Copyright
©The Author(s) 2023.
World J Gastrointest Oncol. Apr 15, 2023; 15(4): 689-699
Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.689
Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.689
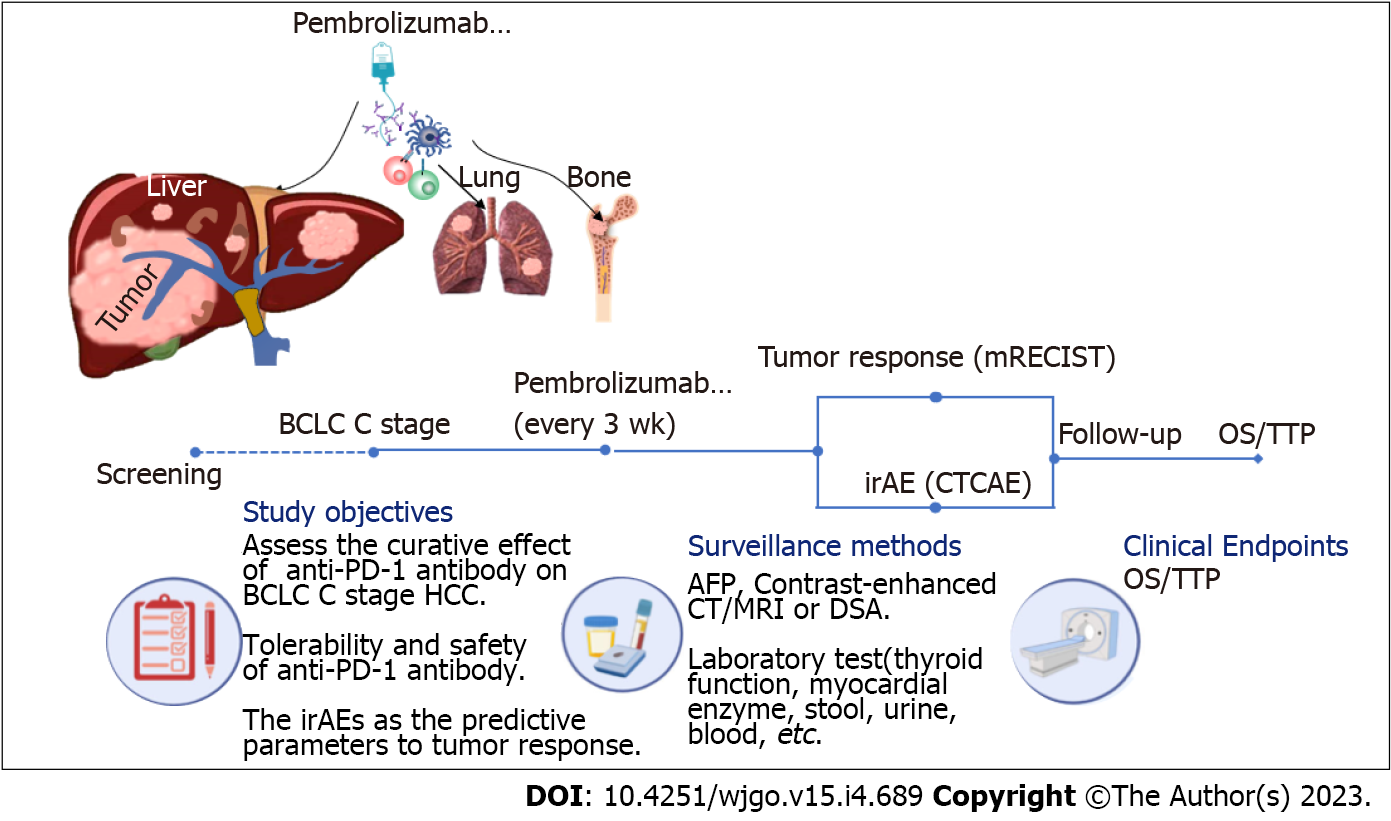
Figure 1 Flow diagram of the study.
mTECIST: Modified response evaluation criteria in solid tumors criteria; CTCAE: Common Terminology Criteria for Adverse Events; HCC: Hepatocellular carcinoma; AFP: Alpha fetoprotein; DSA: Digital subtraction angiography; OS: Overall survival; TTP: Time to progression; BCLC: Barcelona Clinic Liver Cancer; PD-1: Programmed death 1; CT: Computed tomography; MRI: Magnetic resonance imaging; irAE: Immune-related adverse event.
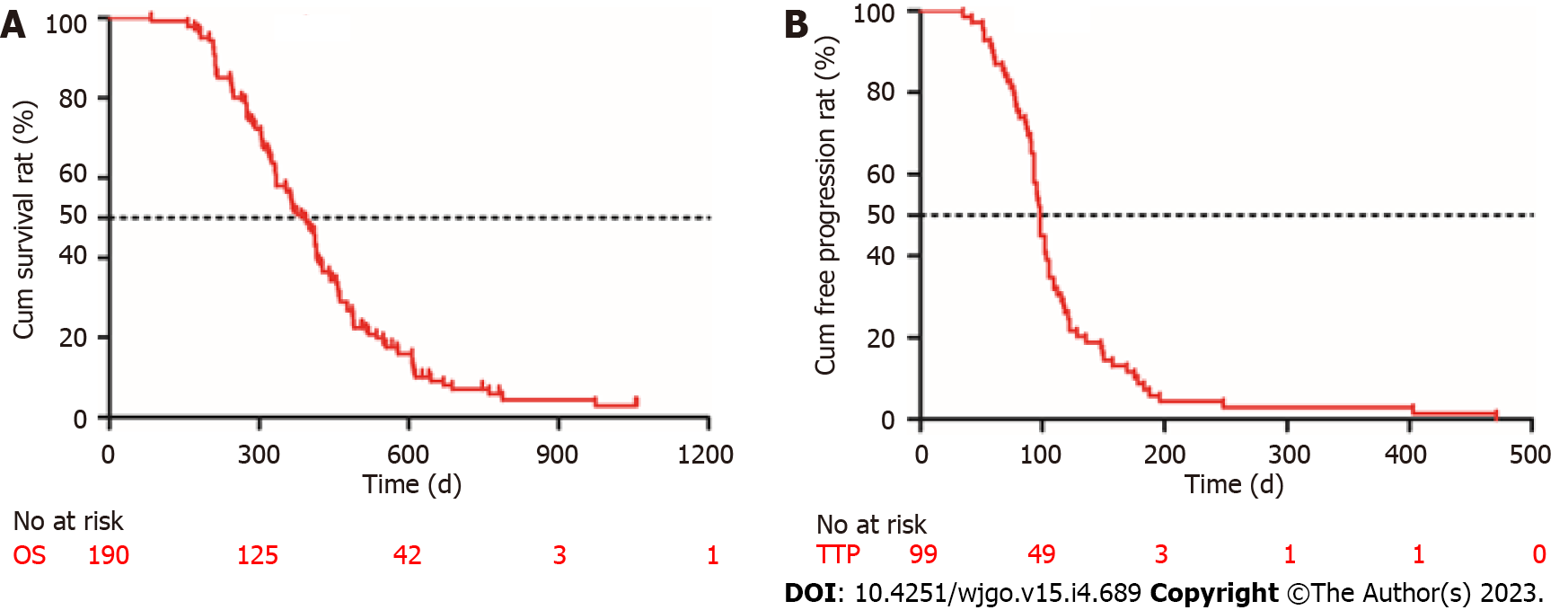
Figure 2 Kaplan-Meier curves describing the overall survival of all patients (n = 190) and the time to progression of patients who achieved disease control (n = 99).
A: Survival curve; B: Time to progression curve. OS: Overall survival; TTP: Time to progression.
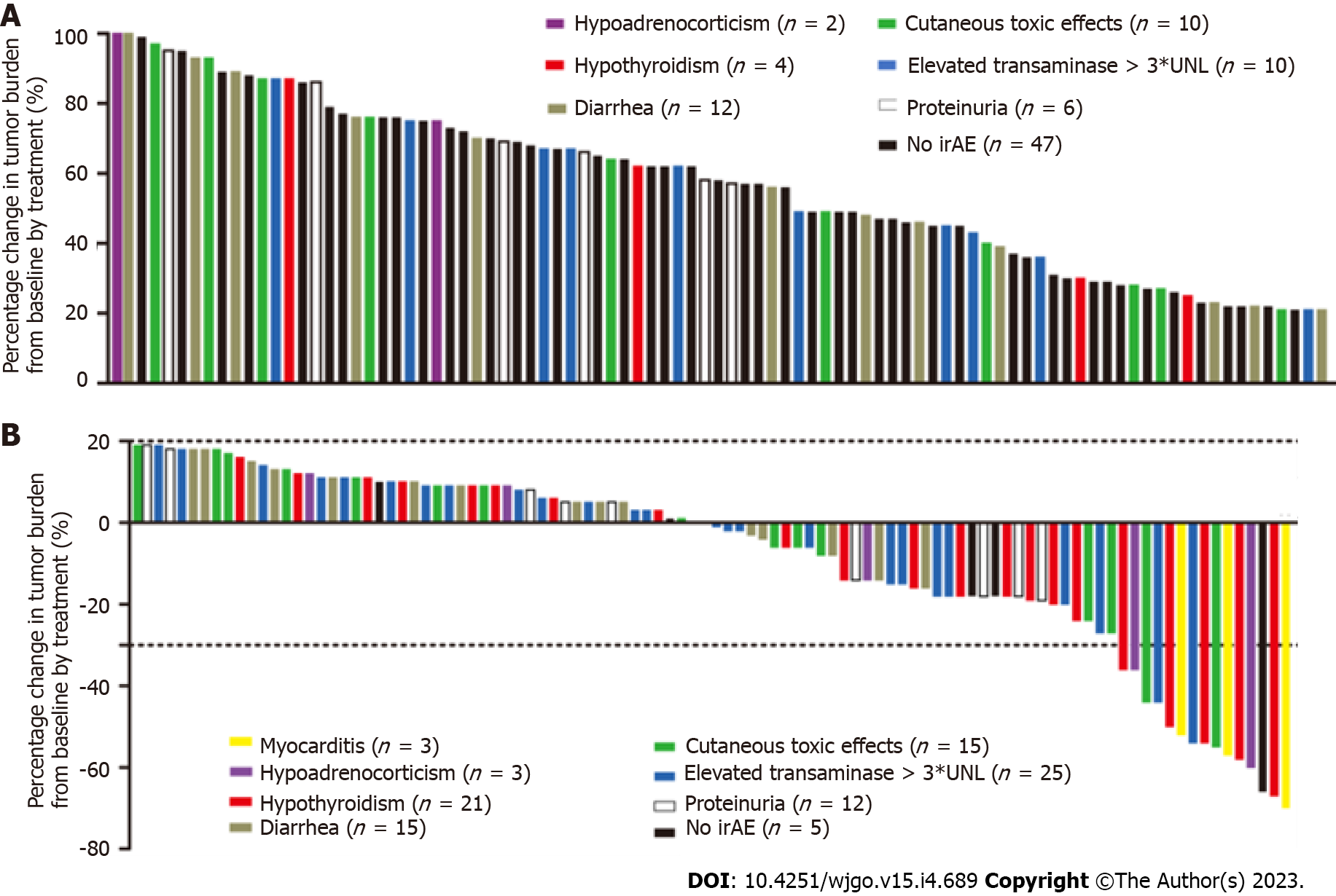
Figure 3 Waterfall plots of percentage change in tumor burden from baseline (n = 190).
The “y” axis represents the percentage change in tumor burden from baseline by treatment. The immune-related adverse events are distinguished by different colors. Negative/positive values represent maximum tumor reduction or minimum tumor increase, respectively. A: 91 patients had progressive disease; B: 99 patients achieved complete response, partial response, or stable disease. irAE: Immune-related adverse event.
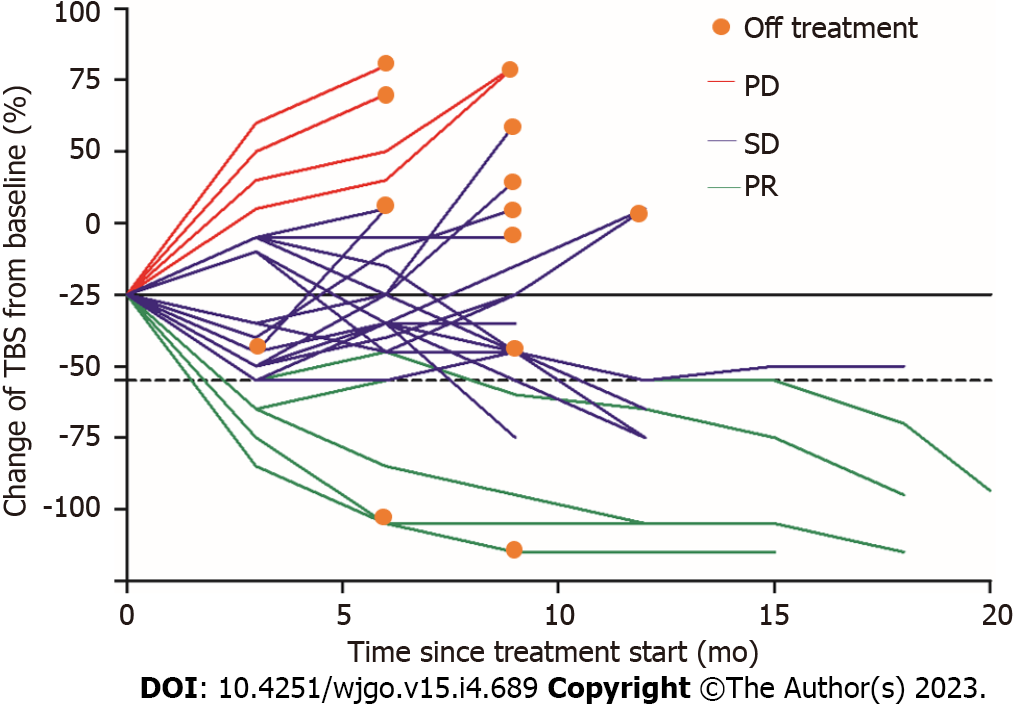
Figure 4 Spider plot displaying tumor response in 25 patients with hypothyroidism.
PD: Progressive disease; SD: Stable disease; PR: Partial response; TBS: Tumor burden score.
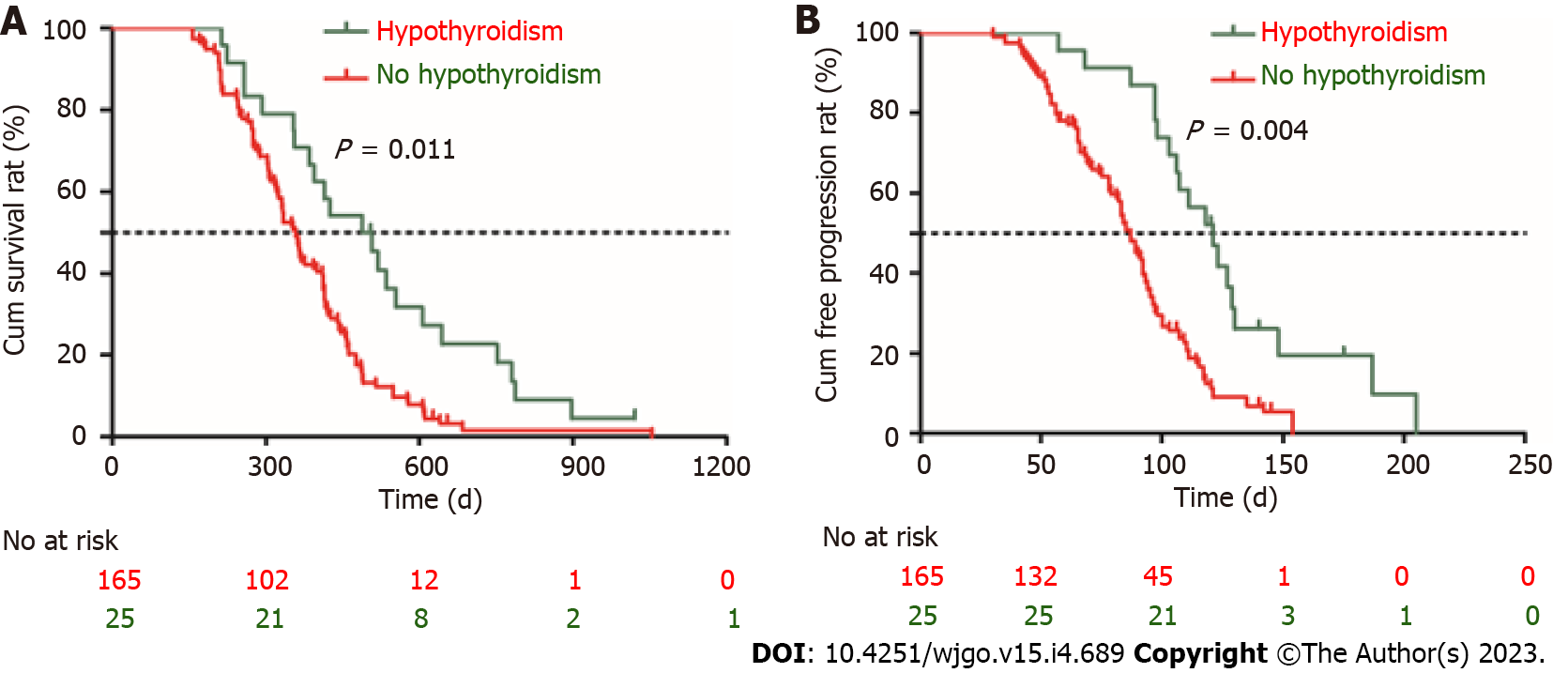
Figure 5 Prognosis comparison between hypothyroidism group and non-hypothyroidism group.
A: Survival curve; B: Time to progression curve.
- Citation: Zhou JM, Xiong HF, Chen XP, Zhang ZW, Zhu LP, Wu B. Correlation between immune-related adverse events and long-term outcomes in pembrolizumab-treated patients with unresectable hepatocellular carcinoma: A retrospective study. World J Gastrointest Oncol 2023; 15(4): 689-699
- URL: https://www.wjgnet.com/1948-5204/full/v15/i4/689.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i4.689