Published online Jan 15, 2019. doi: 10.4251/wjgo.v11.i1.28
Peer-review started: September 18, 2018
First decision: October 15, 2018
Revised: November 21, 2018
Accepted: December 17, 2018
Article in press: December 18, 2018
Published online: January 15, 2019
Processing time: 119 Days and 10.9 Hours
It is unclear whether treatment delay affects the clinical outcomes of chemotherapy in advanced gastric cancer (A-GC).
To assess whether treatment delay affects the clinical outcomes of chemotherapy in A-GC.
This single-center retrospective study examined consecutive patients with A-GC between April 2012 and July 2018. In total, 110 patients with stage IV A-GC who underwent chemotherapy were enrolled. We defined the wait time (WT) as the interval between diagnosis and chemotherapy initiation. We evaluated the influence of WT on overall survival (OS).
The mean OS was 303 d. The median WT was 17 d. We divided the patients into early and elective WT groups, with a 2-wk cutoff point. There were 46 and 64 patients in the early and elective WT groups, respectively. Compared with the elective WT group, the early WT group had significantly lower albumin (Alb) levels and higher neutrophil/lymphocyte ratios and C-reactive protein (CRP) levels but not a lower performance status. The elective WT group underwent more combination chemotherapy than did the early WT group. OS was different between the two groups (230 d vs 340 d, respectively). Multivariate analysis revealed that higher CRP levels, lower Alb levels and monotherapy were significantly related to a poor prognosis. To minimize potential selection bias, patients in the elective WT group were 1:1 propensity score matched with patients in the early WT group; no significant difference in OS was found (303 d vs 311 d, respectively, log-rank P = 0.9832).
A longer WT in patients with A-GC does not appear to be associated with a worse prognosis.
Core tip: Generally, most patients with advanced cancer have various additional conditions that are due to the advanced cancer, unlike patients who are candidates for surgery. It is currently unclear whether a delay in the initiation of chemotherapy in patients with advanced gastric cancer (A-GC) leads to adverse outcomes. In the present study, we evaluated the impact of treatment delay on clinical outcomes in patients with A-GC. Using propensity score matched analysis to minimize potential selection bias, we found that there was no significant difference in overall survival between patients in the early waiting time (WT) and elective WT groups. A longer WT in patients with A-GC does not appear to be associated with a worse prognosis.
- Citation: Nishida T, Sugimoto A, Tomita R, Higaki Y, Osugi N, Takahashi K, Mukai K, Matsubara T, Nakamatsu D, Hayashi S, Yamamoto M, Nakajima S, Fukui K, Inada M. Impact of time from diagnosis to chemotherapy in advanced gastric cancer: A Propensity Score Matching Study to Balance Prognostic Factors. World J Gastrointest Oncol 2019; 11(1): 28-38
- URL: https://www.wjgnet.com/1948-5204/full/v11/i1/28.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i1.28
Gastric cancer (GC) is the fifth most common malignancy[1] and the third most common cause of cancer-related deaths[2] worldwide. The 5-year survival rate of early GC can exceed 90%[3]. However, once GC has reached an advanced stage and curative resection is no longer indicated, it has a poor prognosis. Patients with inoperable or metastatic advanced GC (A-GC) should be considered candidates for chemotherapy[4], which has been shown to improve survival and quality of life compared with supportive care alone[5]. Before starting chemotherapy, patients with A-GC must be precisely evaluated for metastases and phenotype by imaging studies, endoscopy, and pathology, including biomarker status. In addition, performance status (PS), comorbidities, and organ function must always be taken into consideration before treatment. Recently, chemotherapy strategies for malignancies have needed to assess the potential benefit of molecularly targeted therapy at the time of diagnosis because trastuzumab has a more marked effect in the HER-2-positive subgroup of tumors[6]. Therefore, patients with A-GC will have to wait to have imaging tests or to receive the pathological reports, including information about biomarkers; thus, they have to wait to begin their treatment, not unlike patients with lung cancer. The wait time (WT) between the diagnosis and the initiation of chemotherapy for patients with malignancies can be considered a quality indicator for cancer concern because it negatively influences the patients’ quality of life, resulting in psychological distress. Consequently, WT is associated with oncologic outcomes; the effect of WT may depend on the type of malignancy.
There have been some reports regarding the effect of WT on patients with gastrointestinal cancer, most of which have been related to patients who underwent surgery[7-9] or adjuvant chemotherapy[10]. It is believed that the early initiation of chemotherapy after surgery might improve survival because surgical intervention and tumor removal might result in an increased number of circulating tumor cells and accelerated micrometastases[11]. However, advanced cancer without surgical indication already has not only micrometastases but also overt metastatic tumor lesions. In most clinical trials for advanced cancer, overall survival (OS) is defined as the interval between randomization or the start of chemotherapy until death from any cause, and the WT before starting chemotherapy is not evaluated. In the clinical setting, the length of the WT before starting chemotherapy varies depending on the precise diagnosis, evaluation of the stage, and assessment of the patient’s background, including comorbidities or social issues. Generally, most patients with advanced cancer have various additional conditions due to the advanced cancer, unlike patients who are candidates for surgery. It is currently unclear whether a delay in the initiation of chemotherapy in patients with A-GC leads to adverse outcomes. In the present study, we evaluated the impact of treatment delay on clinical outcomes in patients with A-GC.
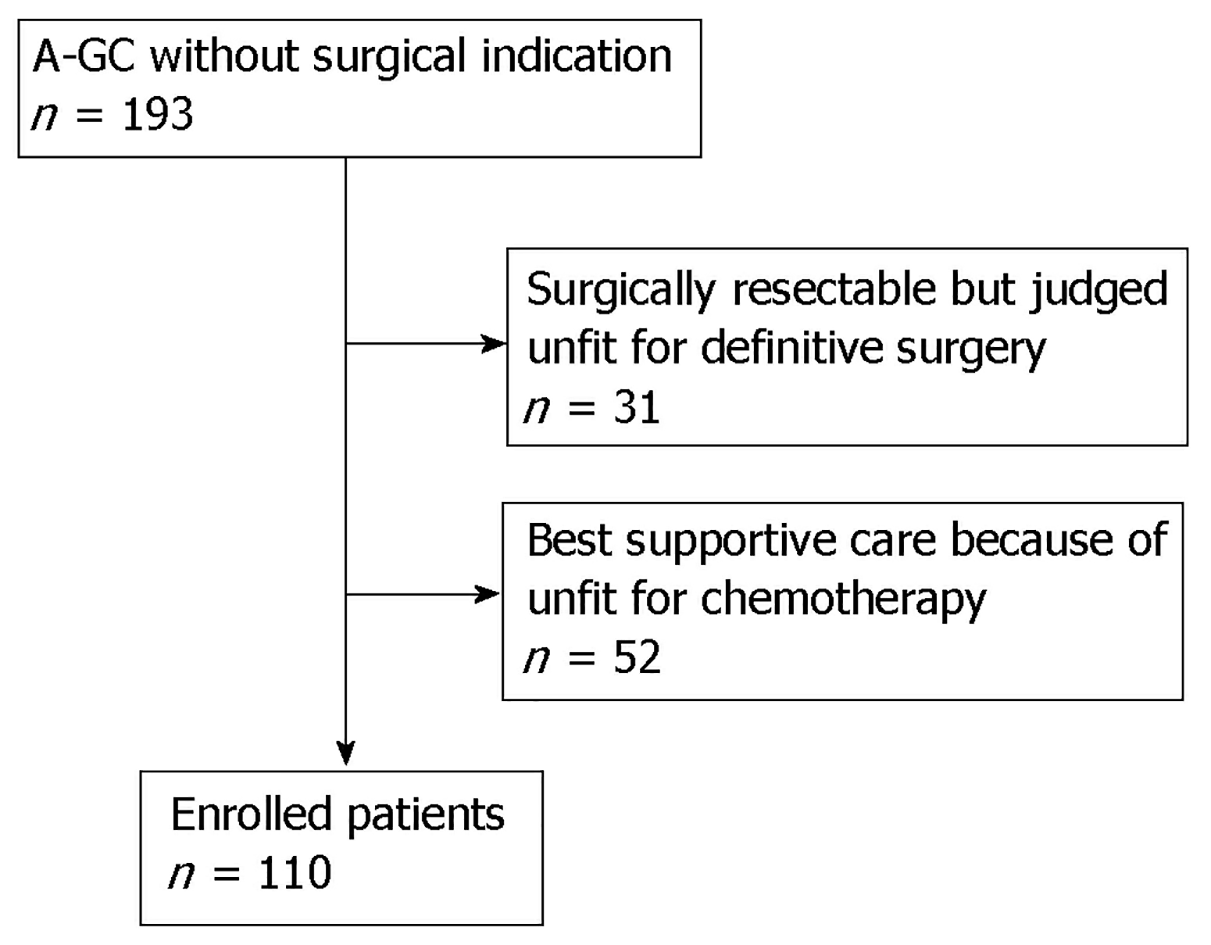
This single-center retrospective study examined consecutive patients with A-GC at Toyonaka Municipal Hospital between April 2012 and July 2018. During the study period, 193 patients diagnosed with A-GC without surgical indication visited our Department. We excluded 31 patients who were surgically resectable but judged unfit for definitive surgery, and 52 patients received supportive care because they were unfit for chemotherapy. Ultimately, 110 patients with stage IV (UICC-TNM classification) A-GC were enrolled in this study. In the present study, the chemotherapy regimen during follow-up was determined by the physician based on the Japanese GC treatment guidelines (ver. 3[12], ver. 4[13]). Chemotherapy regimens with doublet or triplet cytotoxic agents were defined as combination therapies, and regimens with a single agent were defined as monotherapies. Trastuzumab and ramucirumab were not counted in the number of drugs.
We defined the WT as the interval between diagnosis and the initiation of chemotherapy. The date of diagnosis was used as the date of the first detection of A-GC by esophagogastroduodenoscopy. In cases of patients with cancer recurrence, the date of recurrence detection by computed tomography was considered the date of diagnosis. We evaluated these individuals to assess the influence of WT on OS using a Cox proportional hazards model.
Patients who received chemotherapy were evaluated for their response to treatment according to the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). The present study was conducted in accordance with the Declaration of Helsinki, and approval was obtained from the Institutional Review Board of the Toyonaka Municipal Hospital (2018-06-06). This is a retrospective study involving human data that was previously collected and did not require the additional recruitment of human subjects; thus, the need for informed consent was waived via the opt-out method of our hospital website.
The latest follow-up occurred in July 2018. OS was calculated from the date of the initial clinical diagnosis of A-GC until death from any cause or the last available follow-up date. Surviving patients were censored on the date of their last follow-up visit.
The following factors were collected from medical records at the time of A-GC diagnosis: Patient demographics [age, sex, Eastern Cooperative Oncology Group (ECOG) PS]; primary tumor location; histological type; serum albumin (Alb) level; C-reactive protein (CRP) level; neutrophil/lymphocyte ratio (NLR); estimated glomerular filtration rate (eGFR); the presence of tumor markers [serum carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9]; the number of metastatic organs, including lymph nodes; and the Charlson comorbidity index score[14].
The medians and interquartile ranges (IQRs) are expressed for continuous variables. Categorical variables are reported as frequencies (percentages). Differences in variables were examined using the Wilcoxon signed-rank test or the χ2 test. OS was estimated using the Kaplan-Meier method and compared using the log-rank test. To assess the influence of WT on survival, we evaluated whether factors affected the prognosis by univariable and multivariable analyses using Cox proportional hazard models, providing hazard ratios (HRs) with 95% confidence intervals (CIs). Then, we calculated propensity scores for prognosis with those significant factors and created 1:1 matched study groups with a 0.05 caliper width to minimize the impact of potential selection bias. All reported P values were two-sided, and P < 0.05 was considered significant. Statistical analyses were performed using JMP statistical software (ver. 13.1. 0, SAS Institute Inc., Cary, NC, United States).
Figure 1 is a study flow chart. There were 193 patients with A-GC hospitalized at our hospital. Of those 193 patients, 31 patients had resectable cancer but were judged unfit for definitive surgery, and 52 patients received supportive care because they were unfit for chemotherapy. Ultimately, 110 patients were enrolled in this study.
The patient characteristics are shown in Table 1. The median WT from diagnosis to chemotherapy initiation was 17 d. Therefore, we set the time of 2 wk as the cutoff point to define patients as belonging in either the early WT group or the elective WT group. The early WT group was defined those having a WT of fewer than 14 d. The elective WT group was defined as those having a WT equal to or longer than 14 d. There were 46 patients in the early WT group and 64 patients in the elective WT group. The median WTs in the early WT and elective WT groups were 10 and 24 d, respectively.
| Characteristics | Total | Early WT | Elective WT | P value |
| No. | 110 | 46 | 64 | |
| Disease status Unresectable/ recurrent | 103/7 | 43/3 | 60/4 | 1.000 |
| Waiting time (WT), d, median (IQR) | 17 (12.5, 26) | 10 (7, 13) | 24 (19. 36) | P < 0.0001 |
| Male sex, n, % | 79, 72% | 34, 74% | 45, 70% | 0.6743 |
| Age, yr, median (IQR) | 70 (64, 78) | 71 (64, 80) | 67 (62, 75) | 0.1490 |
| BMI, median (IQR) | 20.3 (18.6, 22.8) | 21.1 (19.0, 22.8) | 19.9 (18.4, 22.9) | 0.5298 |
| Diabetes mellitus, n, % | 15, 14% | 5, 11% | 10, 17% | 0.4158 |
| Hypertension, n, % | 36, 35% | 16, 36% | 20, 34% | 1.000 |
| Dementia, n, % | 2, 2% | 1, 2.2% | 1, 1.7% | 1.000 |
| Chronic kidney disease, n, % | 43, 41% | 19, 42% | 24, 40% | 0.8434 |
| Charlson comorbidity index, median (IQR) | 0 (0, 1) | 0 (0, 0.5) | 0 (0, 1) | 0.3884 |
| Low/medium/high/missing | 73 (70%)/29 (28%)/3 (3%), 5(5%) | 34/9/2/1 | 39/20/1/4 | |
| PS 0-1, n, % | 95, 86% | 41, 89% | 54, 84% | 0.8234 |
| Histology type, intestinal n, % | 40, 36% | 18, 39% | 22, 35% | 0.5228 |
| HER 2 IHC 3+< or FISH (+), n, % | 26, 26% | 12, 26% | 14, 22% | 0.6080 |
| Gastric cardia tumor site, n, % | 25, 23% | 7, 15% | 18, 29% | 0.1130 |
| Target lesion, n, % | 91, 91% | 41, 91% | 50, 91% | 1.000 |
| No. of metastatic lesions ≤ 1, % | 39, 35% | 16, 35% | 23, 36% | 1.000 |
| CEA, median (IQR) | 6.5 (2.2, 40.9) | 6.5 (2.4, 80) | 5.7 (2, 25) | 0.4624 |
| CA19-9, median (IQR) | 22 (5, 810) | 54.5 (4.5, 2289) | 17 (5, 630) | 0.7326 |
| Hb, g/dL, median (IQR) | 11.3 (9.2, 12.7) | 11.3 (9.3, 12.9) | 11.2 (9.1, 12.7) | 0.8995 |
| Alb, g/dL, median (IQR) | 3.3 (3, 3.7) | 3.2 (2.8, 3.5) | 3.4 (3, 3.7) | 0.0129 |
| CRP, mg/dL, median (IQR) | 0.63 (0.15, 2.2) | 1.3 (0.47, 4.6) | 0.47 (0.08, 1.71) | 0.0015 |
| NLR, median (IQR) | 3.8 (2.7, 6.5) | 4.5 (3.1, 9.3) | 3.4 (2.3, 5.4) | 0.0112 |
| Clinical symptoms, yes, % | 93, 84.6% | 41, 89.1% | 52, 81.2% | 0.2970 |
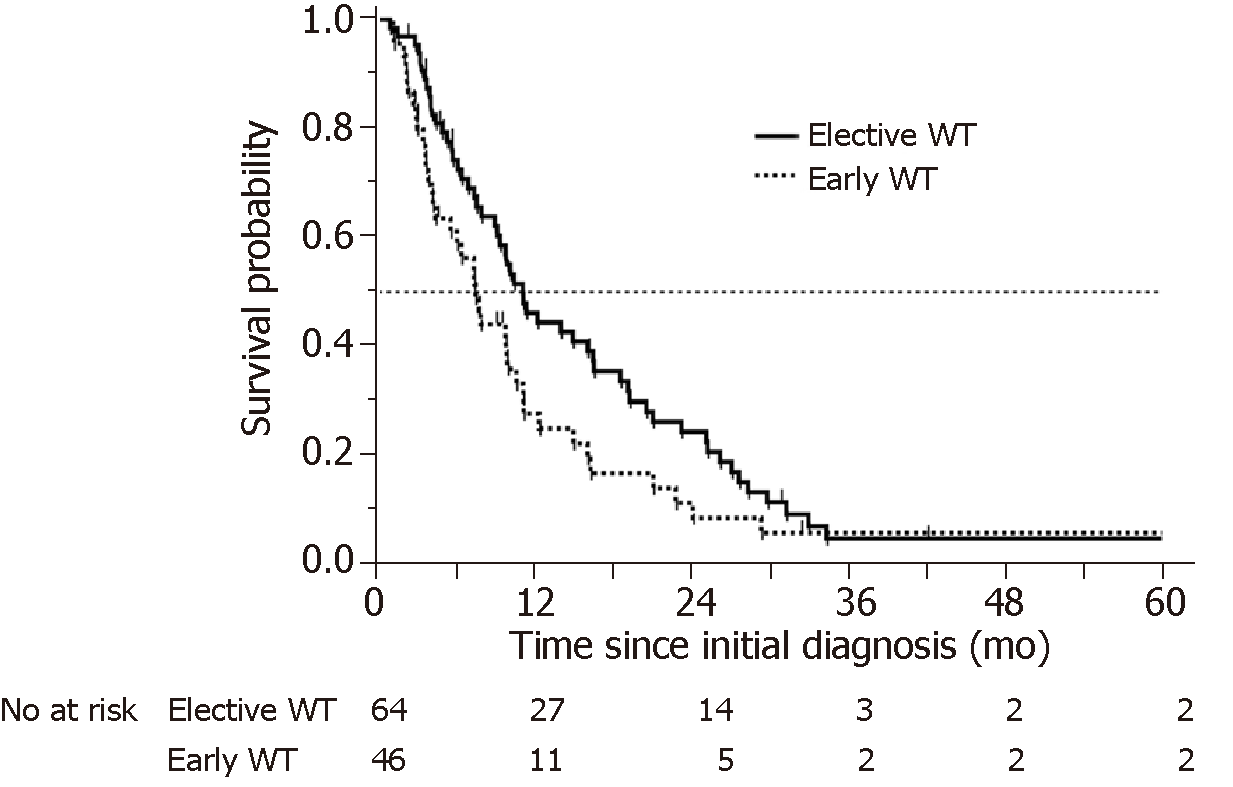
Significantly lower Alb levels and higher NLR and CRP levels were more common in patients in the early WT group. However, there was no significant difference in PS between patients in the early and elective WT groups. There was a difference in OS between the patients in the early and elective WT groups (230 d vs 340 d, respectively, log-rank test P = 0.0537, Wilcoxon test P = 0.0188) (Figure 2).
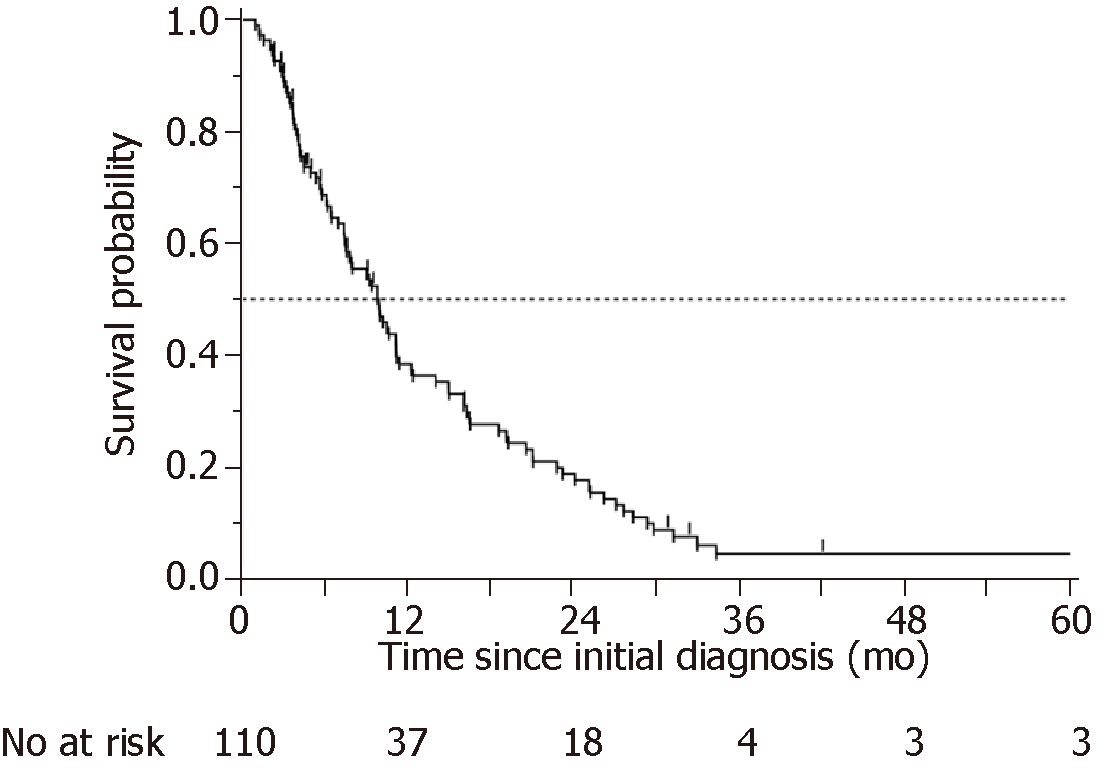
The OS of the 110 patients is plotted in Figure 3. The median OS time (MST) was 303 d. Table 2 shows the details of the chemotherapy regimens and response rates. The response rate (RR) was 22%, and the disease control rate (DCR) was 65%. The RR and DCR in the early WT group were 15% and 52%, respectively, and those in the elective WT group were 27% and 74%, respectively. The PD rate was more than twice as high in the early WT group (22%) as in the elective WT group (9%). Patients in the elective WT group underwent more combination chemotherapy than patients in the early WT group. Second-line treatment was administered to 63.5% of patients; the administration of second-line treatment was not significantly different between the early WT and elective WT groups (59% vs 68%, P = 0.3770).
| Characteristics | Total | Early WT | Elective WT | P value |
| No. | 110 | 46 | 64 | |
| Chemotherapy; combination | 76, 70% | 27, 59% | 49, 78% | 0.0371 |
| Agents | ||||
| 5-FU | 104 | 40, 85% | 64, 93% | 0.3532 |
| Platinum | 79 | 26, 55% | 53, 77% | 0.0100 |
| Irinotecan | 1 | 1, 2.1% | 0, 0% | 1.0000 |
| Taxane | 14 | 8, 17% | 6, 8.7% | 0.2460 |
| Trastuzumab | 19 | 9, 19% | 10, 14% | 0.6110 |
| Ramucirumab | 1 | 0, 0% | 1, 1.5% | 0.4072 |
| Response to first-line chemotherapy | ||||
| CR | 1, 0.91% | 0, 0% | 1, 1.6% | 0.1721 |
| PR | 23, 20.9% | 7, 15.2% | 16, 25% | |
| SD | 47, 42.7% | 17, 37.0% | 30, 46.9% | |
| PD | 16, 14.6% | 10, 21.7% | 6, 9.4% | |
| NE | 23, 20.9% | 12, 26.1% | 11, 17.2% | |
| RR | 24, 21.8% | 7, 15.2% | 17, 26.6% | |
| DCR | 71,64.5% | 24, 52.8% | 47, 73.5% | |
| Second-line chemotherapy, yes1 | 54, 63.5% | 24, 58.5% | 30, 68.2% | 0.3770 |
We evaluated whether differences in clinical factors between the early WT and elective WT groups affected the prognosis of patients with A-GC. The univariate analysis showed that higher CRP and lower Alb levels were significantly associated with poor prognosis, and early WT, higher NLR, and monotherapy were associated with poor prognosis with borderline significance (Table 3). The multivariate analysis included those five characteristics related to poor prognosis; the associations with poor prognosis for higher CRP levels, lower Alb levels, and monotherapy were significant, while those for early WT and higher NLR were not (Table 3).
| Univariate analysis | Multivariate analysis | |||||
| Category | HR | 96%CI | P value | HR | 96%CI | P value |
| Early WT | 1.502 | 0.982-2.28 | 0.0605 | 1.132 | 0.721-1.760 | 0.5857 |
| CRP | 1.12 | 1.050-1.185 | 0.0010 | 1.091 | 1.001-1.173 | 0.0335 |
| NLR | 1.03 | 0.99-1.057 | 0.0563 | 0.990 | 0.954-1.021 | 0.5520 |
| Alb | 0.514 | 0.354-0.750 | 0.0005 | 0.539 | 0.357-0.818 | 0.0038 |
| Chemotherapy (combination) | 0.669 | 0.431-1.065 | 0.0893 | 0.504 | 0.408-0.841 | 0.0094 |
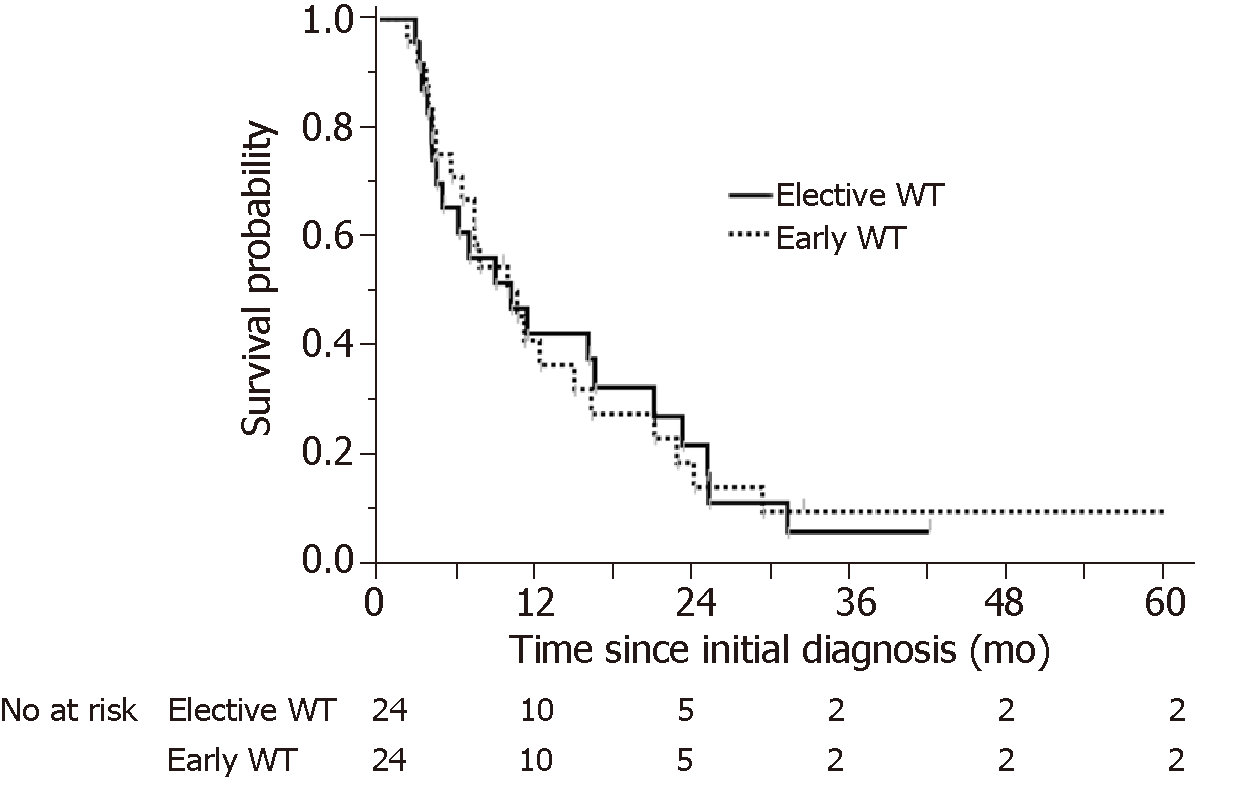
To minimize the impact of potential selective bias, patients in the elective WT group were matched to patients in the early WT group using 1:1 propensity score matching including CRP level, Alb level, and monotherapy, which were the factors identified as significantly affecting the poor prognosis of A-GC. We obtained a total of 48 matched patients in both the early WT group and the elective WT group. Table 4 shows the results of the propensity score matching. After propensity matching, there were no significantly different characteristics between the early and elective WT groups. OS was evaluated in the propensity-matched patients with A-GC in the early and elective WT groups. There was no significant difference in OS between the early and elective WT groups (303 d vs 311 d, respectively, log-rank P = 0.9832) (Figure 4).
| Propensity-matched cohort | |||
| Characteristics | Early WT | Elective WT | P value |
| No. | 24 | 24 | |
| Disease status Unresectable/recurrent | 23/1 | 20/4 | 0.3475 |
| Waiting time (WT), d, median (IQR) | 12 (8, 13) | 22.5 (19.3, 33.8) | < 0.0001 |
| Male sex, n, % | 18, 75% | 18, 75% | 1.0000 |
| Age, years, median (IQR) | 68 (61, 72) | 73.5 (63, 79) | 0.1635 |
| BMI, median (IQR) | 20.7 (18.8, 22.5) | 20.9 (19.3, 23.2) | 0.5727 |
| Diabetes mellitus, n, % | 3, 13% | 3, 13% | 1.0000 |
| Hypertension, n, % | 10, 42% | 9, 39% | 1.0000 |
| Dementia, n, % | 1, 4% | 1, 4% | 1.000 |
| Chronic kidney disease, n, % | 9, 38% | 10 42% | 0.7702 |
| Charlson comorbidity index score, median (IQR) | 0 (0, 0) | 0 (0, 0) | 0.9611 |
| Low/medium/high/missing | 20, 2, 2, 0 | 19, 3, 1, 1 | 0.7348 |
| PS 0-1, n, % | 23, 96% | 20, 87% | 0.5347 |
| Histology type, intestinal, n, % | 8, 33% | 12, 50% | 0.3442 |
| HER 2 IHC 3+< or FISH (+), n, % | 7, 29% | 8, 33% | 1.0000 |
| Gastric cardia tumor site, n, % | 2, 8% | 7, 29% | 0.1365 |
| Target lesion, n, % | 23, 96% | 19, 86% | 0.3364 |
| No. of metastatic lesions ≤ 1 | 9, 38% | 10, 42% | 0.8541 |
| CEA, median (IQR) | 6.7 (2.2, 91) | 8.8 (4.9, 75.5) | 0.7175 |
| CA19-9, median (IQR) | 86 (3, 851) | 27 (7, 6583) | 0.6282 |
| Hb, g/dL, median (IQR) | 11.3 (9.7, 12.9) | 11 (7.7, 12.3) | 0.5226 |
| Alb, g/dL, median (IQR) | 3.3 (2.9, 3.6) | 3.2 (3, 3.7) | 0.8848 |
| CRP, mg/dL, median (IQR) | 0.59 (0.26, 1.97) | 0.48 (0.13, 1.94) | 0.5707 |
| NLR, median (IQR) | 3.9 (3.0, 6.7) | 3.1 (2.4, 5.0) | 0.1576 |
| Monotherapy; combination | 7; 17 | 7; 17 | 1.0000 |
Generally, most solid cancers grow slowly. Therefore, waiting a few weeks for pathological reports, blood test results, including tumor marker results, or the results of imaging tests, such as CT scans, MRI scans, ultrasounds, or endoscopic examinations, does not usually affect the effectiveness of the treatment. Once a cancer gains a rapid growth phenotype and reaches an advanced stage beyond the indication of curative treatment, it is unknown how a delay of treatment affects the clinical outcomes. OS is generally counted starting from the time of randomization in cases of clinical trials or the time of the initiation of chemotherapy, and the WT between the diagnosis and the initiation of chemotherapy is not evaluated. In the case of patients involved in clinical trials, there may be a substantial interval until patients are referred to a special hospital. The tumor may progress during the WT of patients with advanced cancer who intend to participate in clinical trials and who may be excluded from those trials if their condition worsens. Therefore, the WT in clinical trials may select for patients with a good prognosis or slow progression and underestimate the OS time from diagnosis in the real world. In fact, it is ethically impossible to evaluate the impact of WT on prognosis in a randomized clinical trial.
In addition to tumor progression, a patient with advanced cancer feels anxiety during the WT. Longer WTs have negative effects on patients’ anxiety, mental well-being, satisfaction, physical functioning, and quality of life. Regarding esophageal or GC, Song et al[15] reported that the WT to treatment may have different mental health consequences for patients depending on their past psychiatric vulnerabilities. Consequently, those consequences may affect their prognosis, although it is difficult to evaluate the impact of WT. In the present study, a longer WT before initiating chemotherapy for patients with A-GC was not associated with a worse prognosis. Our institution is a medium-volume hospital in an urban area of Osaka Prefecture, Japan, and it is a designated cancer hospital. Of the patients with A-GC in our hospital, 27% received best supportive care during the study period. Therefore, we selected only patients who were fit for chemotherapy, such as those in high-volume cancer centers.
Regarding resectable solid cancer, Yun et al[9] recently reported that treatment delays of more than 1 mo are not associated with poor prognosis for patients with stomach, colon, pancreatic, or lung cancer but are associated with poor prognosis for patients with rectal and breast cancer in high-volume centers. In contrast, in low- to medium-volume centers, treatment delay is associated with poor prognosis for patients with all types of cancer[9]. Khorana et al[16] investigated the number of days between diagnosis and the first treatment for persons with early-stage solid tumors diagnosed from 2004 to 2013 and reported on 3672561 patients. They found that longer delays between diagnosis and initial treatment were associated with worse OS for patients with stages II and II breast, lung, renal, and pancreatic cancers and patients with stage II colorectal cancer, with an increased risk of mortality of 1.2% to 3.2% per week of delay, after adjusting for comorbidities and other variables. A prolonged time to treatment initiation of more than 6 wk was associated with substantially worsened survival[16]. In contrast, Visser et al[7] reported that WT has no impact on long-term outcomes in patients with esophageal cancer treated by either surgery alone or neoadjuvant chemotherapy followed by surgery. Based on these findings, a long WT for resectable solid cancer might be associated with a worse prognosis, but this association might depend on the volume of cancer patients in the particular hospital. However, there have been few reports on whether a delay in the initiation of chemotherapy for unresectable solid cancer will lead to adverse outcomes among patients[17].
Although there is no recommendation for the appropriate pretreatment evaluation interval in patients with A-GC, some practice guidelines for patients with lung cancer recommend the rapid evaluation, diagnosis, and treatment of patients with suspected lung cancer[18-20]. The guideline states that patients with an abnormal shadow on chest X ray or suspected lung cancer should undergo chest computed tomography within 2 wk[18]. Patients referred to a specialist for a complete examination should expect a consultation within 2 wk. The British Thoracic Society states that pathology results should be available within 2 wk of the complete examination[20]. In lung cancer, many biomarkers must be evaluated before deciding on the appropriate treatment. In the future, the evaluation of HER2 status and other biomarkers will mean additional time before the initiation of treatment in patients with GC. Therefore, a recommendation must be developed regarding an appropriate evaluation interval before starting chemotherapy in patients with A-GC.
In the present study, there were significantly lower Alb levels and higher CRP and NLR levels in patients in the early WT group than in those in the elective WT group, and combination chemotherapy was more predominant in the elective WT group than in the early WT group. These findings indicate that A-GC patients in the early WT group had an elevated systemic inflammatory response (SIR). It has been previously reported that an elevated SIR is a predictive marker for poor prognosis in patients with malignancies[21]. We also reported that elevated levels of inflammatory factors are related to poor prognosis in elderly patients with A-GC[22]. Consequently, patients in the early WT group had a poorer prognosis than those in the elective WT group in the present study. After matching the patients for confounding factors affecting prognosis, there was no significant difference in OS between the early and elective WT groups when the cutoff time was set at 2 wk. We used a cutoff value of 2 because the median WT from diagnosis to the initiation of chemotherapy was 17 d. In addition, we used the Kaplan-Meier method to preliminarily evaluate 3 groups with the following WTs: Less than 2 wk, 2-4 wk, and more than 4 wk. These results indicated that the survival curves of those with WTs of 2-4 wk and more than 4 wk nearly overlapped (Supplementary Figure). We believe that an adequate cutoff depends on the type of cancer. Therefore, we have to explore the specific regimen needed to address the elevated SIR but to maintain the PS; for example, triplet combination chemotherapy might be used to treat patients with pancreatic cancer or right-sided colon cancer.
The present study had several limitations due to its retrospective nature. First, it suffers from selection bias of WT. Physicians usually hurry to initiate chemotherapy for urgent cases, such as symptomatic cases or those with large tumor volume. Although propensity score matching analysis was used, it cannot enough remove selection bias for a single center and a small sample size study. However, Elimova et al[17] also reported that asymptomatic patients with delayed therapy (≥ 4 wk) had a good OS compared with patients with early therapy (< 4 wk), but the difference was not significant. They concluded that asymptomatic patients with delayed therapy had no detrimental effect on OS, suggesting that the timing of therapy can be based on patient selection. Second, the treatment strategy, including the WT or regimen, depended on the physician’s decision. Third, we did not assess whether the WT could influence other domains of cancer patients’ overall well-being. In the future, we need to evaluate how waiting for cancer treatment affects the mental health of patients.
In conclusion, the longer WT for patients with A-GC did not appear to be associated with a worse prognosis than the shorter WT. However, we must always evaluate patients as rapidly as possible to reduce the patient’s anxiety.
It is unclear whether treatment delay affects the clinical outcomes of chemotherapy in advanced gastric cancer (A-GC). It is ethically impossible to evaluate the impact of the waiting time (WT) on prognosis in a randomized clinical trial.
It is currently unclear whether a delay in the initiation of chemotherapy in patients with A-GC leads to adverse outcomes. In the present study, we evaluated the impact of treatment delay on clinical outcomes in patients with A-GC.
This single-center retrospective study examined consecutive patients with A-GC between April 2012 and July 2018. In total, 110 patients with stage IV A-GC who underwent chemotherapy were enrolled.
We defined the WT as the interval between diagnosis and chemotherapy initiation. We evaluated the influence of WT on overall survival (OS).
The mean OS was 303 d. The median WT was 17 d. We divided the patients into early and elective WT groups, with a 2-wk cutoff point. There were 46 and 64 patients in the early and elective WT groups, respectively. Compared with the elective WT group, the early WT group had significantly lower albumin (Alb) levels and higher neutrophil/lymphocyte ratios (NLR) and C-reactive protein (CRP) levels but not a lower performance status (PS). The elective WT group underwent more combination chemotherapy than the early WT group. OS was different between the two groups (230 d vs 340 d, respectively). Multivariate analysis revealed that higher CRP levels, lower Alb levels, and monotherapy were significantly related to a poor prognosis. To minimize potential selection bias, patients in the elective WT group were 1:1 propensity score matched with patients in the early WT group; no significant difference in OS was found (303 d vs 311 d, respectively, log-rank P = 0.9832).
A longer WT in patients with A-GC does not appear to be associated with worse prognosis.
The longer WT for patients with A-GC did not appear to be associated with a worse prognosis compared with the shorter WT. However, we must always evaluate patients as rapidly as possible to reduce the patient’s anxiety.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Abdel-Hamid SMM, Lee SW, Li C, Maric I, Senchukova MA S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | International Agency for Research on Cancer. Estimated number of incident cases, both sexes, worldwide (top 10 cancer sites) in 2012. 2012; Available from: http://gco.iarc.fr/today/online-analysis-multi-bars?mode=cancermode_population=continentspopulation=900sex=0cancer=29type=0statistic=0prevalence=0color_palette=default. |
| 2. | International Agency for Research on Cancer. Estimated number of deaths, both sexes, worldwide (top 10 cancer sites) in 2012. 2012; Available from: http://gco.iarc.fr/today/online-analysis-multi-bars?mode=cancermode_population=continentspopulation=900sex=0cancer=29type=1statistic=0prevalence=0color_palette=default. |
| 3. | Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, Nishihara A, Nakanishi F, Nakahara M, Ogiyama H, Kinoshita K, Yamada T, Iijima H, Tsujii M, Takehara T. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 4. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1118] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 5. | Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903-2909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 896] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 6. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5328] [Article Influence: 355.2] [Reference Citation Analysis (3)] |
| 7. | Visser E, van Rossum PS, Leeftink AG, Siesling S, van Hillegersberg R, Ruurda JP. Impact of diagnosis-to-treatment waiting time on survival in esophageal cancer patients - A population-based study in The Netherlands. Eur J Surg Oncol. 2017;43:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Grotenhuis BA, van Hagen P, Wijnhoven BP, Spaander MC, Tilanus HW, van Lanschot JJ. Delay in diagnostic workup and treatment of esophageal cancer. J Gastrointest Surg. 2010;14:476-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, Choi IJ, Kim YW, Park SJ, Kim JH, Lee DH, Yoon SJ, Jeong SY, Noh DY, Heo DS. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012;23:2731-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, Hamilton W, Hendry A, Hendry M, Lewis R, Macleod U, Mitchell ED, Pickett M, Rai T, Shaw K, Stuart N, Tørring ML, Wilkinson C, Williams B, Williams N, Emery J. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer. 2015;112 Suppl 1:S92-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 725] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 11. | McCulloch P, Choy A, Martin L. Association between tumour angiogenesis and tumour cell shedding into effluent venous blood during breast cancer surgery. Lancet. 1995;346:1334-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 13. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1915] [Article Influence: 239.4] [Reference Citation Analysis (1)] |
| 14. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38318] [Article Influence: 1008.4] [Reference Citation Analysis (0)] |
| 15. | Song H, Fang F, Valdimarsdóttir U, Lu D, Andersson TM, Hultman C, Ye W, Lundell L, Johansson J, Nilsson M, Lindblad M. Waiting time for cancer treatment and mental health among patients with newly diagnosed esophageal or gastric cancer: a nationwide cohort study. BMC Cancer. 2017;17:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Khorana AA, Tullio K, Elson P, Pennell NA, Kalady MF, Raymond D, Klein EA, Abraham J, Grobmyer SR, Monteleone EE, Bolwell BJ. Increase in time to initiating cancer therapy and association with worsened survival in curative settings: A U.S. analysis of common solid tumors. J Clin Oncol. 2017;35:6557-6557. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Elimova E, Shiozaki H, Slack RS, Chen HC, Wadhwa R, Sudo K, Charalampakis N, Hiremath A, Estrella JS, Matamoros A, Sagebiel T, Das P, Rogers JE, Garris JL, Blum MA, Badgwell B, Ajani JA. Early versus Delayed Therapy of Advanced Gastric Cancer Patients--Does It Make a Difference? Oncology. 2015;89:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Del Giudice ME, Young SM, Vella ET, Ash M, Bansal P, Robinson A, Skrastins R, Ung Y, Zeldin R, Levitt C. Guideline for referral of patients with suspected lung cancer by family physicians and other primary care providers. Can Fam Physician. 2014;60:711-716, e376-e382. [PubMed] |
| 19. | Leighl NB, Rekhtman N, Biermann WA, Huang J, Mino-Kenudson M, Ramalingam SS, West H, Whitlock S, Somerfield MR. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32:3673-3679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998;53 Suppl 1:S1-S8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Imrie CW. Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology. 2015;15:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Sugimoto A, Nishida T, Takahashi K, Mukai K, Matsubara T, Hayashi S, Yamamoto M, Nakajima S, Fukui K, Inada M. Chemotherapy and survival benefit in elderly patients with advanced gastric cancer. J Clin Oncol. 2017;35:45-45. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |