Published online Oct 15, 2019. doi: 10.4251/wjgo.v11.i10.887
Peer-review started: March 26, 2019
First decision: July 31, 2019
Revised: September 3, 2019
Accepted: September 10, 2019
Article in press: September 10, 2019
Published online: October 15, 2019
Processing time: 209 Days and 20.8 Hours
Early diagnosis of hepatocellular carcinoma (HCC) is necessary to improve the prognosis of patients. However, the currently available tumor biomarkers are insufficient for the early detection of HCC. Acylcarnitine is essential in fatty acid metabolic pathways. A recent study reported that a high level of acylcarnitine may serve as a useful biomarker for the early diagnosis of HCC in steatohepatitis (SH) patients. In contrast, another study reported that the level of acetylcarnitine (AC2) - one of the acylcarnitine species - in non-SH patients with HCC was decreased vs that reported in those without HCC.
To investigate the usefulness of acylcarnitine as a biomarker for the early diagnosis of HCC in non-SH patients.
Thirty-three non-SH patients (14 with HCC and 19 without HCC) were enrolled in this study. Blood samples were obtained from patients at the time of admission. The levels of acylcarnitine and AC2 in the serum were determined through tandem mass spectrometry. The levels of vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR-2) were determined by enzyme-linked immunosorbent assay. Univariate and multivariate analyses were used to determine early diagnostic factors of HCC.
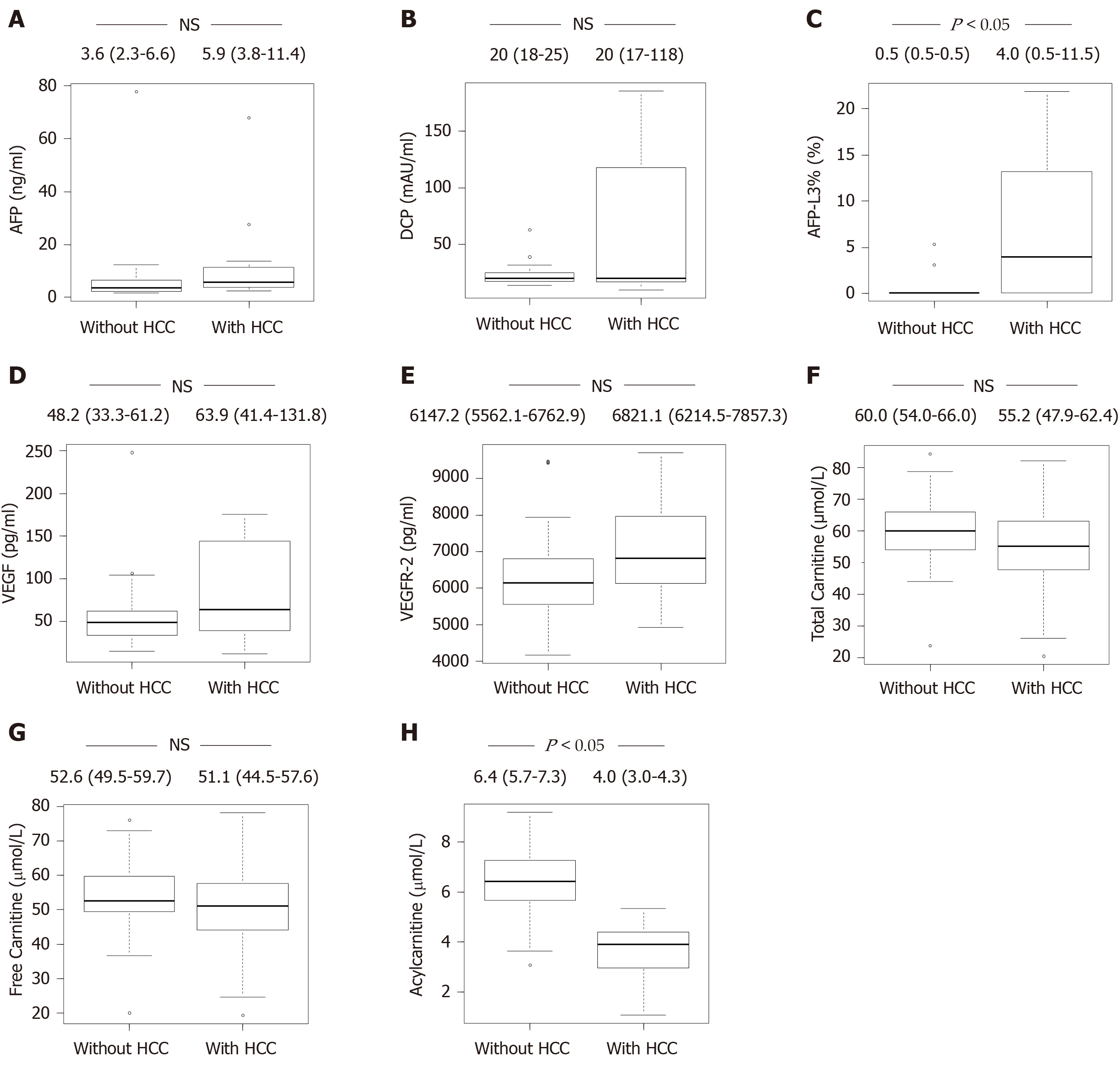
The level of acylcarnitine was significantly lower in non-SH patients with HCC vs those without HCC (P < 0.05). In contrast, the level of lens culinaris agglutinin-reactive fraction of α-fetoprotein (AFP) - AFP-L3% - was significantly higher in non-SH patients with HCC vs those without HCC (P < 0.05). However, the levels of total carnitine, free carnitine, AFP, des-γ-carboxy prothrombin, VEGF, and VEGFR-2 were not different between patients with and without HCC. The multivariate analysis showed that a low level of acylcarnitine was the only independent factor for the early diagnosis of HCC. The patients with a low level of AC2 had a significantly higher level of VEGF vs those with a high level of AC2 (P < 0.05).
The metabolic pathways of fatty acids may differ between SH HCC and non-SH HCC. Further studies are warranted to investigate these differences.
Core tip: There is an urgent clinical need for the early diagnosis of hepatocellular carcinoma (HCC) in cirrhotic patients to improve prognosis. A recent study reported that a high level of acylcarnitine may be a useful biomarker for the early diagnosis of HCC in steatohepatitis (SH) patients. However, the level of acylcarnitine was significantly lower in non-SH patients with HCC than in those without HCC. Multivariate analysis showed that a low level of acylcarnitine was the only independent early diagnostic biomarker for non-SH HCC. Thus, the fatty acid metabolic pathways in SH HCC and non-SH HCC patients may be different.
- Citation: Takaya H, Namisaki T, Kitade M, Shimozato N, Kaji K, Tsuji Y, Nakanishi K, Noguchi R, Fujinaga Y, Sawada Y, Saikawa S, Sato S, Kawaratani H, Moriya K, Akahane T, Yoshiji H. Acylcarnitine: Useful biomarker for early diagnosis of hepatocellular carcinoma in non-steatohepatitis patients. World J Gastrointest Oncol 2019; 11(10): 887-897
- URL: https://www.wjgnet.com/1948-5204/full/v11/i10/887.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i10.887
Hepatocellular carcinoma (HCC) is among the types of cancer with the highest mortality rates worldwide[1,2]. Early diagnosis of HCC is necessary to improve the prognosis of patients. Recently, imaging diagnosis has been used for the detection of HCC at an early stage. However, this approach is limited by the high cost and side effects associated with the use of the contrast medium. Although numerous biomarkers, including α-fetoprotein (AFP), des-γ-carboxy prothrombin (DCP), and lens culinaris agglutinin-reactive fraction of AFP (AFP-L3%) have been developed, they are not useful in the early diagnosis of HCC[3].
Carnitine is a water-soluble compound and an essential nutrient required in fatty acid metabolic pathways such as β-oxidation[4,5]. In humans, approximately 98% of the carnitine is present in the liver, skeletal muscle, heart, and kidneys[4,5]. In the plasma, carnitine is present as free carnitine or acylcarnitine[4,5]. Acyl-coenzyme A (CoA) synthetase catalyzes the conversion of fatty acids and CoA into acyl-CoA. Acyl-CoA is converted to acylcarnitine by carnitine palmitoyltransferase 1 (CPT1) in the outer mitochondrial membrane, while acylcarnitine is converted back to acyl-CoA by CPT2 in the inner mitochondrial membrane[4,5]. This process is followed by β-oxidation. Therefore, CPT1 and CPT2 are involved in the metabolic pathways of fatty acids in the carnitine cycle. Following the downregulation of CPT1, the level of acylcarnitine is decreased. The inverse is observed following the downregulation of CPT2.
A recent study reported that the level of acylcarnitine in steatohepatitis (SH) patients with HCC was increased compared with that reported in SH patients without HCC[6]. In addition, CPT2 was downregulated in SH patients with HCC[6,7]. Hence, a high level of acylcarnitine may serve as a useful biomarker for the early diagnosis of HCC in SH patients. Consequently, a high level of acylcarnitine has been linked to the development of HCC in SH patients. In contrast, another study reported that the level of acetylcarnitine (AC2) - one of the acylcarnitine species - was decreased in non-SH patients with HCC vs those without HCC, the level of AC2 was associated with tumor stage, and the expression of AC2 in HCC tissue was decreased according to tumor stage[8]. In addition, CPT1 was shown to be downregulated in non-SH patients with HCC[8,9]. Therefore, a low level of AC2 has been associated with the development of HCC. Furthermore, a recent study reported that AC2 significantly downregulated the expression of vascular endothelial growth factor (VEGF), VEGF receptor 2 (VEGFR-2), C-X-C motif chemokine 12 (CXCL12) and C-X-C chemokine receptor 4 (CXCR4) in human umbilical vein endothelial cells (HUVECs)[10]. Thus, it was suggested that AC2 possesses anti-angiogenic properties through the VEGF and CXCL12 pathways.
Based on this evidence, it was hypothesized that the fatty acid metabolic pathways in the carnitine cycle may differ between SH HCC and non-SH HCC. Therefore, in the present study, we investigated the relationship between acylcarnitine and non-SH HCC, and assessed the usefulness of acylcarnitine as an early diagnostic biomarker for HCC in non-SH patients.
The levels of acylcarnitine in the serum were evaluated in 40 cirrhotic patients (20 with HCC and 20 without HCC) admitted to Nara medical university from April to November 2016. We excluded patients with alcoholic SH and non-alcoholic SH. Eventually, a total of 33 non-SH patients (14 with HCC and 19 without HCC) were enrolled in this study. The diagnosis of liver cirrhosis was based on physical findings, laboratory tests, and histological criteria, according to the evidence-based clinical practice guidelines for liver cirrhosis established in 2015[11] by The Japan Society of Gastroenterology. All patients underwent blood examination, including for AFP, DCP, and/or AFP-L3%, every 3-4 mo. Moreover, they underwent ultrasound examination, dynamic computed tomography, and/or dynamic magnetic resonance imaging every 4-6 mo. The surveillance, diagnosis, and treatment of HCC was performed in accordance with the clinical practice guidelines for HCC established in 2013[12] by The Japan Society of Hepatology. After diagnosis of HCC, all HCC patients received radiofrequency ablation. None of the patients had infection, ascites, hepatic encephalopathy, uncontrolled gastroesophageal varices, or kidney disease. All patients provided written informed consent prior to their participation in this study.
The levels of free carnitine and acylcarnitine in the serum were determined through tandem mass spectrometry[13] conducted at Sekisui Medical Co., Ltd. (Tokyo, Japan).
The levels of VEGF were determined using commercially available immunoassay kits from RayBiotech, Inc. (Norcross, Georgia, United States), while the levels of VEGFR-2 were determined using immunoassay kits from R and D Systems, Inc. (Minneapolis, Minnesota, United States). The detection limit for the level of VEGF was < 10 pg/mL, while that for the level of VEGFR-2 was < 11.4 pg/mL.
Differences between the groups were analyzed using the Mann-Whitney U test. Correlations were calculated using the Spearman rank test. Categorical data were analyzed using the Fisher’s exact test. Univariate and multivariate analyses were performed to identify early diagnostic factors of HCC. A logistic regression analysis with stepwise selection of variables was applied to determine independent early diagnostic factors of HCC. The data are expressed as median (interquartile range). A two-tailed P < 0.05 denoted statistical significance. Analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0). Specifically, EZR is a modified version of R commander (version 1.6-3), including statistical functions that are frequently used in biostatistics[14].
The characteristics of the patients are shown in Table 1. The median age of the patients was 73 years (range: 66-77 years). The study population included 20 males and 13 females. Twenty patients had hepatitis B, nine had hepatitis C, and four had autoimmune hepatitis. The median maximum tumor size and median total tumor volume were 1.2 cm (range: 1.1-1.3 cm) and 6.3 cm3 (range: 5.0-14.2 cm3), respectively. The levels of AFP, DCP, AFP-L3%, VEGF, and VEGFR-2 in the serum were 4.7 ng/mL (range: 2.5-10.0 ng/mL), 20.0 mAU/mL (range: 17.0-29.3 mAU/mL), 0.5% (range: 0.5-3.7%), 50.1 pg/mL (range: 33.4-91.5 pg/mL), and 6537.2 pg/mL (range: 5687.9-7622.9 pg/mL), respectively.
| Variable | Total (n = 33) | Patients without HCC (n = 19) | Patients with HCC (n = 14) | P value |
| Age (yr) | 73 (66–77) | 73 (68–77) | 72 (66–78) | NS |
| Sex (male ⁄ female) | 20 ⁄ 13 | 9 ⁄ 10 | 11 ⁄ 3 | NS |
| HBV/HCV/AIH | 20/9/4 | 11/5/3 | 9/4/1 | NS |
| Albumin (g/dL) | 4.2 (3.8-4.5) | 4.4 (4.3-4.6) | 4.0 (3.8-4.1) | < 0.05 |
| Total bilirubin (mg/dL) | 0.8 (0.7-1.2) | 0.8 (0.8-1.2) | 0.9 (0.6-1.2) | NS |
| Aspartate aminotransferase (IU/L) | 29 (25-38) | 29 (26-34) | 28 (24-4) | NS |
| Alanine aminotransferase (IU/L) | 24 (17-36) | 21 (17-34) | 27 (19-38) | NS |
| Alkaline phosphatase (IU/L) | 314 (233-442) | 314 (232-451) | 343 (2252-432) | NS |
| γ-glutamyl transpeptidase (IU/L) | 34 (24-45) | 34 (24-43) | 38 (24-63) | NS |
| Prothrombin time (%) | 83 (75-90) | 81 (77-86) | 88 (68-96) | NS |
| Child–Pugh score | 5.0 (5-6) | 5.0 (5-5) | 5.0 (5-6) | NS |
| Platelet count (× 104⁄μL) | 12.9 (9.2-15.1) | 11.9 (9.1-14.5) | 13.6 (9.9-16.2) | NS |
| Maximum tumor size (cm) | 1.2 (1.1-1.3) | |||
| Total tumor volume (cm3) | 6.3 (5.0-14.2) | |||
| UICC TNM stage (stage 1/stage 2/stage 3) | 1/9/4 |
The level of acylcarnitine (Figure 1H) was significantly lower in non-SH patients with HCC compared with that reported in non-SH patients without HCC (P < 0.05). In addition, the level of AFP-L3% (Figure 1C) was significantly higher in non-SH patients with HCC than that observed in non-SH patients without HCC (P < 0.05). However, the levels of AFP, DCP, VEGF, VEGFR-2, total carnitine, and free carnitine (Figures 1A, B, D, E, F, and G, respectively) were not different between the patients with HCC and those without. Acylcarnitine was directly correlated with albumin (r = 0.494, P < 0.05). However, acylcarnitine was not correlated with tumor makers, including AFP-L3%.
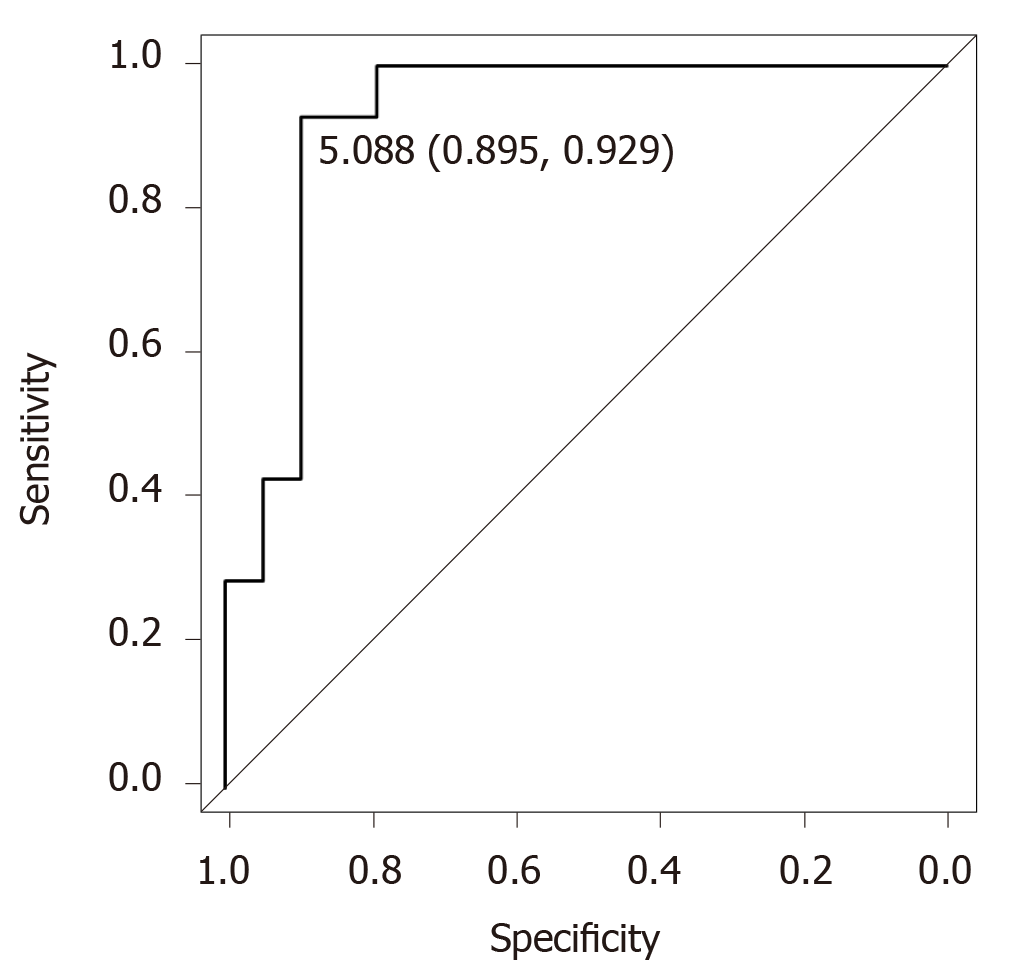
The univariate analysis showed that acylcarnitine and AFP-L3% was associated with the early diagnosis of HCC (Table 2). We performed a univariate analysis using acylcarnitine, AFP-L3%, DCP, VEGF and VEGFR-2 to identify early diagnostic factors of HCC. These factors demonstrated a P < 0.2 in the univariate analysis. Notably, the multivariate analysis identified acylcarnitine as a useful early diagnostic biomarker of HCC (Table 2). Receiver operating characteristic (ROC) analysis revealed that the cutoff value was 5.088, the specificity was 89.5%, the sensitivity was 92.9%, and the area under the curve (AUC) was 0.925 (Figure 2).
| Variable | OR (95%CI) | P value |
| Univariate analysis | ||
| AFP > 10 ng/mL | 1.67 (0.332-8.37) | 0.535 |
| DCP > 40 mAU/mL | 8.00 (0.776-82.5) | 0.0806 |
| AFP-L3% > 5% | 1.35 (0.999-1.84) | 0.0221 |
| VEGF > 60pg/mL | 2.67 (0.630-1.3) | 0.183 |
| VEGFR-2 > 6500 pg/mL | 2.83 (0.666-12.0) | 0.159 |
| Total carnitine (per 1 μmol/L increase) | 0.979 (0.933-1.03) | 0.380 |
| Free carnitine (per 1 μmol/L increase) | 0.991 (0.943-1.04) | 0.710 |
| Acylcarnitine (per 1 μmol/L increase) | 0.0865 (0.0158-0.475) | 0.0049 |
| Multivariate analysis | ||
| Acylcarnitine (per 1 μmol/L increase) | 0.0941 (0.00137-0.646) | 0.0162 |
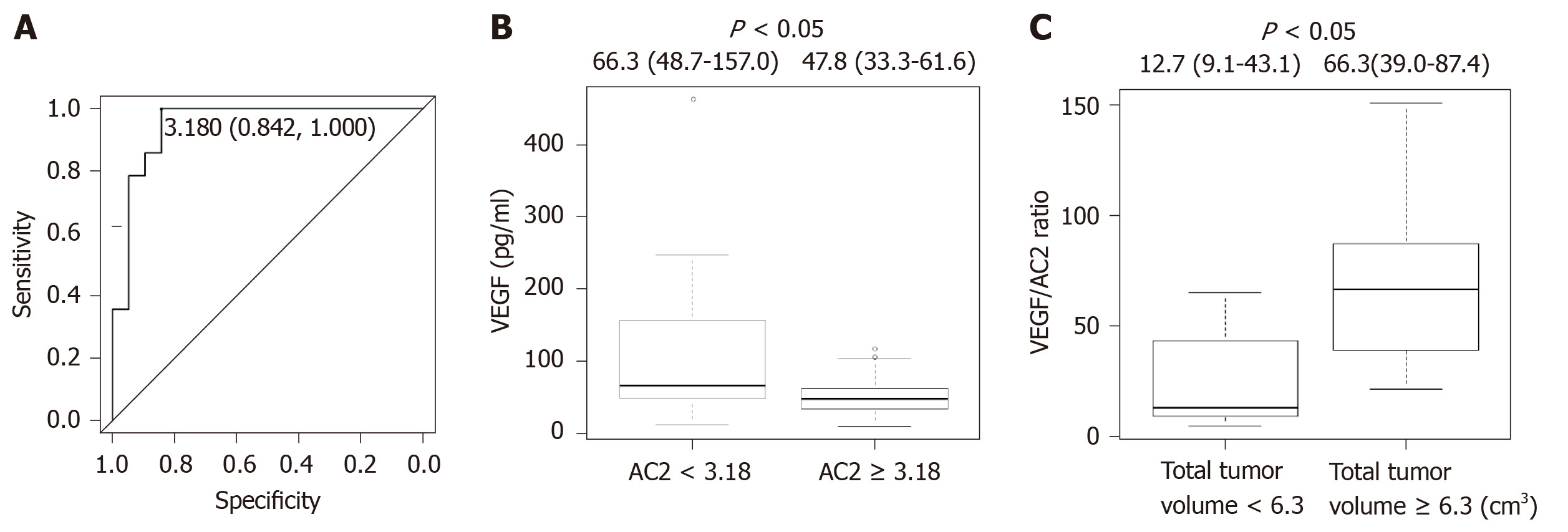
The levels of AC2, hexanoylcarnitine (AC6), octanoylcarnitine (AC8), decanoylcarnitine (AC10), dodecanoylcarnitine (AC12), myristoleylcarnitine (AC14:1), and octadecanoylcarnitine (AC18) were significantly lower in non-SH patients with HCC compared with those reported in non-SH patients without HCC (all P < 0.05) (Table 3). However, the levels of propionylcarnitine (AC3), butyrylcarnitine (AC4), isovalerylcarnitine (AC5), glutarylcarnitine (AC5DC), 3-hydroxy isovalerylcarnitine (AC5OH), myristoylcarnitine (AC14), palmitoylcarnitine (AC16), 3-hydroxy palmitoylcarnitine (AC16OH), oleoylcarnitine (AC18:1), and 3-hydroxy octadecenoylcarnitine (AC18:1OH) were not different between the two groups of patients (Table 3). In addition, the levels of short-chain fatty acids (SCFAs) (i.e., AC2, AC3, AC4, AC5, AC5DC, AC5OH, and AC6), medium-chain fatty acids (MCFAs) (i.e., AC8 and AC10), and long-chain fatty acids (LCFAs) (i.e., AC12, AC14, AC14:1, AC16, AC16OH, AC18, AC18:1, and AC18:1OH) were significantly lower in non-SH patients with HCC than those observed in non-SH patients without HCC (all P < 0.05) (Table 3). The patients were categorized into two groups, according to the ROC cutoff value for AC2 (low, < 3.18; and high, ≥ 3.18; Figure 3A). The patients with AC2 < 3.18 had a significantly higher level of VEGF vs those with AC2 ≥ 3.18 (Figure 3B). The patients with HCC were categorized into two groups according to the median cutoff value for total tumor volume (low, < 6.3 and high, ≥ 6.3). The HCC patients with a total tumor volume of ≥ 6.3 had a significantly higher VEGF/AC2 ratio compared with those with a total tumor volume of < 6.3 (Figure 3C). These results indicated that AC2 may be associated with VEGF and HCC progression in non-SH patients.
| Variable | Patients without HCC (n = 19) | Patients with HCC (n = 14) | P value |
| Acetylcarnitine (AC2) | 3.96 (3.33–4.92) | 2.06 (1.335–2.255) | < 0.05 |
| Propionylcarnitine (AC3) | 0.339 (0.2875–0.4115) | 0.3375 (< 0.24–0.473) | NS |
| Butyrylcarnitine (AC4) | < 0.1 (< 0.1–0.1) | 0.0795 (< 0.1–0.196) | NS |
| Isovalerylcarnitine (AC5) | < 0.06 (< 0.06–0.1085) | < 0.06 (< 0.06–0.0787) | NS |
| Glutarylcarnitine (AC5DC) | < 0.05 (< 0.05–0.05) | < 0.05 (< 0.05–0.05) | NS |
| 3-hydroxy isovalerylcarnitine (AC5OH) | < 0.1 (< 0.1–0.1) | < 0.1 (< 0.1–0.1) | NS |
| Hexanoylcarnitine (AC6) | 0.0531 (< 0.05–0.0596) | < 0.05 (< 0.05–0.05) | < 0.05 |
| Octanoylcarnitine (AC8) | 0.176 (0.1425–0.2375) | 0.0871 (0.0622–0.11025) | < 0.05 |
| Decanoylcarnitine (AC10) | 0.335 (0.256–0.432) | 0.1315 (0.099675–0.171) | < 0.05 |
| Dodecanolycarnitine (AC12) | 0.105 (0.0849–0.1335) | 0.02725 (0–0.06195) | < 0.05 |
| Myristoylcarnitine (AC14) | < 0.06 (< 0.06–0.06) | < 0.06 (< 0.06–0.06) | NS |
| Myristoleylcarnitine (AC14:1) | 0.185 (0.134–0.2325) | 0.07825 (0.06305–0.09855) | < 0.05 |
| Palmitoylcarnitine (AC16) | 0.133 (0.1255–0.1485) | 0.116 (0.10425–0.1310) | NS |
| 3-hydroxy palmitoylcarnitine (AC16OH) | < 0.03 (< 0.03–0.03) | < 0.03 (< 0.0 –0.03) | NS |
| Octadecanoylcarnitine (AC18) | 0.0429 (0.03245–0.04775) | 0.03315 (0.006625–0.039525 | < 0.05 |
| Oleoylcarnitine (AC18:1) | 0.871 (0.6825–1.0350) | 0.721 (0.61275–0.83775) | NS |
| 3-hydroxy octadecenoylcarnitine (AC18:1OH) | < 0.025 (< 0.025–0.02615) | < 0.025 (< 0.025–0.025) | NS |
| Short-chain fatty acids (AC2–AC6) | 4.3362 (3.83995–5.561750) | 2.4316 (1.86085–2.994125) | < 0.05 |
| Medium-chain fatty acids (AC8 + AC10) | 0.50600 (0.40700–0.69100) | 0.21385 (0.16545–0.28075) | < 0.05 |
| Long-chain fatty acid (AC12–AC18:1OH) | 1.3668 (1.106550–1.5871) | 0.9425 (0.836625–1.1087) | < 0.05 |
Our present study reported that acylcarnitine may serve as a useful early diagnostic biomarker for non-SH HCC. A recent study reported that the level of AC2 was decreased in non-SH patients with HCC vs those without HCC. In addition, AC2 was associated with the tumor-node-metastasis stage of HCC in non-SH patients[8]. Acylcarnitine may be associated with the development and progression of HCC. Furthermore, angiogenesis plays an important role in the development and progression of HCC that were related to VEGF and VEGFR-2 because the VEGF and VEGFR-2 levels of patients were increased with the development and progression of HCC[15-18]. A previous study reported that AC2 suppresses the synthesis of VEGF and VEGFR-2 in HUVECs[10]. In addition, adhesion to the extracellular matrix, migration, and invasion are key steps in the neovascularization of cancer. AC2 suppresses these processes in HUVECs through inhibition of CXCL12 and CXCR4. Notably, CXCL12 and CXCR4 induce angiogenesis[10,19] and the tumor escapes from immune surveillance[19]. Therefore, based on this evidence, AC2 may suppress the development and progression of HCC through CXCL12 and CXCR4, as well as VEGF and VEGFR-2. Further studies are warranted to investigate the relationship between AC2 and angiogenic factors such as CXCL12 and CXCR4.
HCC is a type of cancer induced by inflammation. Inflammation leads to oxidative stress, causing genomic damage and promoting hepatocarcinogenesis[20,21]. Hence, oxidative stress plays an important role in the development and progression of HCC. A recent study reported that the expression of CPT1 in the carnitine cycle was downregulated by oxidative stress (i.e., CPT1 inactivated by H2O2in vitro)[22]. Therefore, the level of acylcarnitine may decrease in non-SH patients with HCC through oxidative stress as a consequence of CPT1 downregulation in the carnitine cycle.
Our present and previous findings reported that the level of acylcarnitine was decreased in non-SH patients with HCC vs that detected in non-SH patients without HCC[8]. However, in SH patients with HCC, the level of acylcarnitine was increased compare with that reported in SH patients without HCC[6]. A recent study reported that the expression of CTP2 was downregulated in SH patients with HCC through suppression of peroxisome proliferator-activated receptor-α (PPAR-α), that is related to the development and progression of SH and HCC[6,23]. Furthermore, the downregulation of CPT2 induces activation of c-Jun N-terminal kinase, while AC18:1 - one of the LCFAs- promotes the activation of signal transducer and activator of transcription 3 (STAT3)[6]. The activation of c-Jun N-terminal kinase, activation of STAT3, and suppression of PPAR-α induce the development and progression of HCC. In contrast, a previous study reported that the levels of AC18:1 and AC16 - one of the LCFAs - were decreased in non-SH patients with HCC vs those measured in non-SH patients without HCC[24]. Moreover, the study showed that LCFAs suppress the growth of various types of cancer (e.g., breast, prostate, etc.) in vivo[25,26]. In addition, our present study demonstrated that the levels of LCFAs in non-SH patients with HCC were decreased. Our present findings further show that the levels of MCFAs and SCFAs were decreased in non-SH patients with HCC compared with those observed in non-SH patients without HCC. Of note, a previous study reported that MCFAs and SCFAs suppress the growth of various tumors (e.g., colorectal, skin, breast, etc.) in vitro through downregulation of the c-Myc, Hippo-Yap pathway and/or Mitogen-Activated Protein Kinase signaling[27-29] that induce the development and progression of HCC. In other words, the metabolic pathways of fatty acids in the carnitine cycle may differ between SH HCC and non-SH HCC. Further investigation is required to determine these differences.
Several biomarkers[30], including AFP, DCP, and AFP-L3%, have been used for the early diagnosis of HCC. However, these examinations are associated with high cost and limited practicality in a clinical setting. In our present study, it was reported that acylcarnitine is a more useful early diagnostic biomarker of non-SH HCC compared with AFP, DCP, and AFP-L3%.
Notably, the present study was characterized by limitations. These were the small sample size and absence of pathophysiological data. Cirrhotic patients with HCC occasionally develop renal dysfunction. Moreover, a previous study reported that the level of acylcarnitine is decreased in patients with renal dysfunction compared with that measured in patients without renal dysfunction[31]. Therefore, treating physicians should note that renal dysfunction may affect the value of acylcarnitine, when the latter is used as a biomarker for the early diagnosis of HCC. In addition, tumor markers are typically used for the diagnosis and anti-tumor effect of treatment. Thus far, it has not been clarified whether acylcarnitine is a useful biomarker for the effectiveness of treatment in non-SH HCC, and future studies should address this.
In conclusion, a low level of acylcarnitine is an independent early diagnostic biomarker for non-SH HCC. Moreover, the level of AC2 is associated with that of VEGF. Based on these findings, we anticipate that the development of new diagnostic approaches for HCC may involve acylcarnitine.
This work was completed with the help of Ms. Yoshie Nakai.
Although numerous biomarkers, including α-fetoprotein (AFP), des-γ-carboxy prothrombin, and AFP-L3%, have been developed for early diagnosis of hepatocellular carcinoma (HCC), they are not useful in the early diagnosis of HCC.
The fatty acid metabolic pathways in the carnitine cycle may differ between steatohepatitis (SH) HCC and non-SH HCC.
This study aimed to investigate the usefulness of acylcarnitine as a biomarker for the early diagnosis of HCC in non-SH patients.
Thirty-three non-SH patients (14 with HCC and 19 without HCC) were enrolled in this study. Blood samples were obtained from patients at the time of admission. The levels of acylcarnitine and acetylcarnitine in the serum were determined using tandem mass spectrometry. Univariate and multivariate analyses were used to determine early diagnostic factors of HCC.
The level of acylcarnitine was significantly lower in non-SH patients with HCC compared with those without HCC (P < 0.05). The multivariate analysis showed that a low level of acylcarnitine was the only independent factor for the early diagnosis of HCC.
A low level of acylcarnitine is an independent early diagnostic biomarker for non-SH HCC. Moreover, the level of acetylcarnitine is associated with that of VEGF.
We anticipate that the development of new diagnostic approaches for HCC may involve acylcarnitine.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abadi ATB, Yuan YS S-Editor: Dou Y L-Editor: A E-Editor: Qi LL
| 1. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1872] [Article Influence: 208.0] [Reference Citation Analysis (4)] |
| 2. | Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver. 2016;10:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 3. | Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573-10583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 294] [Cited by in RCA: 382] [Article Influence: 38.2] [Reference Citation Analysis (7)] |
| 4. | Adeva-Andany MM, Calvo-Castro I, Fernández-Fernández C, Donapetry-García C, Pedre-Piñeiro AM. Significance of l-carnitine for human health. IUBMB Life. 2017;69:578-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta. 2016;1863:2422-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 578] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 6. | Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, Tanaka Y, Tateishi R, Hikiba Y, Misumi K, Tanaka M, Hayashi A, Shibahara J, Fukayama M, Arita J, Hasegawa K, Hirschfield H, Hoshida Y, Hirata Y, Otsuka M, Tateishi K, Koike K. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67:1493-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Lin M, Lv D, Zheng Y, Wu M, Xu C, Zhang Q, Wu L. Downregulation of CPT2 promotes tumorigenesis and chemoresistance to cisplatin in hepatocellular carcinoma. Onco Targets Ther. 2018;11:3101-3110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Lu Y, Li N, Gao L, Xu YJ, Huang C, Yu K, Ling Q, Cheng Q, Chen S, Zhu M, Fang J, Chen M, Ong CN. Acetylcarnitine Is a Candidate Diagnostic and Prognostic Biomarker of Hepatocellular Carcinoma. Cancer Res. 2016;76:2912-2920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Chen S, Wang C, Cui A, Yu K, Huang C, Zhu M, Chen M. Development of a Genetic and Clinical Data-Based (GC) Risk Score for Predicting Survival of Hepatocellular Carcinoma Patients After Tumor Resection. Cell Physiol Biochem. 2018;48:491-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Baci D, Bruno A, Bassani B, Tramacere M, Mortara L, Albini A, Noonan DM. Acetyl-l-carnitine is an anti-angiogenic agent targeting the VEGFR2 and CXCR4 pathways. Cancer Lett. 2018;429:100-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Fukui H, Saito H, Ueno Y, Uto H, Obara K, Sakaida I, Shibuya A, Seike M, Nagoshi S, Segawa M, Tsubouchi H, Moriwaki H, Kato A, Hashimoto E, Michitaka K, Murawaki T, Sugano K, Watanabe M, Shimosegawa T. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 12. | Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K; Liver Cancer Study Group of Japan. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3:458-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 489] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 13. | Boemer F, Detilleux J, Cello C, Amory H, Marcillaud-Pitel C, Richard E, van Galen G, van Loon G, Lefère L, Votion DM. Acylcarnitines profile best predicts survival in horses with atypical myopathy. PLoS One. 2017;12:e0182761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 13279] [Article Influence: 1106.6] [Reference Citation Analysis (0)] |
| 15. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Kitade M, Yamazaki M, Tsujinoue H, Masaki T, Fukui H. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, Girardi L, Cillo U, Burra P, Giacomin A, Farinati F. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. 2008;103:914-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 17. | Salum GM, Bader El Din NG, Ibrahim MK, Anany MA, Dawood RM, Khairy A, El Awady MK. Vascular Endothelial Growth Factor Expression in Hepatitis C Virus-Induced Liver Fibrosis: A Potential Biomarker. J Interferon Cytokine Res. 2017;37:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Takaya H, Kawaratani H, Tsuji Y, Nakanishi K, Saikawa S, Sato S, Sawada Y, Kaji K, Okura Y, Shimozato N, Kitade M, Akahane T, Moriya K, Namisaki T, Mitoro A, Matsumoto M, Fukui H, Yoshiji H. von Willebrand factor is a useful biomarker for liver fibrosis and prediction of hepatocellular carcinoma development in patients with hepatitis B and C. United European Gastroenterol J. 2018;6:1401-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Ghanem I, Riveiro ME, Paradis V, Faivre S, de Parga PM, Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am J Transl Res. 2014;6:340-352. [PubMed] |
| 20. | Fu Y, Chung FL. Oxidative stress and hepatocarcinogenesis. Hepatoma Res. 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN, Bartosch B, Isaguliants MG. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget. 2017;8:3895-3932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 22. | Setoyama D, Fujimura Y, Miura D. Metabolomics reveals that carnitine palmitoyltransferase-1 is a novel target for oxidative inactivation in human cells. Genes Cells. 2013;18:1107-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Barrero MJ, Camarero N, Marrero PF, Haro D. Control of human carnitine palmitoyltransferase II gene transcription by peroxisome proliferator-activated receptor through a partially conserved peroxisome proliferator-responsive element. Biochem J. 2003;369:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Xiao JF, Varghese RS, Zhou B, Nezami Ranjbar MR, Zhao Y, Tsai TH, Di Poto C, Wang J, Goerlitz D, Luo Y, Cheema AK, Sarhan N, Soliman H, Tadesse MG, Ziada DH, Ressom HW. LC-MS based serum metabolomics for identification of hepatocellular carcinoma biomarkers in Egyptian cohort. J Proteome Res. 2012;11:5914-5923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Khadge S, Thiele GM, Sharp JG, McGuire TR, Klassen LW, Black PN, DiRusso CC, Cook L, Talmadge JE. Long-chain omega-3 polyunsaturated fatty acids decrease mammary tumor growth, multiorgan metastasis and enhance survival. Clin Exp Metastasis. 2018;35:797-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Liang P, Henning SM, Guan J, Grogan T, Elashoff D, Olefsky JM, Cohen P, Aronson WJ. Role of Host GPR120 in Mediating Dietary Omega-3 Fatty Acid Inhibition of Prostate Cancer. J Natl Cancer Inst. 2019;111:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Narayanan A, Baskaran SA, Amalaradjou MA, Venkitanarayanan K. Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int J Mol Sci. 2015;16:5014-5027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Shinohara H, Taniguchi K, Kumazaki M, Yamada N, Ito Y, Otsuki Y, Uno B, Hayakawa F, Minami Y, Naoe T, Akao Y. Anti-cancer fatty-acid derivative induces autophagic cell death through modulation of PKM isoform expression profile mediated by bcr-abl in chronic myeloid leukemia. Cancer Lett. 2015;360:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Thirunavukkarasan M, Wang C, Rao A, Hind T, Teo YR, Siddiquee AA, Goghari MAI, Kumar AP, Herr DR. Short-chain fatty acid receptors inhibit invasive phenotypes in breast cancer cells. PLoS One. 2017;12:e0186334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Chauhan R, Lahiri N. Tissue- and Serum-Associated Biomarkers of Hepatocellular Carcinoma. Biomark Cancer. 2016;8:37-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Goek ON, Döring A, Gieger C, Heier M, Koenig W, Prehn C, Römisch-Margl W, Wang-Sattler R, Illig T, Suhre K, Sekula P, Zhai G, Adamski J, Köttgen A, Meisinger C. Serum metabolite concentrations and decreased GFR in the general population. Am J Kidney Dis. 2012;60:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |