Published online Dec 15, 2019. doi: 10.4251/wjgo.v11.i12.1193
Peer-review started: June 18, 2019
First decision: July 31, 2019
Revised: August 13, 2019
Accepted: September 10, 2019
Article in press: September 10, 2019
Published online: December 15, 2019
Processing time: 179 Days and 18.6 Hours
Focal nodular hyperplasia (FNH) has very low potential risk, and a tendency to spontaneously resolve. Hepatocellular adenoma (HCA) has a certain malignant tendency, and its prognosis is significantly different from FNH. Accurate identification of HCA and FNH is critical for clinical treatment.
To analyze the value of multi-parameter ultrasound index based on logistic regression for the differential diagnosis of HCA and FNH.
Thirty-one patients with HCA were included in the HCA group. Fifty patients with FNH were included in the FNH group. The clinical data were collected and recorded in the two groups. Conventional ultrasound, shear wave elastography, and contrast-enhanced ultrasound were performed, and the lesion location, lesion echo, Young’s modulus (YM) value, YM ratio, and changes of time intense curve (TIC) were recorded. Multivariate logistic regression analysis was used to screen the indicators that can be used for the differential diagnosis of HCA and FNH. A ROC curve was established for the potential indicators to analyze the accuracy of the differential diagnosis of HCA and FNH. The value of the combined indicators for distinguishing HCA and FNH were explored.
Multivariate logistic regression analysis showed that lesion echo (P = 0.000), YM value (P = 0.000) and TIC decreasing slope (P = 0.000) were the potential indicators identifying HCA and FNH. In the ROC curve analysis, the accuracy of the YM value distinguishing HCA and FNH was the highest (AUC = 0.891), which was significantly higher than the AUC of the lesion echo and the TIC decreasing slope (P < 0.05). The accuracy of the combined diagnosis was the highest (AUC = 0.938), which was significantly higher than the AUC of the indicators diagnosing HCA individually (P < 0.05). This sensitivity was 91.23%, and the specificity was 83.33%.
The combination of lesion echo, YM value and TIC decreasing slope can accurately differentiate between HCA and FNH.
Core tip: The prognosis of hepatocellular adenoma (HCA) is significantly different from focal nodular hyperplasia (FNH). Accurate identification of HCA and FNH is of great significance. This study explored the accuracy of the combined detection of HCA and FNH by conventional ultrasound, shear wave elastography, and contrast-enhanced ultrasound. The combination of lesion echo, Young’s modulus value and time intense curve decreasing slop in multi-parameter ultrasound index based on logistic regression has high clinical guiding value for the differential diagnosis of HCA and FNH.
- Citation: Wu M, Zhou RH, Xu F, Li XP, Zhao P, Yuan R, Lan YP, Zhou WX. Multi-parameter ultrasound based on the logistic regression model in the differential diagnosis of hepatocellular adenoma and focal nodular hyperplasia. World J Gastrointest Oncol 2019; 11(12): 1193-1205
- URL: https://www.wjgnet.com/1948-5204/full/v11/i12/1193.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i12.1193
Focal nodular hyperplasia (FNH) is a proliferative lesion caused by vascular malformation, which is very common in the clinic. The potential risk is extremely low, most of FNH will not be malignant, and it has a tendency to spontaneously resolve[1-3]. Hepatocellular adenoma (HCA) is a rare liver tumor, which is prone to secondary hemorrhage and has a tendency towards malignant transformation. Although it can be treated conservatively after diagnosis, it must be closely monitored. Surgical resection is required once it is considered to be malignant, and its prognosis is also significantly different from FNH[4-6]. Therefore, accurate identification of HCA and FNH is essential for clinical treatment. However, patients with HCA or FNH have no obvious clinical symptoms, and tumor markers are not significantly expressed in FNH or HCA[7-9]. Ultrasound is a routine imaging diagnostic tool for diagnosing liver diseases. Although traditional two-dimensional ultrasound and Doppler ultrasound are difficult to distinguish, recent research has revealed that contrast-enhanced ultrasound (CEUS) has a certain value in this identification[10,11]. CEUS can clearly identify FNH and HCA through stellate scars in the central part of small FNH[12]. However, due to the low incidence of HCA, the CEUS performance of FNH is not typical. Hence, the value of CEUS in identifying HCA and FNH is limited currently[13,14]. In recent years, real-time shear wave elastography (SWE) technology can non-invasively and accurately obtain the absolute elasticity value of diseased tissues, and has been applied to the differential diagnosis of benign and malignant liver-occupying lesions[15-17]. To date, however, SWE has less application in identifying HCA and FNH. In the study of Gerber et al[18], the hardness of FNH was higher than HCA, but the sample size was insufficient and needed further verification[18]. Therefore, this study recruited HCA and FNH patients as research subjects. It systematically analyzed the different performances of conventional ultrasound, SWE and CEUS between HCA and FNH, and explored the value of ultrasound multi-parameter indicators in the differential diagnosis of HCA and FNH. Furthermore, a logistic regression model was used to establish a combined diagnosis of ultrasound multi-parameter indicators. The value of multi-parameter combined identification of HCA and FNH was explored to provide useful information for the follow-up treatment of HCA.
Patients diagnosed with HCA or FNH in Yinzhou Hospital affiliated with Ningbo University School of Medicine from January 2017 to September 2019 were recruited. Inclusion criteria were as follows: (1) Diagnosed as HCA or FNH by surgical pathology or biopsy; and (2) Underwent color Doppler ultrasound, SWE, and CEUS examination, as well as obtained complete imaging data before surgery. Patients with malignant tumors or severe liver, kidney, heart, and brain dysfunction were excluded. A total of 31 patients with HCA who met the criteria were divided into the HCA group, including eight males and 23 females. The age range was 20-42 years old, with an average of 27.29 ± 9.87 years. A total of 50 FNH patients were divided into the FNH group, including 24 males and 26 females. The age range was 18-years-old to 48-years-old, with an average of 28.09 ± 10.57 years. The age, gender, history of viral infection, and history of cirrhosis was recorded in the two groups. All patients gave informed consent for the study, and the study was approved by the Ethics Committee of Yinzhou Hospital affiliated with Ningbo University School of Medicine.
Liver function and serological examination: Liver function analysis and serological examination were performed on both groups. The patients were kept on a light diet, and fasted for 8 h before examination. Blood was taken from the elbow vein in the morning. Liver function and serological parameters of the two groups were determined using a Bayerl 650 automatic biochemical analyzer. Liver function indicators included alanine aminotransferase (ALT), aspartate aminotransferase (AST), and glutamyl transpeptidase (GGT). Serological indicators included albumin, total bilirubin (Tbil), prothrombin time (PT), and serum ferritin (SF).
Tumor markers: The alpha-fetoprotein (AFP) index of the two groups was examined and recorded by chemiluminescence immunoassay. The kit was purchased from Siemens Medical Diagnostics Products (Shanghai) Co., Ltd. and operated in strict accordance with its instructions. The AFP reference values were as follows: < 20 μg/L indicated normal; 25-100 μg/L indicated a low-level increase; 100-500 μg/L indicated a medium-level increase; > 500 μg/L indicated a high-level increase[19].
Conventional ultrasound: An Esaote MyLab 90 (Esaote Group, Italy) color Doppler ultrasound system with 1-8 MHz frequency was used in this study. The patient was placed in a supine position with arms raised to expose the abdomen. An intercostal space scan was applied with a conventional two-dimensional ultrasound mode, and the image was adjusted after detecting the lesion to avoid rib and lung interference. The best display area of the lesion was located. Lesion location, internal echo, echo uniformity, lesion property, morphology, border, posterior echo, surrounding capsule and microcalcification were recorded. Then, the color Doppler mode was switched to observe the blood supply in the center of and surrounding the lesion.
Ultrasound elastography: SWE studies were performed using the Aixplorer™ ultrasound system (SuperSonic Imagine S.A., Aix-en-Provence, France) with a 6-8 MHz probe. The location of the lesion was determined after routine ultrasound examination. We first set the size of the sampling frame to completely cover the lesion. Meanwhile, we avoided the heart and abdominal large blood vessels. After the sampling frame was set, the patient was asked to hold for 3 s to save the SWE image. In the SWE image, the hard tissue is represented by red, and the soft tissue is represented by blue[20]. The Young’s modulus (YM) value (kPa) and YM ratio were measured at the end of the examination.
CEUS examination: An Esaote MyLab 90 (Esaote Group, Italy) color Doppler ultrasound system with 1-4 MHz frequency was used for CEUS. The mechanical index was set to 0.19 and the dynamic range was 80 dB. The contrast agent was used from SonoVue (Braeeo CO., LTD). At the beginning, 25 mg of contrast medium was dissolved in 5 ml of normal saline. A 2.4 mL suspension was then injected via the superficial vein of the elbow. Finally, 5 ml of normal saline was used for washout. The intensive manifestations of lesions in the arterial phase, portal venous phase, and delayed phase were continuously observed in real time. The software can automatically plot the TIC curve and record the following parameters: Background intensity (BI), peak intensity (PI), enhancement time (ET), peak intensity Change (PI-BI), TIC increasing slope, and TIC decreasing slope.
Analysis was performed using Statistical Product and Service Solutions (SPSS 19.0) software. The measurement data were expressed as x ± s, and comparisons were performed using an independent sample t test. The count data were expressed in case or percentage, and the chi-square test was used for comparison. Multivariate logistic regression analysis was used to screen the potential identification indexes of HCA and FNH. An ROC curve was established to analyze the accuracy of the identification of HCA and FNH. The value of the combined indicators based on a logistic regression model for distinguishing HCA and FNH were explored. The difference was considered statistically significant at P < 0.05.
In this study, 31 patients in the HCA group included 31 lesions, and 50 patients in the FNH group included 50 lesions. All patients retained complete clinical, pathological, and ultrasound imaging data.
In the HCA group, six patients had a history of viral infection, and four patents had a history of cirrhosis. The GGT was slightly elevated. ALT, AST, Albumin, Tbil, PT, AFP and SF were normal. Compared with the FNH group, the proportion of females was higher than that of the FNH group (P < 0.05). The other clinical data were similar with those in the FNH group, and the differences were not statistically significant (P > 0.05) (Table 1).
| HCA group, n = 31 | FNH group, n = 50 | t/χ2 value | P value | |
| Age | 27.29 ± 9.87 | 28.09 ± 10.57 | 0.339 | 0.735 |
| Gender, male/female | 8/23 | 24/26 | 3.994 | 0.047 |
| History of viral infection | 6% | 9% | 0.023 | 0.879 |
| History of cirrhosis | 4% | 6% | 0.014 | 0.904 |
| ALT, U/L | 24.83 ± 12.94 | 28.76 ± 15.38 | 1.185 | 0.239 |
| AST, U/L | 29.83 ± 12.85 | 26.92 ± 13.64 | 0.954 | 0.343 |
| GGT, U/L | 38.32 ± 14.08 | 30.84 ± 10.36 | 1.529 | 0.183 |
| Albumin, g/L | 48.23 ± 3.82 | 47.93 ± 3.58 | 0.482 | 0.528 |
| Tbil, μmol/L | 9.38 ± 4.83 | 10.63 ± 5.63 | 0.392 | 0.682 |
| PT, s | 12.86 ± 0.86 | 12.67 ± 0.74 | 1.055 | 0.295 |
| AFP, μg/L | 14.05 ± 5.97 | 12.39 ± 4.29 | 1.454 | 0.150 |
| SF, μg/L | 89.35 ± 35.24 | 96.32 ± 43.2 | 0.755 | 0.452 |
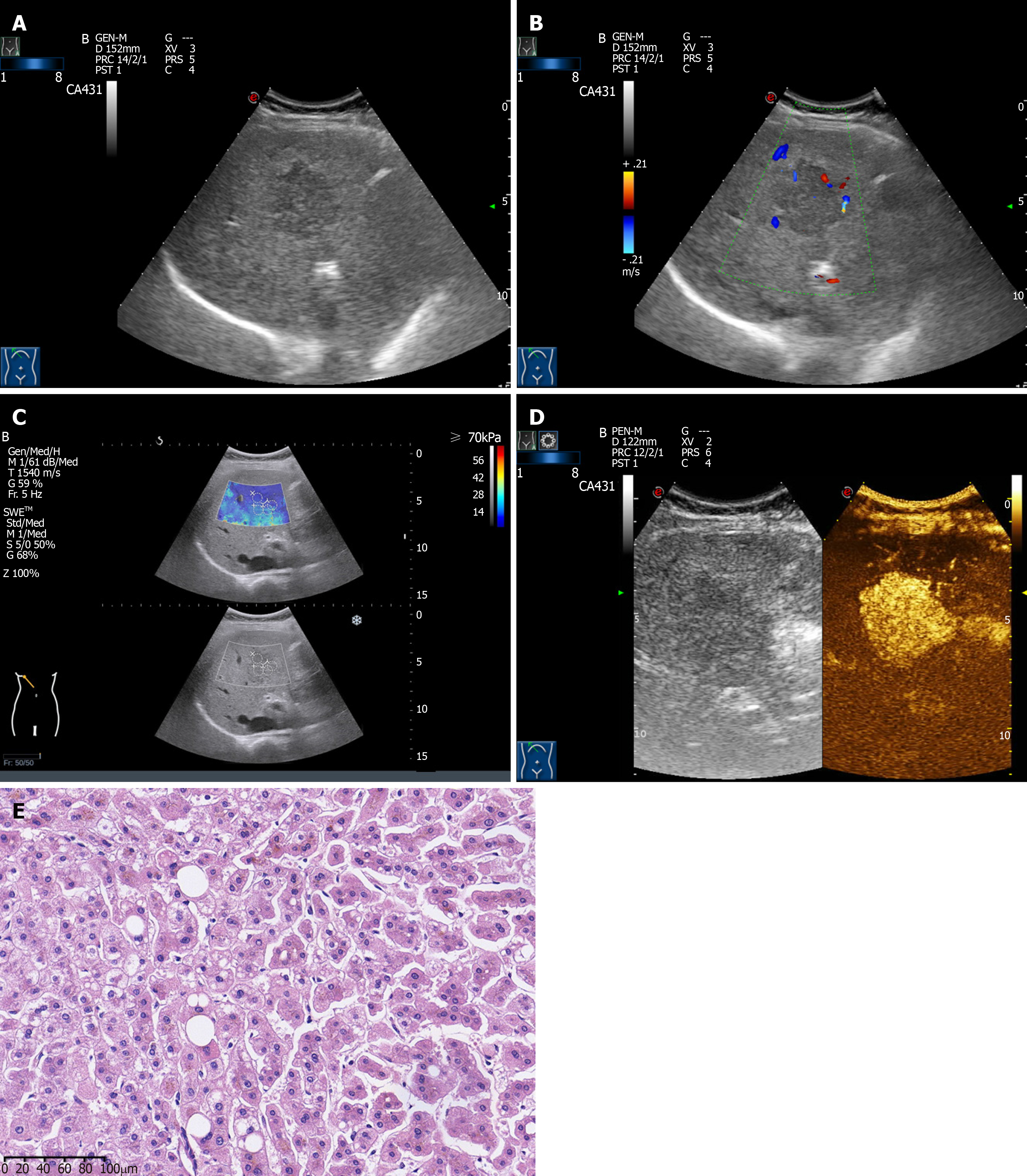
The ultrasound images of HCA showed solid lesions with round or elliptical shapes, with clear borders and high internal echoes in most cases (Figure 1A). Blood flow signals were common in most HCA lesions (Figure 1B). SWE showed that the hardness of HCA was slightly higher than that of normal surrounding liver tissue (Figure 1C). CEUS showed a rapid and significant increase in the arterial phase, a fast washout in the portal venous phase, and an equal or low enhancement in the delayed phase (Figure 1D). In terms of pathology, the gross specimens found abundant blood vessels on the surface of the tumors. The specimens were dark purple, uniform, and a few specimens were accompanied by necrosis or bleeding in the center. Microscopically, the tumor cells were similar to the surrounding normal liver cells, but there was no portal area, portal vein and small bile duct branches in the HCA. Kupffer cells, nuclear fission phase, and complete bile duct structure were lacking in the HCA (Figure 1E).
By comparing the ultrasound features between the HCA and FNH groups, it can be found that the HCA lesions were mostly hyperechoic, while the FNH lesions were mostly hypoechoic. In the SWE feature, the hardness of the HCA lesion was less than that of the FNH lesion. In CEUS performance, the enhancement of HCA was mostly peripheral-centered filling, with low enhancement in the delayed phase, and hemorrhagic necrosis in some HCA lesions. The enhancement of FNH was mostly radioactively filled from the center to the periphery, with slightly high or equal enhancement in the delayed phase. Some stellate scars were found in some FNH lesions. Quantitative analysis of the ultrasound characteristics of the two groups is shown in Table 2. The high echo ratio, solid lesion ratio, and TIC decreasing slope in the HCA group were significantly higher than those in the FNH group. The YM value, YM ratio, and area under the curve (AUC) were significantly lower than those in the FNH group. The differences were statistically significant (P < 0.05). The differences in lesion location, lesion boundary, lesion capsule, microcalcification, posterior echo, blood flow, PI-BI, ET, and TIC increasing slope were similar, and there is no statistical significance (P > 0.05).
| HCA group, n = 31 | FNH group, n = 50 | t/χ2 value | P value | ||
| Diameter in cm | 3.37 ± 1.87 | 3.29 ± 1.58 | 0.206 | 0.837 | |
| Lesion location | Liver right lobe | 24 | 36 | 0.293 | 0.589 |
| Liver left lobe | 7 | 14 | |||
| Lesion echo | Low | 9 | 31 | 11.637 | 0.009 |
| Equal | 5 | 9 | |||
| High | 14 | 10 | |||
| Mixed | 2 | 0 | |||
| Lesion property | Solid | 18 | 16 | 6.202 | 0.045 |
| Cystic | 9 | 23 | |||
| Mixed | 3 | 11 | |||
| Lesion morphology | Regular | 27 | 44 | 0.014 | 0.904 |
| Irregular | 4 | 6 | |||
| Lesion boundary | Clear | 27 | 47 | 1.155 | 0.282 |
| Unclear | 4 | 3 | |||
| Lesion capsule | With capsule | 30 | 46 | 0.753 | 0.386 |
| Without capsule | 1 | 4 | |||
| Lesion internal echo | Uniform | 26 | 43 | 0.069 | 0.793 |
| Non-uniform | 5 | 7 | |||
| Microcalcification | No | 30 | 48 | 0.032 | 0.858 |
| Yes | 1 | 2 | |||
| Posterior echo | No echo attenuation | 31 | 48 | 1.271 | 0.260 |
| Echo attenuation | 0 | 2 | |||
| Lesion blood flow | No blood flow | 7 | 20 | 4.870 | 0.088 |
| Spotted blood flow | 18 | 27 | |||
| Strip blood flow | 6 | 3 | |||
| SWE | YM value in kPa | 14.39 ± 7.28 | 29.27 ± 12.38 | 6.065 | 0.000 |
| YM ratio | 3.73 ± 1.14 | 4.89 ± 1.99 | 2.770 | 0.007 | |
| TIC Quantitative analysis | PI-BI, dB | 21.84 ± 8.83 | 20.18 ± 9.38 | 0.791 | 0.431 |
| ET, s | 18.02 ± 5.88 | 20.27 ± 8.39 | 1.306 | 0.195 | |
| TIC increasing slop | 1.84 ± 0.62 | 1.72 ± 0.23 | 1.241 | 0.218 | |
| TIC decreasing slop | 0.31 ± 0.09 | 0.14 ± 0.07 | 9.510 | 0.000 | |
Multivariate logistic regression analysis was performed to explore the potential indicators identifying HCA. The results showed that gender, lesion property and YM ratio had no significant effect on the identification of HCA. The differences were not statistically significant (P > 0.05). The lesion echo (P = 0.000), YM value (P = 0.000) and TIC decreasing slope (P = 0.000) were potential indicators for identifying HCA (Table 3).
| B | SE | Wald | P value | OR | 95%CI | ||
| Lower limit | Upper limit | ||||||
| Gender | 0.284 | 0.354 | 2.394 | 0.086 | 1.328 | 0.664 | 2.658 |
| Lesion echo | 0.977 | 0.157 | 7.967 | 0.000 | 2.657 | 1.953 | 3.614 |
| Lesion property | 0.427 | 0.964 | 1.234 | 0.137 | 1.532 | 0.232 | 10.135 |
| YM value | - 0.289 | 0.108 | 6.567 | 0.000 | 0.749 | 0.606 | 0.926 |
| YM ratio | - 0.154 | 0.135 | 1.436 | 0.134 | 0.857 | 0.658 | 1.117 |
| TIC decreasing slope | 1.673 | 0.284 | 5.829 | 0.000 | 5.328 | 3.054 | 9.296 |
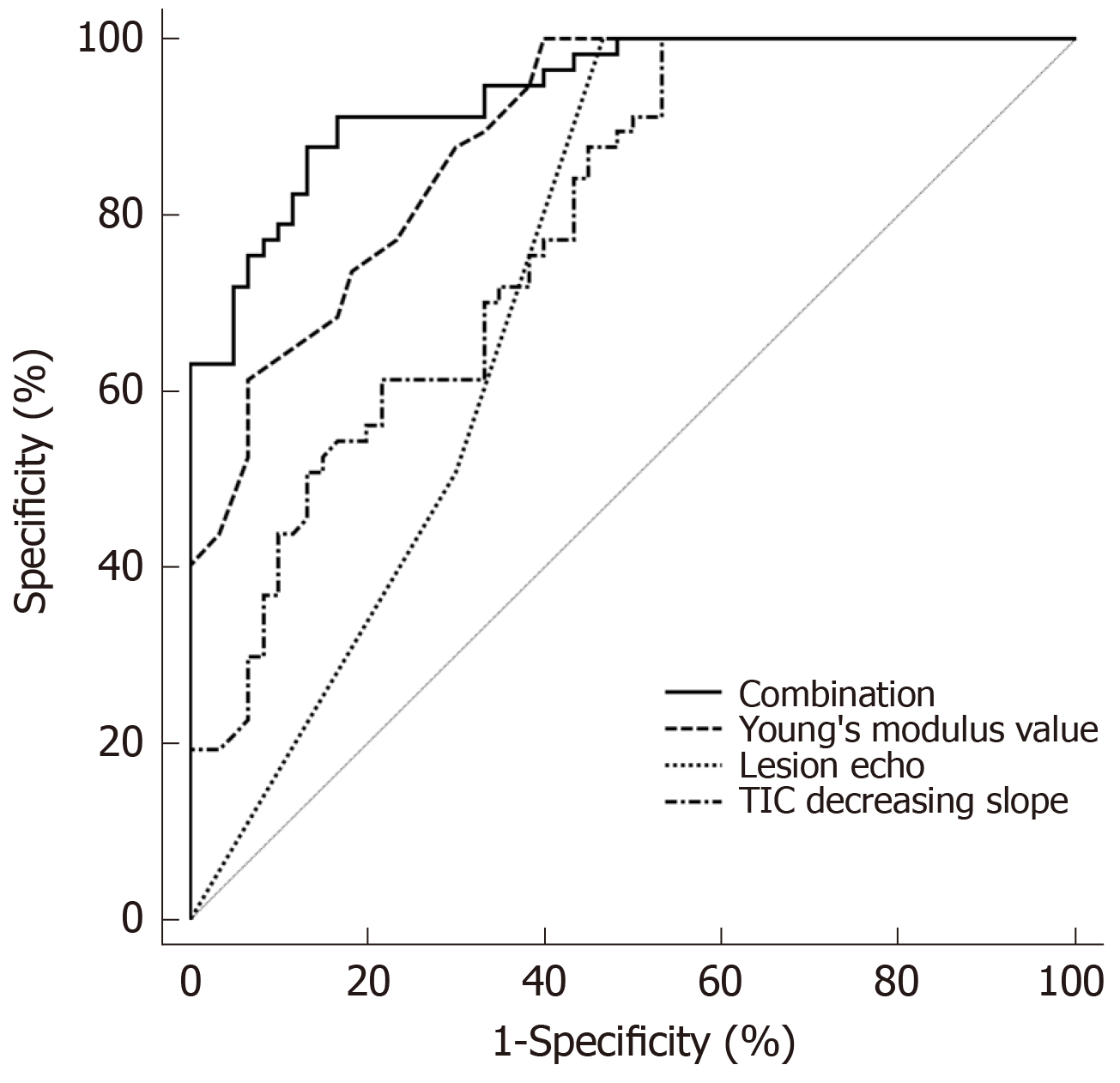
The AUC of each ultrasound indicator distinguishing HCA and FNH was different. The prediction accuracy of the YM value was the highest (AUC = 0.891). The sensitivity was 92.16%, but the specificity was low (73.28%). The AUC of TIC decreasing slope and lesion echo were lower than that of YM value, and the differences were statistically significant (P < 0.05). The accuracy of the combination of three ultrasound indicators identifying HCA and FNH was the highest (AUC = 0.938), which was significantly higher than the AUCs of the three ultrasound indicators individually identifying HCA and FNH. The differences were statistically significant (P < 0.05). The cut-off point was 0.540. Its sensitivity and specificity were 91.23% and 83.33%, respectively (Table 4 and Figure 2).
HCA is a clinically rare benign liver tumor, which often needs to be differentiated from FNH, a non-angiogenic liver benign lesion[21]. Due to the fact that the clinical symptoms of HCA and FNH are not obvious, and the level of related tumor markers are not expressed, it is difficult to differentiate them by physical examination. However, their treatments and prognoses are highly distinct[22,23]. FNH is a proliferative lesion caused by vascular malformation, whereas HCA is a dangerous tumor with a tendency to hemorrhage and undergo malignant transformation. Although a needle biopsy can be used for differential diagnosis, it is an invasive procedure and cannot be used as a routine examination. Ultrasound is important for liver examination because of its simple, non-invasive and high diagnostic accuracy. However, gray-scale ultrasound is limited in identifying HCA and FNH[24]. Although CEUS can recognize the stellate scars of FNH and the ischemic necrosis of HCA, the accuracy of CEUS in identifying HCA and FNH is not high due to the low detection rate of stellate scars of FNH[25]. As a new technique that has been widely used in the differential diagnosis of benign and malignant livers[26], SWE can be used for the differential diagnosis of FNH and HCA by detecting their YM. This study explored the accuracy of conventional ultrasound, SWE and CEUS multi-parameter ultrasound indicators to identify HCA and FNH, with an intent to provide useful information for clinical treatment.
HCA is an estrogen-dependent tumor that has been reported in previous studies in young women or men taking steroids[27-29]. In this study, the proportion of female patients with HCA was higher than that of FNH patients. However, there was no significant difference between the two groups in other clinical data, such as the history of viral infection, serological markers for evaluating liver function, and tumor markers (e.g., AFP). It is difficult to identify HCA and FNH solely through clinical data and laboratory examinations[30]. According to pathological diagnostic criteria, we learned that the typical FNH lesions are nodular, with hyperplastic fibrous tissue, small blood vessels and bile duct structures. Stellate scars can be found in some FNH lesions. The tumor cells of HCA are similar to the surrounding normal liver cells. The center of the tumor may be accompanied by necrosis or hemorrhage. Some HCA lesions had scattered and lumen-expanded small blood vessels. However, there is no portal area, portal vein, small bile duct branches, nuclear fission phase, or complete bile duct structures in HCA lesions. The presence of a complete portal system and bile duct structure is a major feature in identifying HCA and FNH. Histopathological examination is an invasive examination and cannot be used as a routine examination, although it can clearly distinguish HCA and FNH. Therefore, it is important to explore a non-invasive and simple imaging examination method to identify HCA and FNH.
This study compared the gray-scale ultrasound, SWE and CEUS indicators between the HCA group and the FNH group in order to explore the potential identification indicators of HCA and FNH. It revealed that the ratio of high echo HCA lesions was higher than that of FNH. We believe that this is due to the fact that HCA tumor cells are rich in glycogen and fat, and the high fat content makes a high echo. The study of Hasab et al[31] found that although HCA lesions were mostly in high echo types, their ultrasound performance also varies. Because the blood supply of HCA lesions is from the surrounding large blood vessels rather than the central artery, necrosis or hemorrhage is likely to occur in the tumor, and the site of necrosis or hemorrhage will appear as a no echo area. In addition, due to fibrous tissue hyperplasia, the stellate scars of FNH also represent as high echo, which is easily confused in the differential diagnosis of conventional ultrasound. The present study also found that lesion boundary, morphology, blood flow signals and other indicators of HCA and FNH were not significantly different. It indicated that the identification of HCA and FNH solely by conventional gray-scale ultrasound was difficult.
The role of CEUS in the diagnosis of FNH has been recognized[32-34]. In the FNH group of the present study, the enhancement mode was mostly radioactively filled from the center to the periphery. The enhancement mode was slightly higher or of equal enhancement in the delayed phase, and some FNH had stellate scars. This is because the FNH blood supply is distributed centrally to the surrounding area, and the blood vessels are derived from the hepatic artery and the portal vein. During the portal vein phase, the contrast agent can be supplemented by the portal venous system, so it is called “fast-forward and slow-out”[35]. The enhancement mode of the HCA group was mostly peripheral-centered filling. Part of the HCA had a filling defect, that is, an ischemic necrotic area. The TIC decreasing slope of the HCA group was significantly higher than that of the FNH group. This is because the HCA tumor is surrounded by abundant peripheral blood vessels, and the branches of the blood vessels are infiltrated into the tumor for feeding. The contrast agent is filled with blood from the periphery to the center. If the lesion is large, the middle region is prone to lack blood supply and become necrotic, forming a filling defect. Because HCA lacks the portal system, its feeding vessels are derived from the hepatic artery. Hence the contrast agent is not supplemented during the portal vein phase. This enhanced mode is called “fast forward and fast out”[36]. In the FNH lesion, the portal vein was absent in the stellate scar, which was formed by fibrous tissue. Thus, the stellate filling defect area would appear in the CEUS. Nevertheless, HCA and FNH can be initially identified based on the difference in the two groups of CEUS findings. Guo et al[37] have found that the typical CEUS enhancement of FNH can also be found in HCA. Choi et al[38] also reported misdiagnosis in cases of HCA by CEUS[38]. Therefore, it is not accurate to identify HCA and FNH solely by utilizing CEUS.
SWE has been widely used in the differential diagnosis of benign and malignant liver lesions in recent years[39,40]. It was applied in the differential diagnosis of HCA and FNH in this study. The results found that the YM value and YM ratio in the HCA group were significantly lower than those in the FNH group, indicating that the lesion hardness in the HCA group was significantly lower than that in the FNH group. This is because most FNHs have stellate scars formed by coarse fibers. These scars divide the tissue into multiple small nodules. There is hyperplastic fibrous tissue, thickened blood vessel walls and hyperplastic bile ducts among the small nodules. Therefore, the fibrous tissue content in FNH is increased, and the tissue hardness is increased[41]. On the other hand, HCA is mainly composed of hepatocytes rich in glycogen and lipids. The fibrous tissue content is rare. Moreover, HCA lesion lack portal vein structure and bile ducts, often including ischemic necrosis. These all lead to a decrease in the hardness value of the HCA lesion[42-44].
According to the analysis results of the ROC curve in this study, gray-scale ultrasound, SWE and CEUS had their own advantages and disadvantages in identifying HCA and FNH. The lesion echo, YM value, and TIC decreasing slope could be used to identify HCA and FNH. Among them, YM value has the highest accuracy in identifying HCA. The AUC of HCA was 0.891, and the sensitivity was 92.16%. This suggested that the differential diagnosis of HCA and FNH by YM value is not prone to missed diagnosis. However, its specificity is low (73.28%), suggesting that the differential diagnosis of the YM value would result in a certain misdiagnosis rate. The AUC of the three indicators were all < 0.9, indicating that the accuracy of gray-scale ultrasound, SWE and CEUS for individually identifying HCA and FNH is limited. Therefore, this study combined the lesion echo, YM value, and TIC decreasing slope based on the logistic regression model. The combined diagnosis found that the AUC reached 0.938, which was higher than the AUC of these single indicators, indicating that the accuracy of the combined identification of the three indicators was the best. The combination of lesion echo, YM value and TIC decreasing slop in the differential diagnosis of HCA and FNH will hopefully be used in the clinic.
There are still shortcomings in this study. The SWE in this study was susceptible to the depth of the lesion and the operator. Hence, there is an inevitable error in the results. In addition, there is limited attention to this disease in the clinic because HCA is relatively rare and less malignant[45-50]. Thus, several patients in this study were not included because of incomplete clinical data, resulting in a limited sample size. Following this study, we expect to conduct further research on HCA in multiple centers, which will provide more useful information for clinical diagnosis through analyzing more specific data.
In conclusion, the combination of lesion echo, YM value and TIC decreasing slop in multi-parameter ultrasound indicators based on logistic regression has high clinical guiding value for the differential diagnosis of HCA and FNH.
Hepatocellular adenoma (HCA) is prone to secondary hemorrhage, and has a certain tendency towards malignant transformation. It needs to be closely observed and surgically removed if necessary. Focal nodular hyperplasia (FNH), which often needs to be differentiated, is a vascular malformation lesion, which is not a true tumor and has a tendency to spontaneously resolve, so conservative treatment can be adopted. The treatment methods and prognosis of them are quite different, but they are not easy to clinically identify. Therefore, it is of great significance to explore effective identification methods for them.
Current studies have shown that biochemical indicators do not have obvious advantages in identifying HCA and FNH. In imaging methods, it is difficult to distinguish the difference by using ultrasound. Recent studies have shown that contrast enhanced ultrasound (CEUS) can be used to diagnose HCA, but the diagnostic accuracy of FNH is low. It revealed that the value of differential diagnosis using conventional ultrasound, or CEUS individually, is limited. In recent years, shear wave elastography (SWE) has been widely used in the identification of benign and malignant tumors in the liver, but there are few applications for the differential diagnosis of HCA and FNH. Therefore, methods for identifying HAC and FNH are still lacking in the clinic.
In order to explore effective methods for identifying HCA and FNH, we will analyze the routine clinical indicators, including Doppler ultrasound, CEUS and SWE, in HCA and FNH patients. Logistic regression analysis will be used to analyze the significance of combined diagnosis of multi-parameter ultrasound indicators for improving the differential diagnosis of HCA and FNH.
The study included 31 patients with HCA, and 50 patients with FNH. The clinical data of the two groups were recorded, and conventional ultrasound, CEUS, and SWE examinations were performed, and the ultrasound parameters such as lesion position, boundary echo, value and ratio of Young’s modulus (YM), slope of TIC curve, etc were recorded. Multivariate regression analysis was used to screen potential indicators for the differential diagnosis of HCA and FNH. A ROC curve was used to evaluate the accuracy of potential indicators in differential diagnosis. A logistic regression model was used to establish a combination to explore the accuracy of differential diagnosis.
Multivariate regression analysis showed that lesion echo (P = 0.000), YM value (P = 0.000) and TIC decreasing slope (P = 0.000) were the potential indicators for identifying HCA and FNH. The accuracy of differential diagnosis of YM value is the highest, but its AUC is still less than 0.9. It is suggested that although the lesion echo, YM value and TIC decreasing slope were the influencing factors of HCA, the accuracy of differential diagnosis using conventional ultrasound, SWE and CEUS alone was limited. Further logistic regression results showed that the accuracy of the combined diagnosis of three indicators (AUC = 0.938) was significantly higher than the AUC of lesion echo (AUC = 0.676), YM value (AUC = 0.891), and TIC decreasing slope (AUC = 0.785). It is suggested that the accuracy of the combination of the three indicators is the best. The combined diagnosis of multi-parameter ultrasound can significantly improve the accuracy of differential diagnosis between HCA and FNH.
Multi-parameter ultrasound in the differential diagnosis of HCA and FNH plays an important role. The combination of lesion echo, YM value and TIC decreasing slope can significantly improve the accuracy of differential diagnosis.
In order to avoid the limitation of HCA patient cases, as well as the influence of the depth of the lesion and operator in SWE defection. This study plans to further develop a multicenter study on HCA to improve diagnosis accuracy. The multi-center large sample study will further reveal the role of multi-parameter ultrasound in the differential diagnosis of HCA and FNH, and further improve the accuracy of the diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ryan EM, Shaun C, Satya R S-Editor: Zhang L L-Editor: Filipodia E-Editor: Qi LL
| 1. | Zhang G, Wang M, Duan F, Yuan K, Li K, Yan J, Chang Z. Transarterial embolization with bleomycin for symptomatic hepatic focal nodular hyperplasia. Diagn Interv Radiol. 2017;23:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Roncalli M, Sciarra A, Tommaso LD. Benign hepatocellular nodules of healthy liver: focal nodular hyperplasia and hepatocellular adenoma. Clin Mol Hepatol. 2016;22:199-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Dioguardi Burgio M, Ronot M, Salvaggio G, Vilgrain V, Brancatelli G. Imaging of Hepatic Focal Nodular Hyperplasia: Pictorial Review and Diagnostic Strategy. Semin Ultrasound CT MR. 2016;37:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Nault JC, Couchy G, Balabaud C, Morcrette G, Caruso S, Blanc JF, Bacq Y, Calderaro J, Paradis V, Ramos J, Scoazec JY, Gnemmi V, Sturm N, Guettier C, Fabre M, Savier E, Chiche L, Labrune P, Selves J, Wendum D, Pilati C, Laurent A, De Muret A, Le Bail B, Rebouissou S, Imbeaud S; GENTHEP Investigators, Bioulac-Sage P, Letouzé E, Zucman-Rossi J. Molecular Classification of Hepatocellular Adenoma Associates With Risk Factors, Bleeding, and Malignant Transformation. Gastroenterology. 2017;152:880-894.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 269] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 5. | Nguyen TB, Roncalli M, Di Tommaso L, Kakar S. Combined use of heat-shock protein 70 and glutamine synthetase is useful in the distinction of typical hepatocellular adenoma from atypical hepatocellular neoplasms and well-differentiated hepatocellular carcinoma. Mod Pathol. 2016;29:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Dong Y, Zhu Z, Wang WP, Mao F, Ji ZB. Ultrasound features of hepatocellular adenoma and the additional value of contrast-enhanced ultrasound. Hepatobiliary Pancreat Dis Int. 2016;15:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Henriet E, Abou Hammoud A, Dupuy JW, Dartigues B, Ezzoukry Z, Dugot-Senant N, Leste-Lasserre T, Pallares-Lupon N, Nikolski M, Le Bail B, Blanc JF, Balabaud C, Bioulac-Sage P, Raymond AA, Saltel F. Argininosuccinate synthase 1 (ASS1): A marker of unclassified hepatocellular adenoma and high bleeding risk. Hepatology. 2017;66:2016-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Beppu T, Nakagawa S, Nitta H, Okabe H, Kaida T, Imai K, Hayashi H, Koga Y, Kuramoto K, Hashimoto D, Yamashita YI, Chikamoto A, Ishiko T, Baba H. The Number of Positive Tumor Marker Status Is Beneficial for the Selection of Therapeutic Modalities in Patients with Hepatocellular Carcinoma. J Clin Transl Hepatol. 2017;5:165-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Liu D, Liu P, Cao L, Zhang Q, Chen Y. Screening the key genes of hepatocellular adenoma via microarray analysis of DNA expression and methylation profiles. Oncol Lett. 2017;14:3975-3980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Rübenthaler J, Paprottka KJ, Hameister E, Hoffmann K, Joiko N, Reiser M, Rjosk-Dendorfer D, Clevert DA. Diagnostic accuracy of contrast-enhanced ultrasound (CEUS) in monitoring vascular complications in patients after liver transplantation - diagnostic performance compared with histopathological results. Clin Hemorheol Microcirc. 2017;66:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kono Y, Lyshchik A, Cosgrove D, Dietrich CF, Jang HJ, Kim TK, Piscaglia F, Willmann JK, Wilson SR, Santillan C, Kambadakone A, Mitchell D, Vezeridis A, Sirlin CB. Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS®): the official version by the American College of Radiology (ACR). Ultraschall Med. 2017;38:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Huf S, Platz Batista da Silva N, Wiesinger I, Hornung M, Scherer MN, Lang S, Stroszczynski C, Fischer T, Jung EM. Analysis of Liver Tumors Using Preoperative and Intraoperative Contrast-Enhanced Ultrasound (CEUS/IOCEUS) by Radiologists in Comparison to Magnetic Resonance Imaging and Histopathology. Rofo. 2017;189:431-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography. 2018;37:25-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Taimr P, Bröker MEE, Dwarkasing RS, Hansen BE, de Knegt RJ, De Man RA, IJzermans JNM. A Model-Based Prediction of the Probability of Hepatocellular Adenoma and Focal Nodular Hyperplasia Based on Characteristics on Contrast-Enhanced Ultrasound. Ultrasound Med Biol. 2017;43:2144-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Bende F, Sporea I, Sirli R, Popescu A, Mare R, Miutescu B, Lupusoru R, Moga T, Pienar C. Performance of 2D-SWE.GE for predicting different stages of liver fibrosis, using Transient Elastography as the reference method. Med Ultrason. 2017;19:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Dong Y, Sirli R, Ferraioli G, Sporea I, Chiorean L, Cui X, Fan M, Wang WP, Gilja OH, Sidhu PS, Dietrich CF. Shear wave elastography of the liver - review on normal values. Z Gastroenterol. 2017;55:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Gerber L, Fitting D, Srikantharajah K, Weiler N, Kyriakidou G, Bojunga J, Schulze F, Bon D, Zeuzem S, Friedrich-Rust M. Evaluation of 2D- Shear Wave Elastography for Characterisation of Focal Liver Lesions. J Gastrointestin Liver Dis. 2017;26:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. Critical appraisal of Chinese 2017 guideline on the management of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2017;6:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Tian WS, Lin MX, Zhou LY, Pan FS, Huang GL, Wang W, Lu MD, Xie XY. Maximum Value Measured by 2-D Shear Wave Elastography Helps in Differentiating Malignancy from Benign Focal Liver Lesions. Ultrasound Med Biol. 2016;42:2156-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Klompenhouwer AJ, IJzermans JNM. Malignant potential of hepatocellular adenoma. Liver Int. 2017;37:966-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Klompenhouwer AJ, de Man RA, Thomeer MG, Ijzermans JN. Management and outcome of hepatocellular adenoma with massive bleeding at presentation. World J Gastroenterol. 2017;23:4579-4586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Zhang HT, Gao XY, Xu QS, Chen YT, Song YP, Yao ZW. Evaluation of the characteristics of hepatic focal nodular hyperplasia: correlation between dynamic contrast-enhanced multislice computed tomography and pathological findings. Onco Targets Ther. 2016;9:5217-5224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Grieser C, Steffen IG, Kramme IB, Bläker H, Kilic E, Perez Fernandez CM, Seehofer D, Schott E, Hamm B, Denecke T. Gadoxetic acid enhanced MRI for differentiation of FNH and HCA: a single centre experience. Eur Radiol. 2014;24:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, Greiner L, Jäger K, Jong ND, Leen E, Lencioni R, Lindsell D, Martegani A, Solbiati L, Thorelius L, Tranquart F, Weskott HP, Whittingham T; EFSUMB Study Group. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Thiele M, Madsen BS, Procopet B, Hansen JF, Møller LMS, Detlefsen S, Berzigotti A, Krag A. Reliability Criteria for Liver Stiffness Measurements with Real-Time 2D Shear Wave Elastography in Different Clinical Scenarios of Chronic Liver Disease. Ultraschall Med. 2017;38:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Sinclair M, Schelleman A, Sandhu D, Angus PW. Regression of hepatocellular adenomas and systemic inflammatory syndrome after cessation of estrogen therapy. Hepatology. 2017;66:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Taniai-Riya E, Miyajima K, Kakimoto K, Ohta T, Yasui Y, Kemmochi Y, Anagawa-Nakamura A, Toyoda K, Takahashi A, Shoda T. Hepatocellular adenoma with severe fatty change in a male Spontaneously Diabetic Torii rat. J Toxicol Pathol. 2017;30:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Khaoudy I, Rebibo L, Regimbeau JM. Is bariatric surgery a potential new treatment for large inflammatory hepatocellular adenomas in obese patients? Surg Obes Relat Dis. 2018;14:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Reizine E, Amaddeo G, Pigneur F, Baranes L, Legou F, Mulé S, Zegai B, Roche V, Laurent A, Rahmouni A, Calderaro J, Luciani A. Quantitative correlation between uptake of Gd-BOPTA on hepatobiliary phase and tumor molecular features in patients with benign hepatocellular lesions. Eur Radiol. 2018;28:4243-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Hasab Allah M, Salama RM, Marie MS, Mandur AA, Omar H. Utility of point shear wave elastography in characterisation of focal liver lesions. Expert Rev Gastroenterol Hepatol. 2018;12:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Naganuma H, Ishida H, Ogawa M, Watanabe Y, Watanabe D, Ohyama Y, Watanabe T. Focal nodular hyperplasia: our experience of 53 Japanese cases. J Med Ultrason (2001). 2017;44:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Rousseau C, Ronot M, Vilgrain V, Zins M. Optimal visualization of focal nodular hyperplasia: quantitative and qualitative evaluation of single and multiphasic arterial phase acquisition at 1.5 T MR imaging. Abdom Radiol (NY). 2016;41:990-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Sheng R, Palm V, Mayer P, Mokry T, Berger AK, Weiss KH, Longerich T, Kauczor HU, Weber TF. Gadoxetic Acid-Enhanced Hepatobiliary-Phase Magnetic Resonance Imaging for Delineation of Focal Nodular Hyperplasia: Superiority of High-Flip-Angle Imaging. J Comput Assist Tomogr. 2018;42:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Sun XL, Yao H, Men Q, Hou KZ, Chen Z, Xu CQ, Liang LW. Combination of acoustic radiation force impulse imaging, serological indexes and contrast-enhanced ultrasound for diagnosis of liver lesions. World J Gastroenterol. 2017;23:5602-5609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Kong WT, Wang WP, Huang BJ, Ding H, Mao F, Si Q. Contrast-enhanced ultrasound in combination with color Doppler ultrasound can improve the diagnostic performance of focal nodular hyperplasia and hepatocellular adenoma. Ultrasound Med Biol. 2015;41:944-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Guo Y, Li W, Cai W, Zhang Y, Fang Y, Hong G. Diagnostic Value of Gadoxetic Acid-Enhanced MR Imaging to Distinguish HCA and Its Subtype from FNH: A Systematic Review. Int J Med Sci. 2017;14:668-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Choi IY, Lee SS, Sung YS, Cheong H, Lee H, Byun JH, Kim SY, Lee SJ, Shin YM, Lee MG. Intravoxel incoherent motion diffusion-weighted imaging for characterizing focal hepatic lesions: Correlation with lesion enhancement. J Magn Reson Imaging. 2017;45:1589-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Grgurevic I, Bokun T, Salkic NN, Brkljacic B, Vukelić-Markovic M, Stoos-Veic T, Aralica G, Rakic M, Filipec-Kanizaj T, Berzigotti A. Liver elastography malignancy prediction score for noninvasive characterization of focal liver lesions. Liver Int. 2018;38:1055-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 40. | Jiao Y, Dong F, Wang H, Zhang L, Xu J, Zheng J, Fan H, Gan H, Chen L, Li M. Shear wave elastography imaging for detecting malignant lesions of the liver: a systematic review and pooled meta-analysis. Med Ultrason. 2017;19:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Akdoğan E, Yılmaz FG. The role of acoustic radiation force impulse elastography in the differentiation of benign and malignant focal liver masses. Turk J Gastroenterol. 2018;29:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Mulazzani L, Salvatore V, Ravaioli F, Allegretti G, Matassoni F, Granata R, Ferrarini A, Stefanescu H, Piscaglia F. Point shear wave ultrasound elastography with Esaote compared to real-time 2D shear wave elastography with supersonic imagine for the quantification of liver stiffness. J Ultrasound. 2017;20:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Dietrich CF, Dong Y. Shear wave elastography with a new reliability indicator. J Ultrason. 2016;16:281-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Ronot M, Di Renzo S, Gregoli B, Duran R, Castera L, Van Beers BE, Vilgrain V. Characterization of fortuitously discovered focal liver lesions: additional information provided by shearwave elastography. Eur Radiol. 2015;25:346-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Kim JW, Lee CH, Kim SB, Park BN, Park YS, Lee J, Park CM. Washout appearance in Gd-EOB-DTPA-enhanced MR imaging: A differentiating feature between hepatocellular carcinoma with paradoxical uptake on the hepatobiliary phase and focal nodular hyperplasia-like nodules. J Magn Reson Imaging. 2017;45:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Taibbi A, Brancatelli G, Matranga D, Midiri M, Lagalla R, Bartolotta TV. Focal nodular hyperplasia: a weight-based, intraindividual comparison of gadobenate dimeglumine and gadoxetate disodium-enhanced MRI. Diagn Interv Radiol. 2019;25:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Short Version). Ultraschall Med. 2017;38:377-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 49. | Xie LT, Yan CH, Zhao QY, He MN, Jiang TA. Quantitative and noninvasive assessment of chronic liver diseases using two-dimensional shear wave elastography. World J Gastroenterol. 2018;24:957-970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Nault JC, Paradis V, Cherqui D, Vilgrain V, Zucman-Rossi J. Molecular classification of hepatocellular adenoma in clinical practice. J Hepatol. 2017;67:1074-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |