Published online Jan 15, 2019. doi: 10.4251/wjgo.v11.i1.48
Peer-review started: September 28, 2018
First decision: October 15, 2018
Revised: November 26, 2018
Accepted: December 24, 2018
Article in press: December 24, 2018
Published online: January 15, 2019
Processing time: 109 Days and 22.4 Hours
Neoadjuvant chemotherapy has been applied worldwide to improve the survival of patients with gastric adenocarcinoma (GAC). The evaluation of histological regression in primary tumors is valuable for predicting prognosis. However, the prognostic effect of regression change in lymph nodes (LNs) remains unclear.
To confirm whether the evaluation of regression change in LNs could predict the prognosis of GAC patients who received neoadjuvant chemotherapy followed by surgery.
In this study, we evaluated the histological regression of resected LNs from 192 GAC patients (including those with esophagogastric junction adenocarcinoma) treated with neoadjuvant chemotherapy. We classified regression change and residual tumor in LNs into four groups: (A) true negative LNs with no evidence of a preoperative therapy effect, (B) no residual metastasis but the presence of regression change in LNs, (C) residual metastasis with regression change in LNs, and (D) metastasis with minimal or no regression change in LNs. Correlations between regression change and residual tumor groups in LNs and regression change in the primary tumor, as well as correlations between regression change in LNs and clinicopathological characteristics, were analyzed. The prognostic effect of regression change and residual tumor groups in LNs was also analyzed.
We found that regression change and residual tumor groups in LNs were significantly correlated with regression change in the primary tumor, tumor differentiation, ypT stage, ypN stage, ypTNM stage, lymph-vascular invasion, perineural invasion and R0 resection status. Regression change and residual tumor groups in LNs were statistically significant using univariate Cox proportional hazards analysis, but were not independent predictors. For patients who had no residual tumor in LNs, the 5-year overall survival (OS) rates were 67.5% in Group A and 67.4% in Group B. For the patients who had residual tumors in LNs, the 5-year OS rates were 28.2% in Group C and 39.5% in Group D. The patients in Groups A+B had a significantly better outcome than the patients in Groups C+D (P < 0.01). No significant differences in survival were found between Groups A and B, or between Groups C and D.
The existence of residual tumor in LNs, rather than regression change in LNs, is useful for predicting the prognosis after neoadjuvant chemotherapy in GAC patients. In practice, it may not be necessary to report regression change in LNs.
Core tip: Neoadjuvant chemotherapy has been applied worldwide to improve the survival of patients with gastric adenocarcinoma (GAC). In this study, we evaluated the prognostic effect of regression change in lymph nodes (LNs) from 192 GAC patients treated with neoadjuvant chemotherapy. The existence of residual tumor in LNs, rather than regression change in LNs, is useful for predicting prognosis after neoadjuvant chemotherapy in GAC patients. In practice, it may not be necessary to report regression change in LNs.
- Citation: Zhu YL, Sun YK, Xue XM, Yue JY, Yang L, Xue LY. Unnecessity of lymph node regression evaluation for predicting gastric adenocarcinoma outcome after neoadjuvant chemotherapy. World J Gastrointest Oncol 2019; 11(1): 48-58
- URL: https://www.wjgnet.com/1948-5204/full/v11/i1/48.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i1.48
Gastric cancer is the fifth most common malignancy and the third most common cause of cancer mortalities worldwide[1]. Despite the decline of its incidence globally, gastric cancer is still the second most common form of cancer in China[2]. Neoadjuvant chemotherapy may improve the long-term outcome of locally advanced gastric adenocarcinoma (GAC). Earlier investigations have indicated that the evaluation of primary tumor regression could help to predict the prognosis of GAC patients who received neoadjuvant chemotherapy[3,4]. Previously, we evaluated the histological regression of the primary tumors of 192 Chinese patients with GAC (including esophagogastric junction adenocarcinoma) who were treated with neoadjuvant chemotherapy followed by surgery, and we confirmed that the evaluation of histological regression in the primary tumor was valuable for predicting prognosis[5]. However, the prognostic effect of regression change in lymph nodes (LNs) remains unclear. In practice, it is controversial whether pathologists should report regression change in LNs.
Some studies have demonstrated the prognostic effect of regression change in LNs after neoadjuvant therapy for breast cancer, rectal cancer and esophageal cancer[6-8]. There have been few studies on the prognostic effect of regression change in LNs of GAC patients. Kinoshita et al[9] observed regression change in LNs and found no correlation between regression in metastatic LNs and the clinical response of GAC patients; however, they did not analyze the prognostic influence of regression change in LNs. Ott et al[10] briefly reported in a review their unpublished data on the prognostic influence of regression change in the LNs of GAC patients. They showed that regression change in LNs appeared to be less relevant to prognosis in nodal-negative patients than in nodal-positive patients, in whom lymphatic regression seemed to improve prognosis. Additionally, response in LNs was less important than response in the primary tumor. In this study, we aimed to confirm whether the evaluation of regression change in LNs could predict the prognosis of GAC patients who received neoadjuvant chemotherapy followed by surgery.
Between January 2007 and August 2013, all 192 patients who underwent neoadjuvant chemotherapy followed by gastrectomy and esophagogastrectomy surgery with locally advanced GAC (including esophagogastric junction adenocarcinoma) were enrolled in the current study[5]. There were 153 cases of T4 stage, 18 cases of T3 stage and 21 cases of uncertain T staging before neoadjuvant chemotherapy. In addition, there were 117 cases of N+ stage, 14 cases of N0 stage and 61 cases of uncertain N staging before neoadjuvant chemotherapy. The neoadjuvant chemotherapy strategies were not uniform. The drugs most used included oxaliplatin, cisplatin, docetaxel, 5-fluorouracil and Tegafur Gimeracil Oteracil Potassium Capsule. This study was conducted after obtaining approval from the Independent Ethics Committee at the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (No. NCC2013RE-049). Consent was not required from each patient because this was a retrospective study. All procedures were performed in line with the Declaration of Helsinki, 1983.
The survival information was mainly obtained via clinical records, telephone interviews or mails. The overall survival (OS) times were the months from the first day of the neoadjuvant chemotherapy to the day when death occurred or to the last follow-up (September 2015). The progression-free survival (PFS) times were the months from the first day of the neoadjuvant chemotherapy to the day when progression happened or death occurred, or to the last follow-up (September 2015). Eleven patients were lost to follow-up. Three patients who died of surgery complications, and eight patients whose follow-up times after surgery were less than 3 mo, were excluded from the survival analysis. The median follow-up time was 31 mo (4.1-95.3 mo).
The clinicopathological features, including tumor location, tumor size, histological differentiation, Laurén classification, lymph-vascular invasion (LVI) and perineural invasion (PNI), were collected. The primary tumors were staged according to the American Joint Committee on Cancer Staging, 8th Edition[11].
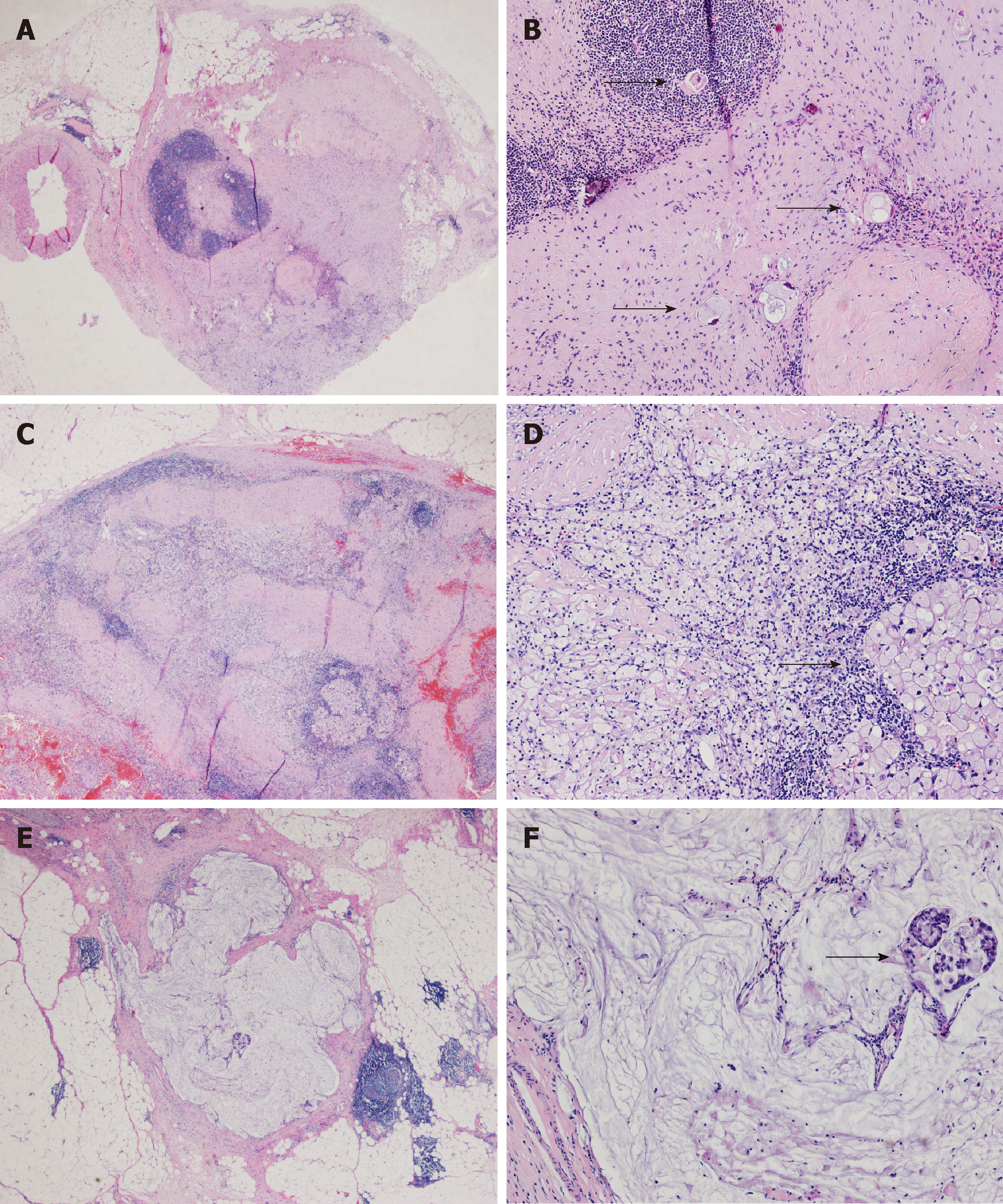
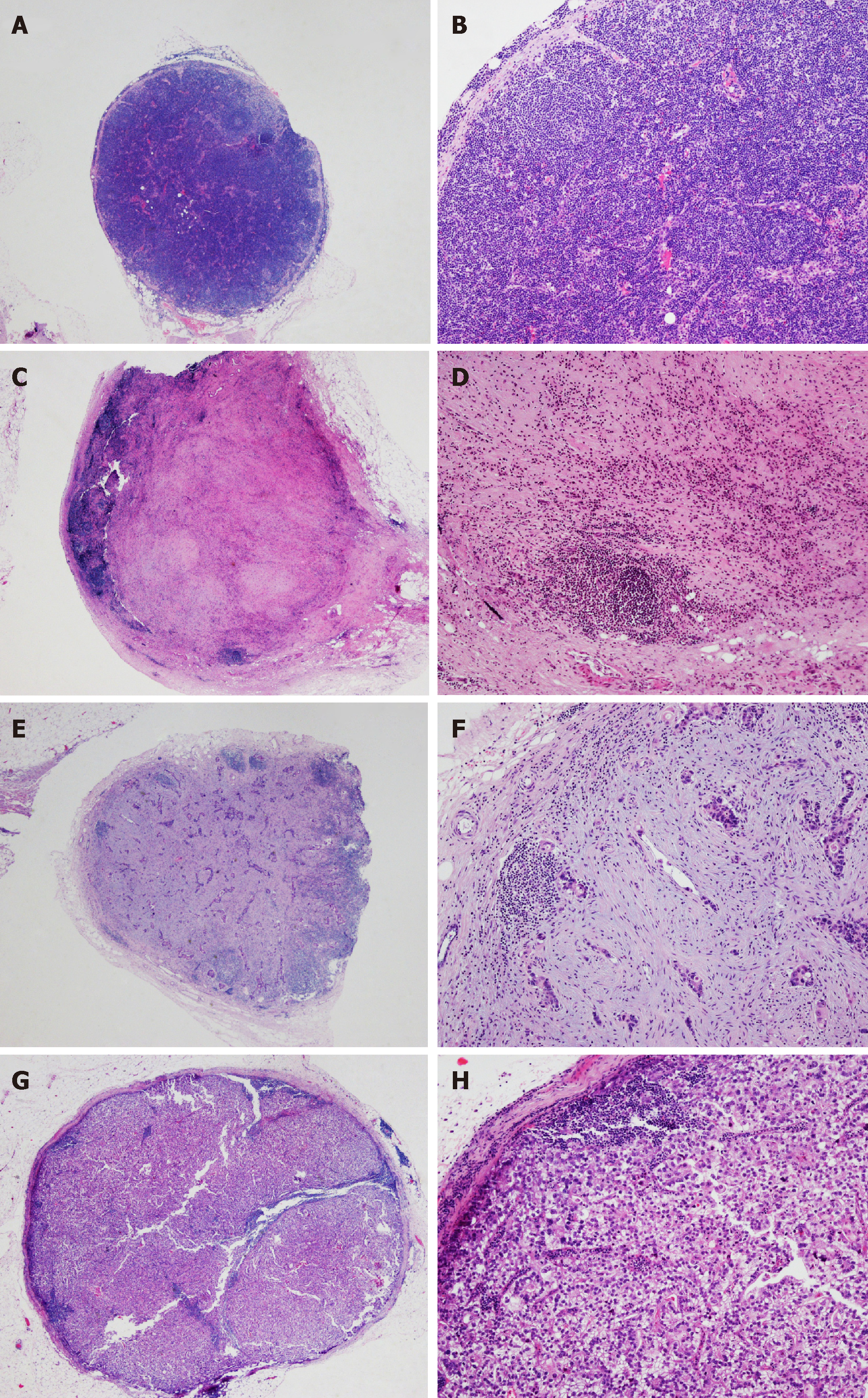
All of the examined LN specimens were embedded in paraffin, and four micrometer tissue sections were stained with hematoxylin and eosin. The median number of resected LNs was 24 (3-58) per case. The histopathological evidence of regression change in LNs was defined as the presence of fibrosis, aggregation of foamy histocytes or accumulation of mucin pools in LN parenchyma[6], which is shown in Figure 1. We classified tumor regression and residual tumor in LNs into four groups: A, true negative LNs with no evidence of a preoperative therapy effect; B, no residual metastasis but presence of regression change in LNs; C, residual metastasis with regression change in LNs; and D, metastasis with minimal or no regression change in LNs (Table 1 and Figure 2). All of the sections were reviewed by three experienced pathologists (Zhu YL, Yue JY and Xue LY). For a controversial diagnosis, three pathologists reviewed the sections on a multi-headed microscope until reaching an agreement.
| Group | Description |
| A | True negative LNs with no evidence of a preoperative therapy effect |
| B | No residual metastasis but presence of regression change in LNs |
| C | Residual metastasis with regression change in LNs |
| D | Metastasis with minimal or no regression change in LNs |
All calculations were performed using SPSS 16.0 software (SPSS Inc, Chicago, IL). Correlations between regression changes in LNs and clinicopathological characteristics were analyzed by χ2 tests. The survival curve and 5 year survival rates of four LN regression change groups were calculated and compared using the Kaplan-Meier method and log rank test. The effects of regression change in LNs on OS and PFS were evaluated by Cox regression analysis. Variables with a two-sided P value of < 0.05 were statistically significant.
The clinicopathological characteristics were tabulated in Table 2. There were 139 (72.4%) male patients and 53 (27.6%) female patients, and the median age was 55 years (ranging from 31 to 77 years). Among all patients, 63 (32.8%) received D1 lymphadenectomy, and 129 (67.2%) received D2 lymphadenectomy. There were 159 (82.8%) patients who reached the status of R0 resection.
| Characteristics | n (%) | Lymph node regression groups | P value | |||
| A, n (%) | B, n (%) | C, n (%) | D, n (%) | |||
| Total | 192 | 30 | 24 | 98 | 40 | |
| Gender | 0.162 | |||||
| Male | 139 (72.4) | 26 (86.7) | 18 (75) | 70 (71.4) | 25 (62.5) | |
| Female | 53 (27.6) | 4 (13.3) | 6 (25) | 28 (28.6) | 15 (37.5) | |
| Age, yr | 0.869 | |||||
| < 55 | 89 (46.4) | 12 (40) | 11 (45.8) | 46 (46.9) | 20 (50) | |
| ≥ 55 | 103 (53.6) | 18 (60) | 13 (54.2) | 52 (53.1) | 20 (50) | |
| Location | 0.876 | |||||
| Esophagogastric junction | 44 (22.9) | 6 (20) | 5 (20.8) | 23 (23.5) | 10 (25) | |
| Proximal gastric | 71 (37) | 13 (43.3) | 9 (37.5) | 32 (32.7) | 17 (42.5) | |
| Distal gastric | 77 (40.1) | 11 (36.7) | 10 (41.7) | 43 (43.9) | 13 (32.5) | |
| Maximal diameter of tumor bed | 0.259 | |||||
| < 4.5 cm | 108 (56.2) | 22 (73.3) | 15 (62.5) | 50 (51) | 21 (52.5) | |
| 4.5-8 cm | 64 (33.3) | 8 (26.7) | 7 (29.2) | 34 (34.7) | 15 (37.5) | |
| > 8 cm | 20 (10.4) | 0 (0) | 2 (8.3) | 14 (14.3) | 4 (10) | |
| Histological differentiation | 0.044 | |||||
| Well-moderately differentiated | 45 (23.4) | 12 (40) | 8 (33.3) | 18 (18.4) | 7 (17.5) | |
| Poorly differentiated | 147 (76.6) | 18 (60) | 16 (66.7) | 80 (81.6) | 33 (82.5) | |
| Laurén classification | 0.336 | |||||
| Intestinal | 77 (40.1) | 17 (56.7) | 11 (45.8) | 37 (37.8) | 12 (30) | |
| Diffuse | 73 (38) | 8 (26.7) | 10 (41.7) | 37 (37.8) | 18 (45) | |
| Mixed | 42 (21.9) | 5 (16.7) | 3 (12.5) | 24 (24.5) | 10 (25) | |
| LVI | < 0.001 | |||||
| Negative | 94 (49) | 21 (70) | 20 (83.3) | 37 (37.8) | 16 (40) | |
| Positive | 98 (51) | 9 (30) | 4 (16.7) | 61 (62.2) | 24 (60) | |
| PNI | < 0.001 | |||||
| Negative | 76 (39.6) | 17 (56.7) | 20 (83.3) | 32 (32.7) | 7 (17.5) | |
| Positive | 116 (60.4) | 13 (43.3) | 4 (16.7) | 66 (67.3) | 33 (82.5) | |
| AJCC ypT category | < 0.001 | |||||
| 0 | 11 (5.7) | 3 (10) | 6 (25) | 2 (2) | 0 (0) | |
| 1 | 20 (10.4) | 5 (16.7) | 6 (25) | 6 (6.1) | 3 (7.5) | |
| 2 | 23 (12) | 5 (16.7) | 5 (20.8) | 10 (10.2) | 3 (7.5) | |
| 3 | 57 (29.7) | 13 (43.3) | 4 (16.7) | 32 (32.7) | 8 (20) | |
| 4 | 81 (42.2) | 4 (13.3) | 3 (12.5) | 48 (49) | 26 (65) | |
| AJCC ypN category | < 0.001 | |||||
| 0 | 54 (28.1) | 30 (100) | 24 (100) | 0 (0) | 0 (0) | |
| 1 | 35 (18.2) | 0 (0) | 0 (0) | 25 (25.5) | 10 (25) | |
| 2 | 52 (27.1) | 0 (0) | 0 (0) | 40 (40.8) | 12 (30) | |
| 3 | 51 (26.6) | 0 (0) | 0 (0) | 33 (33.7) | 18 (45) | |
| AJCC ypTNM stage | < 0.001 | |||||
| 0 | 9 (4.7) | 3 (10) | 6 (25) | 0 (0) | 0 (0) | |
| 1 | 24 (12.5) | 9 (0) | 11 (58.3) | 4 (29.2) | 0 (0) | |
| 2 | 53 (27.6) | 18 (60) | 6 (25) | 20 (20.4) | 9 (22.5) | |
| 3 | 106 (55.2) | 0 (0) | 1 (4.2) | 74 (75.5) | 31 (77.5) | |
| R0 resection | 0.029 | |||||
| Yes | 159 (82.8) | 28 (93.3) | 23 (95.8) | 74 (75.5) | 34 (85) | |
| No | 33 (17.2) | 2 (6.7) | 1 (4.2) | 24 (24.5) | 6 (15) | |
| Mandard-TRG | < 0.001 | |||||
| 1 | 11 (5.7) | 3 (10) | 6 (25) | 2 (2) | 0 (0) | |
| 2 | 23 (12) | 7 (23.3) | 5 (20.8) | 11 (11.2) | 0 (0) | |
| 3 | 40 (20.8) | 8 (26.7) | 9 (37.5) | 21 (21.4) | 2 (5) | |
| 4 | 78 (40.6) | 6 (20) | 4 (16.7) | 47 (48) | 21 (52.5) | |
| 5 | 40 (20.8) | 6 (20) | 0 (0) | 17 (17.3) | 17 (42.5) | |
We reviewed 4864 LNs and found 1087 metastatic LNs with or without the presence of regression change. There were 595 LNs with evidence of regression, including 314 with residual metastasis and 281 with no residual metastasis. Among them, 476 (80%) had fibrosis, 158 (26.6%) had aggregation of foamy cells, and 151 (25.4%) had mucin pools (Table 3). There were 54 (28.1%) patients staged as ypN0, including 30 without regression change in LNs and 24 with regression change in LNs. There were 138 (71.9%) patients staged as pathological ypN+ (ypN1-3), including 98 with regression change in LNs and 40 without regression change in LNs (Table 2).
| Residual metastatic status in LNs | LNs with histopathological regression | |||
| Total | Fibrosis | Foamy cells | Mucin pools | |
| Yes, n = 1087 | 314 | 259 (82.5) | 69 (22) | 78 (24.8) |
| No, n = 3777 | 281 | 217 (77.2) | 89 (31.7) | 73 (26) |
| Total, n = 4864 | 595 | 476 (80) | 158 (26.6) | 151 (25.4) |
Regression change and residual tumor groups in LNs were found to be significantly associated with histological differentiation, ypT stage, ypN stage, ypTNM stage, LVI, PNI, and R0 resection (P < 0.05) (Table 2). It was also correlated with the primary tumor regression grades using the Mandard-TRG system, which was acquired from our previous study[5].
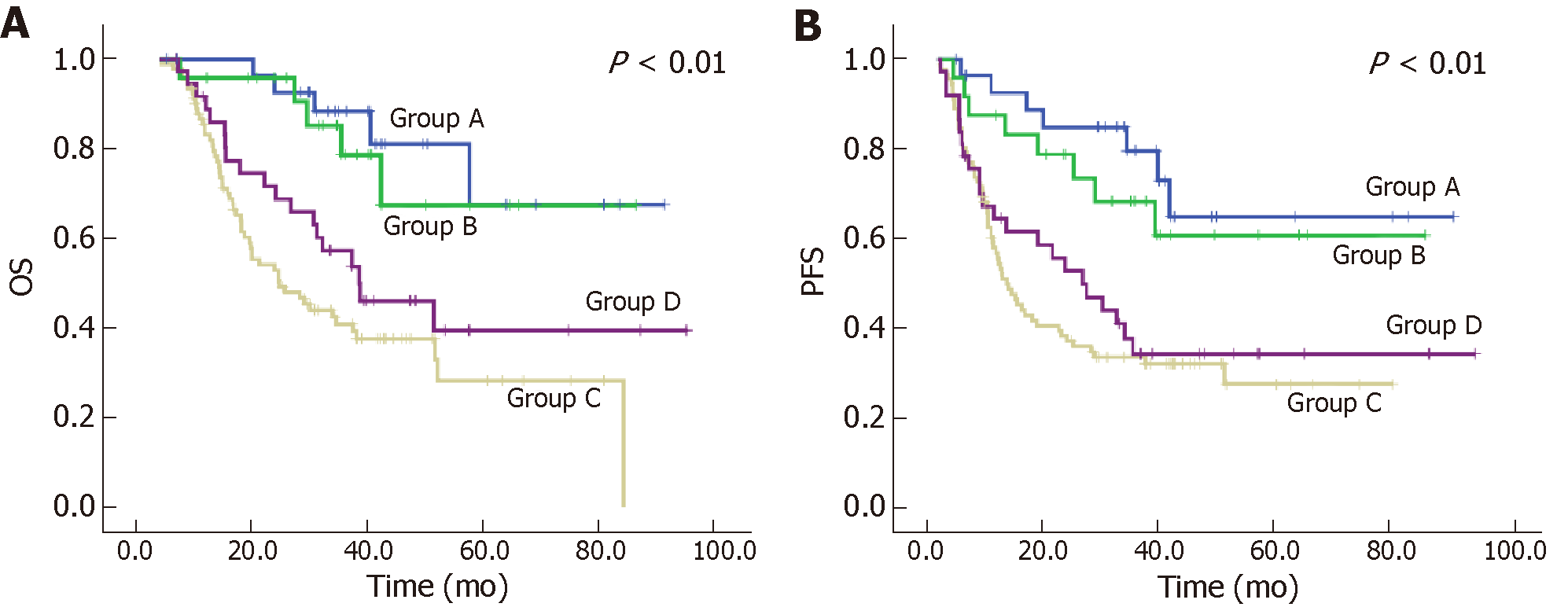
One hundred and eighty-one patients were included in the survival analyses. Regression change and residual tumor groups in LNs were statistically significant using univariate Cox proportional hazards analysis. Postsurgical T stage, R0 resection, and ypN stage were independent predictors for OS, while LVI, R0 resection, and ypN stage were independent predictors for PFS. Regression change and residual tumor groups in LNs was not an independent predictor. Kaplan-Meier analysis and univariate Cox proportional hazards analysis indicated that patients who had residual tumor in the LNs (Group C and Group D, ypN+) had significantly worse outcomes than those patients who had no residual tumor (Group A and Group B, ypN0) in the LNs, both in OS and PFS (Table 4 and 5, respectively). For ypN+ patients, there was no significant difference between the prognosis for Group C and that for Group D (P = 0.127 in OS and P = 0.427 in PFS). The survival curves for regression change and residual tumor groups in LNs are presented in Figure 3.
| Groups based on regression change in LNs | Kaplan-Meier analysis | Univariate Cox proportional hazards analysis | |||
| 5-year OS rate, % | P value1 | HR | 95% CI | P value | |
| A | 67.5 | < 0.01 | 0.276 | 0.103-0.739 | 0.01 |
| B | 67.4 | 0.356 | 0.133-0.955 | 0.04 | |
| C | 28.2 | 1.506 | 0.890-2.548 | 0.127 | |
| D | 39.5 | 1 | |||
| Groups based on regression change in LNs | Kaplan-Meier analysis | Univariate Cox proportional hazards analysis | |||
| 5-year PFS rate, % | P value1 | HR | 95% CI | P value | |
| A | 64.8 | < 0.01 | 0.293 | 0.126-0.684 | 0.005 |
| B | 60.7 | 0.423 | 0.189-0.947 | 0.036 | |
| C | 27.4 | 1.215 | 0.751-1.965 | 0.427 | |
| D | 34.1 | 1 | |||
The use of neoadjuvant chemotherapy for localized advanced GAC promotes the frequency of R0 resection[12-14]. The Japan Clinical Oncology Group recommend neoadjuvant chemotherapy for those patients with “bulky N” status to facilitate surgery[15]. Our previous study also showed that histopathological regression in the primary tumor was significant by univariate survival analysis. Furthermore, the ypN stage was considered as an independent outcome predictor, which is in line with other studies[5,16].
The therapeutic response in either the primary tumor or LN metastasis may be similar to the process of normal tissue injury, clearance and repair. It has been shown that some histological changes (e.g., foamy histiocytes, marked fibrosis and acellular mucin pools) appeared more frequently in resected primary tumor. Additionally, some histological changes (e.g., foamy histiocytes, nodular fibrosis and hyalinosis) appeared more frequently in LNs from GAC patients receiving neoadjuvant chemotherapy when compared to those who were treated with surgery alone. Among them, nodular fibrosis and hyalinosis in LNs were found exclusively in the neoadjuvant chemotherapy group. Foamy histiocytes are believed to be particularly active in the clearance of apoptotic cells. Their presence in patients treated with chemotherapy could be an indication of apoptotic tumor cells induced by the chemotherapeutic agents[17]. The acellular mucin pools were produced from tumor cells that were significantly reduced or, possibly, completely eliminated by preoperative chemotherapeutic agents[18]. Stromal fibrosis can be seen in the metastatic LNs of the breast, gastrointestinal tract and pancreatic carcinomas. Fibrosis may be a result of collagen formation[19].
To our knowledge, there are still few studies on the regression change grading in LNs in GAC. Martin-Romano et al[20] analyzed the prognosis of GAC patients treated with different therapeutic strategies by evaluating the regression change in LNs using the criteria from breast cancer. The grading of LN regression was as follows: A, true negative LN with no evidence of effect from preoperative therapy; B, infiltrated LN with no evidence of any effect from preoperative therapy; C, infiltrated LN with evidence of some degree of histological regression due to preoperative therapy; and D, complete pathological response in a previously infiltrated LN. Patients with complete pathological response in previously infiltrated LNs had a longer 5 year PFS and OS compared with those with infiltrated LNs. No survival differences were observed between patients with truly negative LNs and those with complete pathological response in previously infiltrated LNs in either 5 year PFS or 5 year OS. However, they did not compare the differences between patients with infiltrated LNs with no evidence of any effect and those with evidence of some degree of histological regression from preoperative therapy[20]. In the present study, we also classified regression change and residual tumor in LNs into four groups. Regardless of whether there was regression change in the LNs or not, patients who had residual tumors in the LNs (ypN+) had significantly worse outcomes than those who had no residual tumor in the LNs (ypN0) (P < 0.01). The 5 year OS of the patients who had no tumor (either truly or due to neoadjuvant therapy) in LNs was significantly higher than that of the patients who had residual tumors in LNs, in line with the study of Martin-Romano et al[20]. These data reinforce the idea of the favorable prognostic impact for ypN0 patients, either because patients have truly negative LNs or because preoperative therapy achieved a pathologically complete nodal response. Moreover, we also found that regression change and residual tumor groups in LNs was statistically significant using univariate Cox proportional hazards analysis, but it was not an independent predictor in multivariate Cox proportional hazards analysis. No significant differences in 5 year OS were found between patients with infiltrated LNs with no evidence of any effect and those with evidence of some degree of histological regression from preoperative therapy.
It was reported that the regression change in LNs was the worse prognostic factor in patients with breast, esophageal or rectal cancer[6-8]. However, regression change in LNs does not affect outcome in this study. The reasons of this discrepancy may be different grouping methods used, as well as different LN metastasis rates, different sensitivities to neoadjuvant therapy and different prognosis among different tumors. Newman et al[6] detected that after induction of chemotherapy for breast cancer, patients with negative axillary LNs but without regression change had the best outcome, those with positive axillary LNs but without regression change had the worst outcome, and those with pathological response of metastasis tumor in LNs had an intermediate outcome[6]. Fernández-Aceñero et al[7] found that ypN0 rectal carcinoma cases with regression change in LNs after neoadjuvant therapy showed a significantly worse prognosis when compared to the ypN0 cases without regression change in LNs. Bollschweiler et al[8] stratified esophageal carcinoma regression change in LNs after neoadjuvant therapy into three grades: low risk (no LN metastasis and fewer than 3 LNs with central fibrosis), medium risk (no LN metastasis and central fibrosis in 3 or more LNs, or LN metastasis with a LN ratio of less than 0.05) and high risk (all other cases). They then indicated that the regression change grading in LNs had an important role in survival analysis[8]. In the study of rectal carcinoma, there were 80% patients that had N0 disease[7], while there were 57.5% that had N0 disease in the study of esophageal carcinoma[8]. However, in our study, only 28% patients had no LN metastasis. In addition, 66.2% patients had N1 disease and 16.9% patients had N2 disease in the study of breast carcinoma[6]. While in our study, only 15.6% patients had N1 disease and 53.7% patients had N2 or N3 disease, indicating a worse N stage of GAC patients and a subsequent poor prognosis. Moreover, in our previous study, no significant regression of primary tumor was shown in most GAC patients, indicating their lower sensitivity to neoadjuvant chemotherapy[5]. These aspects may affect the significance of LN regression in the survival analysis of GAC patients.
In conclusion, our study revealed that the existence of residual tumor in LNs, rather than regression change in LNs, is useful for predicting prognosis after neoadjuvant chemotherapy in GAC patients. In practice, it may not be necessary to report regression change in LNs.
Neoadjuvant chemotherapy has been applied to improve the long-term survival of patients with gastric adenocarcinoma (GAC). Although the regression change of primary tumors may affect the prognosis of GAC patients, it is controversial whether evaluation of lymph node (LN) regression change would help predict the outcomes of GAC patients.
Our previous study revealed that the grading of primary tumor regression was valuable for predicting the prognosis of GAC patients. However, the role of regression change in LNs for prognosis remains questionable.
We analyzed the prognostic effect of LN regression change in GAC patients after neoadjuvant chemotherapy, and tried to explore the necessity of LN regression evaluation.
We evaluated the histological regression of resected LNs from 192 GAC patients, and classified regression change and residual tumor in LNs into four groups: (A) true negative LNs with no evidence of a preoperative therapy effect, (B) no residual metastasis but the presence of regression change in LNs, (C) residual metastasis with regression change in LNs, and (D) metastasis with minimal or no regression change in LNs. The effects of regression change and residual tumor groups in LNs on overall survival and progression-free survival were evaluated by Cox regression analysis.
The patients who had no residual tumor in LNs (Groups A+B) had a significantly better outcome than the patients who had residual tumors in LNs (Groups C+D) (P < 0.01), no matter whether they had LN regression change or not. No significant differences in survival were found between Groups A and B, or between Groups C and D.
Our study revealed that patients who had residual tumors in LNs had significantly worse outcomes than patients who had no residual tumors in LNs, independent of whether LN regression change existed or not. Therefore, it may not be necessary to evaluate LN regression change in GAC patients.
This was a single center retrospective study, and the preoperative therapeutic strategies were not uniform. Prospective studies are needed for further investigation.
We thank Lu Yu for providing advice on data presentation for this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nakayama Y, Tanabe S S- Editor: Wang JL L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20516] [Article Influence: 2051.6] [Reference Citation Analysis (20)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13214] [Article Influence: 1468.2] [Reference Citation Analysis (3)] |
| 3. | Becker K, Langer R, Reim D, Novotny A, Meyer zum Buschenfelde C, Engel J, Friess H, Hofler H. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg. 2011;253:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 276] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 4. | Chirieac LR, Swisher SG, Ajani JA, Komaki RR, Correa AM, Morris JS, Roth JA, Rashid A, Hamilton SR, Wu TT. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 387] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 5. | Zhu Y, Sun Y, Hu S, Jiang Y, Yue J, Xue X, Yang L, Xue L. Comparison of five tumor regression grading systems for gastric adenocarcinoma after neoadjuvant chemotherapy: a retrospective study of 192 cases from National Cancer Center in China. BMC Gastroenterol. 2017;17:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Newman LA, Pernick NL, Adsay V, Carolin KA, Philip PA, Sipierski S, Bouwman DL, Kosir MA, White M, Visscher DW. Histopathologic evidence of tumor regression in the axillary lymph nodes of patients treated with preoperative chemotherapy correlates with breast cancer outcome. Ann Surg Oncol. 2003;10:734-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Fernández-Aceñero MJ, Granja M, Sastre J, García-Paredes B, Estrada L. Prognostic significance of tumor regression in lymph nodes after neoadjuvant therapy for rectal carcinoma. Virchows Arch. 2016;468:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 8. | Bollschweiler E, Hölscher AH, Metzger R, Besch S, Mönig SP, Baldus SE, Drebber U. Prognostic significance of a new grading system of lymph node morphology after neoadjuvant radiochemotherapy for esophageal cancer. Ann Thorac Surg. 2011;92:2020-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Kinoshita O, Ichikawa D, Ichijo Y, Komatsu S, Okamoto K, Kishimoto M, Yanagisawa A, Otsuji E. Histological evaluation for chemotherapeutic responses of metastatic lymph nodes in gastric cancer. World J Gastroenterol. 2015;21:13500-13506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Ott K, Blank S, Ruspi L, Bauer M, Sisic L, Schmidt T. Prognostic impact of nodal status and therapeutic implications. Transl Gastroenterol Hepatol. 2017;2:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | American Joint Committee on Cancer. PART III: Upper Gastrointestinal Tract. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare AE, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. New York: Springer 2017; 203-220. |
| 12. | Schirren R, Reim D, Novotny AR. Adjuvant and/or neoadjuvant therapy for gastric cancer? A perspective review. Ther Adv Med Oncol. 2015;7:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688-8696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 951] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 14. | Schuhmacher C, Gretschel S, Lordick F, Reichardt P, Hohenberger W, Eisenberger CF, Haag C, Mauer ME, Hasan B, Welch J, Ott K, Hoelscher A, Schneider PM, Bechstein W, Wilke H, Lutz MP, Nordlinger B, Van Cutsem E, Siewert JR, Schlag PM. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol. 2010;28:5210-5218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 533] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 15. | Kodera Y. Neoadjuvant chemotherapy for gastric adenocarcinoma in Japan. Surg Today. 2017;47:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, Hahne JC, Rugge M, Peckitt C, Nankivell M, Langley R, Ghidini M, Braconi C, Wotherspoon A, Grabsch HI, Valeri N. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016;34:2721-2727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 18. | Perez RO, Habr-Gama A, Nishida Arazawa ST, Rawet V, Coelho Siqueira SA, Kiss DR, Gama-Rodrigues JJ. Lymph node micrometastasis in stage II distal rectal cancer following neoadjuvant chemoradiation therapy. Int J Colorectal Dis. 2005;20:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Aktepe F, Kapucuoğlu N, Pak I. The effects of chemotherapy on breast cancer tissue in locally advanced breast cancer. Histopathology. 1996;29:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Martin-Romano P, Sola JJ, Diaz-Gonzalez JA, Chopitea A, Iragorri Y, Martínez-Regueira F, Ponz-Sarvise M, Arbea L, Subtil JC, Cano D, Ceniceros L, Legaspi J, Hernandez JL, Rodríguez J. Role of histological regression grade after two neoadjuvant approaches with or without radiotherapy in locally advanced gastric cancer. Br J Cancer. 2016;115:655-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |