Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. Apr 15, 2025; 17(4): 97644
Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.97644
Published online Apr 15, 2025. doi: 10.4251/wjgo.v17.i4.97644
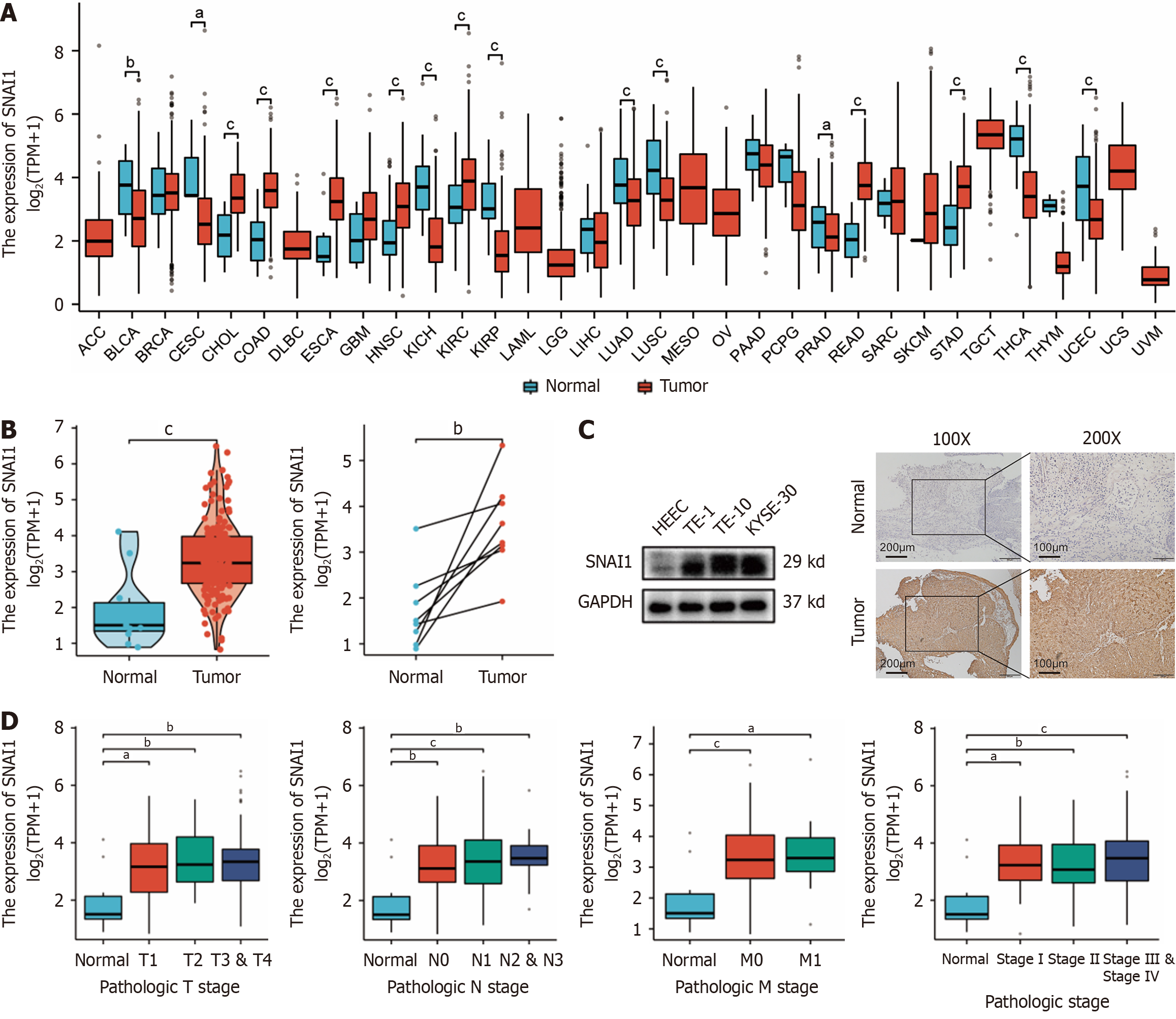
Figure 1 Snail family transcriptional repressor 1 is highly expressed in esophageal cancer and significantly correlates with tumor clinical stage and tumor-node-metastasis stage.
A: Expression of Snail family transcriptional repressor 1 (SNAI1) in various tumors and adjacent tissues; B: Expression of SNAI1 in paired and unpaired esophageal cancer samples compared with that in adjacent tissues; C: Detection of SNAI1 expression in normal esophageal epithelial cells, TE-1, TE-10 and KYSE-30 cells by Western blot and SNAI1 expression in clinical esophageal cancer patient samples compared with that in adjacent tissues by immunohistochemistry; D: Expression of SNAI1 in different tumor-node-metastasis stages and pathological grades. Kruskal-Wallis test was performed. The error bars represent the SD. aP < 0.05; bP < 0.01; and cP < 0.001.
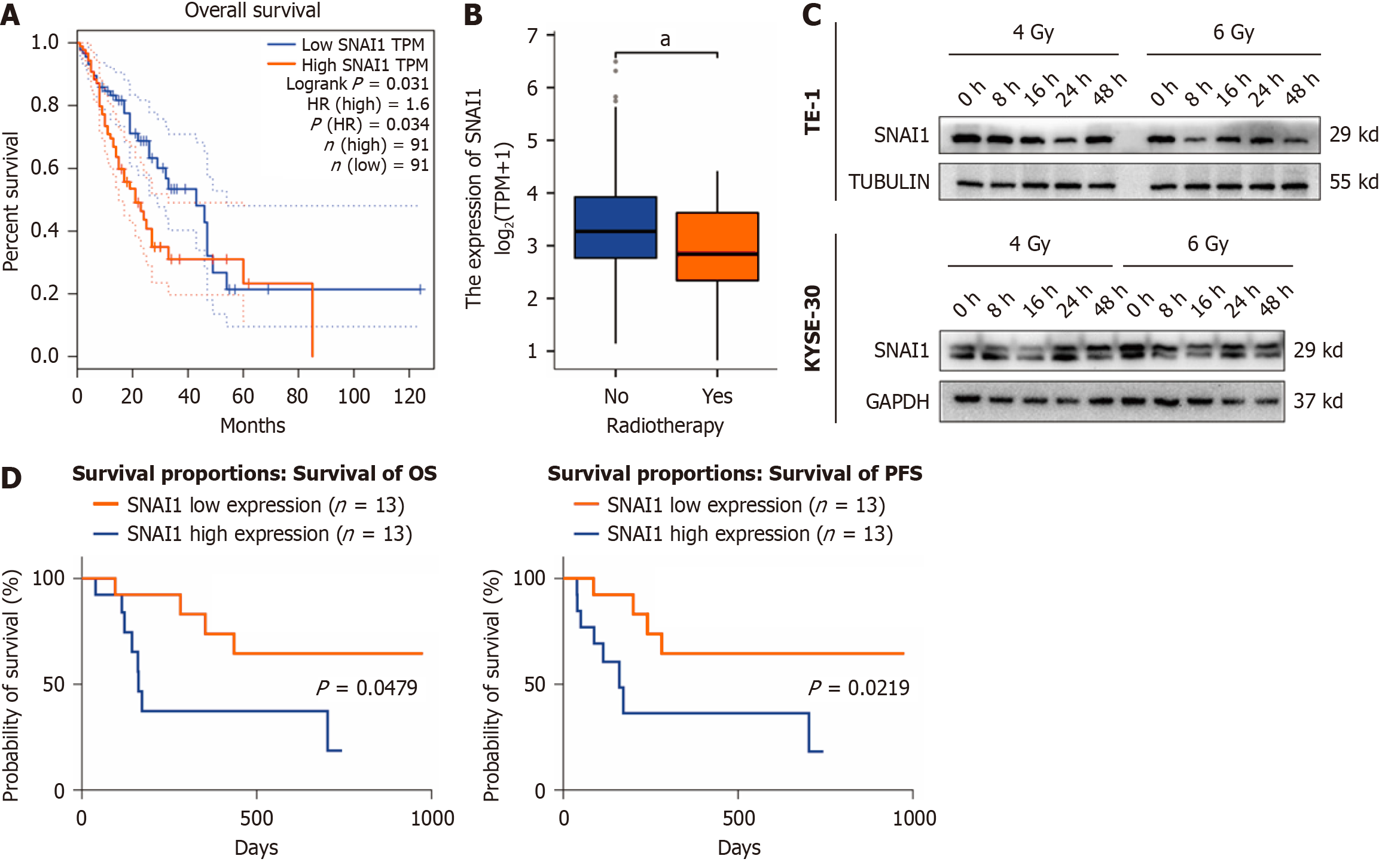
Figure 2 Improved prognosis in esophageal cancer patients with low Snail family transcriptional repressor 1 expression who were receiving radiotherapy.
A: Survival curve showing the relationship between Snail family transcriptional repressor 1 (SNAI1) expression and prognosis in esophageal cancer patients; B: Expression of SNAI1 in esophageal cancer patients with or without radiotherapy; C: Expression of SNAI1 in TE-1 and KYSE-30 cells at different time points after 4 and 6 Gy X-ray irradiation; D: Survival curve correlating SNAI1 expression with radiotherapy outcomes in esophageal cancer patients. Kruskal-Wallis test was performed. The error bars represent the SD. aP < 0.05; OS: Overall survival; PFS: Progression-free survival.
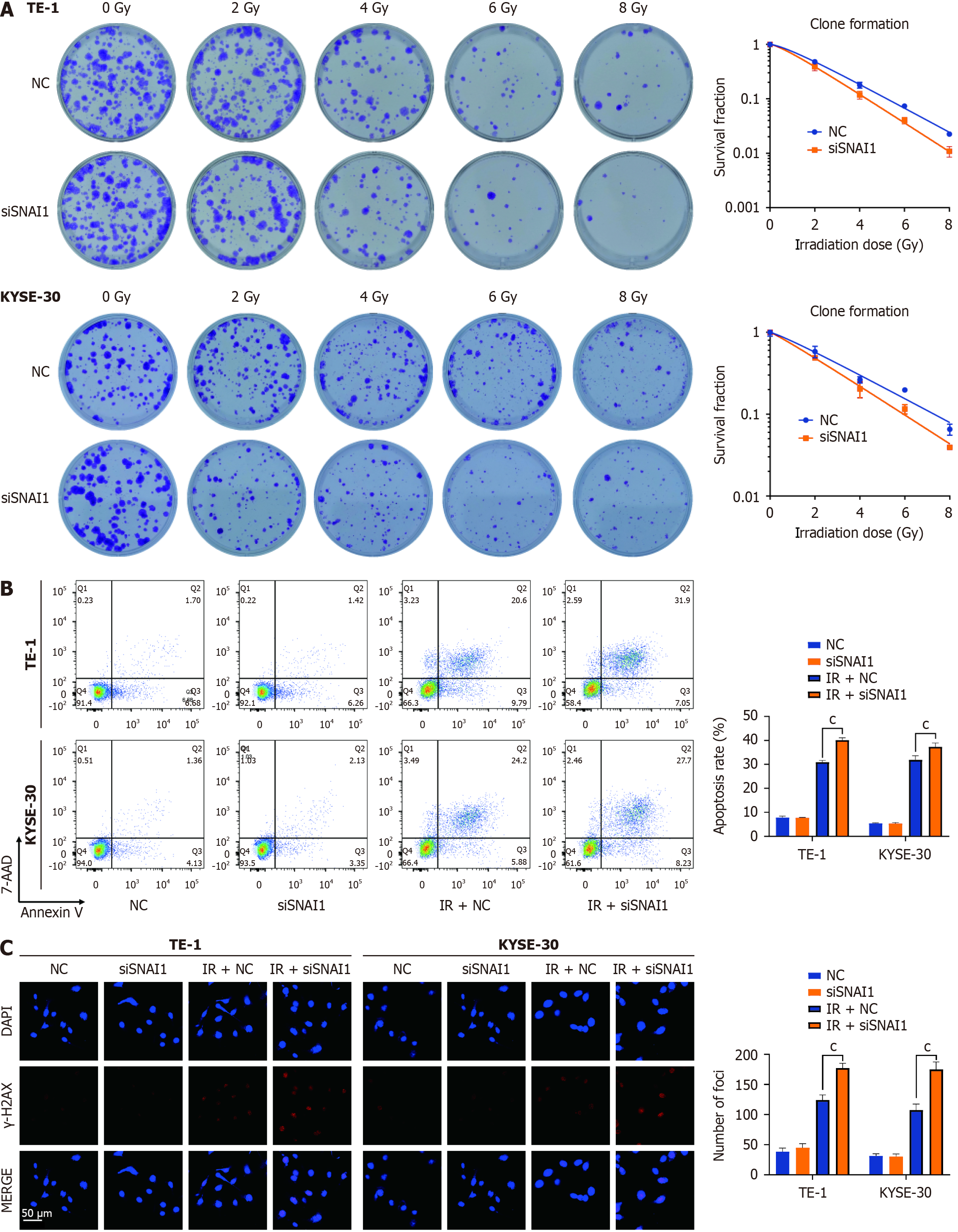
Figure 3 Downregulation of Snail family transcriptional repressor 1 enhances the radiosensitivity of esophageal cancer cells in vitro.
A: Clonogenic formation rates of TE-1 and KYSE-30 cells with different radiation doses in the NC and siSnail family transcriptional repressor 1 (SNAI1) groups; B: Apoptosis rates in TE-1 and KYSE-30 cells in the NC and siSNAI1 groups, with or without 6 Gy X-ray irradiation; C: Expression of γ-H2AX in TE-1 and KYSE-30 cells under NC and siSNAI1 conditions, with or without exposure to 6 Gy X-ray irradiation. ANOVA was conducted. The error bars represent the SD. cP < 0.001.
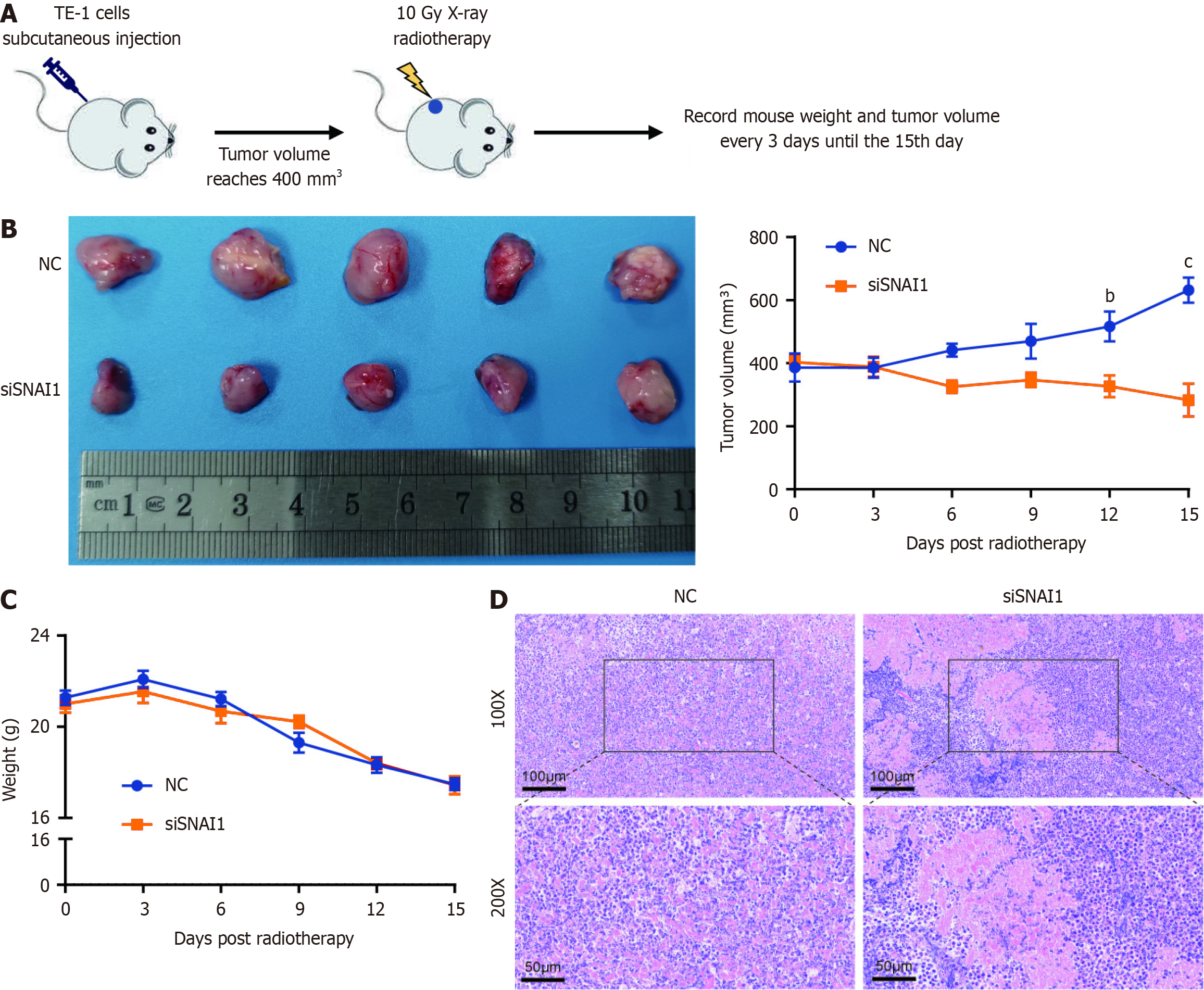
Figure 4 Snail family transcriptional repressor 1 silencing radiosensitizes esophageal cancer in vivo.
A: Construction of xenograft tumors in nude mice and radiation therapy schematic; B: Body weight change curves in the negative control (NC) and siSnail family transcriptional repressor 1 (SNAI1) groups; C: Mouse tumor images and tumor volume changes after radiotherapy in the NC and siSNAI1 groups; D: Hematoxylin & eosin staining of tumor tissues in the NC and siSNAI1 groups. ANOVA was conducted. The error bars represent the SDs. bP < 0.01, and cP < 0.001; NC: Negative control; siSNAI1: Small interfering Snail family transcriptional repressor 1.
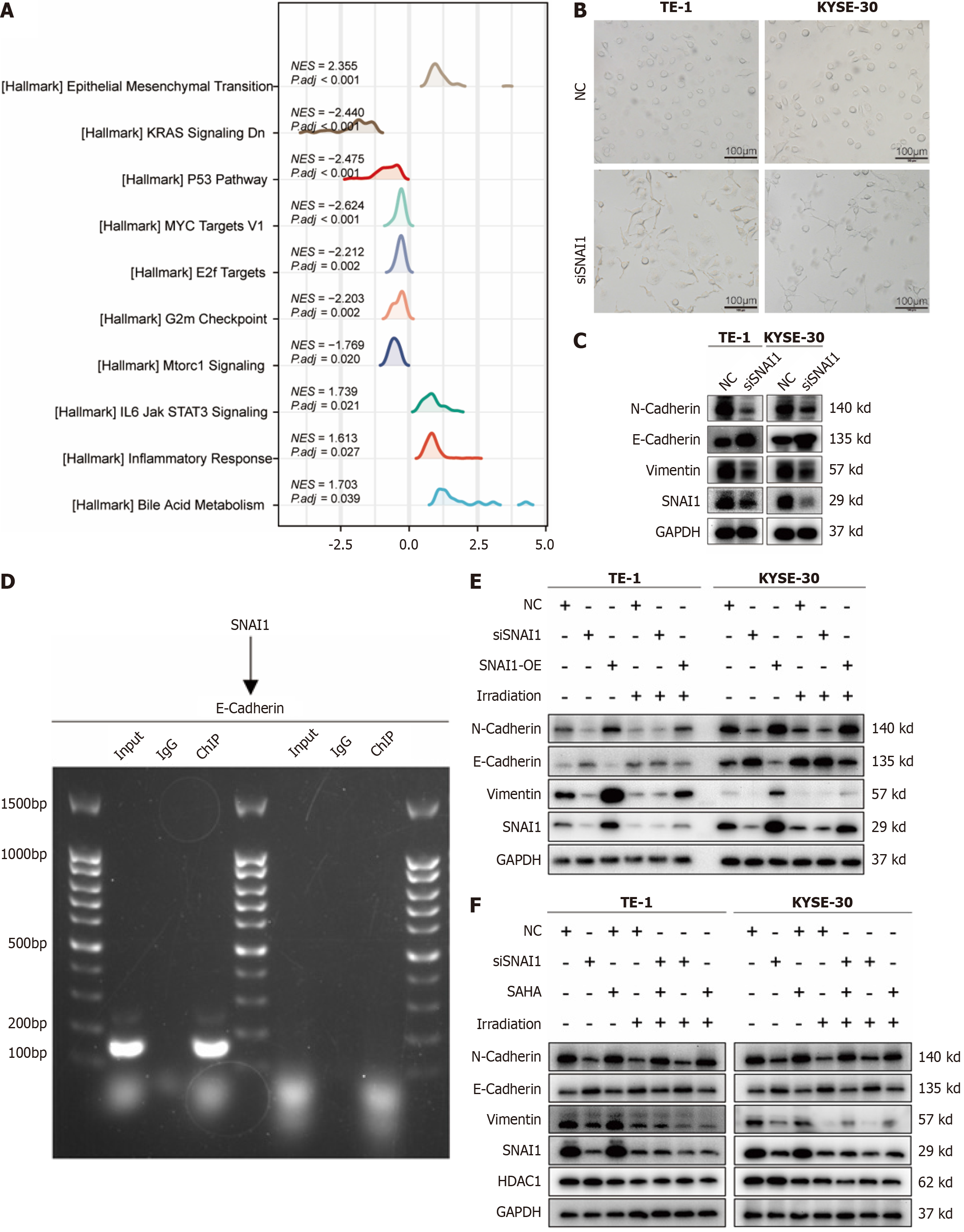
Figure 5 Snail family transcriptional repressor 1 regulates esophageal cancer cell radiosensitivity by epithelial-mesenchymal transition-related deacetylation.
A: Gene set enrichment analysis of Snail family transcriptional repressor 1 (SNAI1) in esophageal cancer; B: Phenotypic changes in TE-1 and KYSE-30 cells upon SNAI1 knockdown; C: Changes in epithelial-mesenchymal transition (EMT) markers upon SNAI1 downregulation in TE-1 and KYSE-30 cells; D: Chromatin immunoprecipitation with subsequent E-cadherin PCR amplification gel electrophoresis; E: Western blot analysis of EMT marker expression in TE-1 and KYSE-30 cells under small interfering SNAI1 (siSNAI1), SNAI1-OE, and irradiation; F: Western blot analysis of EMT marker expression in TE-1 and KYSE-30 cells under siSNAI1, SAHA, and irradiation. NC: Negative control; siSNAI1: Small interfering Snail family transcriptional repressor 1.
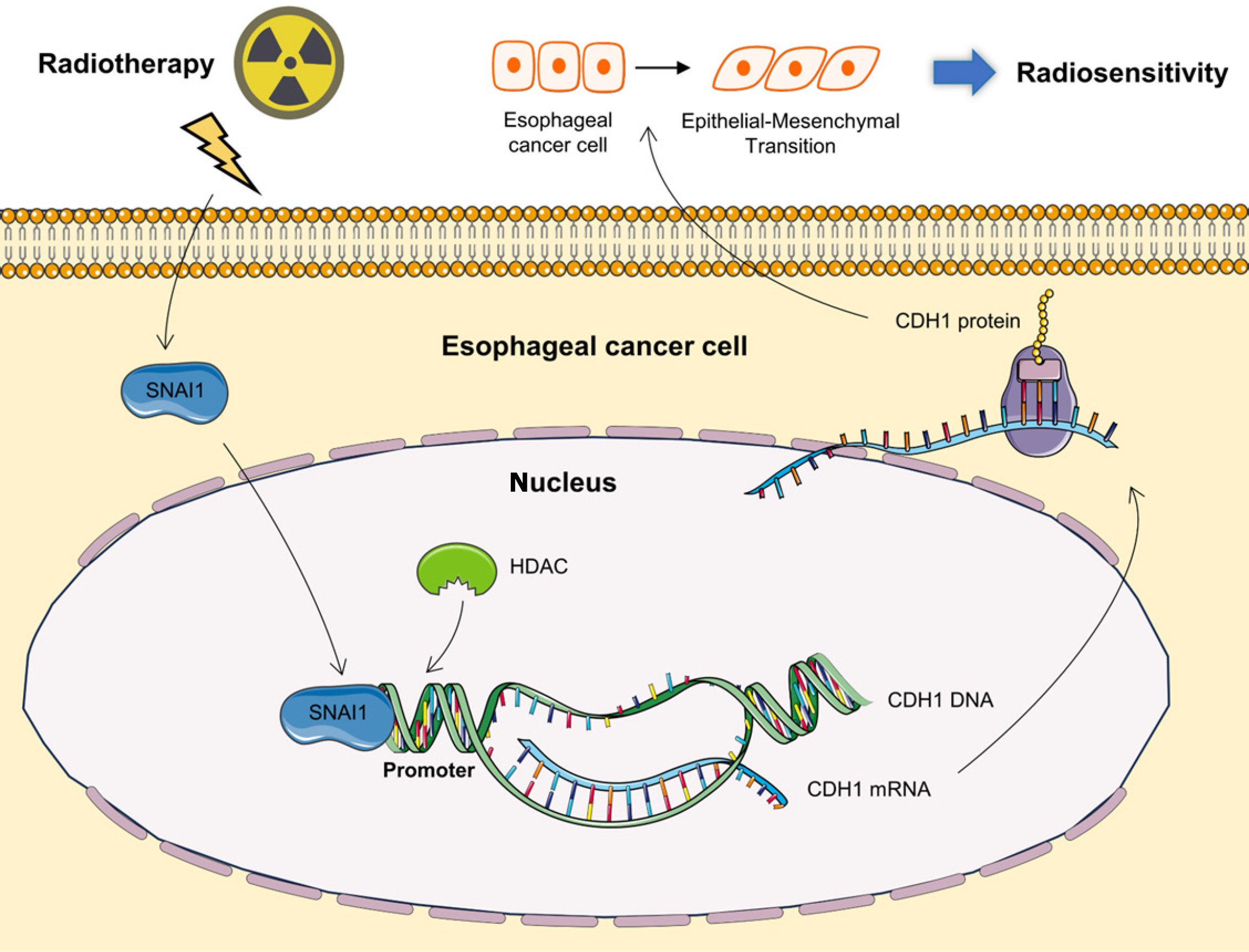
Figure 6 Schematic representation of the ionizing radiation-mediated regulation of radiosensitivity in esophageal cancer cells through Snail family transcriptional repressor 1.
Ionizing radiation influences Snail family transcriptional repressor 1 by diminishing its recruitment at the deacetylation site within the CDH1 promoter region. This attenuation culminates in the upregulation of CDH1 expression, thereby promoting the epithelial-mesenchymal transition process within cells. Ultimately, these molecular events play a pivotal role in shaping the radiosensitivity of esophageal cancer cells. SNAI1: Snail family transcriptional repressor 1.
- Citation: Lv XL, Peng QL, Wang XP, Fu ZC, Cao JP, Wang J, Wang LL, Jiao Y. Snail family transcriptional repressor 1 radiosensitizes esophageal cancer via epithelial-mesenchymal transition signaling: From bioinformatics to integrated study. World J Gastrointest Oncol 2025; 17(4): 97644
- URL: https://www.wjgnet.com/1948-5204/full/v17/i4/97644.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i4.97644