Copyright
©The Author(s) 2020.
World J Gastrointest Oncol. Sep 15, 2020; 12(9): 1065-1072
Published online Sep 15, 2020. doi: 10.4251/wjgo.v12.i9.1065
Published online Sep 15, 2020. doi: 10.4251/wjgo.v12.i9.1065
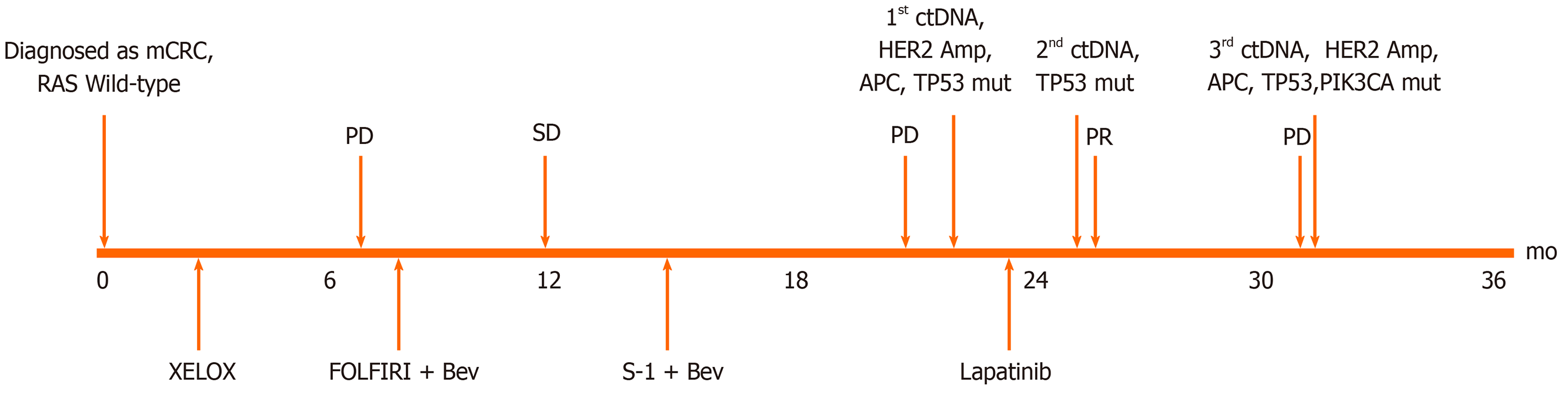
Figure 1 Treatment course.
mCRC: Metastasis colorectal cancer; ctDNA: Circulating tumor DNA; HER2: Human epidermal growth factor receptor 2; APC: Adenomatous polyposis coli; TP53: Tumor protein 53; Amp: Amplification; Mut: mutation; XELOX: Capecitabine and oxaliplatin; Bev: Bevacizumab; FOLFIRI: 5-fluorouracil, leucovorin, and irinotecan; PD: Progressive disease; SD: Stable disease; PR: Partial response; PIK3CA: Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha.
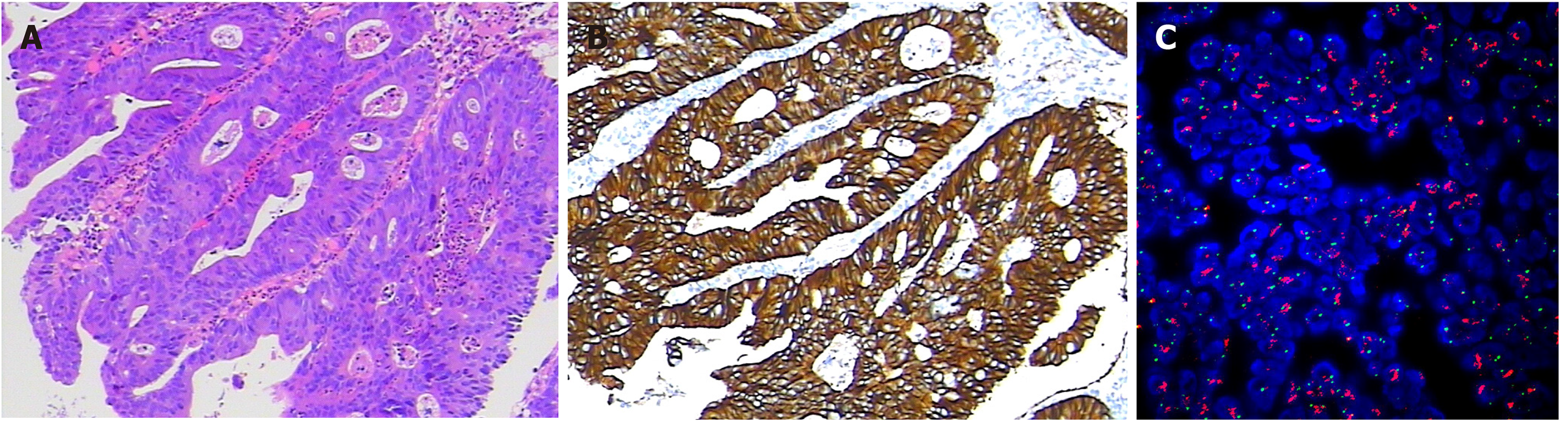
Figure 2 Examination of the tumor tissue.
A: Hematoxylin-eosin staining; B: Immunohistochemical staining for human epidermal growth factor receptor 2 (HER2); C: Fluorescence in situ hybridization for detection of HER2.
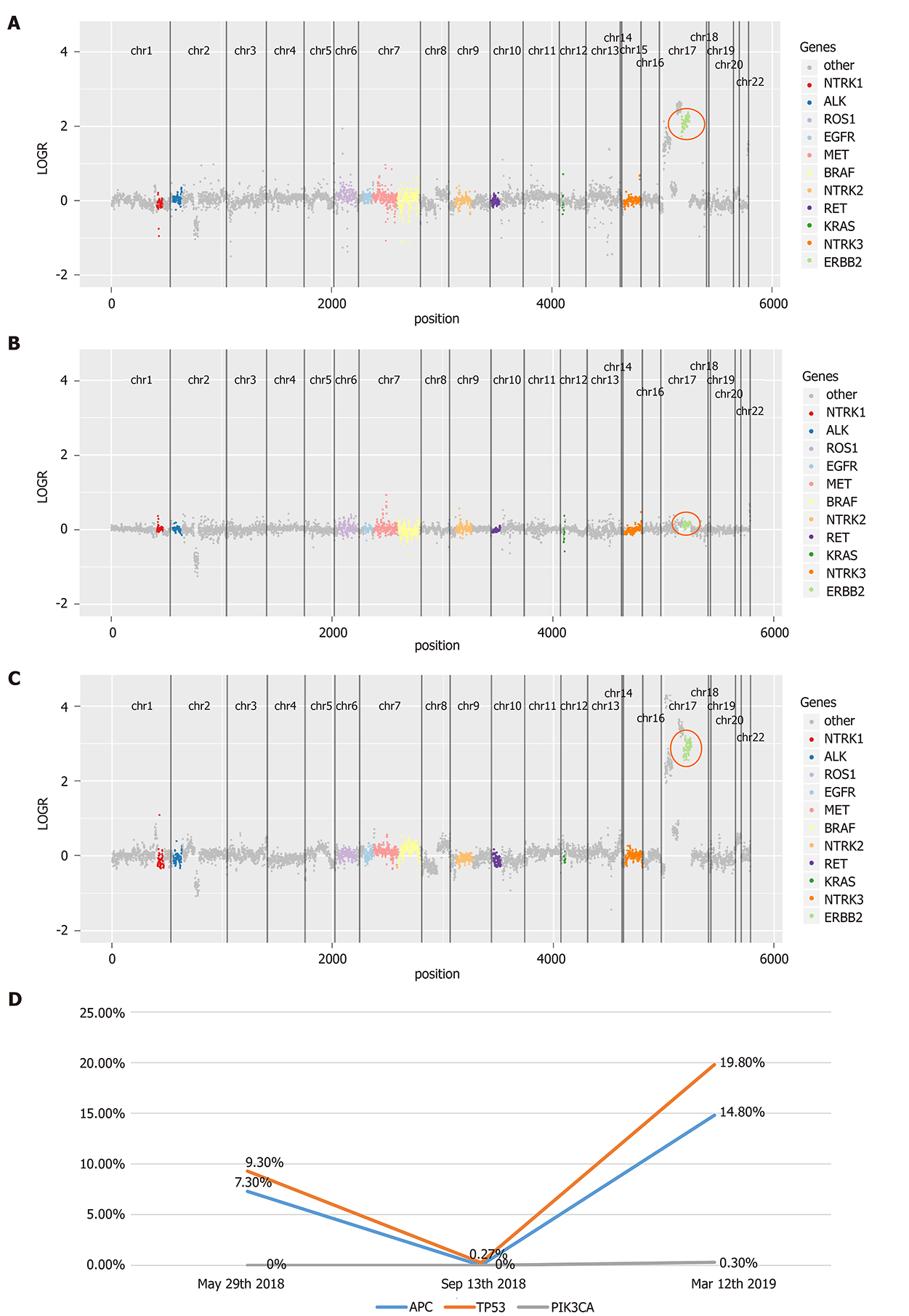
Figure 3 Serial circulating tumor DNA testing for monitoring lapatinib treatment.
A: Human epidermal growth factor receptor 2 (HER2) amplification on May 29, 2018; B: HER2 copy number alterations disappeared after lapatinib treatment on Sep 13, 2018; C: HER2 amplification was detected during disease progression on Mar 12, 2019; D: Mutant allele frequency of APC, TP53, and PIK3CA during lapatinib treatment. HER2: Human epidermal growth factor receptor 2; APC: Adenomatous polyposis coli; TP53: tumor protein 53; PIK3CA: Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha.
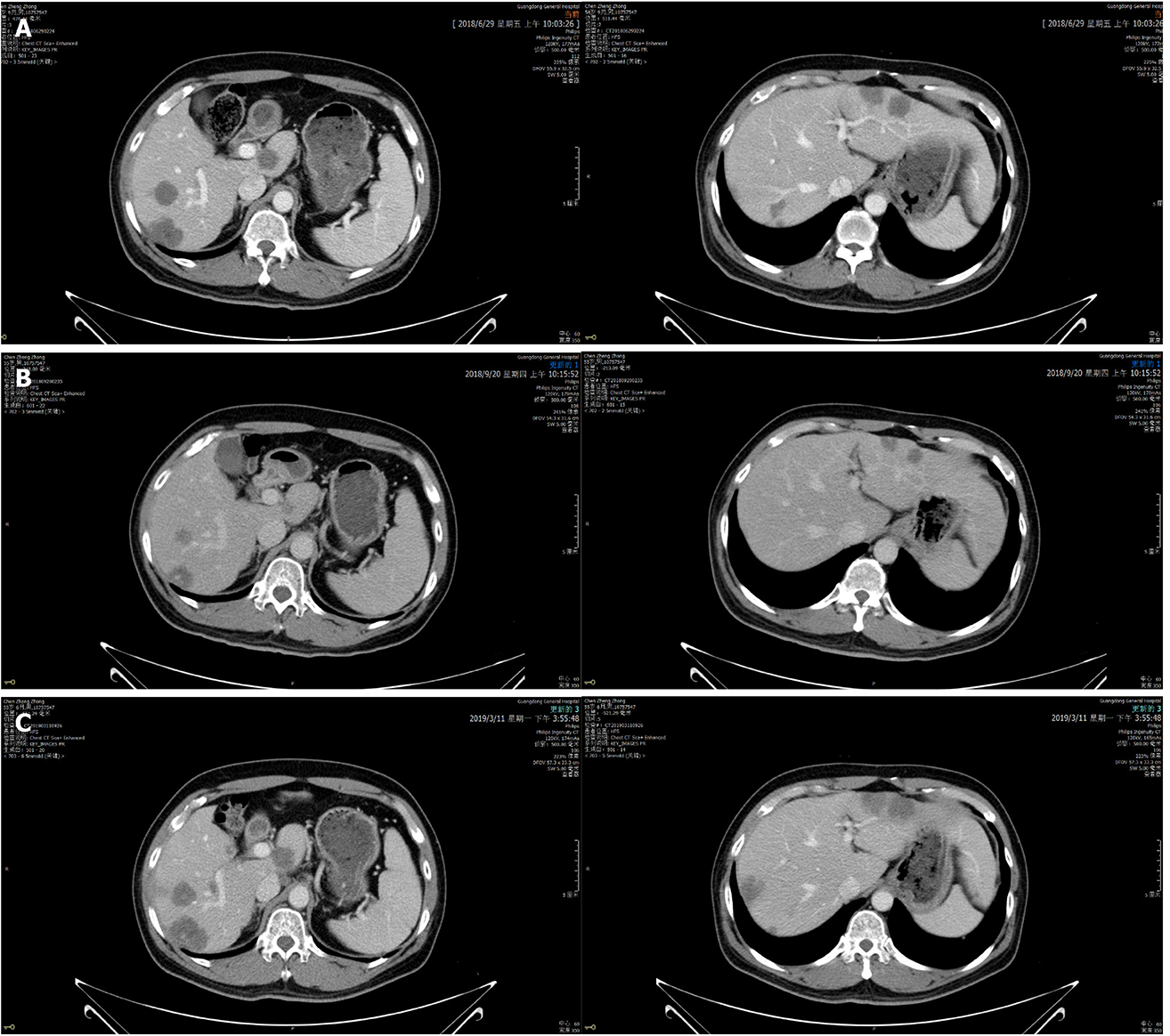
Figure 4 Abdominal enhanced computed tomography images showing that liver lesions changed during lapatinib therapy.
A: Jun 29, 2018: Before lapatinib therapy; B: Sep 20, 2018: 2 mo of lapatinib therapy; C: Mar 11, 2019: Disease progression after lapatinib treatment for 7.9 mo.
- Citation: Guan JL, Liu JH, Wang Q, Cong YW, Chen YX, Huang KF, Huang ML, Huang L. Response of human epidermal growth factor receptor 2-positive colorectal cancer to lapatinib monotherapy: A case report. World J Gastrointest Oncol 2020; 12(9): 1065-1072
- URL: https://www.wjgnet.com/1948-5204/full/v12/i9/1065.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i9.1065