Published online Aug 16, 2022. doi: 10.4253/wjge.v14.i8.487
Peer-review started: February 14, 2022
First decision: April 5, 2022
Revised: April 19, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: August 16, 2022
Processing time: 181 Days and 15.4 Hours
Endoscopic ultrasound (EUS)-guided main pancreatic duct (PD) access may be used when conventional endoscopic retrograde cholangiopancreatography (ERCP) techniques fail. The use of a percutaneous transluminal angioplasty balloon (PTAB), originally developed for vascular interventions, can be used to facilitate transmural (e.g., transgastric) PD access and to dilate high-grade pancreatic strictures.
To describe the technique, efficacy, and safety of PTABs for EUS-guided PD interventions.
Patients who underwent EUS with use of a PTAB from March 2011 to August 2021 were retrospectively identified from a tertiary care medical center supply database. PTABs included 3-4 French angioplasty catheters with 3-4 mm balloons designed to use over a 0.018-inch guidewire. The primary outcome was technical success. Secondary outcomes included incidence of adverse events (AEs) and need for early reintervention.
A total of 23 patients were identified (48% female, mean age 55.8 years). Chronic pancreatitis was the underlying etiology in 13 (56.5%) patients, surgically altered anatomy (SAA) with stricture in 7 (30.4%), and SAA with post-operative leak in 3 (13.0%). Technical success was achieved in 20 (87%) cases. Overall AE rate was 26% (n = 6). All AEs were mild and included 1 pancreatic duct leak, 2 cases of post-procedure pancreatitis, and 3 admissions for post-procedural pain. No patients required early re-intervention.
EUS-guided use of PTABs for PD access and/or stricture management is feasible with an acceptable safety profile and can be considered in patients when conventional ERCP cannulation fails.
Core Tip: Endoscopic ultrasound (EUS)-guided access of the main pancreatic duct (MPD) can be used to perform endotherapy when conventional endoscopic retrograde cholangiopancreatography fails. After access to the MPD is obtained, the tract created between the gastrointestinal lumen and pancreatic duct must be dilated prior to any further intervention. Percutaneous transluminal angioplasty balloons, originally developed for vascular interventions, can be used to access the pancreatic duct effectively and safely, as well as dilate high-grade MPD strictures if needed. Interventional endoscopists should be familiar with these cross-platform balloons as additional tools in the toolbox for EUS-guided MPD endotherapy.
- Citation: AbiMansour JP, Abu Dayyeh BK, Levy MJ, Storm AC, Martin JA, Petersen BT, Law RJ, Topazian MD, Chandrasekhara V. Percutaneous transluminal angioplasty balloons for endoscopic ultrasound-guided pancreatic duct interventions. World J Gastrointest Endosc 2022; 14(8): 487-494
- URL: https://www.wjgnet.com/1948-5190/full/v14/i8/487.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i8.487
Obstruction of the main pancreatic duct (MPD) can occur in the context of chronic inflammation and fibrosis due to a variety of clinicopathologic conditions, including both malignant and benign etiologies (e.g., chronic pancreatitis, post-pancreatic surgery). Obstruction of MPD outflow leads to higher resistance to pancreatic secretions, intraductal hypertension, and ultimately ductal dilation[1,2]. Patients can present with chronic abdominal pain, recurrent pancreatitis, steatorrhea, and unexplained weight loss. Decompression of the PD is the mainstay of treatment for symptomatic patients, and endoscopic therapy has become the preferred treatment modality due to its safety profile when compared to surgery[3,4].
Transpapillary or transanastomotic drainage with endoscopic retrograde cholangiopancreatography (ERCP) remains the preferred approach for endoscopic pancreatic duct access and intervention[5]. While successful in the vast majority of cases, 3% to 10% fail due to inability to cannulate the papilla/anastomosis, obstructive stones, high-grade strictures, and surgically-altered anatomy (SAA) that impacts access to the pancreaticobiliary tree, including surgeries like Roux-en-Y gastric bypass and pancreaticoduodenoctomy[6]. In these cases, endoscopic ultrasound (EUS)-guided pancreatic duct drainage has emerged as a potential salvage approach with a favorable safety profile and technical success rate. Technical and clinical success rates range from 63% to 100% and 76% to 100%, respectively, with adverse event rates ranging from as low as 14% up to 37%[7]. Guidelines recommend consideration of EUS-guided access in multidisciplinary, tertiary care settings when conventional therapy fails[8].
As EUS-guided pancreatic duct access becomes more established among experienced operators, there remains significant variation in technique. Specifically, dilation of the access tract can be performed with a variety of devices and currently published studies include the utilization of hydrostatic balloons, tapered catheters, and electrocautery-enhanced catheters[9,10]. No comparative trials exist comparing the success and complication rates of these devices. The hydrostatic balloons which are currently used were designed for biliary intervention, and their size may increase the risk of complications during pancreatic duct access[11].
Percutaneous transluminal angioplasty balloons (PTAB) are smaller caliber, 3 to 4mm diameter balloons initially designed for vascular interventions but can passed over standard 0.018-inch guidewires for use on endoscopic platforms. Initial case reports described the use of these balloons to treat otherwise impassable biliary strictures[12]. Their size makes them well-suited for dilation of the pancreaticogastrostomy/enterostomy as well as high-grade MPD strictures. Reports describe the use of these devices during ERCP; however, experience during EUS is limited to a handful of reported cases[13,14]. The objective of this study is to describe the use of PTABs during EUS-guided MPD inter
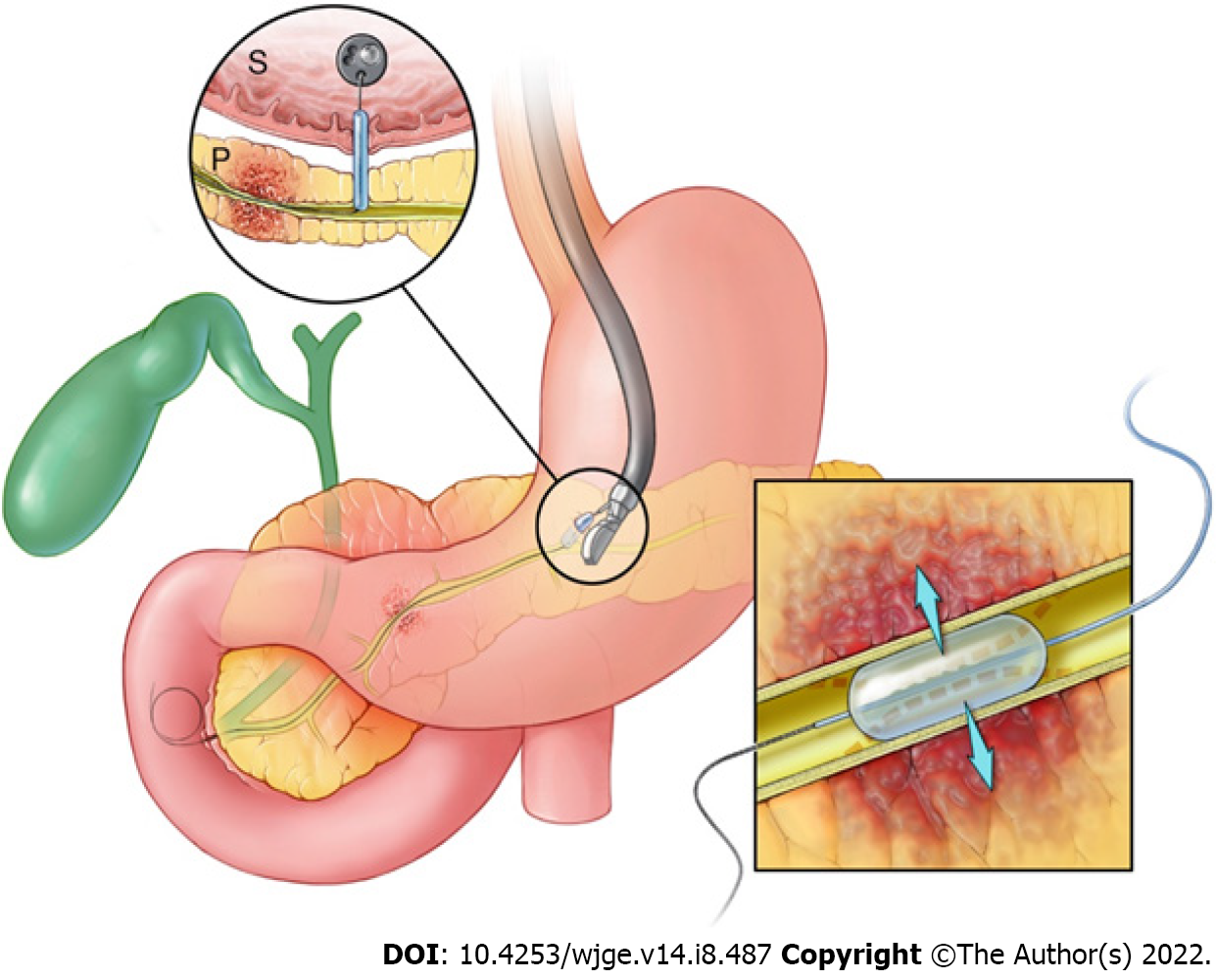
This is a retrospective, single-center cohort study approved by the Institutional Review Board at the Mayo Clinic. Consecutive patients who underwent EUS-guided MPD intervention with use of a PTAB between March 2011 to August 2021 were identified from a single tertiary care center using a supply database. Balloons used included 3 and 4 mm diameter SAVVY™ and SABER™ PTA balloons (Cordis, Santa Clara, CA, United States) which were 20 mm in length. Procedure information was extracted via manual chart review and included procedure indication, inpatient status, preceding ERCP attempts, indication for EUS-guided approach, maximum diameter of the MPD measured intraprocedurally, site of MPD access, and location of balloon dilation (Figure 1). In patients with SAA, the exact procedure was recorded. Patients with post-surgical pancreatic leaks were classified as biochemical leaks, grade B, or grade C according to the International Study Group for Pancreatic Fistula criteria[15].
The primary outcome was technical success defined by successful MPD access and accomplishing the intent of the procedure. If either of these conditions were not met, the procedure was classified as technical failure. Secondary outcomes included procedural related adverse events (AEs) including pain, bleeding, pancreatitis, leak, new fluid collection, perforation, or death as well as need for early reintervention prior to planned follow-up and clinical success. AEs were classified as mild, moderate, or severe based on American Society of Gastrointestinal Endoscopy (ASGE) lexicon[16]. Clinical response was noted at last follow up. Complete response was noted when there was clear documentation that all clinical symptoms fully resolved after intervention, and partial response if it any improvement in severity or frequency was documented. Patients without any benefit were classified as persistent symptoms.
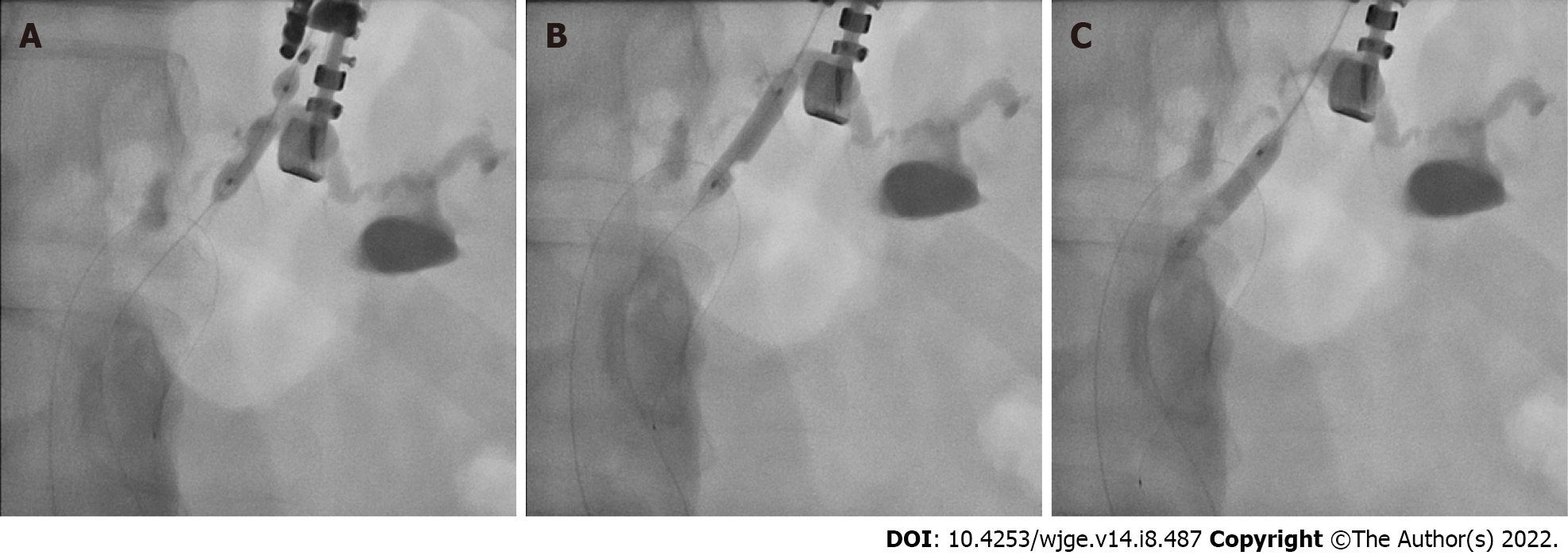
All procedures were performed by EUS- and ERCP-trained interventional endoscopists in a dedicated endoscopy unit with patients under general anesthesia. Due to the retrospective nature of this study, the exact technique used in each case was operator dependent. Generally, a linear-array echoendoscope was passed into the stomach and the MPD was identified. The MPD was preferentially accessed through the gastric wall with an FNA needle (19- to 22-gauge); however, the small bowel was also evaluated as an access point if suitable endosonographic windows for duct puncture were not found in the stomach. After EUS-guided ductal access was achieved, an 0.018-inch guidewire was passed under fluoroscopic guidance into the MPD and through the ampulla/anastomosis when possible. When utilized, the PTAB was then advanced over the guidewire and used to dilate the access tract and/or pancreatic duct stricture prior to any additional intervention, including further dilation or stenting (Figure 2).
Data management, analysis, and visualization was performed using BlueSky Statistics software (version 7.10, BlueSky Statistics LLC, Chicago, IL, United States). Quantitative variables were described with median value and interquartile range (IQR). Categorical data were reported as relative proportions (%).
A total of 23 patients were identified. The median age of the cohort was 55.8 years (IQR 45.0-57.8) with 11 (48%) females and 12 (52%) males. Median body mass index was 25.8 kg/m2 (IQR 23.9-27.5). Procedural indications included chronic pancreatitis in 13 (57%) patients, SAA with stricture in 7 (30%), and SAA with post-operative leak in 3 (13.0%). Of the 10 patients with SAA, 9 had undergone pancreaticoduodenectomy with antrectomy (i.e., Whipple procedure) and 1 had an en-bloc resection of metastatic cervical cancer requiring hepaticogastrostomy with Roux-en-Y reconstruction. The 3 post-operative leaks were identified as nonspecific peripancreatic fluid on computed tomography and confirmed by ERCP. All cases were classified as grade B and none were associated with organ failure or need for operative reintervention. Indications for an EUS-guided approach included 5 cases with inaccessible anastomosis/ampulla (22%), 5 obstructive anastomotic strictures (22%), 2 failed cannulations (9%), 9 proximal obstructions due to stone or stricture (9, 39%), and 2 disconnected pancreatic ducts (9%).
The majority of procedures were performed as an outpatient (n = 18, 78%). Maximum MPD size as measured during EUS was 5.5 mm (IQR 3.7-8.3 mm). Transgastric access was obtained in 22 cases (96%) with 1 pancreaticoenterostomy performed (4%). A 4 mm diameter PTAB was used in 15 cases (65%) with 3 mm balloons used in the remaining 8 (35%). The pancreatic duct was typically accessed through the body (n = 17, 74%) followed by tail (n = 3, 13%), and head (n = 3, 13%). The balloons were primarily used to dilate the access tract in 21 cases (91.3%), of which 9 were then passed into the pancreas and used for PD dilation. Pancreatic duct dilation alone was performed in 2 cases (10%). Dilation with a PTAB was the initial method used in the majority of cases (n = 21, 91%). In the remaining 2 cases, PTAB was used if needle knife access puncture and a dilating catheter was not successful. Further pancreatic duct intervention with dilation was performed in 5 cases (22%) and stenting in 17 (74%). This included 9 transmural stents terminating in the MPD, 8 stents placed through the stomach which traversed the MPD into the small bowel, and 1 retrograde transpapillary stent terminating in the MPD.
Technical success was achieved in 20 cases (87%). All 3 failed cases occurred in patients with chronic, calcific pancreatitis. In 2 of these cases, the procedure failed due to inability to obtain an adequate window for MPD access. The third case failed due to a high-grade MPD stricture with calcified stones that prevented the passage of all devices, including the 4 mm PTAB.
AEs were noted in 6 patients (26%) which were all mild in severity, requiring an unplanned hospital admission for ≤ 3 nights. Additional patient and procedural factors that may have impacted AEs are outlined in Table 1. There was 1 case of pancreatic duct leak identified endosonographically during the procedure, which was self-contained and managed conservatively. Additionally, there were two cases of pancreatitis and 3 cases of post-procedural pain requiring hospital admission. There were no AEs related to bleeding from the access site or perforation.
| Adverse Event | Severity | Additional devices used for tract dilation | Other procedural detail | |
| 1 | Post-procedure pain | Mild1 | None | None |
| 2 | Post-procedure pain | Mild1 | None | Multiple puncture attempts; Needle dislodgement requiring retrieval with forceps |
| 3 | Post-procedure pain | Mild1 | None | Dehiscence of surgical anastomosis noted prior to procedure start |
| 4 | Pancreatic duct leak | Mild1 | Needle knife electrocautery | Electrocautery utilized prior to percutaneous angioplasty balloon dilation; Small, self-contained leak identified sonographically prior to completion of the procedure |
| 5 | Pancreatitis | Mild1 | None | Additional pancreatic duct dilation to 6 mm; Large fragmented pancreatic duct stone cleared in an antegrade fashion with occlusion balloon |
| 6 | Pancreatitis | Mild1 | None | Small endoscopic window with limited mobility; Multiple puncture attempts |
Median post-procedure follow up time was 13.9 mo (IQR 6.9-28.1 mo). No patients required unanticipated, early intervention. In the 20 cases that were technically successful, 14 underwent additional planned interventions prior to stent removal which included routine stent exchange in 7 cases and placement of a parallel stent in the remaining 7. At the time of last follow up, 9 of the 20 (45.0%) technically successful cases were noted to have complete resolution of symptoms, 5 (25.0%) partial resolution, and 3 (15%) persistent symptoms. One patient (4.3%) did not have follow up symptoms documented, and two (8.6%) died during follow up prior to assessment of symptom improvement.
The emergence of interventional EUS has given endoscopists the ability to treat pancreatic duct obstruction even when conventional ERCP fails. These interventions require dilation of the gastro- or enteropancreatic fistula created during EUS-guided pancreatic duct drainage. Given the lack of dedicated devices to facilitate EUS-directed drainage interventions, endoscopists rely on other accessories that were not designed for these interventions. These include hydrostatic pancreaticobiliary dilating balloons, tapered dilating catheters, traction sphincterotome, and diathermy-compatible catheters[13]. PTABs are yet another device that can be used to facilitate access with interventional EUS.
Each technique and device carries its own risk-benefit profile. Axial pressure forces created during dilation with a fixed-diameter catheter, cannula or tapered passage dilator can lead to dissection of the tissue planes. On the other hand, balloon dilation may increase the risk of perforation, leakage, and bleeding due to its “all-or-nothing” approach. Standard endoscopic balloon dilators typically have diameters of 5 to 6 French and were designed primarily for intraductal ERCP-guided interventions. The use of smaller diameter balloons theoretically may allow for controlled dilation of the tract while minimizing the risk of perforation and leak. Notably, all AEs in this cohort were mild, without significant bleeding or perforation. There was one, self-contained pancreatic duct leak, but this occurred in a case where a diathermy catheter was used prior to balloon dilation. Electrocautery devices can result in a delayed-burn effect, increasing the risk of developing serious adverse events[17]. The overall AE rate of 26% may seem high compared to other standard endoscopic procedures but is favorable when compared to the morbidity and mortality associated with surgical alternatives, which include AE rates of up to 30% and 2% mortality[18,19]. Our data is similar to published literature on EUS-guided drainage of the MPD with more conventional ERCP accessories, including one of the largest multicenter studies which reported an AE rate of 20%[12].
Technical success of EUS-guided drainage of the MPD ranges from 50%-100% in the literature, approaching 80%-90% in more recent cohorts with experienced operators[10,12]. A technical success rate of 87% is consistent with the higher end of this range. In a previously published case series on the utilization of PTABs during EUS-guided interventions, a very similar technical success rate of 88% was reported with only one mild adverse event[15]. However, this was a very small cohort of 8 patients, contained only 1 case of chronic pancreatitis with stricture, and details regarding other procedural factors that may have impacted outcomes were limited. In this study, we report on a robust cohort with chronic pancreatitis and post-surgical disease. The majority of PTABs were successfully used as first line EUS-guided therapy, as opposed to salvage therapy when other devices failed. Furthermore, two of the three failures were due to limited mobility and inability to secure a safe window for MPD access, which is a limitation of the procedure itself and not the dilation device used.
This study is limited by its retrospective design with slight variations in patient characteristics and procedural technique. However, this heterogeneity also highlights that PTABs can be used in a wide range of clinical scenarios. Furthermore, procedural outcomes were certainly confounded by patient and technical factors unrelated to PTAB use. This study was not designed to evaluate EUS-guided drainage of the MPD outcomes overall, and additional detail was provided regarding cases of technical failure and AEs to allow for careful evaluation of the role the device played in these outcomes.
This study suggests that PTABs can be used to successfully and consistently access and drain the pancreatic duct while maintaining a high technical success rate without severe AEs. Additional comparative studies are needed to determine optimal technique; however, these cross-platform devices can help address the safety and technical limitations of existing endoscopic devices including larger diameter balloons, fixed diameter catheters, tapered passage dilators, and electrocautery-based devices. Interventional endoscopists should be familiar with these devices as additional tools in the toolbox for EUS-guided MPD endotherapy.
While endoscopic retrograde cholangiopancreatography (ERCP) remains the gold standard for main pancreatic duct (MPD) intervention, endoscopic ultrasound (EUS)-guided MPD access has emerged as a safe and effective alternative when ERCP fails. A key step in EUS-guided intervention is dilation of the tract created between the gastrointestinal lumen and pancreatic duct, however there is limited data regarding the optimal dilation device and technique. Furthermore, current tools were designed primarily for biliary intervention, including hydrostatic balloons, tapered bougies, and electrocautery-enhanced catheters.
A small diameter, hydrostatic balloon would theoretically allow for safe dilation while minimizing the risk of adverse events, however commercially available devices are limited. Percutaneous angioplasty balloons (PTABs) are small diameter balloons that were initially designed for vascular interventions. They can be deployed over a standard guidewire and utilized on endoscopic platforms to dilate the access tract created during EUS-guided access as well as high grade strictures. However, data on the use of these devices is limited to a handful of case reports.
The main objective of this study is to describe the efficacy and safety of PTAB use during EUS-guided MPD access. The primary outcome was technical success with secondary outcomes of clinical success and adverse event rate. The objectives of this study provide key, real-word information on the use of PTABs for clinicians as well as preliminary data to inform future prospective studies.
This is a retrospective, single center cohort study performed at an academic tertiary care center which includes all patients from 2011 to 2021 who underwent EUS-guided MPD which utilized a PTAB. Patients were identified retrospectively from a procedural supply database and clinical information was extracted from the electronic medical record.
A total of 23 cases were identified. Intervention was performed in the setting of chronic pancreatitis in 13 (56%), post-surgical stricture in 8 (35%), and post-surgical leak in 2 (9%). Technical success was achieved in 20 (87%) cases with 6 (26%) adverse events. Adverse events were all mild in severity and included 3 admissions for post-procedural pain, 2 pancreatitis, and 1 pancreatic duct leak.
This study demonstrates that PTABs can be used to consistently access the MPD for EUS-guided interventions with an acceptable safety profile. In the absence of dedicated devices, endoscopists can consider using cross-platform PTABs for initial dilation prior to antegrade interventions.
Further prospective, randomized studies are needed to compare the efficacy and safety of PTABs to other dilating devices and techniques.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American College of Gastroenterology; American Gastroenterological Association; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Dadlani A, United States; Li Q, China; Tantau AI, Romania S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Madsen P, Winkler K. The intraductal pancreatic pressure in chronic obstructive pancreatitis. Scand J Gastroenterol. 1982;17:553-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Bradley EL 3rd. Pancreatic duct pressure in chronic pancreatitis. Am J Surg. 1982;144:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 158] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Catalano MF. Endoscopic treatment of pancreatic duct strictures. Tech Gastrointest Endosc. 1999;1:168-174. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Cohen SA, Siegel JH, Kasmin FE. Treatment of pancreatic strictures. Curr Treat Options Gastroenterol. 2007;10:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Riff BP, Chandrasekhara V. The Role of Endoscopic Retrograde Cholangiopancreatography in Management of Pancreatic Diseases. Gastroenterol Clin North Am. 2016;45:45-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Dawod E, Kahaleh M. Management of Benign and Malignant Pancreatic Duct Strictures. Clin Endosc. 2018;51:156-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Imoto A, Ogura T, Higuchi K. Endoscopic Ultrasound-Guided Pancreatic Duct Drainage: Techniques and Literature Review of Transmural Stenting. Clin Endosc. 2020;53:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 9. | Krafft MR, Croglio MP, James TW, Baron TH, Nasr JY. Endoscopic endgame for obstructive pancreatopathy: outcomes of anterograde EUS-guided pancreatic duct drainage. A dual-center study. Gastrointest Endosc. 2020;92:1055-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Tyberg A, Sharaiha RZ, Kedia P, Kumta N, Gaidhane M, Artifon E, Giovannini M, Kahaleh M. EUS-guided pancreatic drainage for pancreatic strictures after failed ERCP: a multicenter international collaborative study. Gastrointest Endosc. 2017;85:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Chapman CG, Waxman I, Siddiqui UD. Endoscopic Ultrasound (EUS)-Guided Pancreatic Duct Drainage: The Basics of When and How to Perform EUS-Guided Pancreatic Duct Interventions. Clin Endosc. 2016;49:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Baron TH, Poterucha JJ. Use of a small-caliber angioplasty balloon for the management of an impassable choledochocholedochal anastomotic biliary stricture. Liver Transpl. 2008;14:1683-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Hayat U, Freeman ML, Trikudanathan G, Azeem N, Amateau SK, Mallery J. Endoscopic ultrasound-guided pancreatic duct intervention and pancreaticogastrostomy using a novel cross-platform technique with small-caliber devices. Endosc Int Open. 2020;8:E196-E202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Simons-Linares CR, O'Shea R, Chahal P. Severe Primary Sclerosing Cholangitis Biliary Stricture Managed With a Small-Caliber Cardiac Angioplasty Balloon: Looking Outside the Endoscopic Retrograde Cholangiopancreatography Toolbox. ACG Case Rep J. 2019;6:e00141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2964] [Article Influence: 370.5] [Reference Citation Analysis (35)] |
| 16. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1850] [Article Influence: 123.3] [Reference Citation Analysis (1)] |
| 17. | Itoi T, Kasuya K, Sofuni A, Itokawa F, Kurihara T, Yasuda I, Nakai Y, Isayama H, Moriyasu F. Endoscopic ultrasonography-guided pancreatic duct access: techniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Dig Endosc. 2013;25:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Nealon WH, Thompson JC. Progressive loss of pancreatic function in chronic pancreatitis is delayed by main pancreatic duct decompression. A longitudinal prospective analysis of the modified puestow procedure. Ann Surg. 1993;217:458-66; discussion 466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 148] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreaticojejunal decompression for chronic pancreatitis. Strategies for salvage. Arch Surg. 1994;129:374-9; discussion 379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |