16387-16400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 197. | Zhang J, Xu E, Ren C, Yan W, Zhang M, Chen M, Cardiff RD, Imai DM, Wisner E, Chen X. Mice deficient in Rbm38, a target of the p53 family, are susceptible to accelerated aging and spontaneous tumors. Proc Natl Acad Sci U S A. 2014;111:18637-18642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 198. | Yang L, Zhang Y, Ling C, Heng W. RNPC1 inhibits non-small cell lung cancer progression via regulating miR-181a/CASC2 axis. Biotechnol Lett. 2018;40:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 199. | Xu E, Zhang J, Chen X. MDM2 expression is repressed by the RNA-binding protein RNPC1 via mRNA stability. Oncogene. 2013;32:2169-2178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 200. | Ye J, Liang R, Bai T, Lin Y, Mai R, Wei M, Ye X, Li L, Wu F. RBM38 plays a tumor-suppressor role via stabilizing the p53-mdm2 loop function in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 201. | Zhang M, Xu E, Zhang J, Chen X. PPM1D phosphatase, a target of p53 and RBM38 RNA-binding protein, inhibits p53 mRNA translation via dephosphorylation of RBM38. Oncogene. 2015;34:5900-5911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |