Published online Dec 15, 2019. doi: 10.4251/wjgo.v11.i12.1141
Peer-review started: April 3, 2019
First decision: July 31, 2019
Revised: September 4, 2019
Accepted: September 12, 2019
Article in press: September 13, 2019
Published online: December 15, 2019
Processing time: 255 Days and 17 Hours
In recent years, the incidence of gastrointestinal (GI) cancer in China has increased annually. Early detection and appropriate therapy are considered to be the key to treat GI cancer. DNMT1 takes an active part in the advancement of GI cancer, which will change as the disease progresses. But its expression characteristics in the dynamic variations of GI carcinogenesis are still unclear.
To investigate the expression characteristics of DNMT1 in different GI diseases.
We detected the expression of DNMT1 in 650 cases of different GI diseases by immunohistochemistry, including 90 cases of chronic superficial gastritis (CSG), 72 cases of atrophic gastritis with intestinal metaplasia (AG/GIM), 54 cases of low-grade intraepithelial neoplasia (GLIN), 66 cases of high-grade intraepithelial neoplasia (GHIN), 71 cases of early gastric cancer (EGC), 90 cases of normal intestinal mucosa (NIM), 54 cases of intestinal low-grade intraepithelial neoplasia (ILIN), 71 cases of intestinal high-grade intraepithelial neoplasia (IHIN), and 82 cases of early colorectal cancer (ECRC).
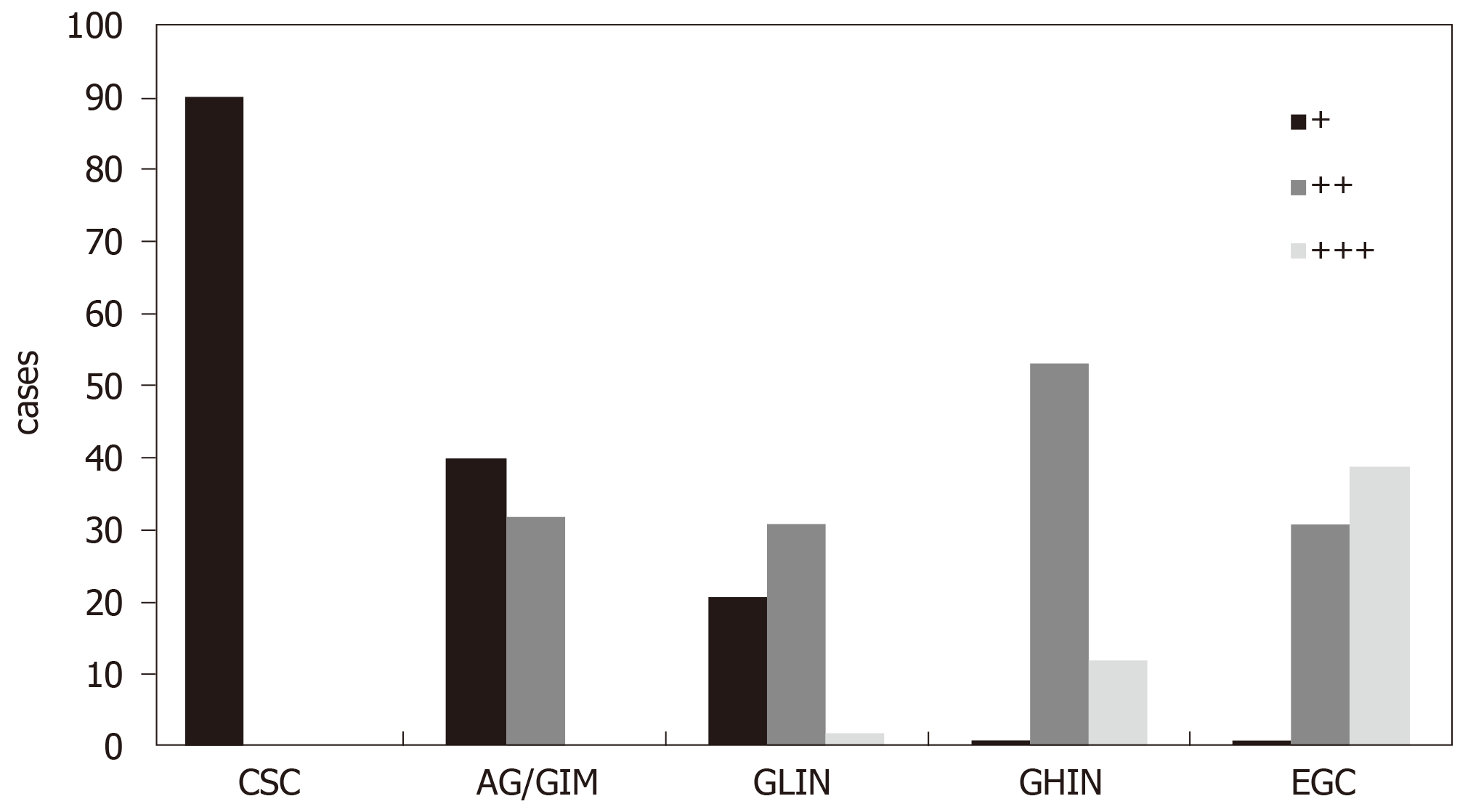
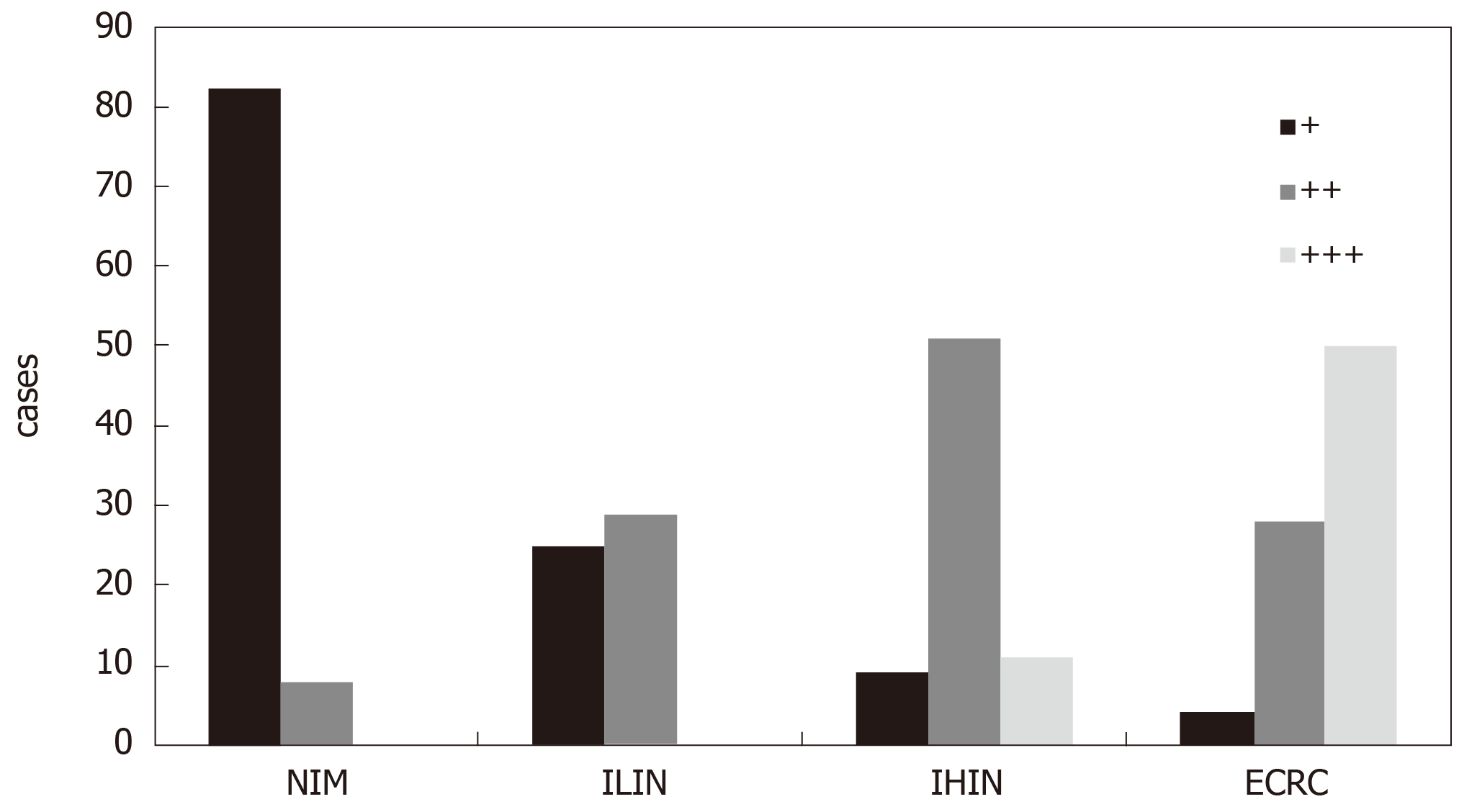
In the CSG group, all cases showed weakly positive or negative expression of DNMT1. However, in other four groups (AG/GIM, GLIN, GHIN, and EGC), the positive expression rate gradually increased with the severity of the diseases; the negative or weakly positive cases accounted for 55.56% (40/72), 38.89% (21/54), 1.52% (1/66), and 1.41% (1/71), respectively. Besides, the moderately positive cases were 44.44% (32/72), 57.41% (31/54), 80.30% (53/66), and 43.66% (31/71), respectively. The strongly positive cases only existed in the GLIN (3.70%, 2/54), GHIN (18.18%, 12/66), and EGC (54.93%, 39/71) groups. The differences between any two groups were statistically significant (P < 0.05). Similarly, in the NIM group, cases with weakly positive expression of DNMT1 were predominant (91.11%, 82/90), and the rest were moderately positive cases (8.89%, 8/90). In the ILIN, IHIN, and ECRC groups, the rates of cases with weak or negative expression of DNMT1 were 46.30% (25/54), 12.68% (9/71), and 4.88% (4/82), respectively; with moderately positive expression were 53.70% (29/54), 71.83% (51/71), and 34.15% (28/82), respectively; and with strongly positive expression were 0.00% (0/54), 15.49% (11/71), and 60.98% (50/82), respectively. The differences between any two groups were also statistically significant (P < 0.05).
The overexpression of DNMT1 protein could effectively predict early GI cancers and severe precancerous lesions, which may have potential clinical application value.
Core tip: The expression of DNMT1 was significantly effective for screening precancerous lesions and cancers. DNMT1 could be a potential marker for the diagnosis of gastric cancer and colorectal cancer. At the same time, the fluctuation of its expression may suggest the progression of gastrointestinal diseases, which could be instructive for further examination and treatment.
- Citation: Ma TM, Sun LP, Dong NN, Sun MJ, Yuan Y. Protein expression trends of DNMT1 in gastrointestinal diseases: From benign to precancerous lesions to cancer. World J Gastrointest Oncol 2019; 11(12): 1141-1150
- URL: https://www.wjgnet.com/1948-5204/full/v11/i12/1141.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i12.1141
The gastrointestinal (GI) tract plays a principal role in the human digestive system, and nearly all the essential nutrients for the human body are absorbed and digested through the GI tract[1]. As a result of unhealthy diet and deteriorating environment, the incidence of GI diseases has increased rapidly[2]. Both gastric cancer (GC) and colorectal cancer (CRC) are malignant GI diseases that require a progressive development process. Early detection and appropriate treatment are the effective measures to delay the GI tumorigenesis[3]. It is well known that common screening methods for GI cancer include gastroscopy, imaging examination, and biomarker examination. Due to the lack of specificity in the early clinical manifestations of GI cancer, the efficiency of imaging examination is not obvious. Endoscopy also has certain limitations due to the invasiveness and the demanding requirements for endoscopists. In comparison, tumor marker detection has several advantages in screening cancers, such as convenient source, good tolerance, and low cost.
DNMT1 is a crucial member during DNA replication, which could copy DNA methylation patterns from the parental DNA strand to the newly synthesized strand[4]. The incidence of cancer cells depends on specific gene silencing mediated by DNA methylation[5], and the primary function of DNMT1 is maintaining and stabilizing gene methylation[6]. When the aberrantly methylated occurs in the mammalian genome, DNMT1 will be transcribed in the nucleus with the expression gradually enhanced, promoting the advancement of DNA methylation and the procession of tumors[7]. Previously, we illustrated that the expression of DNMT1 was significantly higher in GC tissues than in non-GC tissues by meta-analysis[8]. Simultaneously, several studies have also revealed that DNMT1 was strongly expressed in GC and CRC[9,10], suggesting that DNMT1 could be a potential biomarker in screening of GI cancer.
However, the expression of DNMT1 protein in the dynamic variations of GI carcinogenesis, especially in precancerous lesions, is still unclear. In this study, from the perspective of the evolution process of GI cancer, we measured the protein expression of DNMT1 in different stages of GI diseases (from benign to precancerous to cancerous), investigated its expression characteristics, and further analyzed its clinical application value as a warning biomarker in pre-diagnosis of early GI cancer.
A total of 650 patients with different GI diseases diagnosed by histopathology at the Department of Endoscopy and Anorectal Surgery of the First Hospital of China Medical University from August 2012 to December 2017, who had not received preoperative chemotherapy or radiation, were selected. All the pathological diagnoses were made following the updated Sydney Gastritis Classification and World Health Organization Classification of Tumors of the Digestive System[11,12]. Among them, there were 353 cases of different gastric diseases (210 men and 143 women, with an average age of 60.03 years [range 16-88 years]), including 90 cases of chronic superficial gastritis (CSG), 72 cases of atrophic gastritis with intestinal metaplasia (AG/GIM), 54 cases of low-grade intraepithelial neoplasia (GLIN), 66 cases of high-grade intraepithelial neoplasia (GHIN), and 71 cases of early gastric cancer (EGC). In addition, 297 cases of colorectal disease were also contained in this study (156 men and 141 women, with a mean age of 58.27 years [range 20-85 years]), including 90 cases of normal intestinal mucosa (NIM), 54 cases of intestinal low-grade intraepithelial neoplasia (ILIN), 71 cases of intestinal high-grade intraepithelial neoplasia (IHIN), and 82 cases of early colorectal cancer (ECRC). The study protocol was approved by the Human Ethics Review Committee of the First Hospital of China Medical University. Written informed consent was obtained from each participant.
All biopsies were fixed in 10% formalin and embedded in paraffin. After sectioning at 4-micron thickness and mounting on positive-charged glass slides, these specimens were dewaxed by xylene, rehydrated with gradient alcohol, rinsed through tap water, and soaked in phosphate-buffered saline (PBS) solution (PH 7.4) for 10 min. The tissues were treated in boiling citric acid buffer (PH 6.0) for 1.5 min to complete antigen retrieval. Next, each slide was added with 50 μL of peroxidase blocking solution, and incubated at room temperature (25 ± 2 °C) for 20 min before rinsing with PBS solution. Tissue collagen was blocked by the addition of 50 μL of normal goat serum at room temperature for another 20 min. After draining excess liquid, the sections were incubated with primary antibody against DNMT1 (1: 500, Abcam, ab13537, Cambridge, United Kingdom) for 1 h at 37 °C, and rinsed three times with PBS solution. The buffer was then removed from the coverslip, followed by incubation with 50 μL of goat anti-rabbit antibody and 50 μL of streptomyces avidin-peroxidase for 10 min each. Afterwards, 50 μL of fresh DAB (Maixin, Fujian, China) was added per section for 1-1.5 min. Finally, the sections were washed with PBS, counterstained in hematoxylin, blued in running water, dehydrated with gradient alcohol, cleared with xylene, and mounted with neutral gum.
Two independent pathologists who were blinded to the clinicopathologic characteristics of the patients read and scored these immunohistochemical slides with DNMT1-positive expression. A semi-quantitative method was used to evaluate the area and intensity of the staining results. If the scores given by the two pathologists differ by more than one grade, the results would be reconsidered and discussed to determine the final score. The staining intensity of cells from different tissues was scored as 0-3 (I0-3): I0 (no staining), I1 (light yellow), I2 (dark yellow), and I3 (brown or dark brown). The percentage of positive cells was scored as 0-3 (P0-3): P0 (0%); P1 (>0 but ≤1/3), P2 (>1/3 but ≤2/3), and P3 (>2/3). On the basis of semiquantitative scoring, the expression of DNMT1 in the nucleus was assessed using the following formula: IS score = In × Pm. Finally, the protein expression of DNMT1 was graded as follows: negative or weakly positive expression (+), 0-1; moderately positive expression (++), 2-4; and strongly positive expression (+++), 5-9.
Statistical analyses were performed in any two gastric groups and intestinal groups separately, by the rank sum test using SPSS vision 22.0 (IBM SPSS statistics, Armonk, NY, United States) on the basis of DNMT1 positivity, and P < 0.05 was considered statistically significant.
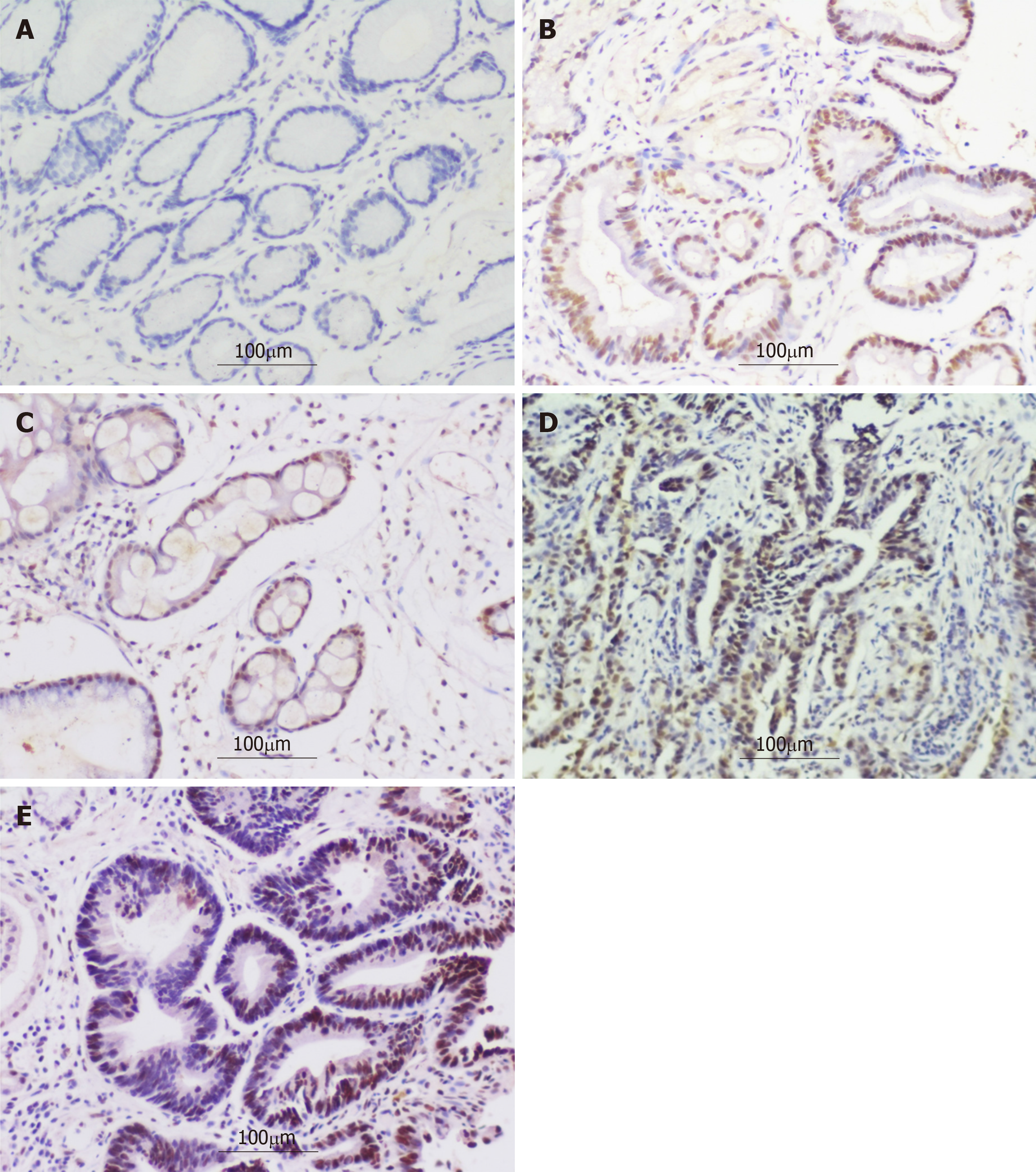
The representative photomicrographs of immunohistochemical staining for DNMT1 in different gastric disease are shown in Figure 1. DNMT1 was hardly expressed in the CSG group (0/90). The negative or weakly positive expression rates of DNMT1 in the AG/GIM and GLIN groups were separately 55.56% (40/72) and 38.89% (21/54), and the moderately positive expression rates were not very high, being 44.44% (32/72) and 57.41% (31/54), respectively. The strongly positive expression was scarcely in these two groups, with 0.00% (0/72) and 3.70% (2/54), respectively. In the GHIN group, the expression of DNMT1 was mainly moderately-positive, which accounted for 80.30% (53/66). There was still one negative or weakly positive case (1.52%, 1/66), and 12 strongly positive cases (18.18%, 12/66). In the GC group, the number of cases with strongly positive expression was the highest among the five groups (39/71, 54.93%). In addition, this group also had 1 case with negative or weakly positive expression of DNMT1 (1.41%) and 31 cases with moderately positive (43.66%) (Figure 2). The differences between any two groups were statistically significant (P < 0.05) (Table 1).
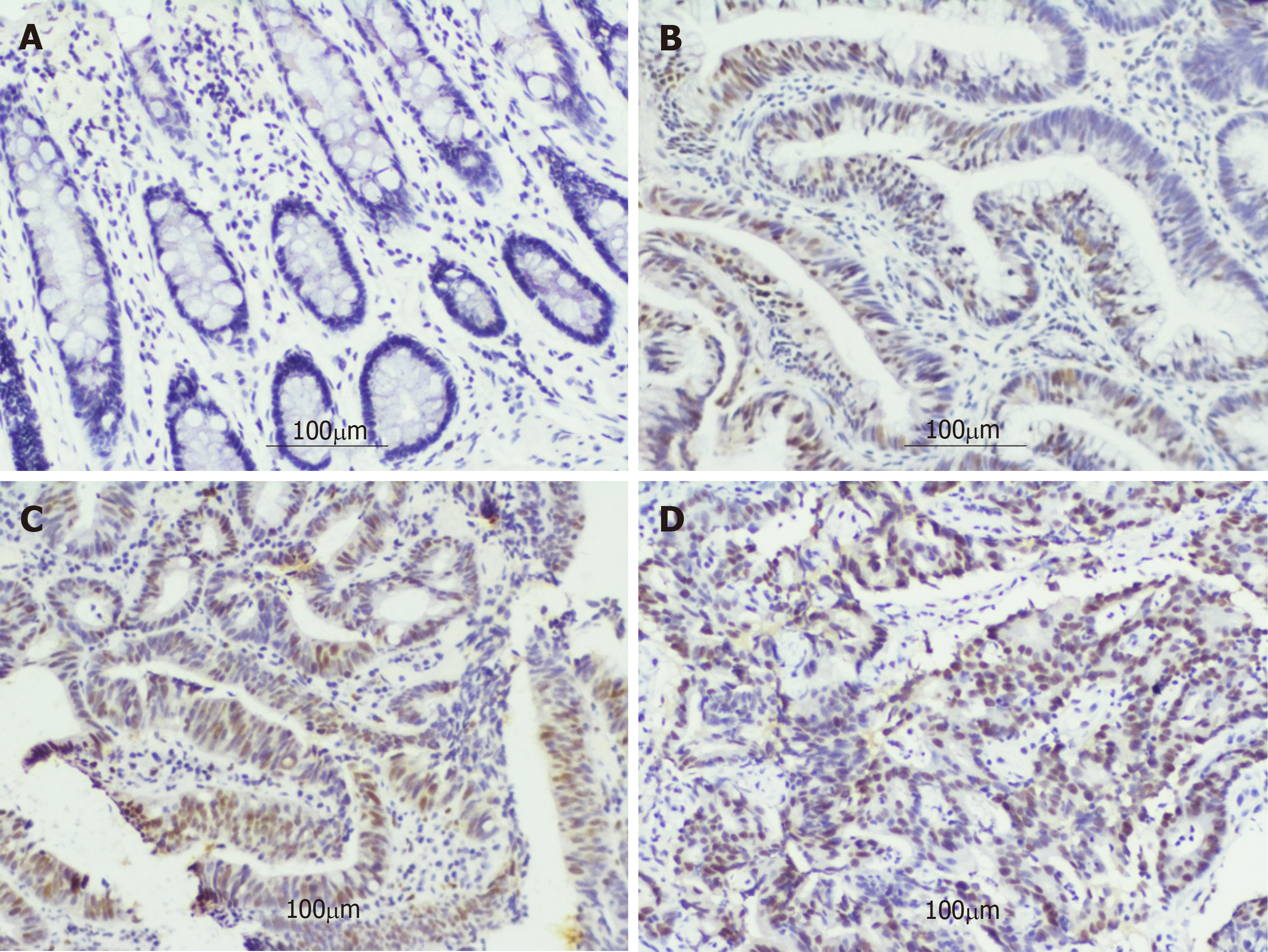
The typical photomicrographs of immunohistochemical staining for DNMT1 in different intestinal diseases are shown in Figure 3. The negative or weakly positive expression rates of DNMT1 protein in the NIM and ILIN groups were 91.11% (82/90) and 46.30% (25/54), and the moderately positive rates were 8.9% (8/90) and 53.7% (29/54), respectively. But there were no strongly positive expression in either group. In the IHIN group, the cases with moderately positive expression were predominant, accounting for 71.83% (51/71). The proportions of weakly positive cases and strongly positive cases in this group were almost the same, being 12.68% (9/71) and 15.49% (11/71), respectively. The majority of cases in the ECRC group had strongly positive expression, accounting for 60.98% (50/82), while those with negative or weakly positive expression accounted for 4.88% (4/82). The remaining part was 34.15% (28/82) (Figure 4). The differences between any two groups were statistically significant (P < 0.05) (Table 2).
The occurrence of both GC and CRC has a long-term evolving process of “normal-dysplasia-cancer”[13,14]. Accurate identification in early stage of carcinogenesis presents a positive effect on risk intervention and prognostic management[15]. DNA methylation is a kind of pivotal epigenetic modification, which can be apparently altered in precancerous lesions[16]. As a key part in maintaining the process of methylation, the level of DNMT1 expression could be changed to varying degrees in most tumors and even earlier conditions[17]. In this research, we recorded the expression features of DNMT1 protein in different GI diseases, interpreted the dynamic changes of DNMT1 from benign to precancerous to cancer in a full disease chain, and explored its warning role for GI cancer identification.
Considering the evolution of GC, five groups of specimens (including CSG, AG/GIM, GLIN, GHIN, and EGC) were collected to analyze the protein expression of DNMT1 in the present study. In accordance with the results, we could figure out that DNMT1 was hardly expressed in CSG tissues, but its expression was gradually up-regulated in AG/GIM and GLIN tissues, and much higher in the GHIN and EGC groups than in the others. This indicated that the expression level of DNMT1 protein increases with the severity of gastric diseases. Intestinal metaplasia is an important histopathological transformation in atrophic gastritis, which is deemed as a precancerous lesion of GC[18]. Certain abnormalities in tissue structures and physiological functions may turn out in this period. If the interventions can be taken in a timely manner, this situation could be reversed during disease progression[19]. Therefore, the identification of AG/GIM with potential malignant transformation would effectively reduce the possibility of GC. It should be on high alert if DNMT1 has positive expression in order to avoid missing the optimal timing of diagnosis and treatment[20]. Intraepithelial neoplasia (IN), especially high-grade intraepithelial neoplasia (HIN), is a non-invasive intramucosal neoplasia with high cellular and structural atypia, which was defined as carcinoma in situ or intramucosal carcinoma in Japan[21]. This study evidenced that the expression of DNMT1 was obviously elevated in the GHIN and EGC groups, testifying that the overexpression of DNMT1 may be an initial event of GC. Our experiments demonstrated that DNMT1 expression had a close relationship with the development of GC, which can be used to monitor the progression of gastric diseases dynamically, and help to distinguish GHIN and GC from benign gastric diseases to some extent.
Studies[22,23] have proved that colon cancer cells cannot survive in the absence of abnormal promoter DNA methylation, and the complete knockdown of DNMT1 would result in the death of a large number of cancer cells. They proposed a strong correlation between DNMT1 and CRC cell viability. We observed the expression of DNMT1 in the NIM, ILIN, IHIN, and ECRC groups. The results showed that the level of DNMT1 protein expression progressively increased with the severity of intestinal lesions. In the NIM group, the positive expression rate of DNMT1 was the lowest. In the ILIN group, there were only weakly or moderately positive cases, and few strongly positive cases. However, when the pathological lesion progressed to the IHIN or ECRC stage, the positive expression rate of DNMT1 was much higher. This trend in intestinal diseases was basically consistent with that in gastric diseases. The precancerous lesions were regarded as the penultimate parts of CRC[24]. Accurate diagnosis, management, and monitoring of the disease at this stage and the adoption of appropriate treatments are very essential to decrease the incidence of cancer[25]. The up-regulation of DNMT1 can usually precede the appearance of DNA methylation[26], which is instructive for estimating the malignancy of precancerous lesions.
The previous classification and assessment of tumors were usually based on the lesion location (like lung cancer, breast cancer, etc.) or morphological characteristics (like adenocarcinoma, squamous carcinoma, etc.), not reflecting the nature of the disease. Recently, discerning tumors by markers rather than sources has become a theory that more and more scholars advocated, which means the understanding of cancer has risen to a molecular field[27]. In May 2017, the United States Food and Drug Administration approved Keytruda for the treatment of MSI/dMMR solid tumors[28]. This was the first anti-tumor therapy according to genetic biomarker, representing a milestone in cancer treatment. Implementing same or similar diagnosis and follow-up treatment for various tumors based on specific gene targets can provide a new idea for cancer research, and realize the precise medical concept of “diagnose and treat different diseases in same ways”[29]. Changes in genomic DNA methylation status could cause chromosomal instability or abnormal gene expression, leading to tumorigenesis eventually[30]. DNMT1 could be significantly overexpressed in the primary stages of many cancers[31-33], and thus be utilized as a common marker for most tumors and precancerous lesions. Our study simultaneously detected the expression of DNMT1 in various gastric and colorectal diseases from benign to precancerous to cancer, with a consistent trend observed. It gave a proof that DNMT1 could be a common marker for these two cancers, and the fluctuation of its expression may prompt the progression of GI diseases. The quantity of DNMT1 protein ascended with the aggravation of mucosal lesions, which is of vital importance not only for the early diagnosis of GI cancer, but also for further clinical treatment. Targeted therapy for GI cancer with DNMT1 overexpression would become the future research direction[34].
In summary, this is the first comprehensive study explaining the expression trend of DNMT1 from benign to precancerous to cancer, and discovering the inseparable relationship between DNMT1 overexpression and GI diseases. In cancer or HGIN tissues, DNMT1 is expressed greatly higher than that in non-cancer mucosa or LGIN tissues, which suggests that DNMT1 could discriminate between cancerous tissues and non-cancer tissues, as well as evaluate the extent of precancerous lesions possibly, warning the patients for further examination and treatment. DNMT1 could be used as a potential biomarker for early detection of GI cancer.
In recent years, the incidence of gastrointestinal (GI) cancer in China has increased annually. Early detection and appropriate therapy are considered to be the key to treating GI cancer. Unfortunately, at present, the early detection rates of gastric cancer (GC) and colorectal cancer (CRC) are both less than 10% of all diagnosed cases on average. Previously, we illustrated that the expression of DNMT1 was significantly higher in GC tissues than in non-GC tissues by meta-analysis. Simultaneously, several studies have also revealed that DNMT1 is strongly expressed in GC and CRC, suggesting that DNMT1 could be a potential biomarker in screening of GI cancer. However, the expression of DNMT1 protein in the dynamic variations of GI carcinogenesis, especially in precancerous lesions, is still unclear.
In this study, from the perspective of the evolving process of GI cancer, we measured the expression of DNMT1 protein in different stages of GI diseases (from benign to precancerous to cancerous), investigated its expression characteristics, and further analyzed its clinical application value as a warning biomarker in diagnosis of early GI cancer.
Although we only used patient tissues as our samples to detect the dynamic variation of DNMT1 protein expression in different gastrointestinal diseases, we consider DNMT1 may be used as part of a future serological test to assess the severity of patients with gastrointestinal disorders and to prompt them to complete more tests, which could help to discover the disease as soon as possible and take appropriate treatment next step.
A total of 650 patients with different gastrointestinal diseases diagnosed pathologically, who had not received preoperative chemotherapy or radiation, were enrolled in our study. Among them, there were 353 cases of different gastric diseases, including 90 cases of chronic superficial gastritis, 72 cases of atrophic gastritis with intestinal metaplasia (AG/GIM), 54 cases of low-grade intraepithelial neoplasia (GLIN), 66 cases of high-grade intraepithelial neoplasia (GHIN), and 71 cases of early gastric cancer (EGC). In addition, 297 cases of colorectal diseases were also contained in our experiment, including 90 cases of normal intestinal mucosa (NIM), 54 cases of intestinal low-grade intraepithelial neoplasia (ILIN), 71 cases of intestinal high-grade intraepithelial neoplasia (IHIN), and 82 cases of early colorectal cancer (ECRC). Immunohistochemistry (IHC) was used to detect the expression of DNMT1 in these cases. Statistical analysis was performed in any two gastric groups and intestinal groups separately, by rank sum test on the basis of DNMT1 positivity, and P < 0.05 was considered statistically significant.
In accordance with the results, we could figure out that DNMT1 was hardly expressed in chronic superficial gastritis tissues, but its expression was gradually up-regulated in atrophic gastritis with intestinal metaplasia and low-grade intraepithelial neoplasia tissues, and much higher in high-grade intraepithelial neoplasia and early gastric cancer. This indicated that the expression level of DNMT1 protein increases with the severity of gastric diseases. In different intestinal diseases, the changes in DNMT1 expression were consistent with the dynamic trend in gastric diseases. In the normal intestinal mucosa group, the positive expression rate of DNMT1 was the lowest. And in the intestinal low-grade intraepithelial neoplasia group, there were many weakly or moderately positive cases, and few strongly positive cases. However, in the high-grade intraepithelial neoplasia or early colorectal cancer stage, the positive expression rate of DNMT1 was much higher. In conclusion, the up-regulation of DNMT1 can usually precede the appearance of DNA methylation, which is instructive for estimating the malignancy of precancerous lesions.
DNMT1 can not only distinguish between normal tissues and cancerous tissues, but also assess the extent of precancerous lesions. With the aggravation of GI mucosal lesions, the expression of DNMT1 is rapidly increasing, which is of great significance for the early diagnosis of GI cancer. DNMT1 is a gene with significantly differential expression in gastrointestinal diseases, and could be used for early diagnosis of GI cancer and guidance of clinical treatment.
The present study suggested that the expression level of DNMT1 in the gastrointestinal mucosa significantly correlates with disease progression, thus being used as an early warning sign of cancer. In the future, the feasibility of serum DNMT1 expression detection as an early warning sign of gastrointestinal risks can be investigated.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Senchukova M, Youssef Mohamed S S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Qi LL
| 1. | Johnstone C, Hendry C, Farley A, McLafferty E. The digestive system: part 1. Nurs Stand. 2014;28:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Baba Y, Ishimoto T, Kurashige J, Iwatsuki M, Sakamoto Y, Yoshida N, Watanabe M, Baba H. Epigenetic field cancerization in gastrointestinal cancers. Cancer Lett. 2016;375:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Vedeld HM, Goel A, Lind GE. Epigenetic biomarkers in gastrointestinal cancers: The current state and clinical perspectives. Semin Cancer Biol. 2018;51:36-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2332] [Cited by in RCA: 2983] [Article Influence: 248.6] [Reference Citation Analysis (0)] |
| 5. | Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1448] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 6. | Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet. 2001;10:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 645] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 7. | De Carvalho DD, Sharma S, You JS, Su SF, Taberlay PC, Kelly TK, Yang X, Liang G, Jones PA. DNA methylation screening identifies driver epigenetic events of cancer cell survival. Cancer Cell. 2012;21:655-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 8. | Ma T, Li H, Sun M, Yuan Y, Sun LP. DNMT1 overexpression predicting gastric carcinogenesis, subsequent progression and prognosis: a meta and bioinformatic analysis. Oncotarget. 2017;8:96396-96408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Li H, Li W, Liu S, Zong S, Wang W, Ren J, Li Q, Hou F, Shi Q. DNMT1, DNMT3A and DNMT3B Polymorphisms Associated With Gastric Cancer Risk: A Systematic Review and Meta-analysis. EBioMedicine. 2016;13:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Huang C, Liu H, Gong XL, Wu L, Wen B. Expression of DNA methyltransferases and target microRNAs in human tissue samples related to sporadic colorectal cancer. Oncol Rep. 2016;36:2705-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Minalyan A, Benhammou JN, Artashesyan A, Lewis MS, Pisegna JR. Autoimmune atrophic gastritis: current perspectives. Clin Exp Gastroenterol. 2017;10:19-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Lam AK. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr Pathol. 2017;28:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 13. | Li ML, Zhang JC, Li SG, Wu WG, Rao LH, Dong P, Gu J, Lu JH, Zhang L, Ding QC, Wu XS, Mu JS, Yang JH, Zhang WJ, Chen L, Liu YB. Characteristic gene expression profiles in the progression from normal gastric epithelial cells to moderate gastric epithelial dysplasia and to gastric cancer. Chin Med J (Engl). 2012;125:1777-1783. [PubMed] |
| 14. | Xi HQ, Zhang KC, Li JY, Cui JX, Zhao P, Chen L. Expression and clinicopathologic significance of TUFM and p53 for the normal-adenoma-carcinoma sequence in colorectal epithelia. World J Surg Oncol. 2017;15:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Grizzi F, Basso G, Borroni EM, Cavalleri T, Bianchi P, Stifter S, Chiriva-Internati M, Malesci A, Laghi L. Evolving notions on immune response in colorectal cancer and their implications for biomarker development. Inflamm Res. 2018;67:375-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Gkountela S, Zhang KX, Shafiq TA, Liao WW, Hargan-Calvopiña J, Chen PY, Clark AT. DNA Demethylation Dynamics in the Human Prenatal Germline. Cell. 2015;161:1425-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 261] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Dan J, Chen T. Genetic Studies on Mammalian DNA Methyltransferases. Adv Exp Med Biol. 2016;945:123-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Busuttil RA, Boussioutas A. Intestinal metaplasia: a premalignant lesion involved in gastric carcinogenesis. J Gastroenterol Hepatol. 2009;24:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5074] [Cited by in RCA: 4879] [Article Influence: 212.1] [Reference Citation Analysis (0)] |
| 20. | Zhang ZB. The best treatment method for low grade dysplasia of gastric mucosa. Herald of Medicine. 2016;35:61-64. |
| 21. | Rokutan H, Abe H, Nakamura H, Ushiku T, Arakawa E, Hosoda F, Yachida S, Tsuji Y, Fujishiro M, Koike K, Totoki Y, Fukayama M, Shibata T. Initial and crucial genetic events in intestinal-type gastric intramucosal neoplasia. J Pathol. 2019;247:494-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2489] [Cited by in RCA: 2431] [Article Influence: 110.5] [Reference Citation Analysis (0)] |
| 23. | Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3397] [Cited by in RCA: 3722] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 24. | Bénard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J Gastroenterol. 2018;24:124-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 228] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (7)] |
| 25. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3134] [Cited by in RCA: 3541] [Article Influence: 393.4] [Reference Citation Analysis (0)] |
| 26. | Kurdyukov S, Bullock M. DNA Methylation Analysis: Choosing the Right Method. Biology (Basel). 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 387] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 27. | Duffy MJ, Lamerz R, Haglund C, Nicolini A, Kalousová M, Holubec L, Sturgeon C. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int J Cancer. 2014;134:2513-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 28. | Cho J, Kang SY, Kim KM. MMR protein immunohistochemistry and microsatellite instability in gastric cancers. Pathology. 2019;51:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Zheng GQ, Wang Y, Gu Y. [On the Conditionality of "Disease" in Treating Different Diseases with the Same Method]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:517-520. [PubMed] |
| 30. | Benetatos L, Vartholomatos G. On the potential role of DNMT1 in acute myeloid leukemia and myelodysplastic syndromes: not another mutated epigenetic driver. Ann Hematol. 2016;95:1571-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Yu Z, Xiao Q, Zhao L, Ren J, Bai X, Sun M, Wu H, Liu X, Song Z, Yan Y, Mi X, Wang E, Jin F, Wei M. DNA methyltransferase 1/3a overexpression in sporadic breast cancer is associated with reduced expression of estrogen receptor-alpha/breast cancer susceptibility gene 1 and poor prognosis. Mol Carcinog. 2015;54:707-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Etoh T, Kanai Y, Ushijima S, Nakagawa T, Nakanishi Y, Sasako M, Kitano S, Hirohashi S. Increased DNA methyltransferase 1 (DNMT1) protein expression correlates significantly with poorer tumor differentiation and frequent DNA hypermethylation of multiple CpG islands in gastric cancers. Am J Pathol. 2004;164:689-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 33. | Feng Y, Wang J, Wu Y, Feng X, Yu Y. [Protein expressions of HDAC1 and DNMT1 in non-small-cell lung cancer and its clinical significance]. Zhonghua Yi Xue Za Zhi. 2014;94:596-598. [PubMed] |
| 34. | Rahman MM, Qian ZR, Wang EL, Yoshimoto K, Nakasono M, Sultana R, Yoshida T, Hayashi T, Haba R, Ishida M, Okabe H, Sano T. DNA methyltransferases 1, 3a, and 3b overexpression and clinical significance in gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2010;41:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |