Published online Jan 15, 2019. doi: 10.4251/wjgo.v11.i1.39
Peer-review started: September 18, 2018
First decision: October 18, 2018
Revised: October 29, 2018
Accepted: December 4, 2018
Article in press: December 5, 2018
Published online: January 15, 2019
Processing time: 120 Days and 1.7 Hours
To clarify the prognostic significance of preoperative albumin-to-alkaline phosphatase ratio (AAPR) in cholangiocarcinoma (CCA) subjects receiving surgery.
In this retrospective study, we included 303 CCA patients receiving surgery without preoperative therapy between 2002 and 2014. Clinicopathological characteristics (including AAPR) were analyzed to determine predictors of post-operative overall survival and recurrence-free survival (RFS). In addition, univariate and multivariate Cox proportional hazards models were conducted, followed by application of time-dependent receiver operating curves to identify the optimal cut-off.
Univariate and multivariate analyses revealed both decreased overall survival [hazard ratio (HR): 2.88, 95%CI: 1.19-5.78] and recurrence-free survival (HR: 2.31, 95%CI: 1.40–3.29) in patients with AAPR < 0.41 compared to those with AAPR ≥ 0.41. The optimal cut-off of AAPR was 0.41. Of the 303 subjects, 253 (83.5%) had an AAPR over 0.41. The overall 1-, 3- and 5-year survival rates were 70.2%, 38.0% and 16.5%, respectively in the low (< 0.41) AAPR group, which were significantly lower than those in the high (≥ 0.41) AAPR group (81.7%, 53.9%, and 33.4%, respectively) (P < 0.0001). Large tumor size, multiple tumors, and advanced clinical stage were also identified as significant predictors of poor prognosis.
Our outcomes showed that AAPR was a potential valuable prognostic indicator in CCA patients undergoing surgery, which should be further confirmed by prospective studies. Moreover, it is necessary to investigate the mechanisms concerning the correlation of low AAPR with poor post-operative survival in CCA patients.
Core tip: Certain combinations of clinical features and laboratory indexes have been validated as prognostic indicators, including albumin to gamma-glutamyltransferase ratio, neutrophil-to-lymphocyte ratio as well as platelet to albumin ratio. Nevertheless, albumin-to-alkaline phosphatase ratio (AAPR), a novel indicator for the prognosis of hepatocellular carcinoma, has not been examined in cholangiocarcinoma (CCA). Hence, it is intriguing to confirm the potential application of AAPR in CCA. Our findings demonstrated that AAPR is a potential prognostic indicator in CCA subjects undergoing surgery.
- Citation: Xiong JP, Long JY, Xu WY, Bian J, Huang HC, Bai Y, Xu YY, Zhao HT, Lu X. Albumin-to-alkaline phosphatase ratio: A novel prognostic index of overall survival in cholangiocarcinoma patients after surgery. World J Gastrointest Oncol 2019; 11(1): 39-47
- URL: https://www.wjgnet.com/1948-5204/full/v11/i1/39.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i1.39
Cholangiocarcinoma (CCA), initially described by Durand Fardel in 1840, is an aggressive cancer that originates from biliary epithelial cells[1,2]. CCA represents the second most common primary hepatic carcinoma, accounting for 3% of all gastrointestinal malignancies and 10%-25% of liver malignancies[3,4]. Moreover, in recent decades, the incidence of CCA has been rising. Intriguingly, the epidemiology of extrahepatic CCA and intrahepatic CCA are distinct, with a decreasing incidence of the former, but an increasing incidence of the latter in certain regions worldwide, including the United Kingdom and the United States[5]. To be specific, the incidence of intrahepatic CCA has increased by 165% in the past 20 years, while that of extrahepatic CCA has decreased by 14% in the United States[6]. In addition, the prognosis of CCA is very poor. The relative 1-, 3-, and 5-year survival rates have been reported to be 25%, 9.7% and 6.8%, respectively, with no improvement in recent decades[7,8].
The following factors have been determined to be of prognostic significance: multifocal disease, increased carbohydrate antigen 19-9 level, tumor, node and metastasis (commonly known as TNM) staging classification, lymph node involvement, margin-positive resection and vascular evasion. In addition, certain combinations of clinical features and laboratory indexes have been validated as prognostic indicators, including albumin to gamma-glutamyltransferase ratio, neutrophil-to-lymphocyte ratio as well as platelet to albumin ratio[9-12]. Nevertheless, albumin-to-alkaline phosphatase ratio (AAPR), a novel indicator for the prognosis of hepatocellular carcinoma (HCC), has not been examined in CCA[13]. Hence, it is intriguing to examine the potential application of AAPR in CCA.
Therefore, a retrospective cohort study was performed to identify the prognostic significance of AAPR in CCA subjects, followed by investigation of the correlation of AAPR with other clinicopathological features.
In this retrospective study, we enrolled 303 patients with histologically diagnosed CCA from Peking Union Medical College Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China. The majority of CCA patients from Beijing were admitted to Peking Union Medical College Hospital (PUMCH) for diagnosis and therapy.
We obtained a list of CCA patients diagnosed from 2002 to 2014 by utilizing the patient informatics dataset of PUMCH; we enrolled those receiving curative resection that were pathologically confirmed. Curative dissection was considered as complete macroscopic removal of tumor, which was conducted in 156 subjects (51.5%).
The calculation of AAPR of all subjects was based on preoperative blood value. Univariate Cox proportional hazards model was conducted of overall survival (OS) and recurrence-free survival (RFS). Specifically, OS was determined in all subjects (n = 303), while RFS was assessed only in those receiving curative surgery (n = 156). We examined the following factors with respect to OS and RFS: age, gender, hepatitis C virus (HCV), hepatitis B virus (HBV), alkaline phosphatase level (ALP), serum albumin level (ALB), tumor size, number of tumors, TNM stage (in accordance with the 8th American Joint Committee on Cancer staging system) and curative resection. Of note, curative resection was eliminated in evaluating RFS. Pre-operative laboratory indexes were acquired, and contiguous variables were included into the model.
This study gained approval from the Institutional Review Board of PUMCH, and all participants signed written informed consent.
The primary endpoint was OS, defined as the duration from surgery to CCA-associated mortality. The secondary endpoint was RFS, defined as the duration from surgery to recurrence. Receiver operating curve (ROC) was employed to identify the optimal cut-off value of AAPR. Chi-squared test or Fisher’s exact test was employed for baseline characteristics comparison. Survival data were compared by Kaplan-Meier curve, along with log-rank test. Univariate and multivariate Cox proportional hazards regression models were performed for hazard ratios with corresponding 95%CIs. A two-sided P value < 0.05 was considered statistically significant. SPSS version 20.0 (SPSS, Chicago, IL, United States) was employed for all statistical analysis.
The baseline clinical characteristics are presented in Table 1. In total, 303 CCA cases were enrolled in this study. The median age was 59 (range 29–83) years old. Overall, 168 subjects were males and 135 were females, with a male-to- female ratio of 1.2:1. Of the 303 CCA patients, 10.23 % (31 subjects) and 1.65 % (five subjects) were positive for HBV and HCV infection, respectively. The median follow-up duration was 21.0 mo. In total, 177 patients passed away during the study, with an estimated median OS of 16.8 mo (range: 1.0-75.0 mo). The 1-year, 3-year and 5-year survival rates were 75.6%, 48.7% and 26.8%, respectively.
| Characteristic | Total (n = 303) |
| Age, yr | |
| < 60 | 150 (49.5) |
| ≥ 60 | 103 (50.5) |
| Sex | |
| Female | 135 (44.55) |
| Male | 168 (55.46) |
| Infectious diseases | |
| HBV | 31 (10.23) |
| HCV | 5 (1.65) |
| Serum albumin level [median (cIQ values)] | 38.7 (25.0 - 49.0) |
| ALP [median (IQ values)] | 315 (42.0 - 6778.0) |
| AAPR | |
| ≥ 0.41 | 253 (83.5) |
| < 0.41 | 50 (16.5) |
| Tumor size | |
| < 3 | 233 (76.9) |
| ≥ 3 | 70 (23.1) |
| Number of tumors | |
| 1 | 278 (91.7) |
| ≥ 2 | 25 (8.3) |
| Curative resection | 156 (51.5) |
| Stage (III + IV) | 98 (32.3) |
Time-dependent ROC curve was employed to examine the optimal cut-off of AAPR for prognosis. Consequently, an AAPR of 0.41 was considered as the optimal cut-off value for predicting prognosis, with a sensitivity of 0.735 and specificity of 0.829. According to the cut-off value, 253 and 50 subjects were assigned to the high- and low-AAPR groups, respectively.
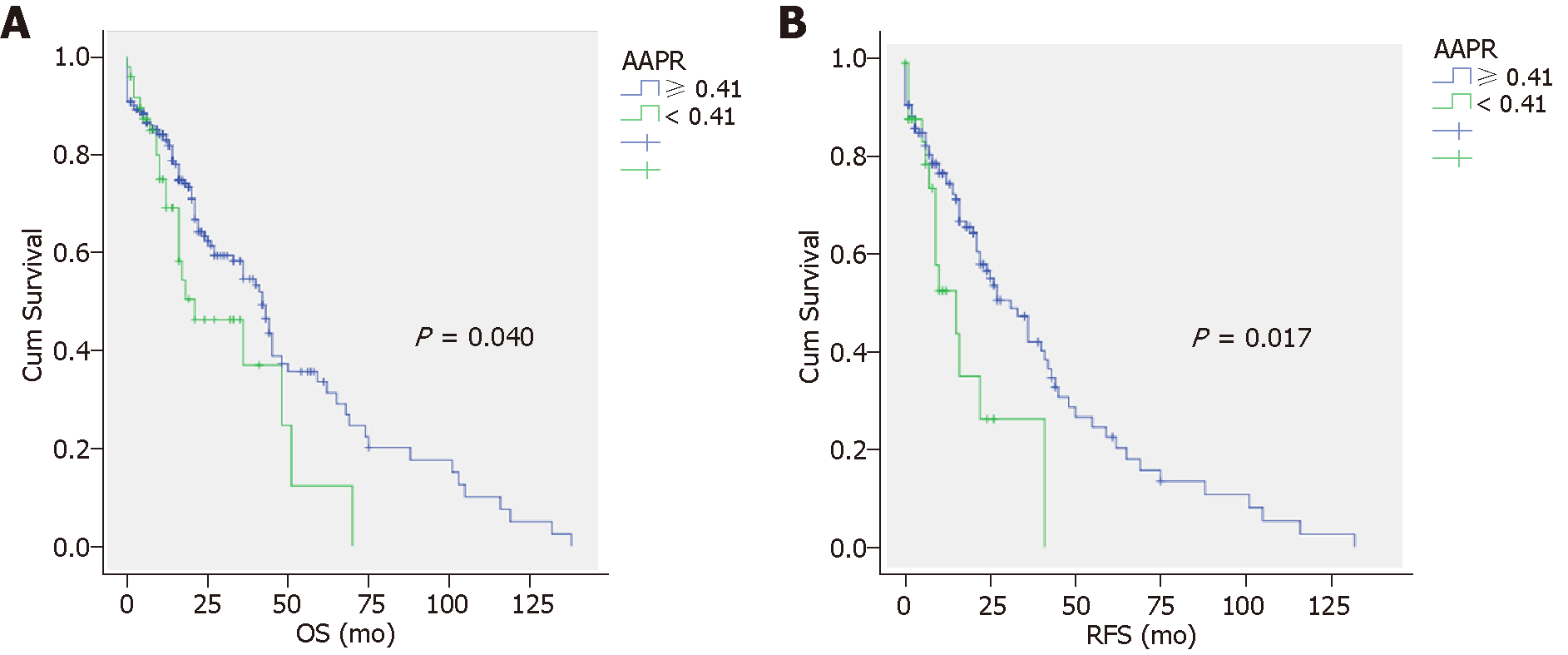
The OS and RFS of patients in both AAPR groups are displayed in Figure 1. Specifically, the overall 1-, 3-, and 5-year survival rates were 70.2%, 38.0%, and 16.5%, respectively in the low (< 0.41) AAPR group, which were significantly lower than those in the high (≥ 0.41) AAPR group (81.7%, 53.9%, and 33.4%, respectively) (P < 0.0001) (Figure 1A). The RFS was significantly prolonged in the high AAPR group compared to the low AAPR group (P = 0.017) (Figure 1B). The median OS and RFS in the high AAPR groups were 48.5 (95%CI: 40.5-56.4) and 39.2 mo (95%CI: 31.1-47.3), respectively. The median OS and RFS in the low AAPR groups were 30.3 mo (95%CI: 21.3-39.3) and 18.5 mo (95%CI: 11.1-25.8), respectively.
The significant prognostic indicators for OS determined by univariate and multivariate analyses are demonstrated in Table 2. Low AAPR level, large tumor size, HBV infection, multiple tumors, advanced clinical stage, and non-curative resection were identified as predictors of poor prognosis (Table 2).
| Variable | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age, yr | 1.3 (0.6–2.1) | 0.650 | ||
| ≥ 60 | ||||
| ≥ 60 | ||||
| Sex | 0. 98 (0.64-1.76) | 0.300 | ||
| Female | ||||
| Male | ||||
| Infectious diseases | ||||
| HBV | 1.85 (1.10-2.47) | P < 0.001 | 1.31 (1.42–1.92) | < 0.001 |
| HCV | 1.67 (0.23-12.03) | 0.486 | ||
| Serum albumin level, g/L | 0.84 (0.59-1.21) | 0.278 | ||
| ≥ 35 | ||||
| < 35 | ||||
| ALP, ng/mL | 2.46 (0.98-6.18) | 0.054 | ||
| ≥ 20 | ||||
| < 20 | ||||
| AAPR | 3.56 (1.28-9.92) | 0.035 | 2.88 (1.19-5.78) | 0.002 |
| ≥ 0.41 | ||||
| < 0.41 | ||||
| Tumor size, cm | 3.14 (1.80-6.05) | 0.021 | 2.71 (1.72-4.79) | 0.012 |
| ≥ 5 | ||||
| < 5 | ||||
| Number of tumors | 2.82 (1.17-4.28) | 0.013 | 2.30 (1.95-3.20) | < 0.001 |
| ≥ 2 | ||||
| 1 | ||||
| Curative resection | 0.49 (0.17-0.85) | 0.007 | 0.35 (0.29-0.42) | 0.001 |
| Yes | ||||
| No | ||||
| Stage (III + IV) | 2.02 (1.34–3.12) | < 0.001 | 1.81 (1.242.49) | < 0.001 |
The significant indicators for RFS indicated by univariate and multivariate analyses are shown in Table 3. Low AAPR level, large tumor size, multiple tumors, and advanced clinical stage were identified as significant predictors of poor prognosis (Table 2).
| Variable | Univariate | Multivariate | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age, yr | 1.31 (0.89-2.91) | 0.381 | ||
| ≥ 60 | ||||
| ≥ 60 | ||||
| Sex | 1.0 (0.5–1.8) | 0.300 | ||
| Female | ||||
| Male | ||||
| Infectious diseases | ||||
| HBV | 1.91 (0.73-5.21) | 0.212 | ||
| HCV | 2.45 (0.61–5.21) | 0.374 | ||
| Serum albumin level, g/L | 0.89 (0.34-2.34) | 0.171 | ||
| ≥ 35 | ||||
| < 35 | ||||
| ALP, ng/mL | 1.96 (0.73–5.32) | 0.212 | ||
| ≥ 20 | ||||
| < 20 | ||||
| AAPR | 2.52 (1.38–4.75) | 0.002 | 2.31 (1.40–3.29) | < 0.001 |
| ≥ 0.41 | ||||
| < 0.41 | ||||
| Tumor size, cm | 3.62 (1.73–7.69) | 0.010 | 1.92 (1.70–2.16) | < 0.001 |
| ≥ 5 | ||||
| < 5 | ||||
| Number of tumors | 2.47 (1.21–5.23) | 0.009 | 2.20 (1.41–4.87) | 0.002 |
| ≥ 2 | ||||
| 1 | ||||
| Stage (III + IV) | 2.73 (1.81–3.55) | 0.003 | 2.32 (1.26–3.53) | < 0.001 |
In this research, we investigated the prognostic significance of AAPR in CCA patients receiving surgery. This is the first study analyzing the association of AAPR with CCA. The results indicate that AAPR is an independent prognostic factor for CCA subjects, and those with AAPR levels under 0.41 have decreased OS and RFS.
Liver function examination is a cost-effective and easy-to-obtain laboratory examination in clinical practice. Serum ALB and ALP levels are the two major indexes, the former a general reflection of hepatic protein synthesis, which are affected by primary chronic liver function damage, as well as the systemic immune response of liver cancer or the inflammatory microenvironment[14]. ALB is the most abundant serum protein, and it is capable of stabilizing cell growth and DNA replication and maintaining diverse biochemical variations, which plays an antioxidant role in tumorigenesis[15]. Hence, low ALB levels not only indicate insufficient liver function, but reflect a lack of human defense capabilities, thereby leading to great infectious lesions and a poor response to anti-cancer therapeutics. ALB has been considered a common biomarker to predict the survival rate of diverse malignant tumors, including colorectal cancer, HCC, and prostate cancer[16-18]. ALP is a hydrolase enzyme that is ubiquitously expressed, but at higher levels in liver, bile duct, bone, kidney and placenta[19]. Pregnancy or certain pathological situations, including CCA, biliary cirrhosis, liver injury, liver cancer as well as bone metastasis, cause increased ALP levels[20-22]. ALP level is an independent prognostic indicator for HCC patients[23]. In addition, ALP is included as a parameter in the Chinese University Prognostic Index system, which is one of the staging systems used to predict survival[24]. The concept of AAPR was initially proposed by Anthony, who demonstrated AAPR as an independent prognostic indicator for OS and disease-free survival in HCC patients undergoing radical operation[25]. Therefore, we speculated that a combination of ALB and ALP might harbor novel prognostic significance, which could better be used to predict survival of CCA subjects. In this study, AAPR (cut-off value: 0.41) was utilized for survival prediction in CCA subjects. As a result, univariate and multivariate analyses demonstrated that an AAPR under 0.41 was related to poor prognosis. Moreover, a low AAPR might indicate malnutrition status, suppressed immunity, or serious lesions (including liver or bone metastasis).
Our study has several strengths. Firstly, to our knowledge, this is the first study concerning the validation of the prognostic value of AAPR in CCA subjects undergoing surgery. Our results may be of interest to CCA researchers and be of help to clinicians aiming to develop approaches to prevent the development of CCA. Secondly, ALB and ALP are both simple but different and objective variables, which are more easily applied in clinical practice. Moreover, we examined the correlation of AAPR with other clinicopathological features, which indicated that advanced clinical stage, low AAPR level, large tumor size and multiple numbers of lesions were significantly associated with poor prognosis.
However, there were certain limitations in our study. Firstly, the single-center property and retrospective design were major limitations. Despite strict adherence to inclusion and exclusion criteria, selection bias might remain in this retrospective study. Secondly, the cut-off NLR values might not have been optimal, and external validation is still needed. Thirdly, the absence of validation cohorts contributed to the failed validation of AAPR as an independent factor for CCA subjects. AAPR is a ratio of ALB and ALP, the low level of which might indicate a condition of inactive immune response, liver failure, malnourishment as well as formation of invasive cancer, all of which might be correlated with the poor prognosis of CCA. More basic research and prospective studies are necessary to further elucidate the molecular mechanism underlying the relationship between AAPR and prognosis.
In conclusion, our findings indicated that AAPR is a potential prognostic indicator in CCA subjects undergoing surgery. In view of its low cost, usability and prognostic power, AAPR should be included in future research projects and clinical trials. However, larger scale, multi-center studies are required for further validation.
Several staging systems and various serum markers have been investigated to provide prognostic information, including the tumor, node and metastasis staging system, margin-positive resection, lymph node metastasis, multifocal disease, an elevated carbohydrate antigen 19-9 level, and vascular involvement. Some combined indexes of clinical characteristics and laboratory biomarkers have also been demonstrated to be prognostic factors, such as the platelet to albumin ratio, neutrophil-to-lymphocyte ratio and albumin to gamma-glutamyltransferase ratio. However, the albumin-to-alkaline phosphatase ratio (AAPR), which is a novel prognostic factor for hepatocellular carcinoma (HCC), has not yet been studied in cholangiocarcinoma (CCA).
The AAPR has been recently revealed as a prognostic index for HCC, whereas its role in CCA remains unclear.
To clarify the prognostic value of the preoperative blood AAPR in patients undergoing surgery for CCA. The results may be of interest to CCA researchers and of help to clinicians aiming to develop a means to prevent the development of CCA. Besides, serum albumin levels and alkaline phosphatase levels are both simple but differentiated and objective variables, which are more easily applied in clinical practice.
We conducted a retrospective cohort study that included 303 patients with histologically confirmed CCA from Peking Union Medical College Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China. Using the patients’ informatics database of Peking Union Medical College Hospital, we compiled a list of all patients who had been diagnosed with CCA between 2002 and 2014. The patients who had undergone a primary attempt at curative resection and whose diagnoses were confirmed by pathological examination were involved in this study. Curative resection was defined as complete macroscopic removal of the tumor and was performed in 156 patients (51.5%). The AAPR of all the patients in this study was calculated on the basis of preoperative blood value. Univariate analysis in overall survival (OS) and recurrence-free survival (RFS) was performed using the Cox proportional hazards model. The OS was evaluated in all 303 patients, and the RFS was evaluated in the 156 patients who underwent curative resection. The primary endpoint was OS, which was calculated from the date of surgery to the date of cholangiocarcinoma-associated death. The secondary endpoint was RFS, which was calculated from the date of surgery to the date of recurrence. The most appropriate cut-off values of AAPR were determined by receiver operating characteristic curve. Baseline characteristics were compared using Chi-squared test or Fisher’s exact test. Survival data were calculated using the Kaplan-Meier method and were compared using the log-rank test. Univariate and multivariate survival analyses were conducted using the Cox proportional hazards regression methodology.
The overall 1-, 3-, and 5-year survival rates were 70.2%, 38.0%, and 16.5% in the low (< 0.41) AAPR group and 81.7%, 53.9%, and 33.4% in the high (≥ 0.41) AAPR group, which was a significant difference (P < 0.0001). The median OS (95%CI) and RFS (95%CI) in the AAPR ≥ 0.41 group were 48.5 mo (40.5-56.4) and 39.2 mo (31.1-47.3), respectively. In the AAPR < 0.41 group, the median OS (95%CI) and RFS (95%CI) were 30.3 mo (21.3-39.3) and 18.5 mo (11.1-25.8), respectively. The recurrence-free survival rate was significantly higher in the high AAPR group than in the low AAPR group (P = 0.017). The main limitation of this study is its single-center and retrospective design. More prospective studies and basic research are still needed to further elucidate the molecular mechanism of AAPR related to prognosis.
In this study, we focused exclusively on the prognostic value of the AAPR in patients with CCA receiving surgery. This is the first study focusing on validating the prognostic potential of AAPR in patients with CCA receiving surgery. Its results may be of interest to CCA researchers and of help to clinicians aiming to develop a means to prevent the development of CCA. Albumin and alkaline phosphatase are both simple but differentiated and objective variables, which are more easily applied in clinical practice. Moreover, this study investigated the relationship between AAPR and other clinical factors. The present study indicated that low AAPR level, large tumor size, multiple tumors, and advanced clinical stage were identified as significant predictors of poor prognosis. To our knowledge, this is the first study to analyze the correlation between the AAPR and CCA. The results indicate that the AAPR is an independent prognostic indicator for patients with CCA; patients with an AAPR less than 0.41 exhibited inferior OS and RFS. Both univariate analysis and multivariate analysis showed that an AAPR less than 0.41 is associated with poor prognosis. A low AAPR may reflect the patient’s malnutrition status, suppressed immunity, and relatively severe disease condition, such as liver or bone metastasis.
Our present study suggested that AAPR is a potentially valuable prognostic index in patients with CCA receiving surgery. In view of its low cost, usability and prognostic power, AAPR should be included in the design of future clinical trials and research projects. A lack of validation cohorts limits the further confirmation of AAPR as an independent indicator for CCA patients. Larger scale and multi-center research is still warranted for further confirmation.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Awad Z, Ishibashi H S- Editor: Wang JL L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 210] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 848] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 5. | Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 310] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 6. | Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 7. | Lepage C, Cottet V, Chauvenet M, Phelip JM, Bedenne L, Faivre J, Bouvier AM. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Pawlik TM. Intrahepatic cholangiocarcinoma: from diagnosis to treatment. Hepatobiliary Surg Nutr. 2017;6:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Saito N, Shirai Y, Horiuchi T, Sugano H, Shiba H, Sakamoto T, Uwagawa T, Yanaga K. Preoperative Platelet to Albumin Ratio Predicts Outcome of Patients with Cholangiocarcinoma. Anticancer Res. 2018;38:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Lin Q, Lin ZH, Chen J, Lin JX, Li X, Jiang JR, Ma XK, Wu DH, Chen ZH, Dong M, Wei L, Wang TT, Ruan DY, Lin ZX, Wen JY, Wu XY, Huang MS. Prognostic significance of preoperative albumin-to-globulin ratio in patients with cholangiocarcinoma. Curr Res Transl Med. 2017;65:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Jing CY, Fu YP, Shen HJ, Zheng SS, Lin JJ, Yi Y, Huang JL, Xu X, Zhang J, Zhou J, Fan J, Ren ZG, Qiu SJ, Zhang BH. Albumin to gamma-glutamyltransferase ratio as a prognostic indicator in intrahepatic cholangiocarcinoma after curative resection. Oncotarget. 2017;8:13293-13303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Tan DW, Fu Y, Su Q, Guan MJ, Kong P, Wang SQ, Wang HL. Prognostic Significance of Neutrophil to Lymphocyte Ratio in Oncologic Outcomes of Cholangiocarcinoma: A Meta-analysis. Sci Rep. 2016;6:33789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Cai X, Chen Z, Chen J, Ma X, Bai M, Wang T, Chen X, Wu D, Wei L, Li X, Lin Q, Wen J, Ruan D, Lin Z, Dong M, Wu X. Albumin-to-Alkaline Phosphatase Ratio as an Independent Prognostic Factor for Overall Survival of Advanced Hepatocellular Carcinoma Patients without Receiving Standard Anti-Cancer Therapies. J Cancer. 2018;9:189-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Tanriverdi O. A discussion of serum albumin level in advanced-stage hepatocellular carcinoma: a medical oncologist's perspective. Med Oncol. 2014;31:282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 16. | Gion T, Taketomi A, Shimada M, Shirabe K, Hasegawa H, Takenaka K, Sugimachi K. Perioperative change in albumin messenger RNA levels in patients with hepatocellular carcinoma. Hepatology. 1998;28:1663-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Heys SD, Walker LG, Deehan DJ, Eremin OE. Serum albumin: a prognostic indicator in patients with colorectal cancer. J R Coll Surg Edinb. 1998;43:163-168. [PubMed] |
| 18. | Chi KN, Kheoh T, Ryan CJ, Molina A, Bellmunt J, Vogelzang NJ, Rathkopf DE, Fizazi K, Kantoff PW, Li J, Azad AA, Eigl BJ, Heng DY, Joshua AM, de Bono JS, Scher HI. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol. 2016;27:454-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 19. | Kaplan MM. Alkaline phosphatase. Gastroenterology. 1972;62:452-468. [PubMed] |
| 20. | Giljaca V, Stimac D, Gluud C. Are levels of alkaline phosphatases and bilirubin surrogate markers of outcomes of patients with primary biliary cirrhosis? Gastroenterology. 2015;148:860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Mayne PD, Thakrar S, Rosalki SB, Foo AY, Parbhoo S. Identification of bone and liver metastases from breast cancer by measurement of plasma alkaline phosphatase isoenzyme activity. J Clin Pathol. 1987;40:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Al Mamari S, Djordjevic J, Halliday JS, Chapman RW. Improvement of serum alkaline phosphatase to < 1.5 upper limit of normal predicts better outcome and reduced risk of cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol. 2013;58:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 23. | Yu MC, Chan KM, Lee CF, Lee YS, Eldeen FZ, Chou HS, Lee WC, Chen MF. Alkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence? J Gastrointest Surg. 2011;15:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 452] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 25. | Chan AW, Chan SL, Mo FK, Wong GL, Wong VW, Cheung YS, Chan HL, Yeo W, Lai PB, To KF. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers. 2015;2015:564057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |