Published online Jan 15, 2019. doi: 10.4251/wjgo.v11.i1.17
Peer-review started: August 17, 2018
First decision: October 5, 2018
Revised: October 28, 2018
Accepted: December 5, 2018
Article in press: December 5, 2018
Published online: January 15, 2019
Processing time: 152 Days and 7.2 Hours
To evaluate the prognostic significance of perioperative carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels in stage II/III gastric cancer.
From a multi-institutional retrospective database compiled by integrating clinical data from nine institutions, data of 998 patients who underwent curative resection for stage II/III gastric cancer between 2010 and 2014 were retrieved and analyzed. The prognostic impact of the preoperative and postoperative levels and chronological changes in CEA, CA19-9 and their combination were evaluated. To test whether postoperative adjuvant chemotherapy alters the prognostic impact of perioperative CEA and CA19-9 levels, the hazard ratios for mortality were compared between patients who underwent surgery alone and patients who underwent surgery followed by adjuvant chemotherapy.
The prognostic impact of postoperative CEA and CA19-9 was superior to that of the preoperative levels. Multivariable analysis identified high postoperative CEA and CA19-9 levels as independent prognostic factors for overall survival. Disease-free survival rates clearly decreased in a stepwise manner in association with postoperative CEA and CA19-9 levels, and patients with high levels of both markers showed significantly poorer prognosis than other patient groups. When we analyzed perioperative changes in serum CEA and CA19-9 levels, patients with high levels before and after surgery had the worst disease-free survival rates among all patient groups. Patients with normalized CEA levels after surgery had a significantly lower disease-free survival rate than those with normal perioperative levels, whereas patients with normalized CA19-9 levels after surgery had equivalent survival to those with normal perioperative levels. The prognostic impact of high CEA levels was observably smaller in patients who underwent adjuvant chemotherapy than in patients who underwent surgery alone, whereas that of high CA19-9 was greater in patients who underwent adjuvant chemotherapy. High postoperative CEA levels were significantly associated with an increased prevalence of liver, lung and bone recurrences, and high postoperative CA19-9 levels were significantly associated with increased frequencies of lymph node and liver recurrences.
The evaluation of serum CEA and CA 19-9 levels both before and after surgery provides useful information for precise risk stratification after curative gastrectomy.
Core tip: Although the outcomes of patients with advanced gastric cancer have gradually improved with the development of adjuvant therapies, a large number of patients experience disease recurrence after curative gastrectomy. To optimize the management of each individual patient, accurate markers to predict prognosis are needed. In this multicenter dataset analysis, we found that evaluation of the serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels both before and after surgery provides more precise risk stratification of patients with stage II/III gastric cancer. Patients with high postoperative CEA and CA19-9 levels are at high risk of disease recurrence, and intensive postoperative management to detect hematogenous recurrences should be considered.
- Citation: Suenaga Y, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. Prognostic significance of perioperative tumor marker levels in stage II/III gastric cancer. World J Gastrointest Oncol 2019; 11(1): 17-27
- URL: https://www.wjgnet.com/1948-5204/full/v11/i1/17.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v11.i1.17
Gastric cancer has been recognized as a common disease, particularly in East Asia, and is one of the leading causes of cancer-related mortality worldwide[1]. Excellent outcomes are expected from surgery alone when gastric cancer is diagnosed at stage I. However, the estimated clinical courses are quite different in patients with stage II/III gastric cancer[1,3]. Owing to the development of various adjuvant therapies for those cases, treatment outcomes have gradually improved[4,5]. Nevertheless, there certainly remains a patient population with disease recurrence after curative gastrectomy, as indicated by the results of pivotal clinical trials (Supplemental Figure 1[6,7]. The accurate prediction of patient prognosis is an important task in optimizing management for each individual patient. For this purpose, in clinical practice, serum tumor markers are ideal options in terms of cost, convenience, and noninvasiveness.
Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are serum tumor markers that have long been routinely used in the diagnosis and monitoring of gastrointestinal malignancies, and their utility as prognostic predictors has been reported many times[8-10]. In most previous reports, only the preoperative levels of CEA and CA19-9 were evaluated, although alterations in their levels after the resection of primary tumors and regional lymph nodes vary among patients[11-14]. We recently reported that the risk of recurrences can be stratified by examining both the preoperative and postoperative levels of serum CEA and CA19-9. In addition, perioperative CEA levels facilitated the prediction of hematogenous metastasis as an initial recurrent pattern after curative gastrectomy in patients with advanced gastric cancer. However, our previous study suffered from caveats such as being a single institution study with a small sample size and variabilities in the adjuvant treatments given due to the change in standard of care during the acquisition of data. We found previously that postoperative S-1 adjuvant chemotherapy substantially alters prognostic factors after gastrectomy for advanced gastric cancer. Accordingly, the influence of adjuvant chemotherapy implementation should be considered in evaluating the prognostic ability of tumor markers.
From this perspective, we compiled a large-scale multi-institutional retrospective database and analyzed patients who underwent resection of gastric cancer between 2010 and 2014. The purpose of this study was to reappraise the prognostic significance of perioperative serum CEA and CA 19-9 levels in patients with stage II/III gastric cancer.
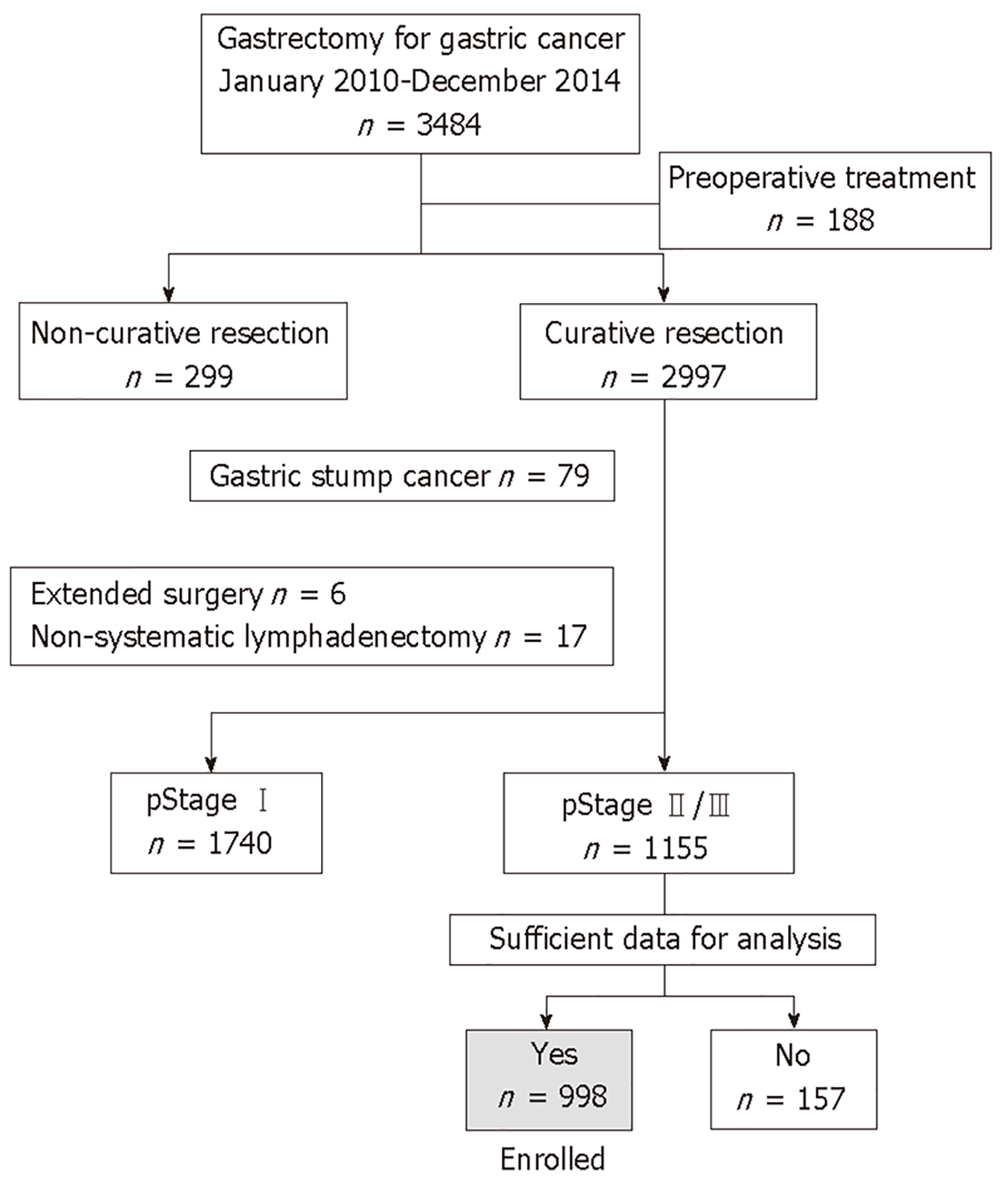
Clinical data from 3484 patients who underwent gastrectomy for gastric cancer between January 2010 and December 2014 were retrospectively collected from medical records at nine institutions. Of these patients, we retrieved 998 patients for subsequent analyses according to the following inclusion criteria: no preoperative treatment, R0 gastrectomy with systematic lymphadenectomy performed according to the Japanese Gastric Cancer Treatment Guidelines[1], pathological stage II/III gastric cancer according to the TNM Classification of Malignant Tumors, 8th Edition[17], and sufficient data for analysis (Figure 1). Patients with gastric stump cancer, patients who underwent extended surgery (e.g., pancreaticoduodenectomy and Appleby’s procedure) and patients with a postoperative follow-up period < 3 mo were excluded. This study conforms to the ethical guidelines of the World Medical Association Declaration of Helsinki–Ethical Principles for Medical Research Involving Human Subjects. Patients provided written informed consent for surgery and the use of clinical data as required by the Institutional Review Board at each participating institute.
The patients underwent gastrectomy with systematic lymphadenectomy according to the Japanese Gastric Cancer Treatment Guidelines[18], and the reconstruction method was determined at the surgeon’s discretion. The patients received postoperative follow-up that included physical examinations, laboratory tests, and enhanced computed tomography (chest and abdominal cavity) once every 6 mo for 5 years or until recurrence[18,19]. S-1 monotherapy for 12 mo or capecitabine plus oxaliplatin for 6 months has been recommended for all patients as a postoperative adjuvant treatment unless contraindicated by the patient’s condition or by patient refusal[20,21]. Treatment after recurrence was determined according to the evidence available at the time of treatment, the patient’s condition, and the patient’s consent.
The baseline levels of serum CEA and CA19-9 were measured within 14 d before gastrectomy. The postoperative CEA and CA19-9 levels were determined 6-10 wk after surgery and before the administration of adjuvant chemotherapy. We employed marker cutoff values (CEA, 5.0 ng/mL; CA19-9, 37 IU/mL) commonly used in Japan to divide patients into the normal and high groups.
The qualitative χ2 and quantitative Mann–Whitney U tests were used to compare the two groups. The differences in survival, hazard ratio (HR), and 95% confidence interval (CI) were calculated using Cox proportional hazards models. Variables with a P value < 0.01 were incorporated into the final model of multivariable regression analysis. Statistical analysis was performed using JMP 10 software (SAS Institute Inc., NC, United States). A P value < 0.05 was considered statistically significant.
The perioperative characteristics of the 998 patients are summarized in Supplemental Table 1. The median values of preoperative serum CEA and CA19-9 were 2.4 ng/mL and 12.0 IU/mL, respectively, and 192 (19.2%) and 162 (16.2%) patients had levels of preoperative serum CEA and CA19-9 higher than the cutoff values. Patients were pathologically diagnosed with stages IIA (n = 236), IIB (n = 222), IIIA (n = 297), IIIB (n = 174), and IIIC (n = 69). The median values of postoperative serum CEA and CA19-9 were 2.2 ng/mL and 8.8 IU/mL, respectively, and 114 (11.4%) and 83 (8.3%) patients had postoperative serum CEA and CA19-9 levels higher than the cutoff values. Postoperative adjuvant chemotherapy was administered to 646 (64.7%) patients. The median postoperative follow-up period was 50.5 months (range 3.5-93.7 mo).
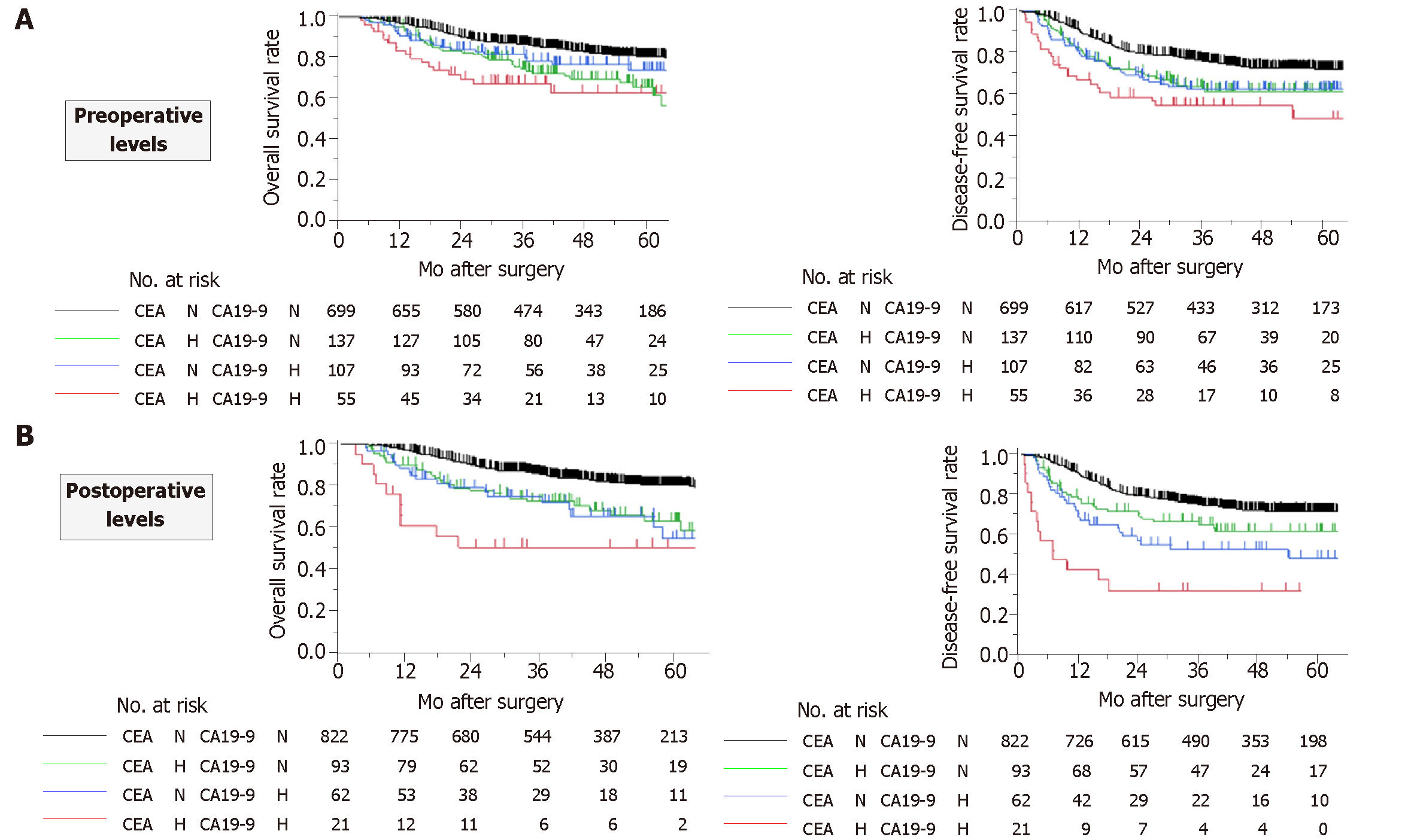
The patients were categorized into the following four groups according to the preoperative levels of CEA and CA19-9: normal serum values for both CEA and CA19-9 (both normal), serum value above the cutoff value for CEA only (CEA high), serum value above the cutoff value only for CA19-9 only (CA19-9 high), and serum values above the cutoff values for both CEA and CA19-9 (both high). Both overall and disease-free survival rates decreased in a stepwise manner from the both normal group to the CEA high and CA19-9 high groups and then to the both high group (Figure 2A). There were significant differences in disease-free survival rates between the both normal group and the CEA high and CA19-9 high groups combined, and also between the CEA high and CA19-9 high groups combined and the both high group. In comparison with the both normal group, the HRs for disease recurrence in the CEA high, CA19-9 high and both high groups were 1.62 (95%CI: 1.17-2.20, P = 0.0044), 1.67 (95%CI: 1.16-2.35, P = 0.0065) and 2.54 (95%CI: 1.63-3.79, P = 0.0001), respectively.
The patients were likewise categorized into four groups according to the postoperative levels of CEA and CA19-9. With respect to overall survival, patients in the both high group had a significantly inferior prognosis than all other groups (Figure 2B). Disease-free survival rates decreased more clearly in a stepwise manner in the following order: both normal, CEA high, CA19-9 high, and both high (Figure 2B). In comparison with both normal group, the HR for disease recurrence for the CEA high, CA19-9 high and both high groups were 1.60 (95%CI: 1.08-2.28, P = 0.0197), 2.38 (95%CI: 1.57-3.47, P = 0.0001) and 5.63 (95%CI: 3.12-9.32, P < 0.0001), respectively.
The prognostic values of the serum CEA and CA19-9 levels before and after surgery are summarized in Table 1. Generally, the predictive performance of the postoperative levels of the markers was superior to that of the preoperative values. Notably, high postoperative CA19-9 demonstrated the highest HR for disease recurrence. Multivariable analysis using a stepwise regression model identified high postoperative CEA and CA19-9 levels, but not preoperative levels, as independent prognostic factors for overall survival (HR 1.93, 95%CI: 1.27–2.87, P = 0.0024 and HR 1.70, 95%CI: 1.10–2.53, P = 0.0188, respectively; Supplemental Table 2.
| Preoperative levels | Postoperative levels | |||||||||||
| Overall survival | Disease-free survival | Overall survival | Disease-free survival | |||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| CEA (> 5 ng/mL) | 2.02 | 1.48-2.73 | < 0.0001 | 1.70 | 1.30-2.20 | 0.0002 | 2.30 | 1.59-3.23 | < 0.0001 | 1.89 | 1.36-2.56 | 0.0002 |
| CA19-9 (> 37 IU/mL) | 1.59 | 1.11-2.23 | 0.0125 | 1.76 | 1.32-2.32 | 0.0002 | 2.61 | 1.72-3.81 | < 0.0001 | 2.76 | 1.96-3.78 | < 0.0001 |
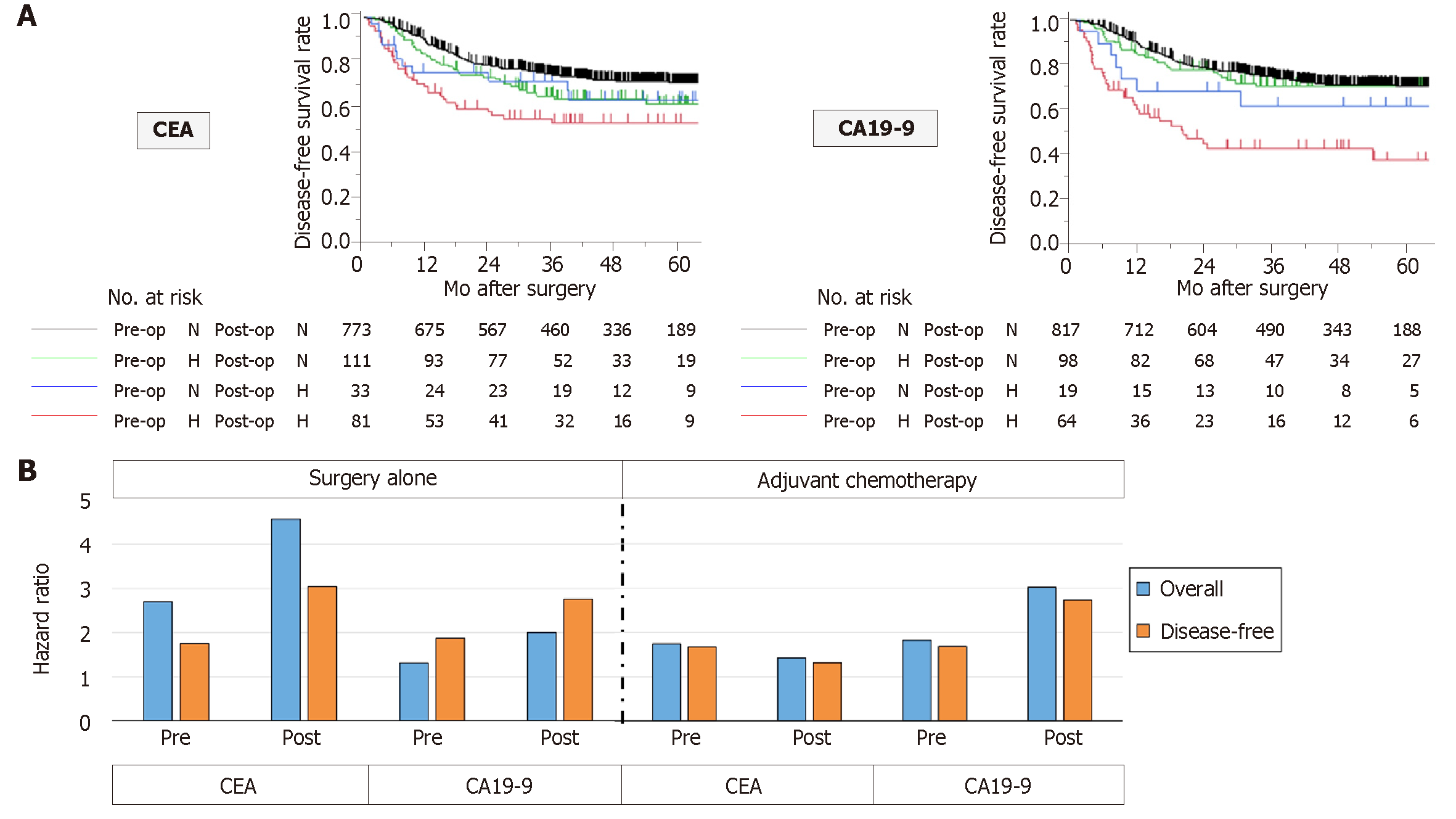
To examine whether perioperative changes in serum tumor marker levels have a superior predictive value to single-point measurement, patients were categorized into four groups according to perioperative levels as follows: Normal levels before and after surgery (normal-normal), high only before surgery (high-normal), high only after surgery (normal-high) and high before and after surgery (high-high). For both CEA and CA19-9 levels, the high-high group had the worst disease-free survival rates among the four groups (Figure 3A). Regarding CEA levels, the high-normal group had a significantly lower disease-free survival rate than the normal-normal group (HR 1.43, 95%CI: 1.00-2.00, P = 0.0477). In contrast, regarding CA19-9, the survival rates of the normal-normal and high-normal groups were equivalent (Figure 3A).
The HRs for overall and disease-free survival of perioperative CEA and CA19-9 levels were determined in patients with and without postoperative adjuvant chemotherapy. Interestingly, the HRs of elevated CEA levels were generally lower in patients who underwent adjuvant chemotherapy than in patients who underwent surgery alone (Figure 3B). In contrast, the HR for death of elevated CA19-9 was greater in patients who underwent adjuvant chemotherapy than in patients who underwent surgery alone (Figure 3B).
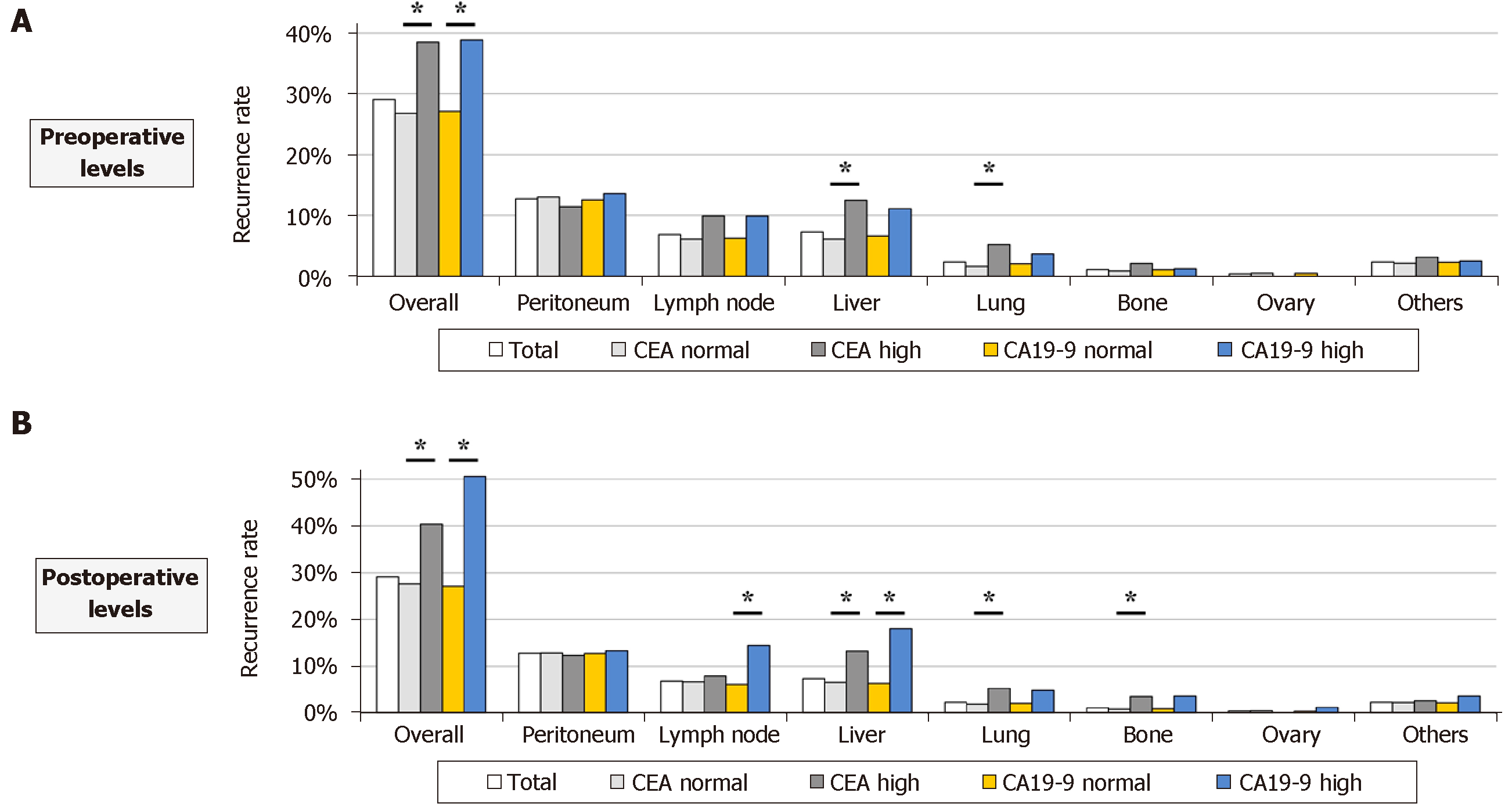
Association between perioperative levels of tumor markers and initial recurrence patterns Patients with high preoperative CEA levels had significantly higher overall recurrence rates than those with normal preoperative CEA levels (38.5% and 26.8%, respectively; Figure 4A). Statistically significant differences were observed in the prevalence of liver and lung recurrences between patients with normal and high preoperative CEA levels (Figure 4A). Similar but clearer trends were found in the analysis of postoperative levels. Patients with high postoperative CEA levels had significantly greater prevalence of liver, lung and bone recurrences than patients with normal postoperative CEA levels (Figure 4B). High postoperative CA19-9 levels were significantly associated with increased frequencies of lymph node and liver recurrences (Figure 4B).
Herein, using a multicenter database, we investigated how the perioperative serum CEA and CA 19-9 values predict the prognosis of patients who underwent curative resection of stage II/III gastric cancer between 2010 and 2014. The postoperative levels of CEA and CA 19-9 were found to be stronger prognostic factors than the preoperative values, and observing both tumor markers over time enabled more precise risk stratification than the single-point measurement of a single marker. In addition, the prognostic relevance of elevated postoperative CEA level was attenuated when adjuvant chemotherapy was administered whereas that of postoperative CA19-9 level was relatively unaffected.
Although various gastric cancer-related molecular markers have been explored in recent years, the classical serum tumor markers CEA and CA 19-9 are still routinely measured in clinical practice[8,22-24]. CEA is one of the cell adhesion factors first identified in human colon cancer tissues by Gold and Freeman in 1965[25]. CA19-9 is a glycolipid secreted antigen, a ligand for E-selectin first identified in the early 1980s, and is expressed in the epithelia of various organs[26]. The mechanisms of elevation of CEA and CA19-9 in the serum have not yet been clarified. For example, a discrepancy has been reported between the tissue and serum expression levels of CEA: serum CEA concentration could be influenced by factors such as tumor differentiation, location of CEA expression within cancer cells, and the degree of vascular invasion rather than the amount of CEA in the tumor tissues per se[9,11,23,27,28]. In any case, measurement of serum CEA and CA 19-9 remain important options in routine practice with respect to the accumulation of data, availability, cost and noninvasiveness, and thus, we sought ways to make maximum use of this information. Although widely used, they are not ideal markers because of their relatively low sensitivity and specificity in the diagnosis and prognosis of gastric cancer when used by preoperative values of a single marker[9,13,29]. According to our data, examining and judging the two markers together in a clinical setting is advisable because the combination of CEA and CA19-9 provided a more precise risk stratification than a single marker. Based on the literatures from Western and Asian countries, serum levels and clinical significance of CEA are almost identical in both areas. CA 19-9 is not expressed in patients who lack the Lewis antigen and the proportions of Lewis negative individuals are approximately 10% both in the Caucasian and Asian population[30,31]. These facts indicated that our findings may be applicable in a broad area of the world.
There have been few reports on the prognostic impact of postoperative CEA and CA19-9 levels in patients with stage II/III gastric cancer[13,14,24]. High postoperative CEA and CA19-9 were identified as independent prognostic factors in multivariable analysis, while the preoperative values were not. In particular, high postoperative CA19-9 showed the highest HR for disease-free survival. Our findings indicated that the postoperative (before adjuvant chemotherapy) measurement of CEA and CA19-9 provides additional information for planning the intensity of postoperative surveillance and treatment. A close correlation between a high postoperative level and prognosis is considered reasonable because high levels after curative gastrectomy with systemic lymph node dissection might represent the existence of remnant micrometastasis outside the surgical field[32,33]. Furthermore, the measurement of postoperative levels gives physicians an opportunity to investigate the perioperative changes in CEA and CA19-9 levels. Only a few studies report the normalization of postoperative tumor markers to be associated with a favorable prognosis in patients with gastric cancer[9,13,24,34]. In this study, patients with normalization of CEA levels after surgery had a better prognosis than patients in whom the postoperative CEA levels remained high but an inferior prognosis to those with normal CEA levels before and after surgery. On the other hand, CA19-9 showed a different trend from CEA, and the survival curve of the population in which CA 19-9 levels normalized after surgery was nearly identical to that of patients with normal CA19-9 levels before and after surgery. Most importantly, lack of normalization of either CEA or CA19-9 indicated poor outcome. From these viewpoints, the perioperative measurement of CEA and CA19-9 would be a more powerful approach than preoperative single-point measurement and is recommended to improve clinical care and the explanation of disease conditions.
We recently reported that the prognostic factors were quite different between gastric cancer patients who underwent surgery alone and those who underwent surgery followed by adjuvant S-1 monotherapy. In that study, high preoperative serum CEA level was a significant prognostic factor in the surgery alone group but not in the postoperative adjuvant S-1 group, suggesting that the prognostic significance of tumor markers may be altered by adjuvant chemotherapy[16]. However, many patients treated in the early 2000s before the standardization of S-1 adjuvant therapy were included in the analysis. In this study, we reexamined the influence of adjuvant chemotherapy on the prognostic impact of CEA and CA19-9 levels, focusing on stage II/III gastric cancer patients treated after the standardization of S-1 adjuvant therapy. The linkage of high CEA levels to a poor prognosis was reduced by adjuvant chemotherapy. Conversely, adjuvant chemotherapy had little influence on the prognostic significance of high CA19-9 levels. These findings should be carefully interpreted because differences in the patient background between the surgery alone and adjuvant chemotherapy groups can be a potential source of selection bias. However, the results suggested that patients with high postoperative CEA levels can expect to benefit from adjuvant chemotherapy.
Consistent with our previous report, high postoperative CEA levels were closely correlated with hematogenous (liver and lung) recurrences. Moreover, high postoperative CA19-9 levels were significantly associated with lymph node and liver recurrences[15]. The 5-year follow-up data of the Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC trial) suggest that postoperative adjuvant S-1 monotherapy contributes to the reduction of peritoneal recurrences rather than hematogenous recurrences[20]. Since the majority of patients underwent S-1 monotherapy as adjuvant chemotherapy, a reasonable speculation is that the prevalence of peritoneal recurrences was reduced by S-1 even in patients with high CEA and/or CA19-9 levels. This situation would be a possible reason for the low correlations between levels of tumor markers and peritoneal recurrence in this study. Nonetheless, our data highlighted that the development of serum markers to accurately predict peritoneal recurrence is another important issue.
Despite use of a database with greater number of patients, the study limitation inherent to the retrospective nature remains unresolved. The appropriate timing of measurement and optimal cutoff values of perioperative tumor markers for maximal risk stratification are unresolved issues. Further discussion could have been possible if data on the levels of CEA and CA19-9 at some other timepoints after the administration of adjuvant chemotherapy were available. Combining the current information with serum CA125 and CA72-4 levels might further improve risk stratification from the viewpoint of predicting peritoneal recurrences[12,14,28,35]. Recently, several candidate molecular markers having the potential to inform molecularly motivated and patient subtype-oriented therapeutic decisions for patients with stage II/III gastric cancer have been emerged as Cheong et al[36] showed that a predictive single patient classifier test based on tissue expression of GZMB, WARS, SFRP4, and CDX1 can identify patients who will benefit from adjuvant chemotherapy. In the future, novel biomarkers for gastric cancer with high sensitivity and specificity are expected to be available after validation in large-size clinical trials.
Taken together, our results indicated that evaluating the combination of serum CEA and CA 19-9 levels both before and after surgery is desirable because these markers have distinct dynamics and prognostic significance. Patients with high postoperative CEA and CA19-9 levels are at high risk of disease recurrence; thus, intensive postoperative management, including whole-body surveillance to detect hematogenous metastasis, should be considered.
There certainly remains a patient population with disease recurrence after curative gastrectomy for advanced gastric cancer. The accurate prediction of patient prognosis is an important task in optimizing management for each individual patient. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are have long been widely used for the diagnosis and monitoring of gastric cancer. However, their performance remains unsatisfactory and further improvement is needed.
In our previous paper, we reported that the risk of recurrences can be stratified by examining both the preoperative and postoperative levels of serum CEA and CA19-9. However, our previous study suffered from caveats such as being a single institution study with a small sample size and variabilities in the adjuvant treatments given due to the change in standard of care during the acquisition of data. Our data should be verified by a larger and modern cohort and the influence of adjuvant chemotherapy implementation should be considered in evaluating the prognostic ability of tumor markers.
To reappraise the prognostic significance of perioperative serum CEA and CA 19-9 levels in patients with stage II/III gastric cancer, we designed a large-scale multi-institutional retrospective database and analyzed patients who underwent resection of gastric cancer between 2010 and 2014.
Data of 998 patients who underwent curative resection for stage II/III gastric cancer between 2010 and 2014 at the nine participating institutions was analyzed. Prognostic impact of the preoperative and postoperative levels and chronological changes in CEA, CA19-9 and their combination were evaluated. The hazard ratios for mortality were compared between patients who underwent surgery alone and patients who underwent surgery followed by adjuvant chemotherapy.
Postoperative levels had better prognostic values compared to preoperative levels. Disease-free survival rates gradually reduced according to postoperative CEA and CA19-9 levels, and patients with high levels of both markers had the worst prognosis. Patients with normalized CEA levels after surgery had a significantly lower disease-free survival rate than those with normal perioperative levels, whereas patients with normalized CA19-9 levels after surgery had equivalent survival to those with normal perioperative levels. The prognostic impact of high CA19-9 was greater in patients who underwent adjuvant chemotherapy.
We herein showed the combination and preoperative measurement of serum CEA and CA 19-9 levels can be a promising tool to predict prognosis of patients with stage II/III gastric cancer. Using a multi-institutional large-size database, our data was successfully refined and more convincing than the previous one.
Serum CEA and CA 19-9 levels have distinct dynamics and prognostic significance. Intensive postoperative management should be considered. The appropriate timing of measurement and optimal cutoff values of perioperative tumor markers for maximal risk stratification are unresolved issues. In the future, novel biomarkers for gastric cancer with high sensitivity and specificity are expected to be available after validation in large-size clinical trials.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Salati M, Soh JS S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [PubMed] [DOI] [Full Text] |
| 2. | Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10:643-655. [PubMed] [DOI] [Full Text] |
| 3. | Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, Fujii T, Nakayama G, Sugimoto H, Koike M, Nomoto S, Murotani K, Fujiwara M, Kodera Y. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer. 2016;19:255-263. [PubMed] [DOI] [Full Text] |
| 4. | Wong RK, Jang R, Darling G. Postoperative chemoradiotherapy vs. preoperative chemoradiotherapy for locally advanced (operable) gastric cancer: clarifying the role and technique of radiotherapy. J Gastrointest Oncol. 2015;6:89-107. [PubMed] [DOI] [Full Text] |
| 5. | Kanda M, Kodera Y, Sakamoto J. Updated evidence on adjuvant treatments for gastric cancer. Expert Rev Gastroenterol Hepatol. 2015;9:1549-1560. [PubMed] [DOI] [Full Text] |
| 6. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, Park SR, Fujii M, Kang YK, Chen LT. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [PubMed] [DOI] [Full Text] |
| 7. | Kanda M, Tanaka C, Kobayashi D, Mizuno A, Tanaka Y, Takami H, Iwata N, Hayashi M, Niwa Y, Yamada S, Fujii T, Sugimoto H, Murotani K, Fujiwara M, Kodera Y. Proposal of the Coagulation Score as a Predictor for Short-Term and Long-Term Outcomes of Patients with Resectable Gastric Cancer. Ann Surg Oncol. 2017;24:502-509. [PubMed] [DOI] [Full Text] |
| 8. | Marrelli D, Pinto E, De Stefano A, de Manzoni G, Farnetani M, Garosi L, Roviello F. Preoperative positivity of serum tumor markers is a strong predictor of hematogenous recurrence of gastric cancer. J Surg Oncol. 2001;78:253-258. [PubMed] [DOI] [Full Text] |
| 9. | Nam DH, Lee YK, Park JC, Lee H, Shin SK, Lee SK, Lee YC, Cheong JH, Hyung WJ, Noh SH, Kim CB. Prognostic value of early postoperative tumor marker response in gastric cancer. Ann Surg Oncol. 2013;20:3905-3911. [PubMed] [DOI] [Full Text] |
| 10. | Wang W, Seeruttun SR, Fang C, Chen J, Li Y, Liu Z, Zhan Y, Li W, Chen Y, Sun X, Li Y, Xu D, Guan Y, Zhou Z. Prognostic Significance of Carcinoembryonic Antigen Staining in Cancer Tissues of Gastric Cancer Patients. Ann Surg Oncol. 2016;23:1244-1251. [PubMed] [DOI] [Full Text] |
| 11. | Park SH, Ku KB, Chung HY, Yu W. Prognostic significance of serum and tissue carcinoembryonic antigen in patients with gastric adenocarcinomas. Cancer Res Treat. 2008;40:16-21. [PubMed] [DOI] [Full Text] |
| 12. | Liu X, Qiu H, Liu J, Chen S, Xu D, Li W, Zhan Y, Li Y, Chen Y, Zhou Z, Sun X. Combined preoperative concentrations of CEA, CA 19-9, and 72-4 for predicting outcomes in patients with gastric cancer after curative resection. Oncotarget. 2016;7:35446-35453. [PubMed] [DOI] [Full Text] |
| 13. | Wang W, Chen XL, Zhao SY, Xu YH, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget. 2016;7:35423-35436. [PubMed] [DOI] [Full Text] |
| 14. | Feng F, Tian Y, Xu G, Liu Z, Liu S, Zheng G, Guo M, Lian X, Fan D, Zhang H. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer. 2017;17:737. [PubMed] [DOI] [Full Text] |
| 15. | Uda H, Kanda M, Tanaka C, Kobayashi D, Inaoka K, Tanaka Y, Hayashi M, Iwata N, Yamada S, Fujii T, Sugimoto H, Murotani K, Fujiwara M, Kodera Y. Perioperative Serum Carcinoembryonic Antigen Levels Predict Recurrence and Survival of Patients with Pathological T2-4 Gastric Cancer Treated with Curative Gastrectomy. Dig Surg. 2018;35:55-63. [PubMed] [DOI] [Full Text] |
| 16. | Kanda M, Murotani K, Kobayashi D, Tanaka C, Yamada S, Fujii T, Nakayama G, Sugimoto H, Koike M, Fujiwara M, Kodera Y. Postoperative adjuvant chemotherapy with S-1 alters recurrence patterns and prognostic factors among patients with stage II/III gastric cancer: A propensity score matching analysis. Surgery. 2015;158:1573-1580. [PubMed] [DOI] [Full Text] |
| 17. | Liu JY, Peng CW, Yang XJ, Huang CQ, Li Y. The prognosis role of AJCC/UICC 8th edition staging system in gastric cancer, a retrospective analysis. Am J Transl Res. 2018;10:292-303. [PubMed] |
| 18. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [PubMed] [DOI] [Full Text] |
| 19. | Kanda M, Shimizu D, Tanaka H, Tanaka C, Kobayashi D, Hayashi M, Iwata N, Niwa Y, Yamada S, Fujii T, Sugimoto H, Murotani K, Fujiwara M, Kodera Y. Significance of SYT8 For the Detection, Prediction, and Treatment of Peritoneal Metastasis From Gastric Cancer. Ann Surg. 2018;267:495-503. [PubMed] [DOI] [Full Text] |
| 20. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [PubMed] [DOI] [Full Text] |
| 21. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [PubMed] [DOI] [Full Text] |
| 22. | Chen S, Feng XY, Li YF, Zhao BW, Zhou ZW, Chen YB. The prognosis of gastric cancer patients with marginally elevated carcinoembryonic antigen (CEA) values after D2 radical gastrectomy. J Surg Oncol. 2013;107:641-645. [PubMed] [DOI] [Full Text] |
| 23. | Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [PubMed] [DOI] [Full Text] |
| 24. | Zhang Q, Qu H, Sun G, Li Z, Ma S, Shi Z, Zhao E, Zhang H, He Q. Early postoperative tumor marker responses provide a robust prognostic indicator for N3 stage gastric cancer. Medicine (Baltimore). 2017;96:e7560. [PubMed] [DOI] [Full Text] |
| 25. | Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467-481. [PubMed] [DOI] [Full Text] |
| 26. | Del Villano BC, Brennan S, Brock P, Bucher C, Liu V, McClure M, Rake B, Space S, Westrick B, Schoemaker H, Zurawski VR Jr. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983;29:549-552. [PubMed] |
| 27. | Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957-971. [PubMed] [DOI] [Full Text] |
| 28. | Kim JH, Jun KH, Jung H, Park IS, Chin HM. Prognostic Value of Preoperative Serum Levels of Five Tumor Markers (Carcinoembryonic Antigen, CA19-9, Alpha-fetoprotein, CA72-4, and CA125) in Gastric Cancer. Hepatogastroenterology. 2014;61:863-869. [PubMed] |
| 29. | He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87. [PubMed] [DOI] [Full Text] |
| 30. | Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266-270. [PubMed] [DOI] [Full Text] |
| 31. | Park JK, Paik WH, Ryu JK, Kim YT, Kim YJ, Kim J, Song BJ, Park JM, Yoon YB. Clinical significance and revisiting the meaning of CA 19-9 blood level before and after the treatment of pancreatic ductal adenocarcinoma: analysis of 1,446 patients from the pancreatic cancer cohort in a single institution. PLoS One. 2013;8:e78977. [PubMed] [DOI] [Full Text] |
| 32. | Kanda M, Shimizu D, Tanaka H, Tanaka C, Kobayashi D, Hayashi M, Takami H, Niwa Y, Yamada S, Fujii T, Sugimoto H, Kodera Y. Synaptotagmin XIII expression and peritoneal metastasis in gastric cancer. Br J Surg. 2018;105:1349-1358. [PubMed] [DOI] [Full Text] |
| 33. | Kanda M, Tanaka H, Shimizu D, Miwa T, Umeda S, Tanaka C, Kobayashi D, Hattori N, Suenaga M, Hayashi M, Iwata N, Yamada S, Fujiwara M, Kodera Y. SYT7 acts as a driver of hepatic metastasis formation of gastric cancer cells. Oncogene. 2018;37:5355-5366. [PubMed] [DOI] [Full Text] |
| 34. | Hasegawa H, Fujitani K, Nakazuru S, Hirao M, Yamamoto K, Mita E, Tsujinaka T. Optimal treatment change criteria for advanced gastric cancer with non-measurable peritoneal metastasis: symptom/tumor marker-based versus CT-based. Anticancer Res. 2014;34:5169-5174. [PubMed] |
| 35. | Wada N, Kurokawa Y, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. The characteristics of the serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer cases. Surg Today. 2017;47:227-232. [PubMed] [DOI] [Full Text] |
| 36. | Cheong JH, Yang HK, Kim H, Kim WH, Kim YW, Kook MC, Park YK, Kim HH, Lee HS, Lee KH, Gu MJ, Kim HY, Lee J, Choi SH, Hong S, Kim JW, Choi YY, Hyung WJ, Jang E, Kim H, Huh YM, Noh SH. Predictive test for chemotherapy response in resectable gastric cancer: a multi-cohort, retrospective analysis. Lancet Oncol. 2018;19:629-638. [PubMed] [DOI] [Full Text] |