Published online Oct 15, 2019. doi: 10.4251/wjgo.v11.i10.925
Peer-review started: March 8, 2019
First decision: April 15, 2019
Revised: July 24, 2019
Accepted: August 26, 2019
Article in press: August 26, 2019
Published online: October 15, 2019
Processing time: 226 Days and 0.1 Hours
Epstein-Barr virus (EBV)-associated carcinoma is a gastric cancer subtype with a morphology characterized by gastric carcinoma with lymphoid stroma (GCLS). Clinicopathological studies have indicated a better prognosis for GCLS than for common gastric carcinomas. Some previous cases of early gastric cancer associated with EBV had been diagnosed by endoscopic resection.
We present two GCLS cases subjected to endoscopic submucosal dissection (ESD) for a definitive diagnosis. A protruded gastric lesion was identified by routine endoscopic examination, but forceps biopsy showed no atypical cells before ESD. The resected specimen showed a poorly differentiated adenocarcinoma with lymphoid cells involving the mucosa and submucosa. The final diagnosis was submucosa-invasive poorly differentiated gastric adenocarcinoma. Accordingly, additional gastrectomy was recommended to obtain a complete cure. One patient underwent additional distal gastrectomy with lymph node dissection, but the other was refused because of cardiovascular complications. Both patients remained in remission for more than half a year. EBV positivity was determined by EBV-encoded RNA in situ hybridization. We also conducted a literature review of cases of early gastric cancer associated with EBV that had been diagnosed by ESD.
Submucosa-invasive GCLS could be dissected using ESD, and EBV positivity should be subsequently assessed to determine whether or not any additional curative surgery is required. Further prospective investigations on the prevalence of lymph node metastasis in EBV-associated carcinoma should be performed to expand the indications for endoscopic resection.
Core tip: Two cases of Epstein-Barr virus (EBV)-associated gastric carcinoma were diagnosed by endoscopic submucosal dissection (ESD). Because of its low frequency of lymph node metastasis, EBV-associated carcinoma can be treated with ESD without additional surgery.
- Citation: Kobayashi Y, Kunogi T, Tanabe H, Murakami Y, Iwama T, Sasaki T, Takahashi K, Ando K, Nomura Y, Ueno N, Kashima S, Moriichi K, Takei H, Fujiya M, Okumura T. Gastric submucosa-invasive carcinoma associated with Epstein-Barr virus and endoscopic submucosal dissection: A case report. World J Gastrointest Oncol 2019; 11(10): 925-932
- URL: https://www.wjgnet.com/1948-5204/full/v11/i10/925.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i10.925
Epstein-Barr virus (EBV), also known as human herpesvirus 4, is associated with Burkitt lymphoma, nasopharyngeal carcinoma, and natural killer/T lymphoma. EBV is also positive in 80%-90% of cases of gastric carcinoma with lymphoid stroma (GCLS), which includes undifferentiated adenocarcinoma with intense lymphoid infiltration[1].
GCLS was first proposed as a separate entity in 1976[2], and lymphoepithelioma-like carcinoma associated with EBV was found in 1990[3,4]. The clinical features of EBV-associated gastric carcinoma include its location in the middle or upper stomach and a superficially depressed or submucosal tumor (SMT)-like appearance. Clinicopathological studies have indicated that EBV-positive carcinomas have a better prognosis and lower rate of lymph node metastasis than EBV-negative carcinomas[1,5,6]. In the Cancer Genome Atlas (TCGA) project, gastric cancers are divided into four subtypes, one of which is positive for EBV. EBV-positive gastric cancer has recently attracted much attention due to dramatic advances in drug therapies such as DNA methylation inhibitors and immune checkpoint inhibitors[7].
EBV-associated GCLS is defined as a poorly differentiated carcinoma admixed with marked subepithelial lymphoid cell infiltration. Because the carcinoma is covered with normal overlying epithelium, an endoscopic biopsy sometimes fails to yield tissue specimens to be pathologically diagnosed as malignant. We performed an endoscopic mucosal dissection (ESD) in two GCLS cases to obtain a definitive diagnosis. EBV positivity in cancer cells was confirmed by EBV-encoded small RNA in situ hybridization. We also reviewed cases of EBV-associated submucosa-invasive GCLS that were subjected to ESD.
Chief complaint: A 72-year-old woman complaining of abdominal discomfort had been treated for chronic gastritis in our hospital.
History of present illness: She had a medical history of eradication of Helicobacter pylori without a family history of gastric malignancy.
Physical examination: There were no abnormal findings on physical examination, and the serum chemistry and complete blood count were normal.
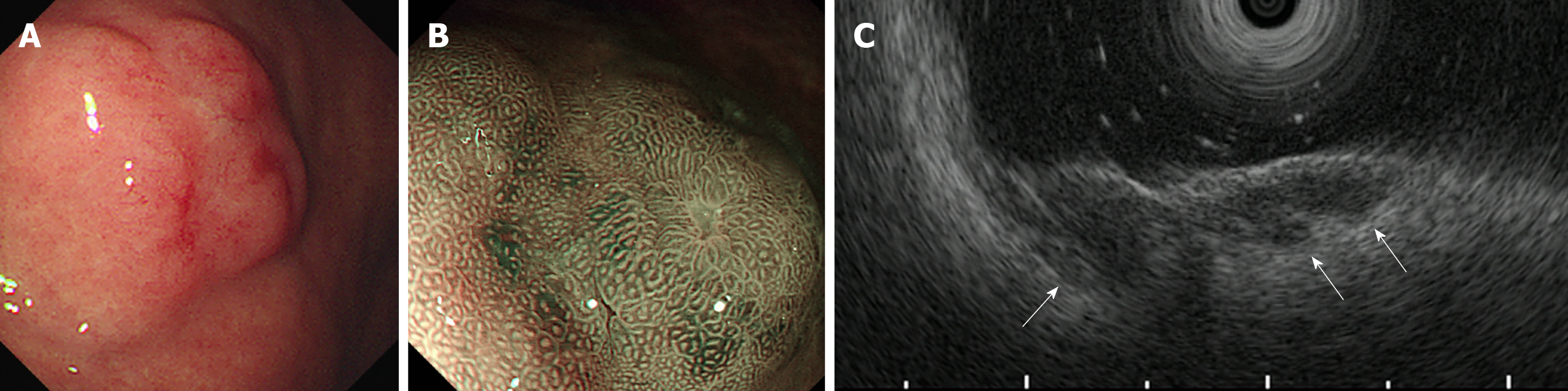
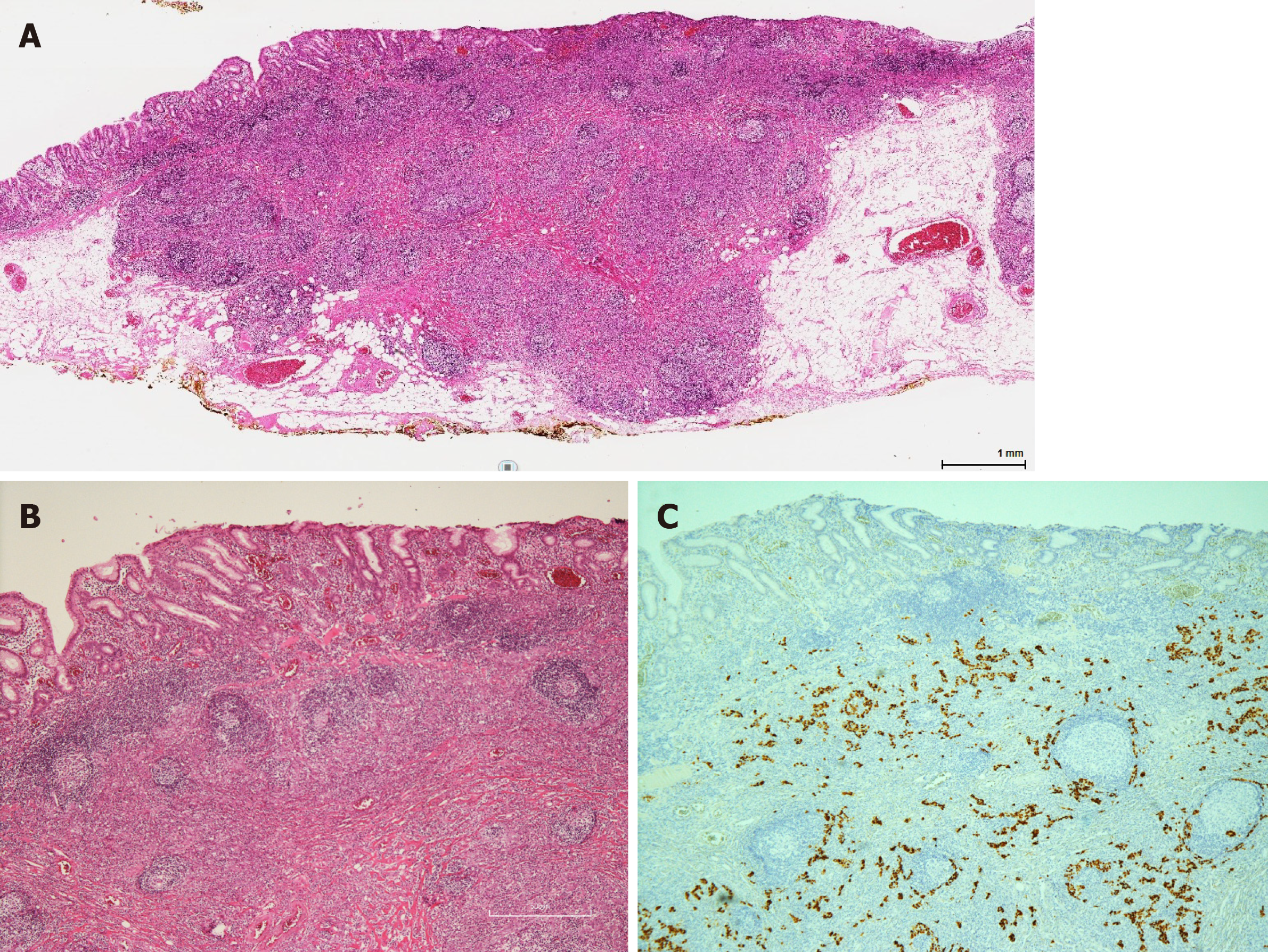
Imaging examinations: She underwent esophagogastroduodenoscopy at her routine check-up. A 20-mm protruding lesion with a central depression was noted in the middle gastric body (Figure 1A). Forceps biopsy showed no atypical cells. Computed tomography (CT) did not revealed gastric tumor or lymph node swelling. Four months later, an endoscopic re-examination revealed no significant difference in findings, with no atypical cells in the biopsy specimen, but subsequent endoscopic ultrasonography indicated a hypoechoic lesion that massively infiltrating the submucosa (Figure 1B and C). Due to a strong suspicion of gastric carcinoma, ESD was performed. The ESD specimen showed a poorly differentiated adenocarcinoma with accompanying prominent lymphoid tissues involving the mucosa and submucosa (Figure 2). Lymphatic invasion was observed, and EBV-encoded RNA (EBER) was detected by in situ hybridization.
Chief complaint: A 73-year-old man was referred to our hospital due to an SMT in the lower gastric body, which had been followed at a city hospital for 4 years.
History of present illness: A follow-up endoscopic examination showed no apparent changes from previous evaluations. The SMT was further examined because the patient needed treatment for myocardial infarction and abdominal aortic aneurysm at a tertiary hospital.
Physical examination: There was no family history of malignancy. No physical finding in his abdomen was observed in our consultation room.
Laboratory examinations: His complete blood count was normal, and most of the blood parameters were within normal range, except for a slight decrease in the renal function test (estimated glomerular filtration rate, 48.5 mL/min).
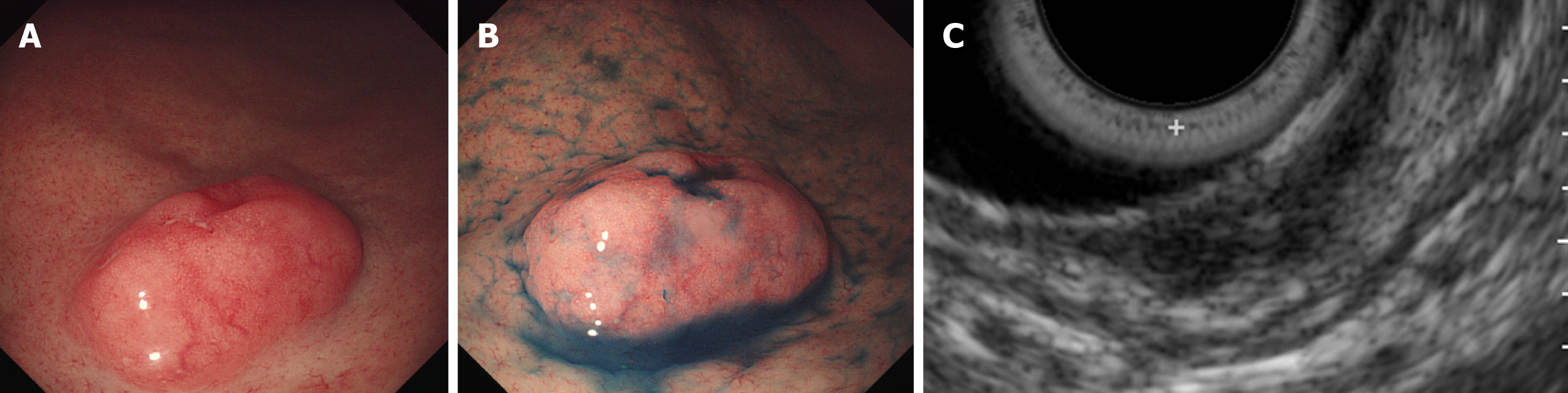
Imaging examinations: Annual endoscopy revealed no significant change for the following 3 years, and no atypical cells were obtained by forceps biopsy. CT did not reveal gastric tumor or lymph node metastasis. However endoscopic ultrasonography demonstrated a multinodular hypoechoic lesion, measuring 1.5 cm in the greatest dimension, with submucosal involvement (Figure 3). GCLS was suspected, and ESD was performed for a definitive pathological diagnosis when the patients was 76 years old. The pathological diagnosis (Figure 4) was a carcinoma with lymphoid stroma, 19 x 16 mm, and infiltrating into the deep submucosa (SM2, 5200 μm). No lymphovascular invasion was detected. EBV was positive in cancer cells based on EBER in situ hybridization.
Submucosa-invasive GCLS, EBV-positive. Both patients were recommended to undergo additional distal gastrectomy because there is a risk of lymph node metastasis when poorly differentiated gastric carcinoma is invading the submucosa.
The patient underwent distal gastrectomy, and no residual tumor or lymph node metastasis was observed in the resected specimen.
The patient refused surgical operation because of his cardiovascular complications, and was hoping for careful medical observation without chemotherapy or radiotherapy.
The patient remained without recurrence at the 14-month follow-up.
The patient underwent re-examination by gastroscopy, and no residual atypical cells were pathologically observed at 12 months after ESD. Helicobacter pylori was positive in the biopsy specimen taken from the gastric body, and eradication therapy was therefore performed.
Helicobacter pylori is the major cause of gastric cancer, and its eradication is recommended to decrease the risk of gastric cancer. An association between EBV and gastric cancer has also been suggested, as viral clonality is observed in proliferating cancer cells[8]. EBV-associated gastric cancer is characterized as poorly differentiated carcinoma with prominent lymphoid infiltration, clearly distinguishable from Helicobacter pylori-associated gastric cancer. As the infection rate with Helicobacter pylori is decreasing worldwide, trends in EBV-associated gastric cancer are now being monitored with interest by many researchers.
TCGA classifies gastric cancer into four subtypes: EBV-positive tumors, microsatellite unstable tumors, genetically stable tumors, and tumors with chromosomal instability[7]. The EBV-positive subtype shows DNA hypermethylation in host cell DNA due to methyltransferase 1 transcription induced by EBV latent membrane protein 2A. It displays amplification of programmed death-ligand 1 (PD-L1) and PD-L2, and the immune tolerance of the neoplasms may be associated with carcinogenesis. While patients with EBV-associated gastric cancer tend to have a good prognosis, the underlying molecular mechanism remains unclear[9,10].
Early GCLS has peculiar clinicopathological features, and its prognosis depends on the EBV infection status[11]. Early GCLS has also been analyzed in relation to lymph node metastasis, and EBV positivity is a predictive marker of a negative lymph node metastasis[12,13]. The risk of lymph node metastasis of mucosal GCLS and that of submucosal GCLS are 0 and 4.0%-10.6%, respectively. Limited to EBV-positive cases, intramucosal gastric cancer displays no lymph node metastasis, as reported by Japanese researchers (Tokunaga et al[14] and Murai et al[15]). Therefore, intramucosal EBV-positive gastric carcinomas could be treated by ESD rather than radical gastric surgery, because these cases are associated with a minimal risk of lymph node metastasis. The association between EBV positivity of submucosa-invasive GCLS and lymph node metastasis has not been previously described. A study in Korea reported that EBV positivity is a favorable risk factor for lymph node metastasis in submucosa-invasive gastric cancer, with a rate of metastasis of 4.7%[9]. The authors suggested that EBV positivity might be considered an additional criterion for the indication of endoscopic resection. We therefore reviewed cases of EBV-positive gastric cancer in which ESD was performed for diagnosis and treatment.
We conducted a database search of PubMed, Scopus and ScienceDirect using the following terms: (“gastric carcinoma” or “gastric cancer”) and (EBV or “Epstein-Barr virus” or “human herpesvirus 4”) and (“endoscopic submucosal dissection” or ESD). An search of reported references was also performed. A total of 9 cases were described with detailed clinical information in six reports[16-21]. Upon the addition of our 2 cases, 11 EBV-positive gastric submucosal cancer cases were ultimately collected by our database search (Table 1). This disease was found to be associated with male sex, proximal location, and depressed or SMT-like appearance. The diagnosis appeared to be difficult, as 5 cases (45.5%) were negative on pathological examination by forceps biopsy. Repeated biopsies failed to yield malignant cells in both of our cases. The depths of the tumor invasion were more than 1 mm into the submucosa from the deepest portion of the muscularis mucosae; however, the horizontal dimension was smaller than 2 cm in most cases. In the five cases that were subjected to subsequent radical gastrectomy with lymph node dissection, no lymph node metastasis was observed. Furthermore, neither local recurrence nor distant metastasis was reported throughout the follow-up periods. Therefore, submucosa-invasive cancer can be excised entirely, allowing us to cure the patients with early gastric cancer. Excessive and unnecessary gastrectomy might therefore be avoided and replaced by minimally invasive endoscopic resection.
| Study | Age (yr), Sex | Diameter (cm) | Lesions | Features | Biopsy diagnosis | ESD diagnosis | EBV | Depth | Additional surgery | Lymph node metasta-sis | Prognosis |
| Gromski et al[16], 2012 | 67, M | 2 × 1.2 | Lower body | Centrally depressed lesion | Chronic gastritis | Lymphoepithelioma-like gastric carcinoma | Positive | SM2 | Not performed | ND | No recurrence for 12 M |
| Lee et al[17], 2012 | 43, M | NA | NA | Multiple elevated erosive lesions with mild central depressi-ons | Adenocarci-noma | Lymphoepithelioma-like gastric carcinoma with lymphoid-rich stroma | Positive | SM2 (1538 μm) | Not performed | ND | No recurrence for 24 M |
| Matsumoto et al[18], 2013 | 58, M | NA | Upper body | Submuco-sal tumor associated with a slightly depressed lesion | Adenocarci-noma | Gastric carcinoma with lymphoid stroma | Positive | SM2 | Formal resection | Negative | NA |
| Lee et al[19], 2014 | 63, M | 2.0 | High body | Elevated lesion that displayed surface hyperemia | Moderately differentiat-ed adenocarci-noma | Lymphoepithelioma-like gastric carcinoma | Positive | SM2 (1800 μm) | Total gastrecto-my | Negative | No recurrence for 48 M |
| 65, M | 1.0 | Low body | Slightly elevated | Moderately differentiat-ed adenocarci-noma | Lymphoepithelioma-like gastric carcinoma | Positive | SM (1800 μm) | Not performed | ND | No recurrence for 32 M | |
| 74, M | 1.5 | Low body | Slightly elevated lesion with central dimpling | A few markedly atypical cells | Lymphoepithelioma-like gastric carcinoma | Positive | SM2 (2500 μm) | Not performed | ND | No recurrence for 28 M | |
| 84, M | 1.5 | Cardia | Large reddish and slightly depressed lesion | Moderately differentiated adenocarci-noma | Lymphoepithelioma-like gastric carcinoma | Positive | SM2 (2300 μm) | Not performed | ND | No recurrence for 27 M | |
| Chen et al[20], 2016 | 50, M | 2.5 × 2.5 | Gastric body | A submuco-sal columnar lesion with surface erosion | Moderate chronic superficial gastritis | Lymphoepitheliomalike gastric carcinoma | Positive | SM | Total radical gastrecto-my | Negative | No recurrence for 12 M |
| Kato et al[21], 2018 | 53, M | 2.0 | Middle body | Subepithe-lial lesion with center depressed | Benign gastric mucosa | Gastric cancer with lymphoid stroma | Positive | SM2 | Distal gastrecto-my | Negative | NA |
| Present cases | 72, F | 2.0 | Middle body | Protruding lesions with central depression | No atypical cells | Gastric carcinoma with lymphoid stroma | Positive | SM2 (> 4000 μm) | Distal gastrecto-my | Negative | No recurrence for 14 M |
| 76, M | 1.5 | Lower body | Submuco-sal tumor | No atypical cells | Gastric carcinoma with lymphoid stroma | Positive | SM2 (5200 μm) | Not performed | ND | No recurrence for 12 M |
The European Society of Gastrointestinal Endoscopy guidelines strongly recommend endoscopic resection for the treatment of superficial gastric neoplastic lesions that possess a very low risk of lymph node metastasis[22]. According to the recommendations of Japanese gastric cancer treatment guidelines, “endoscopic resection is considered for tumors that have a very low possibility of lymph node metastasis and are suitable for en-bloc resection” as a general principle regarding the indications for endoscopic resection[23]. The absolute indication as a standard treatment is well-differentiated adenocarcinoma with no ulceration, T1a depth, and diameter < 2 cm. The expanded indication for undifferentiated-type adenocarcinoma is T1a depth, no ulceration, and diameter < 2 cm. Based on our review of the previous reports of GCLS and EBV-associated carcinomas, endoscopic resection may thus be acceptable for mucosal GCLS even if it is of the undifferentiated-type, regardless of its horizontal diameter. For cases of submucosa-invasive EBV-associated carcinoma, ESD can also be a diagnostic procedure unless a diagnosis of GCLS is confirmed by forceps biopsy.
Further prospective studies into whether or not endoscopic resection can cure EBV-associated carcinomas are required to expand the therapeutic indications for gastric cancer. Positivity for EBV will be a useful predictive marker of lymph node metastasis, which can help us determine the optimal treatment strategy.
Conflict of interest statement: The authors declare no conflict of interest in association with the present study.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yu SP S-Editor: Yan JP L-Editor: A E-Editor: Wu YXJ
| 1. | Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, Yanai H, Sakai K, Suehiro Y, Yamasaki T, Sakaida I. Clinical Importance of Epstein⁻Barr Virus-Associated Gastric Cancer. Cancers (Basel). 2018;10:pii: E167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Watanabe H, Enjoji M, Imai T. Gastric carcinoma with lymphoid stroma. Its morphologic characteristics and prognostic correlations. Cancer. 1976;38:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377-380. [PubMed] |
| 4. | Shibata D, Tokunaga M, Uemura Y, Sato E, Tanaka S, Weiss LM. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Huh CW, Jung DH, Kim H, Kim H, Youn YH, Park H, Kim JW, Choi SH, Noh SH, Kim JH. Clinicopathologic features of gastric carcinoma with lymphoid stroma in early gastric cancer. J Surg Oncol. 2016;114:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Wang HH, Wu MS, Shun CT, Wang HP, Lin CC, Lin JT. Lymphoepithelioma-like carcinoma of the stomach: A subset of gastric carcinoma with distinct clinicopathological features and high prevalence of Epstein-Barr virus infection. Hepatogastroenterology. 1999;46:1214-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4849] [Article Influence: 440.8] [Reference Citation Analysis (2)] |
| 8. | Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: Monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A. 1994;91:9131-9135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 337] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Park JH, Kim EK, Kim YH, Kim JH, Bae YS, Lee YC, Cheong JH, Noh SH, Kim H. Epstein-Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer. Gastric Cancer. 2016;19:1041-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Lim H, Park YS, Lee JH, Son DH, Ahn JY, Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY, Kim JH, Yook JH, Kim BS. Features of Gastric Carcinoma With Lymphoid Stroma Associated With Epstein-Barr Virus. Clin Gastroenterol Hepatol. 2015;13:1738-1744.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Hissong E, Ramrattan G, Zhang P, Zhou XK, Young G, Klimstra DS, Shia J, Fernandes H, Yantiss RK. Gastric Carcinomas With Lymphoid Stroma: An Evaluation of the Histopathologic and Molecular Features. Am J Surg Pathol. 2018;42:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Shin DH, Kim GH, Lee BE, Lee JW, Ha DW, Jeon HK, Baek DH, Song GA, Ahn SJ, Park DY. Clinicopathologic features of early gastric carcinoma with lymphoid stroma and feasibility of endoscopic submucosal dissection. Surg Endosc. 2017;31:4156-4164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Lim H, Lee IS, Lee JH, Park YS, Kang HJ, Na HK, Ahn JY, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY, Kim JH, Kim BS, Yook JH, Kim BS. Clinical application of early gastric carcinoma with lymphoid stroma based on lymph node metastasis status. Gastric Cancer. 2017;20:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Tokunaga M, Land CE. Epstein-Barr virus involvement in gastric cancer: Biomarker for lymph node metastasis. Cancer Epidemiol Biomarkers Prev. 1998;7:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 15. | Murai K, Kakushima N, Sugino T, Yoshida M, Kawata N, Tanaka M, Takizawa K, Muramatu K, Kusafuka K, Bando E, Ono H. Epstein-Barr virus positivity among surgically resected intramucosal gastric cancer. Dig Endosc. 2018;30:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Gromski MA, Miller CA, Lee SH, Lee TH, Chung IK, Park SH, Kim SJ, Cho HD. Gastric lymphoepithelioma-like carcinoma mimicking a subepithelial lesion treated by endoscopic submucosal dissection. Gastrointest Endosc. 2012;76:419-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Lee HL, Kim DC, Lee SP, Lee KN, Jun DW, Lee OY, Han DS, Yoon BC, Choi HS, Hahm JS, Jang KS. Treatment of Epstein-Barr virus-associated gastric carcinoma with endoscopic submucosal dissection. Gastrointest Endosc. 2012;76:913-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Matsumoto T, Shimeno N, Imai Y, Inokuma T. Gastric carcinoma with lymphoid stroma resembling a hypoechoic submucosal tumor. Gastrointest Endosc. 2013;78:164-5; discussion 165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Lee JY, Kim KM, Min BH, Lee JH, Rhee PL, Kim JJ. Epstein-Barr virus-associated lymphoepithelioma-like early gastric carcinomas and endoscopic submucosal dissection: case series. World J Gastroenterol. 2014;20:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Chen M, Yin L, Yao Y, Wang L, Xu G, Zhang X, Lv Y, Sun QI, Fan X, Zou X. Lymphoepithelioma-like gastric carcinoma in a patient with rectal laterally spreading tumor: A case report. Oncol Lett. 2016;11:2491-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Kato M, Hayashi Y, Fukumoto K, Nagai K, Tsujii Y, Shinzaki S, Iijima H, Takehara T. Early gastric cancer with lymphoid stroma presenting as a subepithelial lesion diagnosed by endoscopic submucosal dissection. Clin J Gastroenterol. 2018;11:382-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 23. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1914] [Article Influence: 239.3] [Reference Citation Analysis (1)] |