Copyright
©The Author(s) 2023.
World J Gastrointest Oncol. May 15, 2023; 15(5): 757-775
Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.757
Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.757
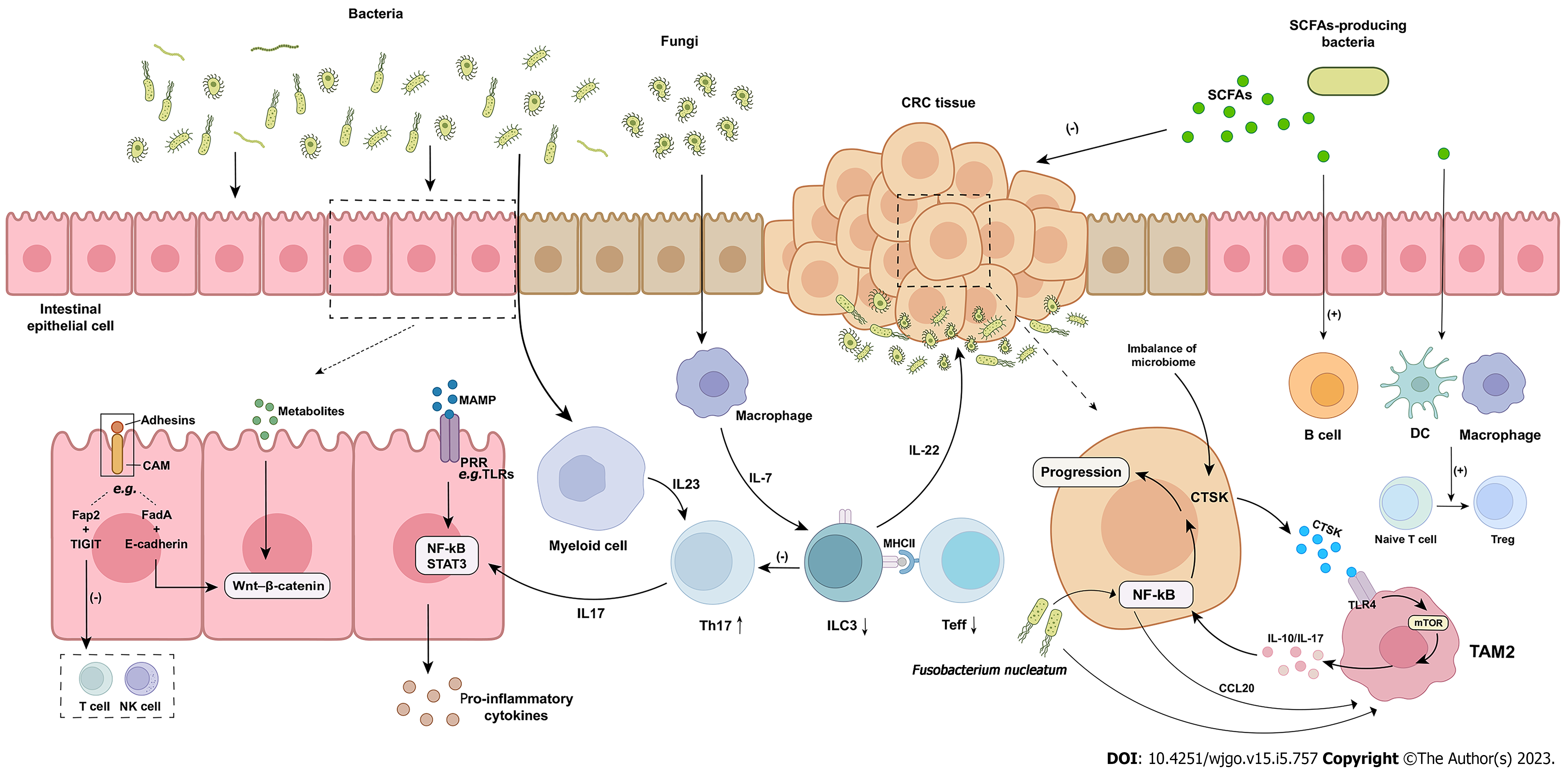
Figure 1 The role of microbiome in the occurrence and development of colorectal cancer.
Microbiome influences the occurrence and development of colorectal cancer through a variety of mechanisms. Microbiome and their metabolites can induce tumorigenesis through direct mutagenesis of intestinal epithelial cells or activation of intracellular carcinogenic signals. Bacterial metabolites can also trigger the release of pro-inflammatory signals, which further promote tumorigenesis. Pathogenic bacteria or their products activate tumor-associated myeloid cells and induce tumor-promoting inflammation. Symbiotic fungi activate the production of interleukin (IL)-7 in macrophages and induce the production of IL-22 in ILC3, leading to tumor progression. ILC3s inhibited Th17 cells and intestinal inflammation. ILC3s decreased significantly and Th17 increased in colorectal cancer. The dialogue between ILC3s, effector T cell and major histocompatibility complex class II is disrupted by colorectal cancer and intestinal inflammation, promoting the progress of colorectal cancer. Fusobacterium nucleatum upregulates the expression of chemokine (C-C motif) ligand 20 through nuclear factor-κB and induces M2 macrophages to polarize and promote tumor metastasis. Cathepsin K mediates tumor invasion and metastasis. Short chain fatty acids directly inhibits tumor cell growth and induces host macrophage, T and B cell responses to protect colitis-induced colorectal cancer. CRC: Colorectal cancer; CAM: Cell adhesion molecule; FadA: Fusobacterium adhesin A; TIGIT: T-cell immunoglobulin and ITIM domain; MAMP: Microbe-associated molecular pattern; PRR: Pattern recognition receptor; NF-κB: Nuclear factor-κB; STAT3: Signal transducer and activator of transcription 3; MHCII: Major histocompatibility complex class II; Teff: Effector T cell; CTSK: Cathepsin K; TLR4: Toll-like receptor 4; TAM: Tumor-associated macrophages; SCFA: Short chain fatty acid; DC: Dendritic cell; IL: Interleukin; NK: Natural killer; CCL20: Chemokine (C-C motif) ligand 20.
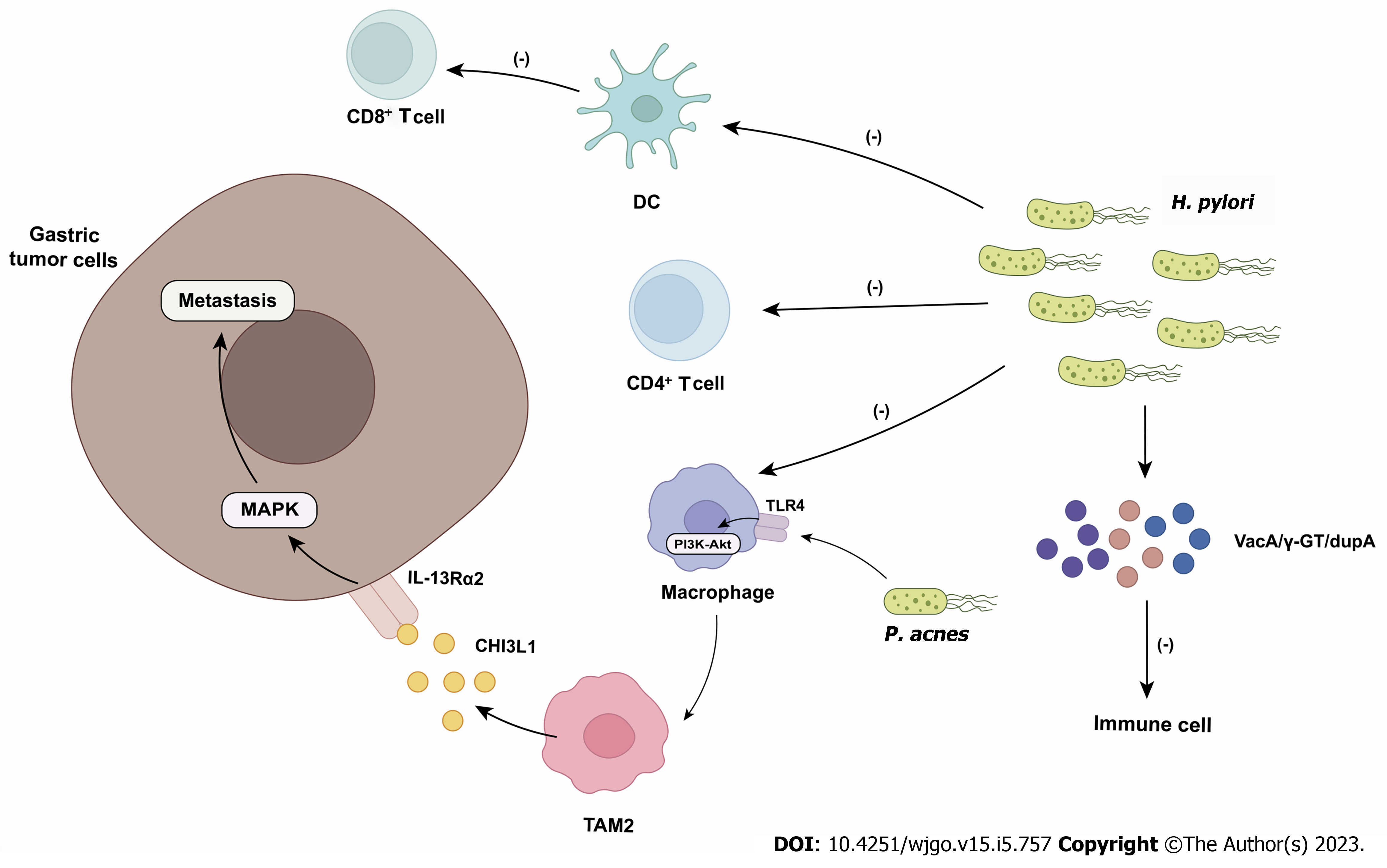
Figure 2 The role of microbiome in gastric cancer.
Helicobacter pylori inhibits the effector functions of CD4+ T cells, dendritic cells and macrophages and suppresses antitumor CD8+ T cell responses by altering the cross-presentation activity of dendritic cells. Helicobacter pylori also produce virulence factors (e.g., VacA, γ-GT, dupA) to impair immune cell activity. Propionibacterium acnes promote M2 polarization of macrophages via toll-like receptor 4/PI3K/Akt signaling. M2 macrophages secrete chitinase 3-like protein 1 that binds specifically to the interleukin-13 receptor α2 chai of tumor cells, triggering the mitogen-activated protein kinase signaling pathway and promoting gastric cancer metastasis. DC: Dendritic cell; TLR4: Toll-like receptor 4; TAM: Tumor-associated macrophages; CHI3L1: Chitinase 3-like protein 1; IL-13Rα2: Interleukin-13 receptor α2 chain; MAPK: Mitogen-activated protein kinase; H. pylori: Helicobacter pylori; P. acnes: Propionibacterium acnes.
- Citation: Lin Q, Guan SW, Yu HB. Immuno-oncology-microbiome axis of gastrointestinal malignancy. World J Gastrointest Oncol 2023; 15(5): 757-775
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/757.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.757