Copyright
©The Author(s) 2022.
World J Gastrointest Oncol. Mar 15, 2022; 14(3): 547-567
Published online Mar 15, 2022. doi: 10.4251/wjgo.v14.i3.547
Published online Mar 15, 2022. doi: 10.4251/wjgo.v14.i3.547
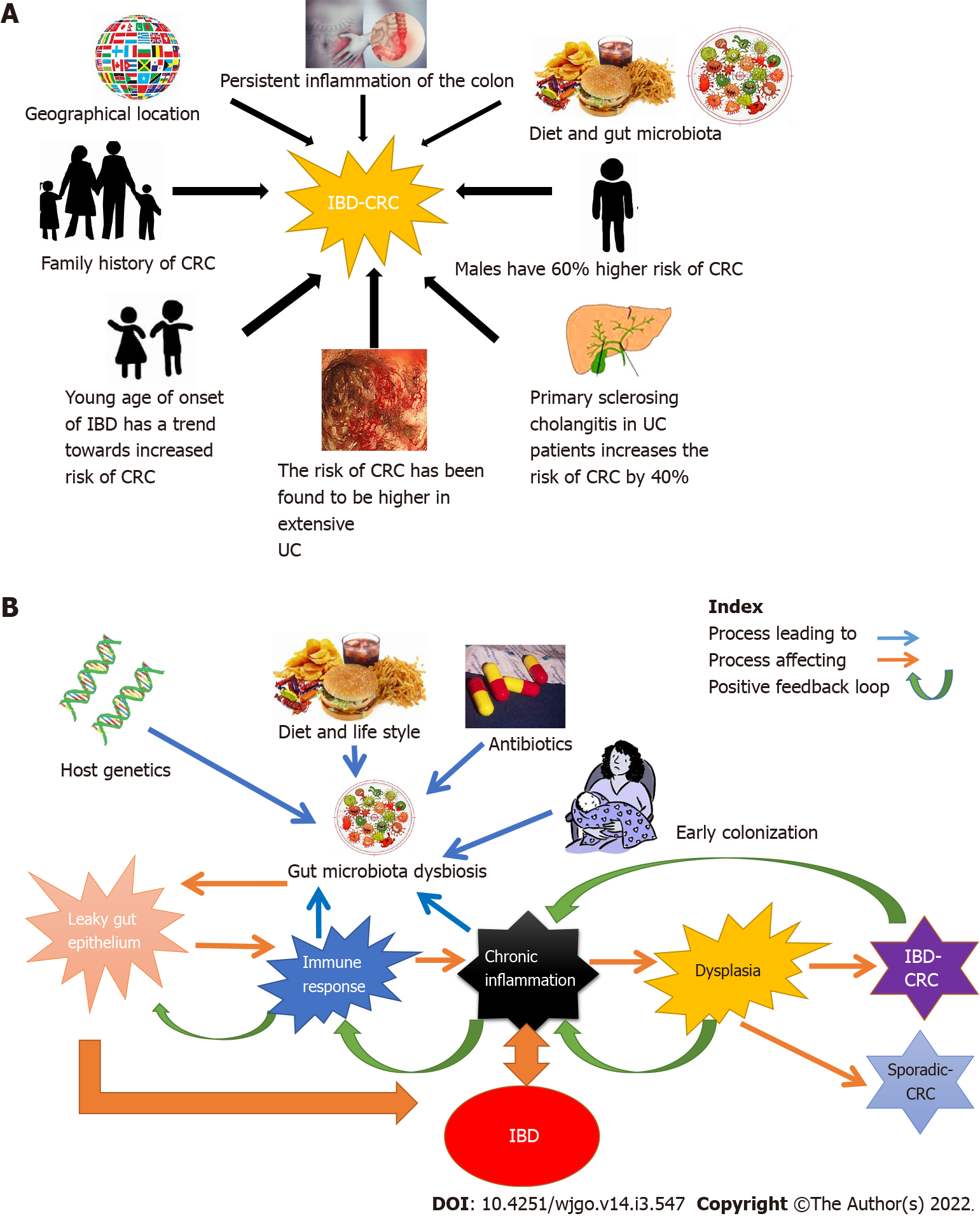
Figure 1 Risk factors leading to the development of inflammatory bowel disease-related colorectal cancer and the role of gut microbiota in inflammatory bowel disease-related colorectal cancer.
A: Risk factors leading to the development of inflammatory bowel disease-related colorectal cancer (IBD-CRC). Risk factors are classified as familial and genetic. The factors are depicted in clockwise order: Genetic factors include a person’s genetic makeup, family history of IBD and rarely monogenic causes of IBD. Younger age at diagnosis, male gender and durations of the disease has been identified as strong risk factors for IBD-CRC in longitudinal studies. The geographical location of the person, their diet, lifestyle, underlying diseases like extensive or left-sided ulcerative colitis, Primary sclerosing cholangitis, and other conditions causing persistent colon inflammation, are also known to increase the risk of development of IBD-CRC; B: Role of gut microbiota in IBD-CRC. Multiple factors such as diet, antibiotic use, and mode of birth and host genetic makeup influence/modulate the gut microbiota. This can lead to microbial dysbiosis mediated intestinal tissue damage causing intestinal barrier leak and mobilization of gut microbiota into host mucosa. The gut microbiota mediated break in the mucosal barrier, in turn, triggers an aggravated immune response leading to chronic inflammation. IBD, driven by an aberrant autoimmune response also leads to inflammation of the gut. This chronic inflammatory state leads to tissue damage causing dysplasia that can progress to cancer over time. CRC: Colorectal cancer; IBD: Inflammatory bowel disease; UC: Ulcerative colitis.
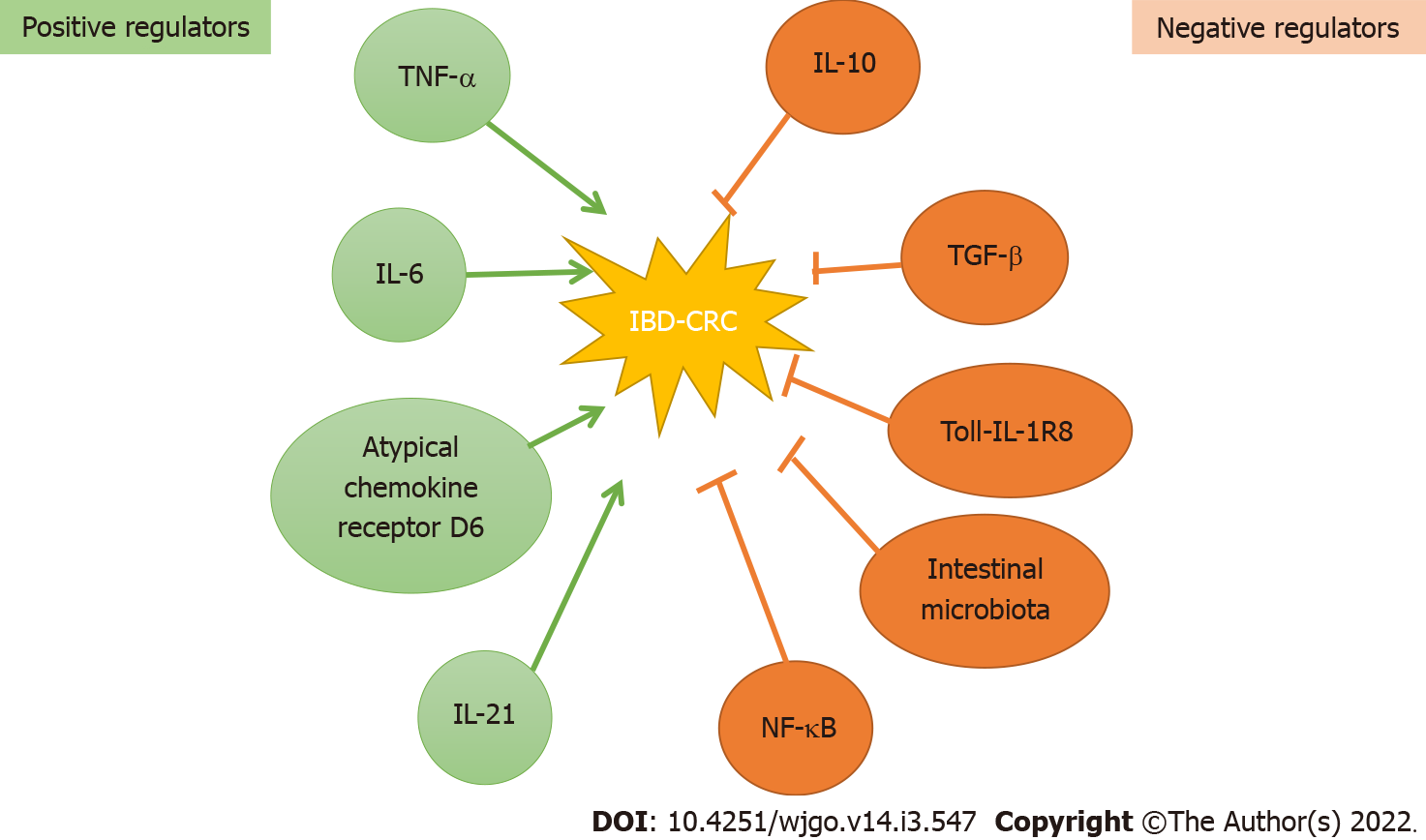
Figure 2 Molecular regulators of development of inflammatory bowel disease-related colorectal cancer.
Over the years numerous biological molecules and pathways have been identified that positively or negatively regulate the development of inflammatory bowel disease-related colorectal cancer. Microbial dysbiosis in conjunction with cytokines [tumor necrosis factor-α, interleukin (IL)-6 and IL-21] and chemokines (atypical chemokine receptor D6) drive intestinal immune response, in turn leading to chronic inflammation, tissue injury, dysplasia and cancer. The negative regulators including cytokines IL-10 and transforming growth factor-β, nuclear factor-κappa beta, Toll-like receptors, along with healthy gut microbiota prevent gut inflammation-mediated tissue injury and promote healing of damaged tissue. NF-κβ: Nuclear factor-κappa beta; TNF-α: Tumor necrosis factor-α; TGF-β: Transforming growth factor β; IL: Interleukin; CRC: Colorectal cancer; IBD: Inflammatory bowel disease.
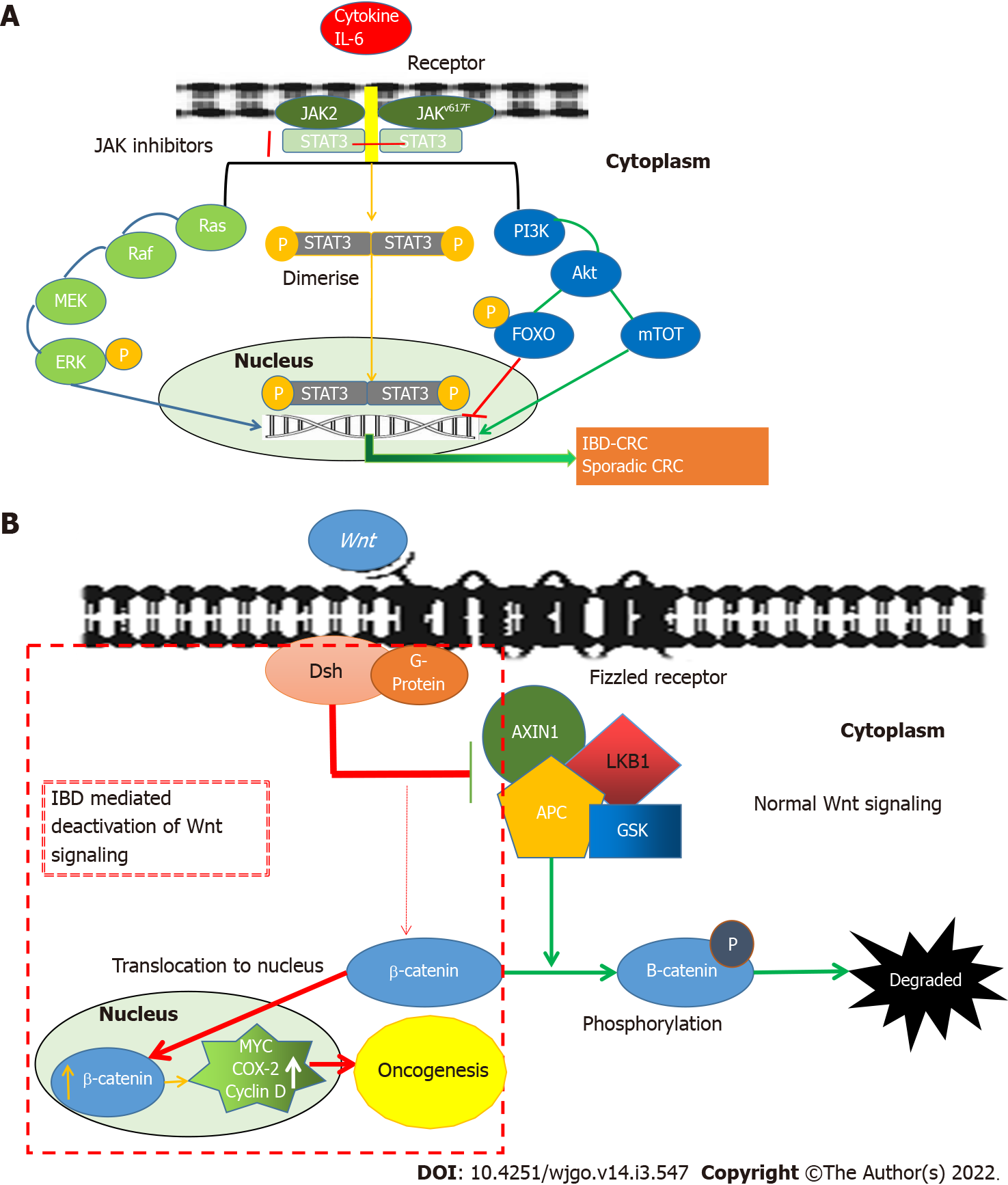
Figure 3 The inflammatory pathways leading to the development of IBD-related colorectal cancer.
A: The role of the JAK/STAT3 pathway in the development of IBD-related colorectal cancer. Various in vivo and in vitro models have shown that the JAK/STAT3 pathway plays a vital role in oncogenesis. The signal transduction of IL-6 involves the activation of JAK, activating transcription factors of the signal transducers and activators of STAT3. This is followed by its phosphorylation, dimerization and nuclear translocation of STAT3, initiating transcription of STAT3 target genes (including cyclin D1, Bcl-xL, c-myc, Mcl1, surviving and VEGF) leading to carcinogenesis. PI3K mediated activation of Forkhead box O3 (FOXO) leads to inhibition of gene transcription, whereas PI3K mediated activation of mTOT leads to oncogene transcription-mediated development of oncogenesis; B: The canonical Wnt-pathway in the development of IBD-related colorectal cancer. The canonical Wnt-pathway (β-catenin mediated Wnt-signaling) regulates proliferation and differentiation of the colonic stem cell in the normal colon. However, the loss of the adenomatous polyposis coli (APC) gene results in the shift of β-catenin from the membrane to the nucleus leading to increased transcription of cyclin D1 and c-myc genes thereby triggering carcinogenesis. IBD: Inflammatory bowel disease; CRC: Colorectal cancer; JAK: Juan kinase; P: Phosphorylation; STAT3: Signal transducer and activator of transcription proteins 3; PI3K: Phosphoinositide-3-kinases; Akt: RAC-alpha serine/threonine-protein kinase; FOXO: Forkhead box; mTOT: Mechanistic target of rapamycin; Ras: Small GTPase; Raf: Rapidly accelerated fibrosarcoma; MEK: Mitogen-activated extracellular signal-regulated kinase; ERK: Extracellular-signal-regulated kinase; Wnt: Wingless and int-1; Dsh: Dishevelled; AXIN: Axin-related protein 1; LKB1: Liver kinase B1; APC: Anaphase-promoting complex; GSK: Glycogensynthase kinase; MYC: C-myc; COX-2: Cyclooxygenase-2.
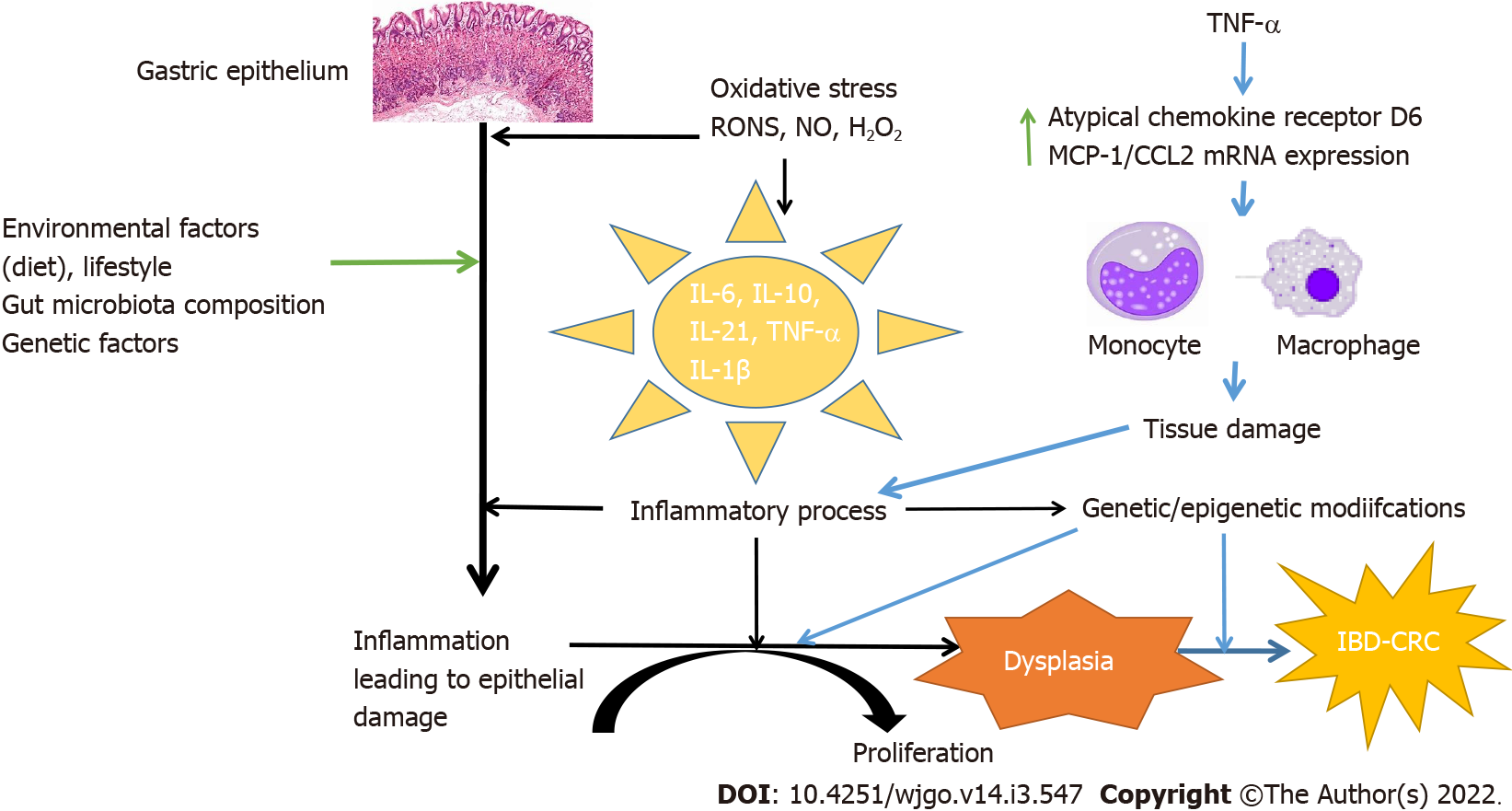
Figure 4 Pathophysiology of inflammatory bowel disease-related colorectal cancer.
The pathophysiology of inflammatory bowel disease-related colorectal cancer (IBD-CRC) is different from sporadic IBD. IBD-CRC follows an “inflammation-dysplasia-carcinoma” sequence instead of the “adenoma-carcinoma” sequence as is seen in sporadic CRC. The pathophysiology associated with inflammation is at the heart of IBD-CRC. Various factors including genetic, familial along with numerous positive and negative molecular regulators and pathways have been identified which influence the development and maintenance of an inflammatory state. Inflammation leads to aberrant immune response leading to a chronic inflammatory state and gut tissue damage. Tissue damage and inflammation lead to dysplasia mediated carcinogenesis. CRC: Colorectal cancer; IBD: Inflammatory bowel disease; TNF-α: Tumor necrosis factor-α.
- Citation: Majumder S, Shivaji UN, Kasturi R, Sigamani A, Ghosh S, Iacucci M. Inflammatory bowel disease-related colorectal cancer: Past, present and future perspectives. World J Gastrointest Oncol 2022; 14(3): 547-567
- URL: https://www.wjgnet.com/1948-5204/full/v14/i3/547.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i3.547