Published online Dec 15, 2019. doi: 10.4251/wjgo.v11.i12.1172
Peer-review started: May 9, 2019
First decision: July 31, 2019
Revised: August 8, 2019
Accepted: September 10, 2019
Article in press: September 10, 2019
Published online: December 15, 2019
Processing time: 228 Days and 14.9 Hours
There has been no study comparing the difference in the failure patterns between patients with or without postoperative radiotherapy (PORT) after esophagectomy for pT3-4N0-3M0 esophageal squamous cell carcinoma (ESCC).
To investigate the difference in the failure patterns of stage pT3-4N0-3M0 ESCC patients with or without PORT.
Patients with stage pT3-4N0-3M0 ESCC, who underwent surgery with or without PORT, were enrolled in this study. The primary endpoint was to investigate the difference in the failure patterns between patients with or without PORT after esophagectomy. The secondary endpoint was to estimate whether patients with stage pT3-4 ESCC could achieve a disease-free survival (DFS) advantage after receiving adjuvant PORT. Statistical analyses were performed by the Kaplan-Meier method, Cox regression model, and Chi-squared test or Fisher’s exact test.
In total, 230 patients with stage pT3-4N0-3M0 ESCC were included in this study. Fifty-six patients who received PORT were screened from a prospective cohort (S + R arm). And 174 patients involving surgery alone were retrospectively selected from July 2006 to October 2014 (S arm). There were no significant differences in the clinical or pathological characteristics of patients between the two arms, except for tumor location (P = 0.031). The failure patterns between the two arms were significantly different (P < 0.001). Patients in the S arm had a significantly higher proportion of locoregional recurrence and a lower proportion of distant metastasis than those in the S + R arm (92.0% vs 35.7%, P < 0.001 and 19.0% vs 75.0%, P < 0.001, respectively). The difference in the median DFS between the two arms was statistically significant (12.7 vs 8 mo, P = 0.048). Univariate analysis and multivariate analysis both demonstrated that the number of lymph node metastases ≥ 3 (HR = 0.572, 95%CI: 0.430-0.762, P < 0.001) was an independent poor prognostic factor for DFS in patients with stage pT3-4N0-3M0 ESCC.
PORT could improve DFS and local control of patients with stage pT3-4N0-3M0 ESCC. However, further studies need to be conducted to control hematogenous metastasis after PORT.
Core tip: This is the first study to compare the difference in the failure patterns for pT3-4N0-3M0 esophageal squamous cell carcinoma (ESCC) patients with or without postoperative radiotherapy (PORT) after esophagectomy. The result showed that PORT could improve disease-free survival and local control of patients with stage pT3-4N0-3M0 ESCC. However, distant metastasis was the main failure pattern after receiving PORT. Further studies need to be conducted to control hematogenous metastasis in the future.
- Citation: Zeng Y, Yu W, Liu Q, Yu WW, Zhu ZF, Zhao WX, Liu J, Wang JM, Fu XL, Liu Y, Cai XW. Difference in failure patterns of pT3-4N0-3M0 esophageal cancer treated by surgery vs surgery plus radiotherapy. World J Gastrointest Oncol 2019; 11(12): 1172-1181
- URL: https://www.wjgnet.com/1948-5204/full/v11/i12/1172.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i12.1172
Esophageal cancer is an aggressive malignancy with high morbidity and mortality[1]. The guidelines of the National Comprehensive Cancer Network[2] recommend that esophagectomy is still the primary choice for esophageal cancer. Neoadjuvant chemoradiotherapy followed by surgery is the standard care for patients with locally advanced esophageal squamous cell carcinoma (ESCC)[3]. Nevertheless, many patients tend to choose surgery as their primary treatment because of the traditional concept in China, although neoadjuvant therapy is recommended by surgeons. Furthermore, most patients who were clinically diagnosed with stage T1-2 disease were pathologically confirmed as having stage T3-4 after surgery owing to the less frequent use of ultrasound endoscopy. However, with a poor survival and high rate of recurrence after esophagectomy[4,5], patients with pT3-4 disease require multidisciplinary adjuvant treatments. Additionally, the efficacy of postoperative radiotherapy (PORT) has been investigated widely. Based on a cancer registry database, patients with esophageal cancer could not benefit from PORT[6]. However, many retrospective studies showed that PORT could apparently improve the locoregional control rate and overall survival (OS) rate for patients with locally advanced ESCC (stage III or with positive lymph node)[7-9].
Locoregional recurrence is the most common pattern of failure after esophagectomy[4,10]. Approximately 80% of recurrence sites of ESCC patients after curative esophagectomy are located in the supraclavicular and superior mediastinal lymph nodes, which are called the T-shape field[11-13]. Chen et al[7] reported that the locoregional recurrence rate for patients receiving PORT was significantly lower than that of patients who had undergone surgery alone. However, no study has compared the difference of the failure patterns between stage pT3-4 patients with or without PORT after esophagectomy.
In that case, this study was administered with the intention of investigating the difference in the failure patterns of patients with stage pT3-4N0-3M0 ESCC after complete resection with or without PORT and estimating whether patients could obtain a DFS benefit from PORT.
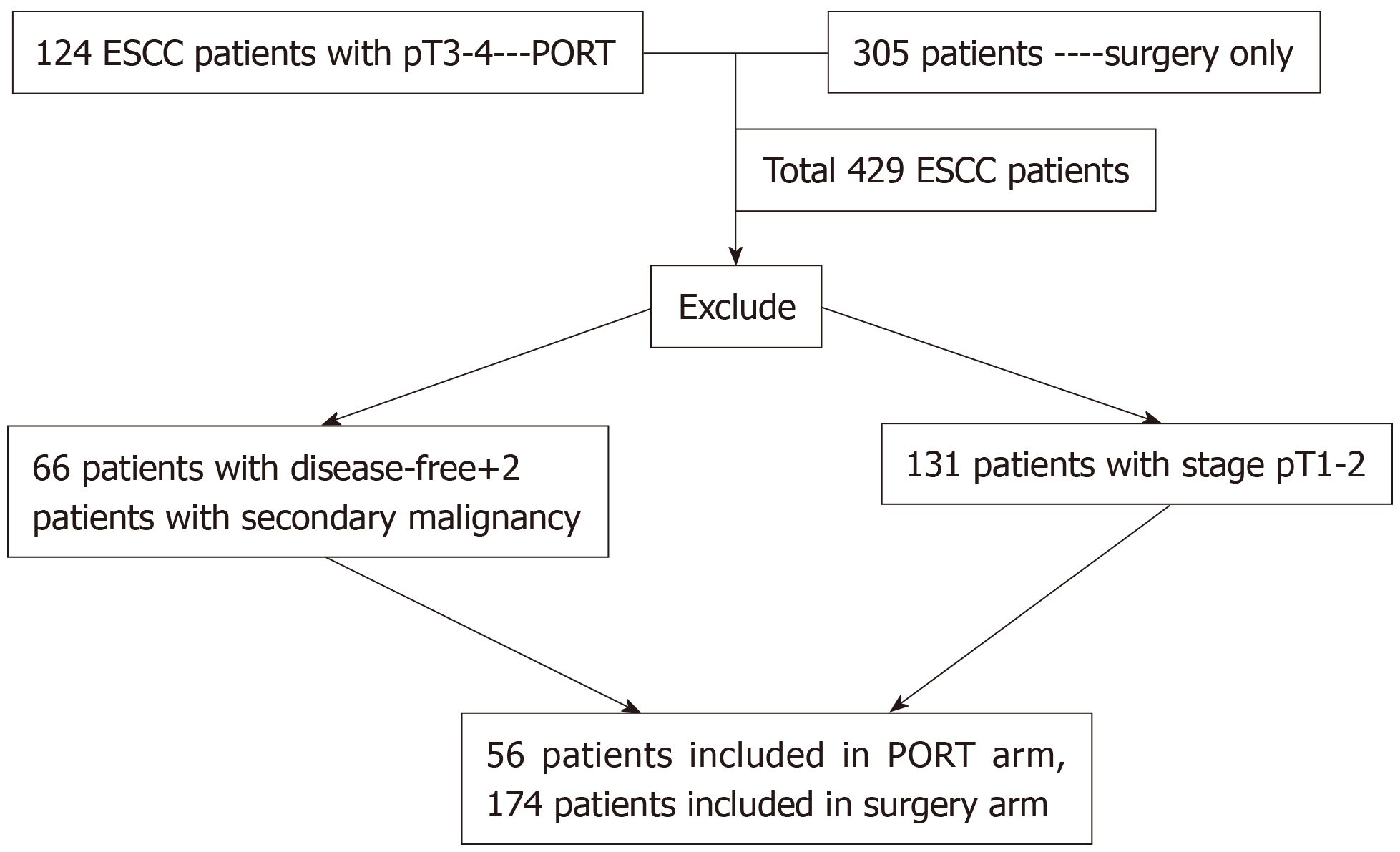
Patients in this study were screened from two treatment centers that included 429 ESCC patients with stage pT1-4N0-3M0. Patients in the S + R arm were screened from a prospective cohort that included 124 patients with stage pT3-T4 ESCC, who had received radiotherapy after surgery[14]. Patients in the S arm were screened from a retrospective cohort including 305 patients who had undergone radical surgery alone[12]. The main eligible inclusion criteria were as follows: (1) A diagnosis of primary thoracic ESCC and pathologically confirmed stage T3-4N0-3M0 disease; (2) Aged 18-75 years; (3) Integrated physical examination, contrast esophagography, enhanced computed tomography of the chest and upper abdomen, and external ultrasonography of the neck or positron emission tomography (PET/CT) conducted before surgery and radiotherapy; (4) Adequate bone marrow, liver and renal functions for patients receiving radiotherapy; and (5) Patients with any patterns of failure during follow-up. The exclusion criteria were as follows: Receiving chemotherapy or radiotherapy before surgery; no failure patterns occurring to the last follow-up; patients with a secondary primary tumor after or during PORT. Additionally, patients who had received adjuvant chemotherapy were not considered in the study. The patient selection procedure is shown in Figure 1.
All the patients in the S + R arm received radiotherapy within 8 wk after surgery. The patients underwent CT-based treatment simulation in the supine position with a thermoplastic mask for immobilization. Five-millimeter-thick images were obtained from the entire neck to the upper abdomen. Intensity-modulated radiotherapy with a 6 MV X-ray linear accelerator was used for external beam radiation. The delineation of the clinical tumor volume (CTV) was based on surgery procedure, CT imaging obtained before and after surgery, and gastrointestinal endoscopy obtained before treatment. The CTV included the primary tumor bed and metastatic lymph nodes or plus bilateral supraclavicular and upper-middle mediastinal lymphatic drainage areas (T-shape field). The planning target volume was defined as CTV plus a 0.8 to 1.0 cm margin. The radiation dose to the primary tumor bed and metastatic lymph nodes was 63 Gy (2.25 Gy/day/fraction, 28 fractions) for patients with stage T4 or 60.2 Gy (2.15 Gy/day /fraction, 28 fractions) for patients with stage T3 disease. The lymphatic drainage area was prescribed a dose of 50.4 Gy (1.8 Gy/day/fraction, 28 fractions). The prescription dose was delivered to the target volume mainly through the mediastinum. Normal tissue dose constraints met the usual requirements: (1) Maximum spinal cord dose ≤ 45 Gy; (2) Lung V20 ≤ 25% and mean lung dose ≤ 15 Gy; and (3) Mean heart dose ≤ 30 Gy.
After surgery or radiotherapy was completed, chemotherapy was scheduled intravenously with cisplatin (25 mg/m2/d, d1–3) and 5-fluorouracil (5-Fu, 600 mg/m2/d, d1–5) or paclitaxel (135-175 mg/m2/d, d1) for four cycles with an interval of 3-4 wk.
Follow-up visits were administered every 3 mo for the first two years after treatment, every 6 mo for the next three years, and then once a year thereafter. Patients in the S + R arm were followed to March 2017. Follow-up investigations included hematological examination, supraclavicular lymph node ultrasonography, contrast esophagography, and enhanced computed tomography of the chest and upper abdomen. When necessary, PET/CT or bone emission computed tomography was required.
This study analyzed the first failure pattern only. The discovery of relapse was mainly based on imaging features. Fine-needle aspiration biopsy and PET/CT were implemented only when necessary. Locoregional recurrence included the tumor bed area, anastomotic stoma relapse, and regional lymph nodes, for which the short axis diameter was at least 1 cm in the CT image, according to the tumor location. Distant metastasis was defined as neoplasms occurring in organs or non-regional lymph node metastasis. The definitions of failure patterns were described in detail in our previous report[12]. Recurrence comprising locoregional and distant failure simultaneously was classified as mixed failure.
Owing to the retrospective characteristics of the S arm, we did not have access to the OS information of over 10% patients. The primary endpoint of this study was to compare the difference in the failure patterns between the two arms. The secondary endpoint was to evaluate whether patients with locally advanced ESCC could achieve DFS benefit from PORT. DFS was calculated from the date of surgery to the date that any type of failure occurred or the date of the last follow-up. The difference in the failure patterns and clinical characteristics was calculated by the Chi-squared test or Fisher’s exact test. The Kaplan-Meier method was used to evaluate the difference in DFS between the two arms. The log-rank test was used to determine the statistical significance of the difference. Univariate analysis and multivariate analysis (Cox proportional hazard regression model) were performed to evaluate the risk factors associated with the prognosis. A P value less than 0.05 (two sided) was considered statistically significant. All statistical analyses were calculated with SPSS (version 22.0 IBM Chicago, United States).
This study included 230 patients. In the prospective cohort of 124 ESCC patients with stage pT3-T4 disease who underwent PORT, 66 patients lived free of disease and 2 patients with secondary primary tumor were excluded. Finally, 56 patients who met the criteria were recruited as the S + R arm from April 2011 to February 2016. We retrospectively screened 305 patients who received surgery alone. Among them, 131 patients with stage pT1-2 disease were excluded and 174 patients with pT3-4 disease suffering failure were enrolled as the S arm from July 2006 and October 2014. Finally, 230 patients were included in this study. All patients in the S + R arm completed the intended radiotherapy treatment plan within 6 wk. There were 60.7% of patients who received sequential chemotherapy after radiotherapy was completed. Others who declined or could not tolerate did not receive chemotherapy. There was no difference in the percentage of patients receiving chemotherapy between the S arm and S + R arm. The baseline clinical and pathologic characteristics of the two arms are shown in Table 1. Differences between the two cohorts in sex, age, pathological differentiation, number of lymph nodes dissected, lymph node involvement, and chemotherapy were not significant, except for tumor location (P = 0.031).
| Variable | S arm (n = 174) | S + R arm (n = 56) | P value |
| Sex | 0.656 | ||
| Male | 159 (91.4) | 49 (87.5) | |
| Female | 15 (8.6) | 7 (12.5) | |
| Age (yr) | 0.056 | ||
| <60 | 106 (60.9) | 42 (75.0) | |
| ≥60 | 68 (39.1) | 14 (25.0) | |
| Pathological differentiation | 0.586 | ||
| Poor | 66 (37.5) | 1 (1.8) | |
| Moderate | 97 (55.7) | 33 (58.9) | |
| Well | 10 (5.7) | 22 (39.3) | |
| Tumor location | 0.031a | ||
| Upper | 15 (8.6) | 3 (5.4) | |
| Middle | 98 (56.3) | 22 (39.3) | |
| Lower | 61 (35.1) | 31 (55.4) | |
| Lymph node dissection | 0.448 | ||
| 2-field resection | 139 (79.9) | 49 (87.5) | |
| 3-field resection | 28 (16.1) | 7 (12.5) | |
| No. of LNs involved | 0.740 | ||
| ≤2 | 113 (64.9) | 35 (62.5) | |
| ≥3 | 61 (35.1) | 21 (37.5) | |
| No. of LNs dissected | 0.569 | ||
| <12 | 2 (1.1) | 1 (1.8) | |
| ≥12 | 172 (98.9) | 55 (98.2) | |
| T stage | 0.075 | ||
| T3 | 164 (94.3) | 48 (85.7) | |
| T4 | 10 (5.7) | 8 (14.3) | |
| Chemotherapy | 0.978 | ||
| Yes | 106 (60.9) | 34 (60.7) |
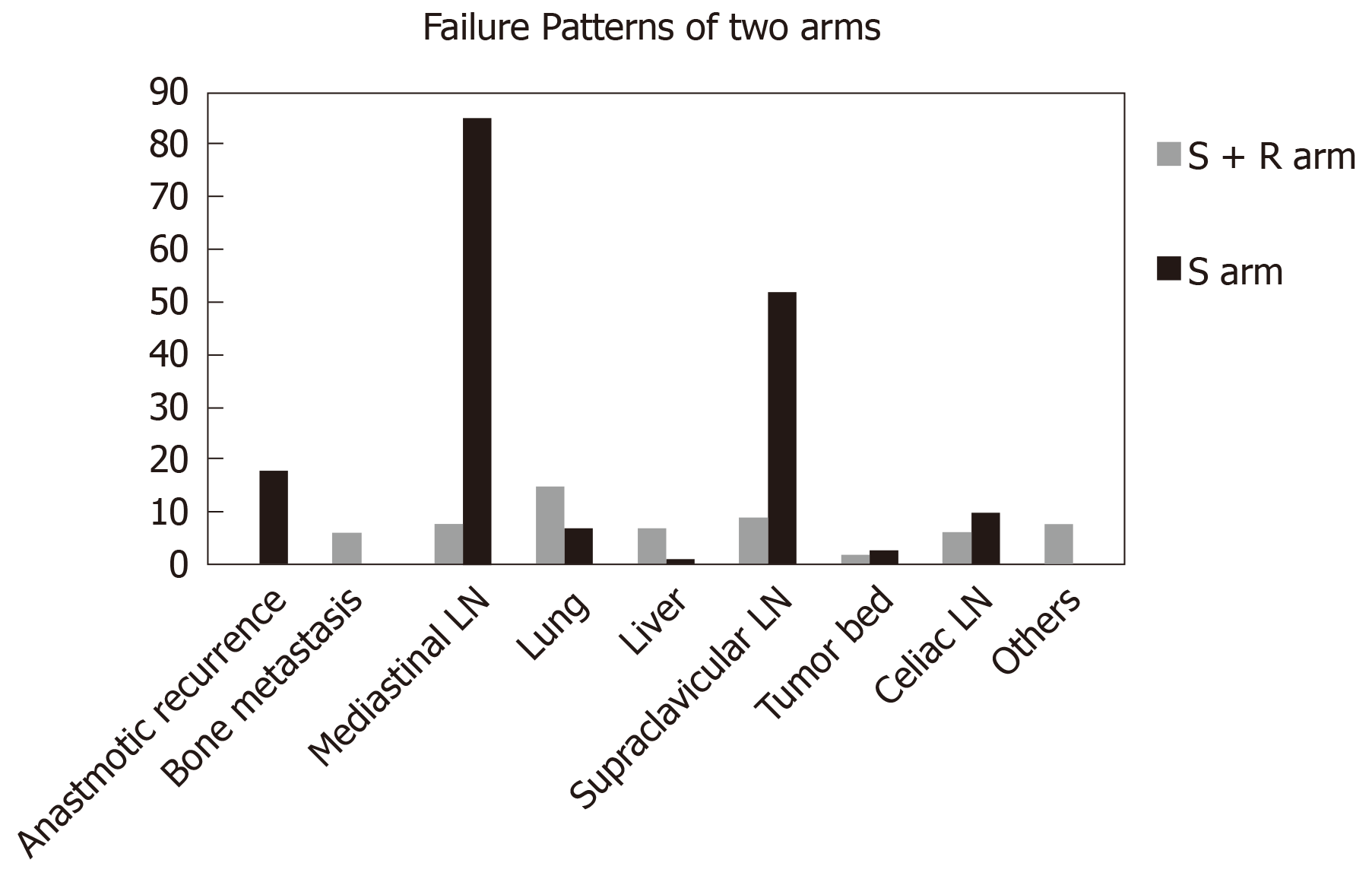
All patients included in this study developed failure during the follow-up. We compared the difference in the constituent ratio of the failure patterns between the two arms. As shown in Table 2, the difference in the failure patterns between the two arms was statistically significant (P < 0.001). Regarding patients with distant metastasis, locoregional recurrence, and mixed failure, 64.3%, 25.0%, and 10.7%, respectively, were in the S + R arm and 8.0%, 81.0%, and 11.0%, respectively, were in the S arm. Patients in the S arm had a significantly higher proportion of locoregional recurrence than patients in the S + R arm (92.0% vs 35.7%, respectively, P < 0.001). However, patients in the S + R arm had a much higher proportion of distant metastasis than patients in the S arm (75.0% vs 19.0%, P < 0.001). The most common regions for locoregional recurrence were the supraclavicular and mediastinal lymph nodes (29.5% and 48.3%, respectively) for the surgery cohort. For the S + R arm, supraclavicular lymph nodes (9/17 patients) were the most common relapse region after PORT. The lungs, liver, and bone [41.7% (14/36 patients), 19.4% (7/36 patients), and 16.7% (6/36 patients), respectively] were the most common metastatic organs for the S + R arm. Distant metastasis mainly occurred in the lungs (7/33), supraclavicular lymph nodes (16/33), and celiac lymph nodes (10/33) for the S arm (Figure 2).
| Type of failure | S arm (n = 174) | S + R arm (n = 56) | P value |
| Failure patterns | < 0.001 | ||
| Distant metastasis | 14 (8.0) | 36 (64.3) | |
| Locoregional recurrence | 141 (81.0) | 14 (25.0) | |
| Mixed failure | 19 (11.0) | 6 (10.7) | |
| Distant metastasis | < 0.001 | ||
| Distant metastasis | 33 (19.0) | 42 (75.0) | |
| Others | 141 (81.0) | 14 (25.0) | |
| Locoregional failure | < 0.001 | ||
| Locoregional recurrence | 160 (92.0) | 20 (78.3) | |
| Others | 14 (8.0) | 36 (21.7) |
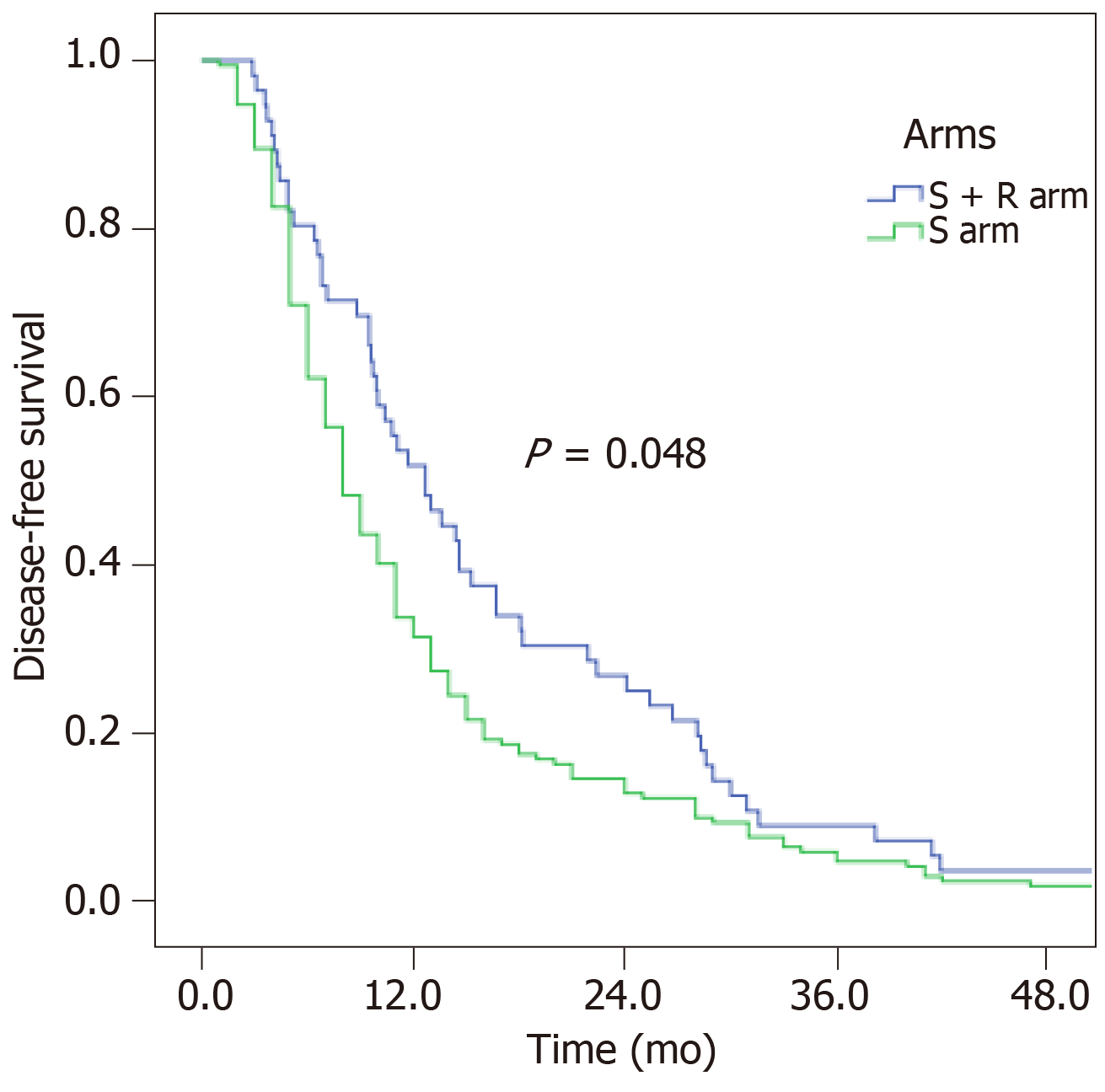
Recurrence occurred almost within two years for both arms. The 1-, 2-, and 3-year DFS rates of the S arm and S + R arm were 33.7%, 14.5%, and 4.7% and 51.8%, 26.8%, and 8.9%, respectively. The DFS was improved from 8 mo to 12.7 mo after receiving PORT. The difference in DFS for the two arms was marginally significant (P = 0.048, Figure 3).
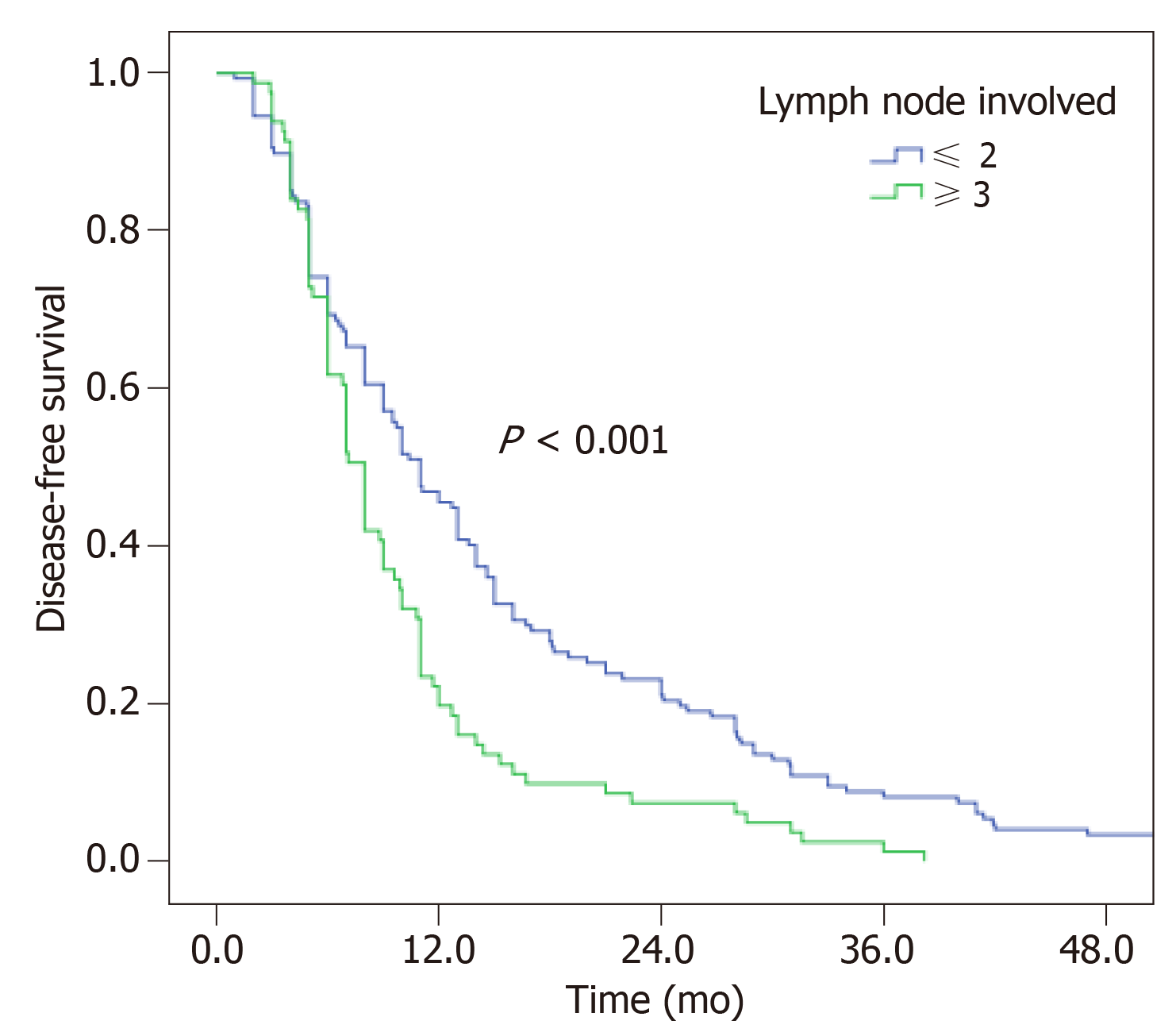
Univariate analysis revealed that only lymph node involvement was associated with DFS for T3-4 ESCC patients (P < 0.001, Figure 4 and Table 3). Patients with the number of lymph node metastases fewer than 2 could achieve a better DFS (11.0 vs 8.0 mo). DFS was not statistically associated with age, sex, tumor location, pathological differentiation, lymph node dissection, number of lymph nodes dissected, and adjuvant chemotherapy.
| Univariate analysis | Multivariate analysis | ||||
| Median DFS (mo) | χ2 value | P value | HR (95%CI) | P value | |
| Sex | 0.841 (0.532-1.328) | 0.457 | |||
| Male | 9.5 | 0.309 | 0.579 | ||
| Female | 8 | ||||
| Age (yr) | 0.712 (0.530-0.957) | 0.024 | |||
| < 60 | 9 | 1.023 | 0.312 | ||
| ≥ 60 | 9.5 | ||||
| Pathological differentiation | 1.128 (0.094-1.934) | 0.071 | |||
| Poor | 8 | 6.377 | 0.095 | ||
| Moderate | 10.4 | ||||
| Well | 9.5 | ||||
| Tumor location | 1.423 (0.849-2.386) | 0.363 | |||
| Upper | 6.6 | 3.277 | 0.194 | ||
| Middle | 9.9 | ||||
| Lower | 8.8 | ||||
| Lymph node dissection | 0.531 (0.222-1.186) | 0.107 | |||
| 2-field resection | 9 | 3.462 | 0.177 | ||
| 3-field resection | 8 | ||||
| No. of LNs involved | 1.843 (1.369-2.482) | <0.001a | |||
| ≤ 2 | 11 | 13.483 | < 0.001 | ||
| ≥ 3 | 8 | ||||
| No. of LN dissected | 1.460 (0.459-4.644) | 0.522 | |||
| < 12 | 7 | 0.656 | 0.418 | ||
| ≥ 12 | 9.5 | ||||
| Chemotherapy | 0.732 (0.552-0.971) | 0.031 | |||
| Yes | 8 | 1.769 | 0.183 | ||
| No | 10 | ||||
| T stage | 0.697 (0.441-1.124) | 0.132 | |||
| T3 | 9 | 0.087 | 0.767 | ||
| T4 | 8 | ||||
| Radiotherapy | - | - | - | 0.667 (0.487-0.915) | 0.012a |
However, multivariate analysis indicated that the number of lymph node metastases ≥ 3 (HR = 1.843, 95%CI: 1.369-2.482; P < 0.001), PORT (HR = 0.667, 95%CI: 0.487-0.915; P = 0.012), age (HR = 0.712, 95%CI: 0.530-0.957; P = 0.024), and chemotherapy (HR = 0.732, 95%CI: 0.552-0.971; P = 0.031) were independent prognostic factors for DFS in patients with stage pT3-4N0-3M0 ESCC (Table 3).
Although many studies have investigated the characteristics of failure patterns for patients with thoracic esophageal carcinoma after esophagectomy, this study is the first to investigate the difference in the failure patterns for stage pT3-4 ESCC patients with or without PORT after radical surgery. Our study found that radiotherapy following esophagectomy was more effective in improving DFS and decreasing locoregional recurrence of patients with stage pT3-4 ESCC. Distant metastasis was the most common failure after receiving PORT. The number of lymph nodes involved over 3, radiotherapy, age, and chemotherapy were significantly associated with disease progression for patients with stage pT3-4 ESCC.
It is known that neoadjuvant chemoradiotherapy is recommended as the standard care for locally advanced ESCC. However, many patients initially diagnosed with T1-2N0 disease before surgery were finally diagnosed as having pT3-4N0-3M0 disease after surgery. Do these patients need to receive adjuvant treatment after surgery? Xiao et al[15] revealed that patients receiving PORT with a prescription dose of 60 Gy could obtain a local control benefit and a higher 5-year OS than patients receiving surgery alone. With the application of 3-dimentional conformality radiotherapy, PORT could bring both local control and OS benefit for patients with stage III and stage T3N0M0 esophageal cancer or patients with positive lymph nodes[16-19]. Additionally, Schreiber et al[18] reported a survival benefit for patients with both stage III ESCC and esophageal adenocarcinoma after receiving PORT. Our result also revealed a better DFS for patients in the S + R arm than those in the S arm.
It is common for patients with esophageal cancer to develop relapse after treatment. Locoregional failure is the most common failure pattern for patients with surgery alone; and mediastinal lymph nodes, especially the upper mediastinal lymph nodes and supraclavicular lymph nodes, are the most common recurrence sites[11-13,20]. This study also showed that approximately 80% of cases with locoregional failure occurred in the T-shape field. By comparing the constituent ratio of failure patterns for patients who underwent surgery with or without PORT, it was showed that locoregional recurrence decreased and distant metastasis became the main failure pattern after receiving PORT for patients with stage T3-4 disease, a finding that agrees with the previous study results[7,21]. Distant metastasis after receiving PORT is a new issue that leads to treatment failure. Considering that no difference was found in the survival of patients receiving adjuvant chemotherapy, whether chemotherapy could further decrease the rate of metastasis needs further research. The reasons that more patients showed distant metastasis may be due to the small number of patients, local control increase, or there were inherent factors making patients prone to distant metastasis. Our recent research identified a potential biomarker for metastasis of ESCC[22], and we will study this topic further to clarify the mechanism of increased distant metastasis after PORT.
Both univariate and multivariate analyses demonstrated that lymph node involvement was significantly associated with the DFS of patients with stage T3-4 disease. Adjuvant chemotherapy may be a favorable factor. Meanwhile, many studies have reported that adjuvant chemotherapy could improve local control and OS or DFS, especially for patients with positive lymph nodes[23,24]. Researchers also reported that postoperative chemoradiotherapy could decrease both locoregional and distant recurrence. However, it must be noted that combined chemoradiation therapy may lead to higher toxicity[25]. Thus, we supposed that combined radiation and chemotherapy sequentially may be effective in improving DFS or even OS.
This study possessed some limitations. First, due to the retrospective nature of this study, it is inevitable that some information was uncontrolled or missed and it is difficult to address some biases. A perspective study should be conducted to answer these questions in the future. Second, patients in the S + R arm were fewer than those in the S arm, possibly influencing DFS; a longer follow-up and more enrolled patients are needed. Third, the study compared the constituent ratio of failure patterns because of the respective characteristics of patients.
In conclusion, PORT could improve DFS and decrease the locoregional recurrence of patients with stage pT3-4N0-3M0 ESCC. However, distant metastasis is the main failure pattern in patients after receiving PORT. Further study needs to be conducted to evaluate how to control hematogenous metastasis.
Postoperative radiotherapy (PORT) could improve the local control of stage T3-4 or lymph node positive esophageal squamous cell carcinoma (ESCC) patients. There was no study comparing the difference of failure patterns after surgery with or without PORT in such patients.
We wanted to investigate the difference of failure patterns in order to guide the following treatment for patients suffering treatment relapse.
To define the difference between patients with stage pT3-4N0-3M0 ESCC with or without PORT after esophagectomy.
Patients with pathologically stage T3-4 ESCC who receive PORT after surgery were included in an S + R arm, and the others without PORT were included in an S arm. This study mainly investigated the difference of failure patterns between the two arms.
This study reported that PORT could decrease locoregional relapse. However, the proportion of distant metastasis in the S + R arm was much more than that in the S arm.
PORT could improve the local control for patients with stage pT3-4 ESCC. Further studies need to be conducted to control hematogenous metastasis.
The treatment of locally advanced ESCC is a hot topic. PORT could decrease locoregional lymph node relapse, but distant metastasis after PORT is the main reason that results in treatment failure. It is urgent to find an effective treatment to control this situation. And now we should explain the main failure patterns after undergoing different treatment strategies.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tarnawski AS S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13199] [Article Influence: 1466.6] [Reference Citation Analysis (3)] |
| 2. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers, 2017. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx. |
| 3. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1814] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 4. | Chen G, Wang Z, Liu XY, Liu FY. Recurrence pattern of squamous cell carcinoma in the middle thoracic esophagus after modified Ivor-Lewis esophagectomy. World J Surg. 2007;31:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062-5067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 752] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 6. | Bilimoria KY, Stewart AK, Tomlinson JS, Gay EG, Ko CY, Talamonti MS, Bentrem DJ. Impact of adjuvant radiation on survival: a note of caution when using cancer registry data to evaluate adjuvant treatments. Ann Surg Oncol. 2007;14:3321-3327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Chen G, Wang Z, Liu XY, Zhang MY, Liu FY. Clinical study of modified Ivor-Lewis esophagectomy plus adjuvant radiotherapy for local control of stage IIA squamous cell carcinoma in the mid-thoracic esophagus. Eur J Cardiothorac Surg. 2009;35:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Xu Y, Liu J, Du X, Sun X, Zheng Y, Chen J, Li B, Liu W, Jiang H, Mao W. Prognostic impact of postoperative radiation in patients undergoing radical esophagectomy for pathologic lymph node positive esophageal cancer. Radiat Oncol. 2013;8:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Chen J, Zhu J, Pan J, Zhu K, Zheng X, Chen M, Wang J, Liao Z. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma esophagus. Ann Thorac Surg. 2010;90:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Mariette C, Balon JM, Piessen G, Fabre S, Van Seuningen I, Triboulet JP. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer. 2003;97:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 305] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Liu J, Cai X, Liu Q, Li H, Cheng Y, Fu X. Characteristics of the local recurrence pattern after curative resection and values in target region delineation in postoperative radiotherapy for lower thoracic esophageal squamous cell cancer. Thorac Cancer. 2017;8:630-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Liu Q, Cai XW, Wu B, Zhu ZF, Chen HQ, Fu XL. Patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma: implications for the clinical target volume design of postoperative radiotherapy. PLoS One. 2014;9:e97225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Cai WJ, Xin PL. Pattern of relapse in surgical treated patients with thoracic esophageal squamous cell carcinoma and its possible impact on target delineation for postoperative radiotherapy. Radiother Oncol. 2010;96:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Cai XW, Zeng Y, Feng W, Liu MN, Yu W, Zhang Q, Liu J, Wang JM, Lv CX, Fu XL. Randomized phase II trial comparing tumor bed alone with tumor bed and elective nodal postoperative radiotherapy in patients with locoregionally advanced thoracic esophageal squamous cell carcinoma. Dis Esophagus. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Xiao ZF, Yang ZY, Liang J, Miao YJ, Wang M, Yin WB, Gu XZ, Zhang DC, Zhang RG, Wang LJ. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg. 2003;75:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 16. | Hsu PK, Huang CS, Wang BY, Wu YC, Hsu WH. Survival benefits of postoperative chemoradiation for lymph node-positive esophageal squamous cell carcinoma. Ann Thorac Surg. 2014;97:1734-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Chen J, Pan J, Zheng X, Zhu K, Li J, Chen M, Wang J, Liao Z. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Schreiber D, Rineer J, Vongtama D, Wortham A, Han P, Schwartz D, Choi K, Rotman M. Impact of postoperative radiation after esophagectomy for esophageal cancer. J Thorac Oncol. 2010;5:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Yang J, Zhang W, Xiao Z, Wang Q, Zhou Z, Zhang H, Chen D, Feng Q, He J, Gao S, Sun K, Liu X, Fang D, Mu J, Wang D, Li Y. The Impact of Postoperative Conformal Radiotherapy after Radical Surgery on Survival and Recurrence in Pathologic T3N0M0 Esophageal Carcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol. 2017;12:1143-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Wang X, Luo Y, Li M, Yan H, Sun M, Fan T. Recurrence pattern of squamous cell carcinoma in the midthoracic esophagus: implications for the clinical target volume design of postoperative radiotherapy. Onco Targets Ther. 2016;9:6021-6027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Zhang W, Liu X, Xiao Z, Zhang H, Chen D, Feng Q, Zhou Z, Lv J, Liang J, Hui Z, Wang L, Yin W, Cheng G, Sun K, Liu X, Fang D, He J. Postoperative intensity-modulated radiotherapy improved survival in lymph node-positive or stage III thoracic esophageal squamous cell carcinoma. Oncol Res Treat. 2015;38:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Cai XW, Yu WW, Yu W, Zhang Q, Feng W, Liu MN, Sun MH, Xiang JQ, Zhang YW, Fu XL. Tissue-based quantitative proteomics to screen and identify the potential biomarkers for early recurrence/metastasis of esophageal squamous cell carcinoma. Cancer Med. 2018;7:2504-2517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Qin RQ, Wen YS, Wang WP, Xi KX, Yu XY, Zhang LJ. The role of postoperative adjuvant chemotherapy for lymph node-positive esophageal squamous cell carcinoma: a propensity score matching analysis. Med Oncol. 2016;33:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Zou B, Pang J, Liu Y, Xu Y, Li L, Zhou L, Zhu J, Huang M, Wang J, Ren L, Gong Y, Lu Y, Chen L, Peng F. Postoperative chemoradiotherapy improves survival in patients with stage II-III esophageal squamous cell carcinoma: An analysis of clinical outcomes. Thorac Cancer. 2016;7:515-521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Wong RK, Malthaner R. Withdrawn. Combined chemotherapy and radiotherapy (without surgery) compared with radiotherapy alone in localized carcinoma of the esophagus. Cochrane Database Syst Rev. 2010;CD002092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |