Copyright
©The Author(s) 2023.
World J Gastrointest Oncol. Feb 15, 2023; 15(2): 251-267
Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.251
Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.251
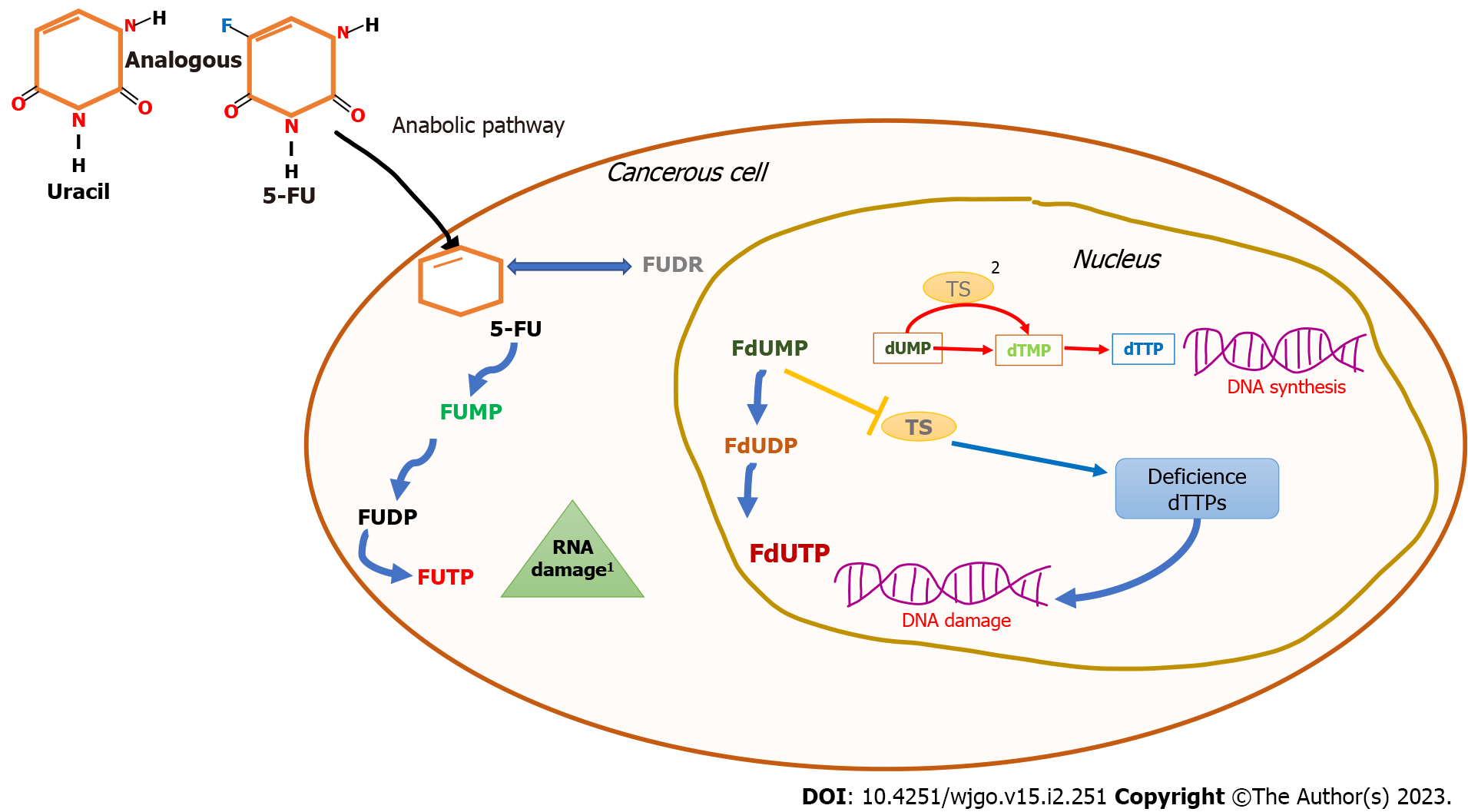
Figure 1 5-Fluorouracil mechanism of action.
The 5-fluorouracil structure is analogous to that of the nucleotide uracil; its ability to disrupt standard RNA processing and function is mediated by three primary metabolites: fluorodeoxyuridine monophosphate, fluorodeoxyuridine diphosphate, and fluorouridine triphosphate. 1: 5-fluorouracil inhibits thymidylate synthase activity by fluorodeoxyuridine monophosphate metabolite binding, blocking the typical substrate deoxyuridine monophosphate that inhibits deoxythymidine monophosphate synthesis leading to deoxythymidine triphosphate imbalance. The consequent result is DNA damage due to a deficiency in its synthesis and its repair; 2: DNA replication and repair are regulated by deoxyuridine monophosphate transition to deoxythymidine monophosphate. This step is coordinated by thymidylate synthase; FUMP: Fluorodeoxyuridine monophosphate; FUDP: Fluorodeoxyuridine diphosphate; FUTP: Fluorouridine triphosphate; dUMP: Deoxyuridine monophosphate; dTMP: Deoxythymidine monophosphate; FUDR: Fluorodeoxyuridine; FdUMP: Fluorodeoxyuridine monophosphate; dTTP: Deoxythymidine triphosphate; FdUDP: Fluorodeoxyuridine diphosphate; FdUTP: Fluorodeoxyuridine triphosphate; 5-FU: 5-Fluorouracil; TS: Thymidylate synthase.
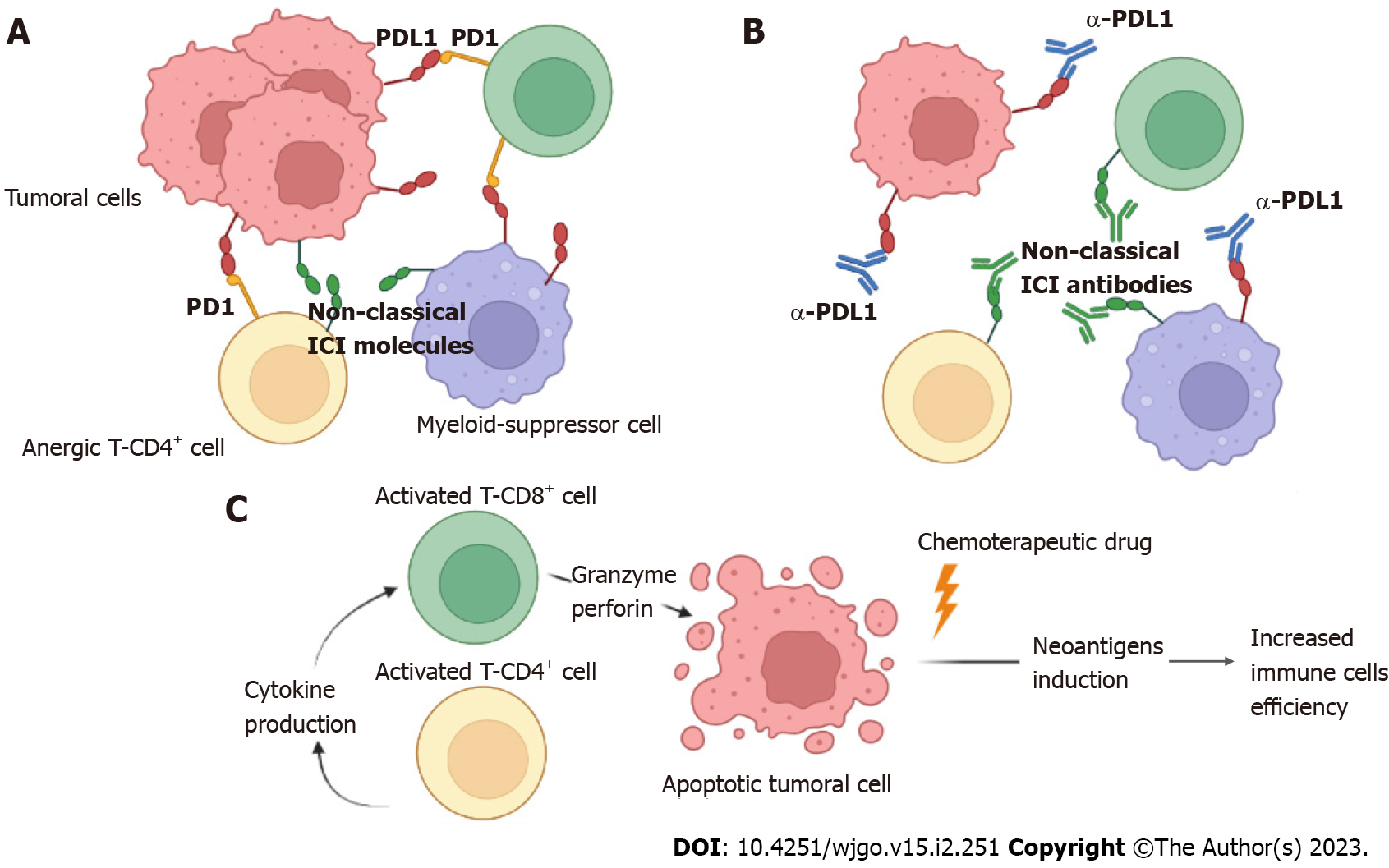
Figure 2 Mechanism of action using either anti-programmed cell death ligand 1 or anti-nonclassical immune checkpoint inhibitor antibodies to increase the effector immune response in colorectal cancer.
A: The programmed cell death ligand 1 molecule expressed on both tumor and myeloid suppressor cells interacts with programmed cell death 1 molecules expressed on exhausted CD8+ and CD4+ T cells, inducing a state of anergy. Additionally, other nonclassical immune checkpoint inhibitor molecules can induce anergy in T cells; B: Anti-programmed cell death ligand 1 antibodies block the interaction of the programmed cell death 1/programmed cell death ligand 1 axis, favoring the return from the state of anergy to exert the effector function of CD4+ and CD8+ T cells, favoring the reduction of the tumor burden. Most likely, nonclassical immune checkpoint inhibitor antibodies may have a similar effect; C: Once CD4+ T cells are activated, they produce cytokines for the efficient activation of CD8+ T cells, which in turn produce granzyme and perforin, inducing apoptosis in tumor cells. The addition of chemotherapeutic drugs increases the induction of neoantigens, favoring immune response activation. PDL1: Programmed cell death ligand 1; PD1: Programmed cell death 1; ICI: Immune checkpoint inhibitor.
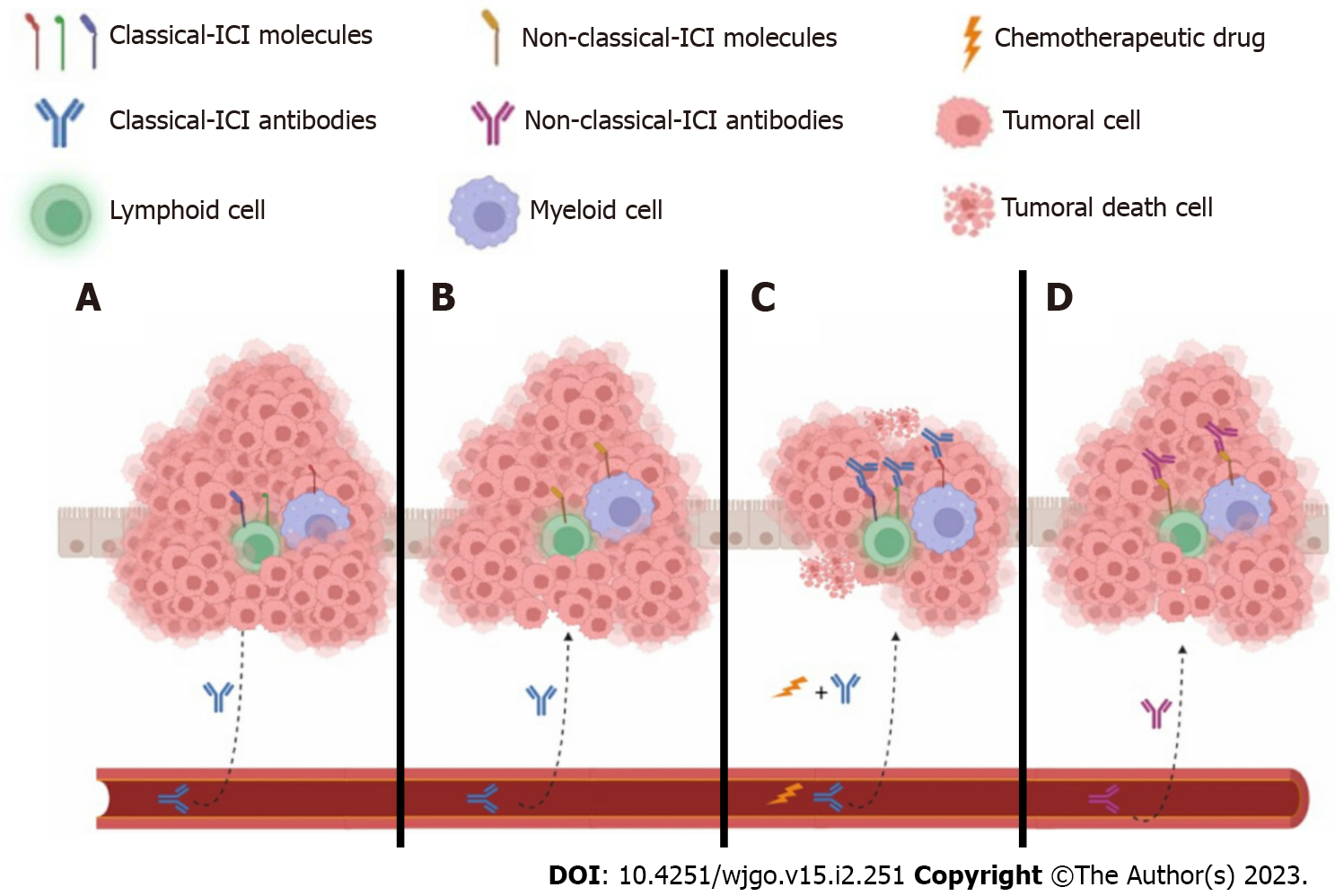
Figure 3 The tumor microenvironment favors or does not allow the access of both classical and nonclassical immune checkpoint inhibitors to their targets in immune cells.
A: The classical immune checkpoint inhibitor (ICI) antibody cannot access its target because immune cells are surrounded by tumor cells, although immune cells may express classical ICIs; B: On the other hand, classical ICIs probably have access to immune cells but may not express the targeting molecules; C: Chemotherapy can induce the death of tumoral cells, favoring the formation of neoantigens that may reactivate immune cells. Additional classical ICI antibodies target individual molecules such as cytotoxic T-lymphocyte-associated protein 4, programmed cell death 1, and programmed cell death ligand 1; D: Finally, immune cells expressing nonclassical ICIs could have effector profiles such as helper T type 1 cells, cytotoxic T cells, M1, or N1 when nonclassical antibodies target them. Additionally, tumor epithelial cells may likely express both classical and nonclassical ICIs. ICI: Immune checkpoint inhibitor.
- Citation: Olguin JE, Mendoza-Rodriguez MG, Sanchez-Barrera CA, Terrazas LI. Is the combination of immunotherapy with conventional chemotherapy the key to increase the efficacy of colorectal cancer treatment? World J Gastrointest Oncol 2023; 15(2): 251-267
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/251.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.251