Published online Aug 16, 2019. doi: 10.4253/wjge.v11.i8.454
Peer-review started: March 4, 2019
First decision: April 11, 2019
Revised: July 8, 2019
Accepted: July 20, 2019
Article in press: July 20, 2019
Published online: August 16, 2019
Processing time: 108 Days and 22.8 Hours
Fine needle aspiration (FNA) is currently the standard of care for sampling pancreatic solid masses by using endoscopic ultrasound (EUS). The accuracy of the technique is reported to be high, especially if coupled with the rapid on site evaluation (ROSE), and it has a high safety profile. However, FNA presents some limitations, such as the small amount of tissue that can be collected and the inability of obtaining a core tissue with intact histological architecture, which is relevant to perform immunohistochemical analysis, molecular profiling and, therefore, targeted therapies. Moreover, the presence of the ROSE by an expert cytopathologist is very important to maximize the diagnostic yield of FNA technique; however, it is not widely available, especially in small centers. Hence, the introduction of EUS fine needle biopsy (FNB) with a new generation of needles, which show a high safety profile too and a satisfying diagnostic accuracy even in the absence of ROSE, could be the key to overcome the limitations of FNA. However, FNB has not yet shown diagnostic superiority over FNA. Considering all the technical aspects of FNA and FNB, the different types of needle currently available, comparisons in term of diagnostic yield, and the different techniques of sampling, a tailored approach should be used in order to determine the needle that is most appropriate for the different specific scenarios.
Core tip: Endoscopic ultrasound guided fine needle aspiration (FNA) is the gold standard for sampling solid pancreatic masses, but the small amount of tissue collected and the need of on site evaluation to maximize the diagnostic yield are some disadvantages. New fine needle biopsy (FNB) needles, with high safety profile and satisfying diagnostic accuracy even in absence of on site evaluation, could overcome FNA limitations. However, FNB has not yet shown a clear diagnostic superiority. Thus, in order to choose the better needle for a given scenario, it is important to know the technical aspects of FNA and FNB, the different sampling techniques, the types of needle available, and their diagnostic performance.
- Citation: Conti CB, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come? World J Gastrointest Endosc 2019; 11(8): 454-471
- URL: https://www.wjgnet.com/1948-5190/full/v11/i8/454.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i8.454
Pancreatic cancer is the fourth leading cause of cancer related fatalities in Western countries[1,2]. Ductal adenocarcinoma (ADK) is considered the main cause of pancreatic mass, but many other neoplasms and benign conditions can be detected in the pancreas. Distinguishing different types of pancreatic masses is an important clinical challenge because the pathological diagnostic confirmation is highly relevant for establishing the best treatment. Endoscopic ultrasound (EUS) guided-fine needle aspiration (EUS-FNA) is currently the standard of care for sampling pancreatic masses, with a diagnostic accuracy ranging in literature from 77% to 95%[3,4].
EUS-FNA is a safe technique, with related morbidity and mortality rates < 1% and complications such as pain (0.38%), bleeding (0.10%), and pancreatitis (0.4%; n = 8246)[5]. There were some concerns about the risk of seeding, but peritoneal carcinomatosis may occur more frequently in patients undergoing percutaneous FNA than those who have EUS-FNA for the diagnosis of pancreatic cancer. The reported risk of seeding during pancreatic tissue acquisition is significantly lower during EUS-guided procedure compared with percutaneous sampling (2.2% vs 16.3%; P < 0.025)[6].
A recent study has indicated that EUS-FNA could be carried out without consequence on efficacy of surgery[7]. Again, the European Society for Medical Oncology guidelines recommended EUS-FNA, especially in doubtful cases. Percutaneous biopsy of the pancreas is contra-indicated in potentially resectable cases[8]. When performing EUS tissue acquisition, the operator should consider several variables that may influence the outcome to maximize the accuracy and reduce adverse events. These include correct EUS assessment of target lesion and type, size of needle, and most suitable sampling technique[9]. Of note, considering strict cytological criteria, EUS-FNA sensitivity has been reported to be as low as 77%, even in expert hands, due to inadequate samples and the presence of extensive necrosis or fibrosis[10,11].
Therefore, rapid on site evaluation (ROSE) by a cytopathologist, firstly described by Hikichi et al[12], has been proposed to improve EUS-FNA diagnostic accuracy by evaluating samples adequacy/cellularity and thus, theoretically, increasing the overall accuracy and reducing needle passes. Unfortunately, ROSE is not widely available, and its real impact on diagnostic accuracy is not well established[13].
Although EUS-FNA is usually adequate for the final diagnosis of pancreatic ADK, it is not able to obtain a core tissue with a preserved architecture, essential for a definite diagnosis of other pancreatic solid tumors and benign conditions[14]. Moreover, cytological samples do not allow immunohistochemistry, phenotyping, and genetic analysis, which are fundamental factors for risk stratification and tailored oncological management. To overcome the aforementioned shortcomings, fine needle biopsy (FNB) was developed in order to guarantee the acquisition of a core tissue, ideally providing a sample with preserved architecture for both histological, immunohistochemical, and genetic profiling.
The aim of this review is to provide an overview about the diagnostic yield of EUS-FNA and FNB for pancreatic masses, to analyze the technical features of the different needles and the different techniques in sampling (e.g., stylet/no stylet; different aspiration methods, needle sizes) in order to provide a small practical guide with reference to the different possible scenarios where EUS guided sampling is performed.
An extensive bibliographic search in PubMed via MeSH was performed using the following key words and free terms: Pancreatic mass, pancreatic cancer, FNA, FNB, endoscopic ultrasound, EUS sampling, EUS needle, comparisons between FNA and FNB, FNB versus FNA, FNB versus FNB, FNA versus FNA needle, AND pancreatic masses. The reference lists from the selected studies were manually examined to identify further relevant reports. Non-English-language papers were excluded.
One recent study of 985 patients with pancreatic masses[15] found that pre-operative EUS-FNA led to “significantly fewer benign lesions resected” compared with the group that underwent surgery without EUS (P = 0.024). Hence, if “tissue is the issue”, the main purpose of EUS is to collect material for pathological evaluation. EUS-guided tissue acquisition of solid pancreatic lesions can be performed using two different methods: FNA and FNB.
Historically, FNA needles were developed only to obtain an adequately representative cellularity of the lesion. Therefore, EUS-FNA does not necessarily retain the stroma and requires the presence of an expert pathologist both for the preparation of the collected specimens and for their interpretation. The ROSE process, done during the procedure in the endoscopy suite, involves the processing of a tissue smear and the evaluation under a light microscope by a trained cytopathologist. An on-site cytopathologist is fundamental to confirm adequate tissue sampling, which increases the diagnostic accuracy, when compared to EUS-FNA performed without ROSE[16]. ROSE reduces the number of needle passes necessary to obtain an adequate specimen and increases the diagnostic capability of the endosonographer through immediate feedback during the procedure[17-18]. Early data from three meta-analyses demonstrated that ROSE was associated with a statistically significant (P < 0.001) improvement in the adequacy rate (average 10%, 95%confidence interval (CI): 5%-24 %)[16,19-20].
Hence, EUS-FNA with ROSE has been considered the reference standard for obtaining high diagnostic accuracy in the biopsy sampling of the pancreas[21]. However, the main limitation of this approach is represented by the cost related to the presence of a dedicated and skilled cytopathologist in the endoscopic room; and although EUS-FNA with ROSE reduces the number of passes necessary to obtain a suitable sample, it seems to increase the overall procedure time, both for the need of specimen processing and for the time requested for the interpretation[22].
However, high quality studies reported conflicting conclusions[23]. Two randomized clinical trials (RCTs) conducted in 2015[23,24] showed no significant difference in the diagnostic yield of malignancy, proportion of inadequate specimens, and accuracy in patients with pancreatic mass undergoing EUS-FNA with or without ROSE. FNA without ROSE was performed using a fixed number of needle passes, which was significantly higher compared to the number of passes needed in the group with on-site pathologist. No difference was reported in terms of complications related to the number of passes in RCTs and meta-analyses
Moreover, high-volume centers had adequacy rates > 90% of the sample without ROSE, suggesting that ROSE should be considered in centers where the specimen adequacy rate is < 90%[25,26]. A meta-analysis published in 2016 compared EUS-FNA with and without ROSE, including RCTs, with a total of 1299 patients[27]. No statistically significant difference was found between the EUS-FNA with or without ROSE in term of diagnostic yield of malignancy or proportion of patients with adequate specimens. The diagnostic sensitivity and specificity between the two groups were also comparable.
Since ROSE is a time-consuming service with poor reimbursement and is not available in many centers, it should not be strongly recommended to provide a ROSE service throughout all centers performing EUS for pancreatic lesions[28].
In order to theoretically overcome these limitations, a new-generation of needles has been developed. FNB-needles were specially designed to obtain a core specimen with preserved tissue architecture. The specimen fragments are not lost or consumed during cell block centrifugation or specimen sectioning, and histological architecture and tissue integrity can be retained in most of the specimens. The FNB needles are the ideal sampling method for solid masses, like subepithelial lesions of the gastrointestinal (GI) tract, lymph nodes, and pancreatic and non-pancreatic lesions (such as liver parenchyma) as FNB allows immunohistochemical testing relevant in many diseases.
The FNB needles procure large volumes of tumor cells and desmoplastic stroma, providing better histological samples with a diagnostic yield exceeding 90%. This observation is important for low volume centers without ROSE or a dedicated cytopathologist because a cell block specimen can be interpreted by any GI pathologist without special expertise in cytopathology. Indeed, a recent systematic review and meta-analysis compared the diagnostic yield of FNA with FNB on solid GI lesions, lymph nodes, and pancreatic lesions, specifically evaluating the diagnostic value of ROSE while comparing the two types of needles[29]. Fifteen studies (n = 1024) were included in the analysis. No significant difference in diagnostic adequacy [Relative risk (RR): 0.98, CI: 0.91-1.06, I2 = 51%] was observed. Although not statistically significant (P = 0.06), FNB without ROSE showed a relatively better diagnostic adequacy. For solid pancreatic lesions only, there was no difference in diagnostic adequacy (RR: 0.96, CI: 0.86-1.09, I2 = 66%), but, in the absence of ROSE, FNB was associated with better diagnostic adequacy (P = 0.02). In terms of both diagnostic accuracy (RR: 0.99, CI: 0.95-1.03, I2 = 27%) and optimal quality core histological sample procurement (RR: 0.97, CI: 0.89-1.05, I2 = 9.6%), there were no significant differences. However, FNB established the diagnosis with fewer passes (Standardized mean difference: 0.93, CI: 0.45-1.42), I2 = 84%). In the presence of ROSE, FNA required relatively fewer passes to establish the diagnosis than in its absence. The authors concluded that FNB without ROSE can replace EUS-FNA with ROSE without loss of diagnostic accuracy[29]. In case of pancreatic mass, when ROSE is unavailable, current European Society of Gastrointestinal Endoscopy guidelines suggest (low quality evidence, weak recommendation) performance of three to four needle passes with an FNA needle or two to three passes with an FNB needle[30].
The evolution of FNB needles started from a Menghini-type 18G core needle, adapted to a prototype 2.8 mm channel convex array echoendoscope[31]. The technical limitation of this needle was the poor penetration into the pancreatic tissue and a consequent poor diagnostic yield. However, that was the first description of EUS-FNB, and it set the stage for all future development.
The first original FNB needle (QuickCore® Biopsy Needle; Cook Medical) was a Tru-Cut needle (Medline Industries) that could be used with echoendoscopes and was introduced in the early 2000s. The Quick-Core was composed of a cannula, a tissue penetrating stylet that can be disposed within the cannula, and a handle mechanism to advance the cannula over the stylet to maintain the cannula capability to move smoothly over the stylet, even when the scope is bent.
However, technical issues included challenges in deploying the spring-loaded tray when the needle was pulled back, especially within the duodenum or in case of not having the specimen be retained. Additionally, a certain track length within the pancreas was needed in order to deploy safely the needle and avoid injury of the pancreatic duct, which can increase the risk of pancreatitis[32].
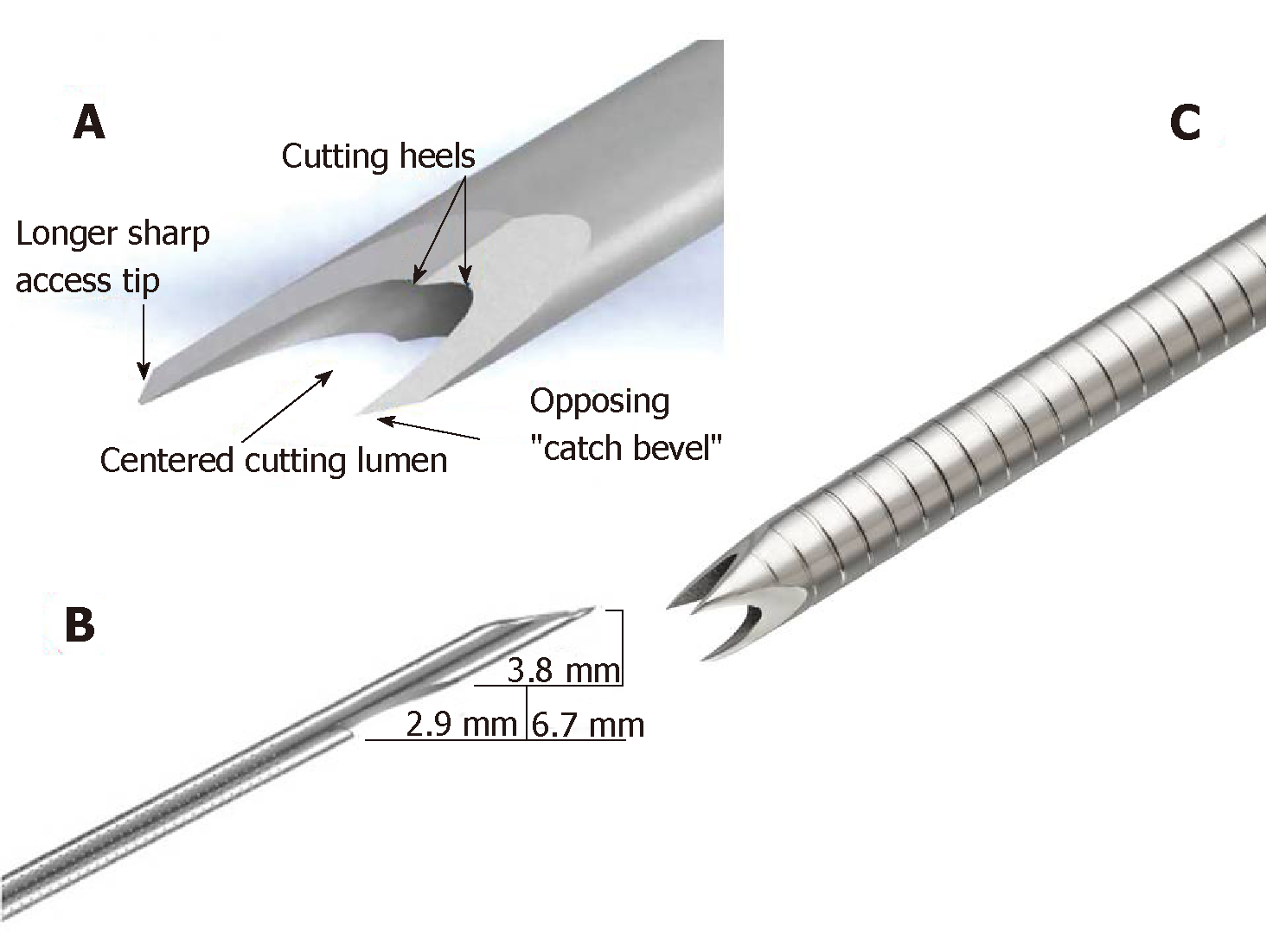
The currently available core biopsy needles can be mainly classified as non-cutting or cutting type, including side-type and the most recently introduced end-type (Figure 1).
The Echo Tip® HD ProCore™ (Wilson-Cook Medical Inc., Winston-Salem, NC, United States) needle was introduced in 2011. It is a cutting, end-side needle. It has two distinct cutting surfaces: the tip and a reverse bevel, just distal to the tip that promotes collection of a core sample during the retrograde movement of the needle within a lesion. The reverse bevel has a potential advantage of increasing tissue acquisition amount while preserving histological architecture. The EchoTipProCore is available in 19 (4.8 French sheath), 22, and 25 gauge (G) (5.2 French sheath). Early published results on the performance of ProCore needles demonstrate high diagnostic accuracy rates (86%-89%)[33-35]. In 2015, a 20 G FNB needle (8 French sheath) was developed to increase the diagnostic accuracy; it was designed to combine a large lumen and enhanced flexibility to facilitate tissue acquisition, even from an angulated endoscope position. According to the manufacturer’s design specifications, this was achieved by coating the sheet of the needle with a smooth and flexible material (polytetra-fluoroethylene). Also, the cutting edges of the needle were changed from a reverse- to a forward-facing bevel, and from a Lancet to a Menghini tip design, in order to decrease resistance when traversing the tissue (Figure 2).
The SharkCore™ (Medtronic Inc., Sunnyvale, CA, United States) is a fork-tip FNB needle with six distal cutting surfaces in an asymmetric design, specifically designed to obtain cohesive units of tissue with intact cell architecture. By minimizing tissue stacking and fracturing, the needle can potentially provide better core samples. This needle is available in 19, 22, and 25 G (8 French sheath).
The SharkCore needle uses the Beacon™ EUS delivery system, which allows needle removal from the sheath, maintaining the position of the sheath in the endoscope and consequently its relation to the lesion. Theoretically, this system could allow the endoscopist to maintain the position or even to replace the needle with one of a different size.
A large and initial multicenter retrospective experience of EUS-guided fine needle biopsies obtained using the SharkCore FNB needle on different solid lesions (pancreas, subepithelial lesion, and lymph node) demonstrated an excellent 88% overall pathologic diagnostic yield with a median number of two passes only. Overall, histological diagnosability and thus pathologic yield for each lesion subtype were as follows: pancreatic lesions 86%, subepithelial lesions 87%, lymph nodes 93%. The needle size did not have an impact on pathologic diagnostic yield, as both 25 G needle and 22 G needle performed at a very high level, 86% and 89%, respectively[36].
The most recently introduced FNB-needle is the Acquire™ needle (Boston Scientific, Marlborough, MA, United States). This is a Franseen needle with a three-plane symmetric cutting surface. This structure of the electropolished tip improves control and stability of the needle and allows penetrating the tissue, minimizing sample tearing and fragmentation. Furthermore, the Acquire needle is made of cobalt-chromium, a material subject to less deformation than stainless steel alloys.
The Acquire core biopsy needle is available in 19 (5.2 French sheath; minimum working channel 2.8 mm), 22 (5 French sheath; minimum working channel 2.4 mm), and 25 G (4.8 French sheath; minimum working channel 2.4 mm).
A multicenter retrospective study of 200 patients undergoing EUS-FNB of solid lesions with Acquire needle showed a high rate of tissue adequacy and tissue core, with no adverse events. The tissue obtained by EUS-FNB was adequate for evaluation and diagnosis by ROSE in 98.5% of cases. In 90% of cases, a core of tissue was obtained[37].
Emerging data suggest that needle aspiration techniques could have a direct effect on the yield of EUS-FNA or EUS-FNB.
Conventionally, when performing EUS-FNA, a negative pressure is applied using suction with a 10 or 20-mL syringe (“standard suction”). In the “high pressure suction”, a negative pressure with a 50-mL syringe is applied during EUS-FNA. To avoid GI contamination of the sample, the stopcock of the syringe is usually closed before needle removal. However, a negative pressure persists in the syringe and can be neutralized by disconnecting the syringe stopcock from the needle port before withdrawing the needle from the lesion.
In the “stylet slow-pull” technique, the stylet is slowly and continuously withdrawn as the needle moves to-and-from within the target lesion, creating minimal negative pressure (about 5% of the force generated with a standard suction technique). The suction and the no suction methods are similar in terms of diagnostic adequacy. However, the suction method applies a lot of pressure, causing more bleeding and more tissue damage, leading to reduced sample quality and an increase in the number of slides used, but it improves both the cellularity and the quality of the aspirate. The capillary action may improve specimen quality by reducing the amount of blood in the aspirated material.
Many trials compared the diagnostic yield of EUS-FNA samples obtained with slow-pull and with standard suction technique[38]. No differences in term of smear cellularity, diagnostic yield, and sufficient histological material obtained were found, but bleeding was significantly higher in the standard suction group (P < 0.001).
In the “wet suction” technique, the needle is preloaded with saline solution in order to replace the column of air with liquid, which is less compressible and transmits better to the needle tip the negative pressure applied to the proximal part of the needle. Therefore, the wet suction technique may be considered a modified standard suction technique. A blinded randomized trial by Attam et al[39] compared the wet technique with the dry technique. The results revealed that the wet technique yielded a significantly higher cellularity (1.82 vs 1.45; P < 0.0003) and a significantly better diagnostic yield (85.5% vs 75.2%; P < 0.035) compared to the dry technique.
The “hybrid technique” consists of preparing the needle as in the wet technique but applying the suction as the dry technique. It has the advantage of having a column of fluid in the needle that guarantees a continuous negative pressure with a 10 cc prevacuum syringe. This avoids the manual suction of the syringe, as performed in the wet technique, while sampling the lesion. A single-center underpowered pilot study compared wet, dry, and hybrid techniques. Considering diagnostic yield, there was no statistically significant difference between the three techniques (hybrid 100%, wet 92%, dry 90%)[39].
The role of the different aspiration techniques when performing FNB was assumed from previous studies on FNA needles. Lee et al[40] carried out a randomized trial enrolling patients (n = 50) with suspected pancreatic malignancy and undergoing EUS-FNB. A 22 G ProCore needle, used without ROSE, was randomized at the use of stylet slow-pull-back technique (group A), standard suction (group B), or non-suction after stylet removal (group C) method. The rate of good or excellent cellularity was highest in group A compared with groups B and C (72% vs 60% vs 50%; P = 0.049). A > 25% rate of blood contamination was prevalent in group B (30% vs 42% vs 10%; P = 0.009). The rate of adequate core-tissue acquisition was not different among the groups (52% vs 34% vs 50%; P = 0.140).
The use of stylet theoretically reduces the sample contamination by the GI cells and clogging of the needle. It also allows an easier escape of the sample from the needle. Unfortunately, the use of the stylet extends the procedure time and reduces the needle flexibility, especially when the scope tip is bent (duodenal position) or if a large needle (19 G) is used.
A 2016 meta-analysis of these studies (five RCTs and two retrospective studies for a total of 5491 specimens) demonstrated no significant difference in the rate of sample adequacy between the stylet group (2135/2504, 85.26 %) and no-stylet group (2609/2987, 87.35 %) (odds ratio: 0.94, 95%CI: 0.79-1.11, P = 0.45). Furthermore, the rate of cellularity > 50 % and the contamination rate and blood contamination rate were not significantly superior in the stylet group when compared with the no-stylet group[41].
Regarding the diagnostic performance (sample adequacy and quality) of FNA needles of different caliber, no significant differences between 22 G versus 25 G needles were found[42,43].
However, conflicting results can be derived from two recent meta-analyses, in terms of the diagnostic sensitivity, specificity, and safety of 22 G and 25 G FNA needles in sampling solid pancreatic lesions[44,45]. Facciorusso et al[44] included seven trials with a total of 732 lesions: 295 lesions were sampled with 22 G needle, 309 were sampled with 25 G needle, and 128 lesions with both needles. Regarding the pooled sensitivity, a non-significant superiority of 25 G needle over 22 G was found [RR: 0.93 (0.91-0.95) vs 0.89 (0.85-0.94) for 25 G and 22 G needle, respectively; P = 0.13], and no difference was observed when considering specificity (P = 0.85). No differences in safety and sample adequacy were found.
Xu et al[45], on the contrary, obtained a higher sensitivity for the 25 G needle in the diagnosis of solid pancreatic lesions. In detail, 11 prospective RCTs were analyzed, including 837 patients of which 412 were sampled with 22 G and 425 with 25 G FNA needle. The 25 G needle was superior in terms of sensitivity [92% (95%CI: 0.89-0.95)] compared to the 22-G needle [88% (95%CI: 0.84-0.91)] in sampling solid pancreatic masses (P = 0.046), whereas the specificity of the two needles were comparable. Importantly, the pooled positive and negative likelihood ratio for the 22 G needle were 12.61 (95%CI: 5.65-28.14) and 0.16 (95%CI: 0.12-0.21), respectively, whereas the pooled positive and negative likelihood ratio for the 25 G needle were 8.44 (95%CI: 3.87-18.42) and 0.13 (95%CI: 0.09-0.18), respectively, with area under the receiver operating curve of 0.97 for the 22 G needle and 0.96 for the 25 G needle.
Similarly, a meta-analysis in 2018 that included four RCTs, with a total of 462 patients (233 sampled by using 25 G needle and 229 by using 22 G needle) highlighted a slight not statistically significant superiority of 25 G needle over 22 G[46]. The diagnostic sensitivity was 93% and 91% for the 25 G and 22 G needle, respectively. The specificity was 87% and 83% for 25 G and 22 G needle, respectively. However, area under the receiver operating curve did not show any statistically significant difference between the two needles (P = 0.497).
Hence, no definitive recommendations over the use of one particular device can be made, as there was no strong superiority of one needle on the other. In addition, a RCT[47] comparing 22 G FNA needles with and without a side port did not find significant differences between them in terms of both diagnostic accuracy and sample adequacy.
Some studies focused on the possibility of obtaining histological samples by using a large caliber needle, such as a 19 G FNA needle, which could preserve the architecture of the tissue. An RCT in 2010 compared the diagnostic accuracy of 19 G needle versus 22 G needle in a cohort of 117 patients with solid pancreatic/peripancreatic masses[48]. EUS-FNA was performed without ROSE. The accuracy of the samples obtained from the body/tail lesion was higher for the 19 G needle (95.0%) than the 22 G (76.7%) (P = 0.031), and the amount of cellular material obtained was significantly higher in the 19 G needle group (P = 0.033). However, the overall diagnostic accuracy was not significantly different (86.7% vs 78.9% for 19 G and 22 G, respectively; P = 0.268).
Moreover, using the 19 G needle could be difficult when sampling pancreatic masses with the scope in the duodenum because of its stiffness and caliber, which could affect the needle flexibility and its diagnostic yield. In a large multicenter prospective study from Attili et al[49], 246 patients with solid lesions (203 cases) or enlarged lymph nodes (43 cases) were examined. The procedure was technically feasible in 228 patients, with an overall procurement yield of 76.8%, which was very low. Considering malignant versus nonmalignant disease, the sensitivity, specificity, and positive/negative likelihood ratios were 70.7% [95%CI: 64.3-76.6, 100% (95%CI: 79.6-100), and 35.3 (95%CI: 2.3-549.8)/0.3 (95%CI: 0.2-0.4)], respectively, with a diagnostic accuracy of 73.6% (95%CI: 67.6-79.0).
The main outcomes considered in the studies that evaluated and compared the performance of FNA versus FNB needles were: safety, diagnostic accuracy, sample adequacy, sample quality, technical performance of the needle, and costs(Table 1). Importantly, no studies found a relevant difference in the safety between FNA and FNB. Therefore, the most important outcome considered in the comparison between the two methods was the diagnostic accuracy.
| Ref | Study design | N°Lesions,pan-creatic | Rose | Needles (G),FNA vs FNB | Overall diagnostic yield | Sample adequacy | Comments |
| [4] | RCT | (56) | Yes | 22 vs 22 Procore | Equivalent | Equivalent | |
| [29] | Meta-analysis (11 observational study and 4 RCTs) | 1024 (mainly pancreatic and lymph nodes) | #6 NO #9 Yes | 19 (only one study); 22 and 25 G vs 22 | Equivalent | Equivalent | in the absence of ROSE, FNB was associated with better diagnostic adequacy (P = 0.02) and FNB required less passes |
| [50] | RCT | 194 (100) | No | 22 vs 22 Procore | 84 vs 90 | Equivalent | Lower n° of passes for FNB vs FNA needle (2 vs 3) |
| [51] | RCT | 377 (249) | Yes | 22 vs 22 Procore | Equivalent | 81.7 vs 92.6 | |
| [52] | RCT | (36) | No | 22 vs 22 Procore | Equivalent | Equivalent | 1.1 passes needed for FNB vs 1.83 passes for FNA (P < 0.05) |
| [53] | Meta-analysis (8 RCT) | 921 | No | 22, 25, and 19 (only one study) G vs 22 | Equivalent | Equivalent | Few passes for FNB |
| [54] | Retrospective | 42 (12) | Yes | 22 or 25 | Equivalent | Equivalent | |
| [55] | Retrospective | (87) | No | 22 vs 22 Franseen | Equivalent | Equivalent | |
| [56] | Retrospective | (76) | No | 22 vs 25 | 32.4 vs 60 | Equivalent | |
| [57] | RCT | (214) | No | 25 vs 25 Procore | Equivalent | 69.4 vs 81 | |
| [58] | RCT | (116) | Yes | 22, 25 vs 22, 25 Procore | Equivalent | Equivalent | Few passes for FNB |
| [59] | Meta-analysis (7 comparative studies and 4 single cohort studies) | 896 (pancreatic and lymph nodes) | Only in 4 studies | 22 and 25 | Equivalent | Equivalent | |
| [60] | RCT | 140 (73) | YES | 19, 22, 25 | 67 vs 90 | Equivalent | Diagnostic yield only for pancreatic masses was equivalent |
| [61] | Prospective comparative | 145 (69) | No | 22 vs 22 Procore | Equivalent | Equivalent | Few passes for FNB |
| [62] | RCT | 58 (16) | No | 22 vs 22 Procore | Equivalent | Equivalent | Few passes for FNB |
| [63] | RCT (13 centers) | 608 (312) | In 7 centers | 25 vs 20 Procore | 44 vs 77 | Equivalent |
The technical aspects and the presence of ROSE, as already stressed, are important issues in the evaluation of the overall results when comparing FNA and FNB. Although the literature evidence did not support a strong superiority of FNB over FNA, most recent studies showed a trend in favor of FNB, especially without ROSE, in terms of specimen adequacy with fewer needle passes. A 2012 RCT compared 22 G FNA without suction (Expect; Boston Scientific, Natick, MA, United States) and 22 G FNB (Echotip ProCore; Cook Endoscopy, Bloomington, IN, United States) performance[4]. Both the procedures were performed with ROSE. This study examined a cohort of 28 FNA and 28 FNB procedures and found no significant difference in terms of median number of passes required to obtain a diagnosis, rate of diagnostic sufficiency reached, complication rates, and rate of obtaining histological core and its quality. The 22 G biopsy needle obtained a diagnostic cytological specimen in 89.3% of patients and histological specimen in 80% of patients; on-site cytological diagnosis was established with biopsy needle in nearly 90% of patients.
Accordingly, Alatawi et al[50] found a similar accuracy of 22 G FNA or 22 G Procore FNB needle in the diagnosis of malignancy, when biopsying pancreatic solid masses (sensitivity of 88.4% vs 97.8%, respectively, specificity of 100% for both methods). However, a lower number of passes was required with FNB needles versus FNA (two passes vs three passes). The use of FNB also improved the histopathological quality of the specimens, in term of slide cellularity and tissue microfragments. These results were obtained by the examination of 100 patients[50].
A large recent RCT conducted by Cheng et al[51] found EUS-FNB samples to be more accurate in diagnosing pancreatic masses than EUS-FNA samples. In detail, they examined 190 pts patients undergoing EUS-FNA (22 G EchoTip Ultra needles; Cook Medical) and 187 pts undergoing FNB (22G EchoTip ProCore needle; Cook Medical) for the sampling of solid masses: pancreatic (249 patients), abdominal (82 patients), and mediastinal (46 patients). For each procedure, four passes with the slow-pull technique were performed. ROSE was available in all cases. Diagnosis was accurate in 91.4% of cases for FNB, whereas it was 80% for FNA cases, based on the final patient diagnoses (P = 0.0015). In the subgroup of pancreatic masses, diagnosis with FNB was accurate in 92.7% of the cases, whereas it was 81.7% for FNA (P = 0.0099). Regarding the cytological analysis of the pancreatic masses, FNB samples accurately identified 88.6% of all pancreatic lesions, whereas FNA samples only accurately identified 79.4% (P = 0.0046).
No significant difference between FNA and FNB needle were found when comparing the performance of the technique without ROSE. An advantage in terms of passes needed to obtain a diagnosis was found with the 22 G FNB needle (Cook EchoTip ProCore) in comparison to 22 G FNA (Olympus, GF UCT 160) when using the suction method without ROSE[52]. This study found an overall diagnostic yield of 83.3% for both techniques (a total of 136 patients), with 1.11 passes versus 1.83 passes (P < 0.05) required when using FNA and FNB, respectively.
Data from a large meta-analysis including eight RCTs (921 cases) supported these results[53], as FNB gave higher specimen adequacy compared to FNA, despite the need of fewer needle passes.
A retrospective review of consecutive patients undergoing FNB sampling and FNA of the same single lesion with the same needle gauge and number of passes without ROSE and another retrospective cohort reviewed a total of 87 consecutive EUS-FNB specimens using either a 22 G Franseen needle (51 patients) or a 22 G FNA needle (36 patients) for sampling pancreatic diseases[54,55]. The diagnostic accuracy of the two methods was statistically comparable, but the median sample area was significantly larger in samples obtained from FNB than those obtained from FNA (4.07 vs 1.31 mm2, P < 0.0001). ROSE was not available in this study. Furthermore, a recent systematic review and a meta-analysis already cited in the previous paragraph showed that FNB required fewer passes to establish the diagnosis than FNA sampling with ROSE[29].
In the studies conducted in centers where ROSE was not available, FNA and FNB seemed to perform similarly[54,55], but FNB allowed for obtaining larger samples with fewer needle passes. These observations open the possibility of using FNB instead of FNA when ROSE is not available, as it maintains the same diagnostic accuracy.
Most of the available studies that compared FNA and FNB investigated the performance of 22 G FNA versus 22 G FNB needles. However, beyond the 22 G needles comparisons, some evidence is available.
A retrospective study examined a cohort of patients sampled with 22 G FNA (Echotip Ultra; Cook Ireland Ltd., Limerick, Ireland) versus a cohort sampled with 25 G FNB needle (Echotip ProCore; Cook Ireland Ltd.) for EUS-guided sampling of solid pancreatic masses without ROSE[56]. Among a total of 76 patients, there were no significant differences in safety, technical success (100% for both), and mean number of passes between the two cohorts (38 patients each). However, interestingly, the 25 G FNB group had a higher amount of both diagnostic cellular material and preservation of tissue architecture than FNA (P = 0.030 and 0.010, respectively), with a better diagnostic yield for specific tumor discrimination compared with the 22 G FNA group (P = 0.018).
Moreover, four RCTs[4,50,57,58] and a meta-analysis including 11 studies and 896 patients[59] compared FNA and reverse bevel needles in patients with solid pancreatic masses. The RCTs evaluated mainly 22 G and 25 G needles. ROSE was available only in some of them[4,58], and they used stylet or suction method[50]. No difference was found in the accuracy of final diagnosis in all studies, but the sample histological quality was higher for reverse bevel than for FNA needles[49,56]. Lee et al[58] found a higher accuracy for samples obtained with reverse bevel needles during the ROSE. A similar observation on the rate of diagnostic samples adequacy for ROSE was found by Aadam et al[60]. Moreover, based on the observations of three RCTs, it seems that reverse bevel needles required fewer passes to obtain adequate samples for histological diagnosis, offering potentially shorter procedure time[49,61,62].
Interestingly, in the recent ASPRO multicenter trial, the authors compared the performance of a commonly used 25 G FNA needle with the new 20 G FNB needle on 608 patients with solid lesion[63]. The 20 G FNB needle outperformed the 25 G FNA needle in terms of histological yield (77% vs 44%; P < 0.001) and diagnostic accuracy (87% vs 78%; P = 0.002), with a 99% technical success rate of the FNB needle.
FNB NEEDLES COMPARISON
With the increasing availability of new FNB needles, some studies have focused on comparing their performances, mainly in term of diagnostic yield comparison.
In detail, a cohort study compared the opposing bevel-tipped needles (22 and 25 G) and reverse-bevel needles (20, 22, and 25 G)[64]. The fanning technique was used for all procedures. Twenty-five gauge needles were used preferentially for transduodenal biopsy. A minimum of three needle passes were performed, and ROSE was not available. A higher diagnostic sensitivity and higher diagnostic overall accuracy for the opposing bevel needle was obtained in comparison with the reverse-bevel needle: 71.1% vs 90.1%; P = 0.0006 and 74% vs 92%; P = 0.0006, respectively. The percentage of samples adequate for histology was 87% for the reverse bevel needle versus 99% for the opposing bevel needle (P = 0.002). Therefore, this study concluded that the opposing bevel tip seems to be superior, in terms of diagnostic performance, compared with a reverse-bevel needle (Table 2).
| Ref | Study design | N°Lesions,pan-creatic | Rose | Needles | Gauge | Diagnostic yield, % | Sample adequacy, % | Comments |
| [64] | Cohort | (201) | No | Opposing bevel vs reverse bevel | 22-25 vs 20-22-25 | 71 vs 90 | 87 vs 99 | Opposing bevel needle resulted superior |
| [65] | Cohort | 194 (100) | Only in 12% of cases | Franseen vs fork tip | 22 | 64 vs 85 | The use of ROSE is a confounding factor | Fork tip seems superior, but the study lack of methodology |
| [66] | RCT | (50) | Yes | Franseen vs fork tip | 22 | > 90%, equivalent | 94 vs 98 | Equivalent |
| [67] | Cohort | (66) | Procore | 22 vs 25 | 87.5 vs 82.1 | 98 vs 95 | Equivalent |
Another recent study compared the diagnostic yield of the Franseen needle with the fork-tip needle[65]. A total of 194 solid lesions were sampled, 100 of them located in pancreas (52%). For solid pancreatic masses, the yield with the Franseen needle was lower [34/53 (64%) in comparison with the fork-tip needle 40/47 (85%), OR 3.4, 9.1-8.9; (P = 0.017)]. At the multivariate analysis the number of passes, the site, and lesion size did not affect the diagnostic yield. However, in this study, one of the endosonographer used the ROSE, and this affected the overall methodology.
An RCT also compared the 22 G Franseen and 22 G fork-tip needles in sampling of pancreatic masses[66]. Fifty patients were sampled using both 22 G Franseen and 22 G fork-tip needles, with randomization of the needle order. Two passes were performed using both needles for cell block, and dedicated passes were performed for ROSE, using both needles until the diagnosis was established. They observed that there was no significant difference in term of surface of total tissue (P = 0.50), retained architecture, diagnostic cell block, and diagnostic adequacy at ROSE (94.0% vs 98.0%; P = 0.32) between Franseen and fork-tip needles, respectively. The authors concluded also that, given their ability to yield diagnostic cell block in greater than 90% of patients, ROSE is not mandatory.
Lastly, in terms of needle performance, no significant difference was found between 22 and 25 G FNB needle in a prospective study[67].
In conclusion, the comparison among the different FNB models available seems to be an interesting topic in the perspective of identifying the perfect needle for histology, but larger comparative studies are needed.
Multiple factors may contribute to the outcomes of pancreatic EUS-guided tissue acquisition, as above reported: Site selection for sampling, sampling technique, location, and nature of the lesion, size and type of needle, ROSE availability, experience of the endosonographer, cytopathologist expertise, and methods of handling and processing the sample.
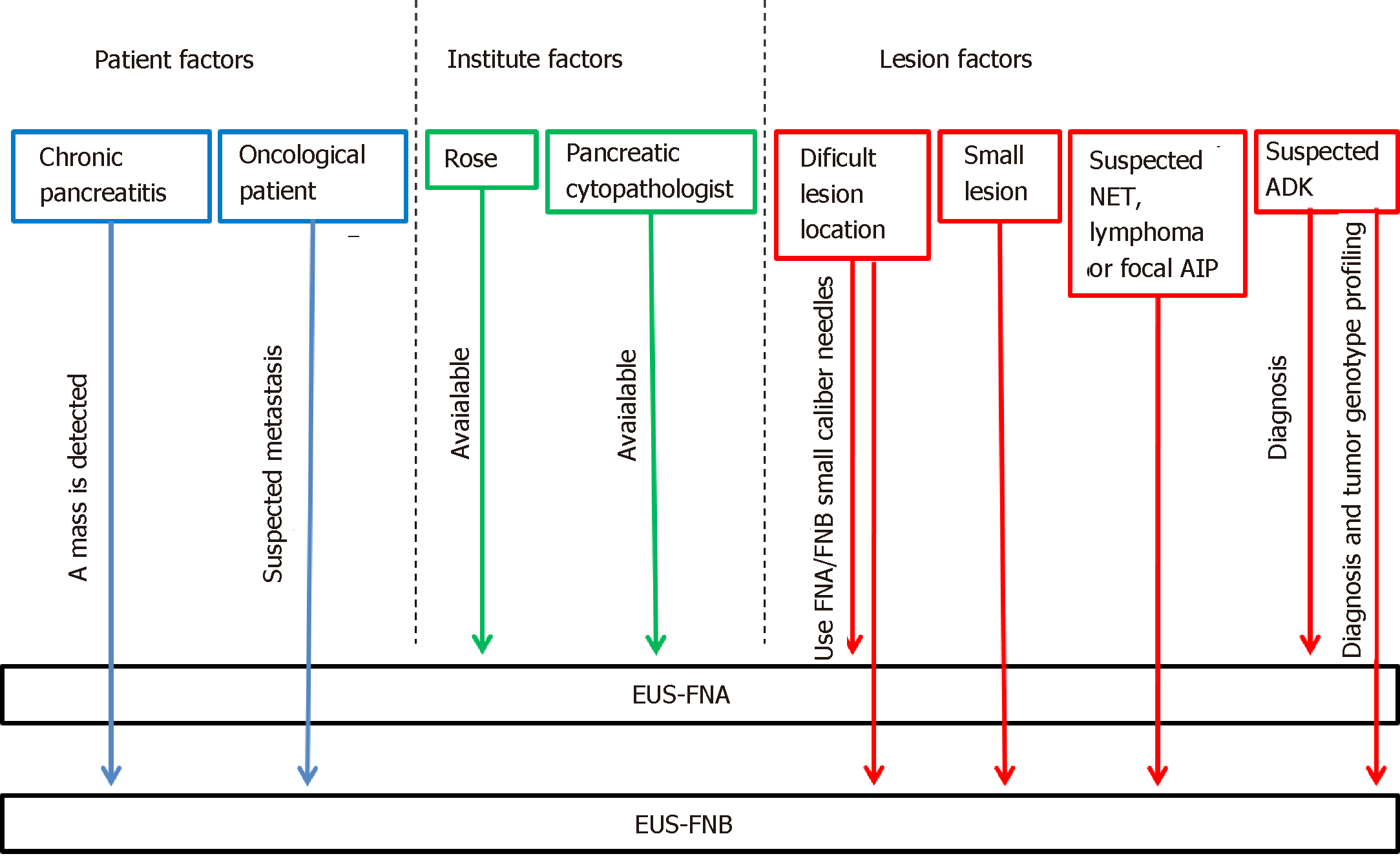
In order to maximize the diagnostic yield of pancreatic masses sampling, we propose a practical guide that takes into account the aforementioned factors and groups them into three main categories. The choice of the needle could be therefore made by combining these factors and their categories (Figure 3).
The three categories we choose are: Lesion related factors; patient-related factors; and institute related factors.
Among the factors linked to the pancreatic lesions, its location is a key factor to consider, for the difficulty of using a needle of greater caliber for lesions located in the head, uncinate process, or on the most distal portion of the tail, where it is more difficult to move the needle from the working channel, with the scope torqued in the duodenum or in the gastric fundus. Even the stylet use is more difficult with larger caliber needles in the case of sampling performed through the duodenum[68,69].
Considering the size of the lesion, approximately 60% of small solid pancreatic lesions ≤ 15 mm are not reported as being histologically consistent with ADK and, therefore, do not require radical surgery[70]. Without preoperative diagnosis, an unacceptably large proportion of patients would be exposed to unnecessary radical surgery, with significant morbidity and mortality. Many studies have reported a correlation between EUS-FNA accuracy and lesion size[11,71-73]. Pancreatic tumors are frequently stiff, accompanied by inflammation and desmoplasia and are thus difficult to penetrate with a needle. Once the needle reaches the target lesion, some limitations may be found, such as the lack of space to perform the back-and forth movement and the displacement of the needle from the lesion during the maneuvers. The lower diagnostic yield of EUS–FNA in small pancreatic lesions may be related to the presence of inflammatory tissue and desmoplastic stroma, which surround and constitute the most of small carcinomas. Agarwal et al[71] reported an increasing sensitivity from 75% to 94% for lesions smaller or larger than 20 mm, respectively. Similarly, another retrospective study reported that EUS-FNA accuracy without ROSE was 71% and 90% for lesions smaller or larger than 30 mm, respectively, and these were significant via multivariate analysis[72]. Siddiqui et al[11] showed that the EUS–FNA sensitivity for pancreatic lesions with < 1 cm size and with 1-2 cm in diameter was 40% and 75.9%, respectively, and the sensitivity strongly correlated with tumor size (P = 0.001). Similarly, the accuracy of EUS-FNA increased directly with the lesion size, ranging from 47% for tumors less than 1 cm in size to 88% for tumors larger than 4 cm (P < 0.05). On the other hand, Fabbri et al[73] suggested that EUS-FNB of small pancreatic lesions (mean lesion size: 16.5 mm) using a 22 G ProCore needle was effective, with a diagnostic accuracy of 82%, and the presence of a tissue core was recorded in 52.9% of the samples. The authors explained the high needle performance on small lesion with the presence of the side fenestration, increasing the efficacy of tissue sampling: the tissue specimens could be collected not only via frontal orifice but also via side fenestration, which remains in the center of the small lesion during repeated needle passages[73].
Taking into account the nature of the lesions, obtaining a tissue histology has been recognized as important for the diagnosis of autoimmune pancreatitis, especially in focal form[74], or in case of Hodgkin lymphoma[75]. Hence, FNB needle should be considered when facing these diagnostic suspects.
Furthermore, although neuroendocrine tumor (NEN) diagnosis and the assessment of the degree of their differentiation with FNA needles are possible[76], the use of FNB needle may be helpful for their definitive diagnosis. In a recent retrospective study of patients with histologically confirmed pancreatic NENs (pan-NENs), Chen et al[77] found that a definitive diagnosis of pan-NENs was possible only in 13/21 (61.9%) of EUS-FNA specimens. Each of the 13 cases with definitive diagnosis showed adequate cell block material, used for ancillary testing, underpinning the need for robust cell block material to render a conclusive determination of pan-NENs. Conversely, in a recent 15-year retrospective study, 30% of false-positive EUS-FNA diagnoses of ADK were proved to be pan-NENs on the resected specimen[78]. The recent study by Witt et al[79] on patients with known or suspected pan-NENs compared EUS sampling with SharkCore® in patients receiving EUS-FNA using a standard needle. The authors confirmed that the FNB needle showed promising results in obtaining suitable tissue for ancillary tests, allowing for more definitive pathologic interpretations.
Moreover, pancreatic ADK genotyping will play an increasingly important role in cancer therapy in the next years. Therefore, tissue histology and the ability to obtain a cell block for additional studies will soon be included among the goals of EUS-sampling. Today, the role of “personalized medicine” in cancer therapy remains a process in evolution, and the amount of tissue needed for molecular profiling still remains to be defined. Although repeatedly smaller amounts of DNA are required to achieve “Next Generation Sequencing”, a current benchmark of adequate tissue is considered as 1 mm of tissue, eight to 10 slides, or 5 × 5 mm surface area, with at least 20% tumor tissue[80]. These expectations could be easily fulfilled by FNB needles (Figure 4).
One of the most relevant issues is the presence of an underlying chronic pancreatitis. Identifying a neoplasia in the setting of chronic pancreatitis can be challenging. This difficulty is compounded by the fact that patients with chronic pancreatitis are at increased risk of developing pancreatic ADK, whereas patients with pancreatic ADK often have focal areas of chronic pancreatitis too. The reported sensitivity of EUS-FNA when sampling solid pancreatic masses in the setting of chronic pancreatitis ranged from 54% to 74%, which is unacceptably low[81,82]. The presence of underlying chronic pancreatitis makes the morphological interpretation of neoplasms even more challenging because of their very similar imaging features. The pancreatitis-induced morphological changes (e.g., lobulations) may mimic a pancreatic mass, while the presence of acoustic shadowing from a calcified stone may reduce the ultrasound’s capability to detect a neoplasm. Again, the coexistence of collateral vascularization in patients with severe chronic pancreatitis makes the EUS sampling even more difficult. On the other hand, when EUS-guided sampling is possible, the pathological interpretation can be hard. Some of the cytological features that may mimic malignancy in chronic pancreatitis are occasional atypical cells, enlarged, single cells with large nuclei, degenerative vacuoles, and occasional mitosis. Diagnosing well-differentiated ADK can be particularly challenging as they tend to lack the typical hyperchromasia, display only minimal architectural disorders, and have only modestly increased nuclear-to-cytoplasmic ratios[83]. The use of contrast harmonic imaging and elastography, doing more FNA passes, repeating the procedure with ROSE, and consulting an experienced pancreatic cytologist may be helpful to improve the overall EUS accuracy. But above all, the use of the new EUS-FNB needles or FNA19 G needles may be considered[84]. Theoretically, a core biopsy yields tissue fragments with an intact histological architecture, which is sometimes required, particularly in patients with chronic pancreatitis and well-differentiated pancreatic ADK when cytology is inconclusive. Currently, although it seems reasonable to use FNB needles in this setting, its role in discriminating pseudotumoral masses from pancreatic cancer in the setting of chronic pancreatitis has not yet been explored.
Again, FNB needle may be preferable in the context of an oncological patient with evidence of focal pancreatic lesion. In these cases, when a solid pancreatic mass is identified, even though it is a single lesion, the possibility of facing a secondary lesion should be considered. The collaboration of an experienced cytopathologist and the use of EUS-FNB needles may facilitate the diagnosis, increasing both the diagnostic accuracy and the quantity of material required; especially for patients requiring complementary immunohistochemical studies[83-87].
Finally, among the institute setting factors, we remember the availability of ROSE and the availability of a pancreatic cytopathologist as key aspects in sampling a pancreatic solid mass (see EUS-FNA accuracy: the role of ROSE on the way to FNB). If both these elements are present in the hospital, the option of FNA needles may be preferable.
EUS-FNA is currently still the standard of care for sampling pancreatic masses with high diagnostic accuracy, especially if coupled with ROSE, and high safety profile. However, FNA presents some intrinsic drawbacks that probably will reduce, in the near future, its use as first line method for tissue acquisition. These include the small amount of tissue with scant cellularity without the ability to guarantee a core tissue with intact histological architecture, which impairs immunohistochemical analysis and molecular profiling. Before long, these two features will become of paramount importance not only to aid definite diagnosis but even to guide tailored personalized oncological therapies. Secondly, FNA requires ROSE to maximize its diagnostic yield, which may prolong procedural time, and is unfortunately not widely available outside referral center.
Second generation FNB needles have shown satisfying diagnostic accuracy even in the absence of on-site pathology, reducing the number of passes required to establish the diagnosis. Nonetheless, FNB has not yet showed a clear undisputed diagnostic superiority over FNA, especially when considering pancreatic masses sampling. Indeed, the 2017 European Society of Gastrointestinal Endoscopy guidelines stated that for routine EUS-guided sampling of solid masses and lymph nodes, FNA and FNB needles are equally recommended (high quality evidence, strong recommendation)[30].
Theoretically the ideal needle should provide specimens with preserved cellular architecture and fulfill the attributes pin-pointed by Lachter[88]. Among them the most relevant should be needle safety, high accuracy (thus reducing false negatives), tip visibility, flexibility, and low cost.
In real practice, the aforementioned attributes are seldom fulfilled by a single kind of needle. The best needle is the one that better complies with the different factors (lesion related, patient related, and institute related), influencing the overall performance of tissue acquisition.
Currently, a “one size fits all” approach should be abandoned in favor of a tailored approach, selecting each time the needle better adaptive to the different specific scenarios. According to our proposed flowchart on needle selection, FNB should be preferred in case of concomitant chronic pancreatitis, diagnosis of focal autoimmune pancreatitis and pan-NENs, pancreatic masses suspected for metastases, need for tumoral genotype profiling, and in cases where ROSE is not available, in order to reduce needle passes.
In the near future, with the development of newly designed core biopsy needles, it is expected that FNB will most probably replace FNA as the standard of care for tissue acquisition.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A, A, A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Takagi T, Hara K, Eysselein V, Sugimoto M, Mastoraki A, de Bree E S-Editor: Dou Y L-Editor: Filipodia E-Editor: Zhou BX
| 1. | Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford). 2008;10:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Chang DK, Merrett ND, Biankin AV; NSW Pancreatic Cancer Network. Improving outcomes for operable pancreatic cancer: is access to safer surgery the problem? J Gastroenterol Hepatol. 2008;23:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Dumonceau JM, Deprez PH, Jenssen C, Iglesias-Garcia J, Larghi A, Vanbiervliet G, DAithal GP, Carrara S, Czakó L, Fernández-Esparrach G, Larghi A, Vanbiervliet G, Fockens P, Ginès À, Havre RF, Hassan C, Vilmann P, van Hooft JE, Polkowski M. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated January 2017. Endoscopy. 2017;49:695-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 4. | Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 5. | Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 294] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, Arnold D; ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 930] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 9. | Rana A, Rana SS. Endoscopic Ultrasound-Guided Tissue Acquisition: Techniques and Challenges. J Cytol. 2019;36:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Turner BG, Cizginer S, Agarwal D, Yang J, Pitman MB, Brugge WR. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 2010;71:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 11. | Siddiqui AA, Brown LJ, Hong SK, Draganova-Tacheva RA, Korenblit J, Loren DE, Kowalski TE, Solomides C. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:3370-3375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 12. | Hikichi T, Irisawa A, Bhutani MS, Takagi T, Shibukawa G, Yamamoto G, Wakatsuki T, Imamura H, Takahashi Y, Sato A, Sato M, Ikeda T, Hashimoto Y, Tasaki K, Watanabe K, Ohira H, Obara K. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Wani S, Mullady D, Early DS, Rastogi A, Collins B, Wang JF, Marshall C, Sams SB, Yen R, Rizeq M, Romanas M, Ulusarac O, Brauer B, Attwell A, Gaddam S, Hollander TG, Hosford L, Johnson S, Kushnir V, Amateau SK, Kohlmeier C, Azar RR, Vargo J, Fukami N, Shah RJ, Das A, Edmundowicz SA. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Levy MJ. Endoscopic ultrasound-guided trucut biopsy of the pancreas: prospects and problems. Pancreatology. 2007;7:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Eguia V, Chiang AL, Doukides TP, Sethi A, Poneros JM, Allendorf JD, Chabot JA, Lightdale CJ, Gonda TA. Potential risks and benefits of preoperative endosonographic evaluation of resectable pancreatic masses. Gastrointest Endosc. 2013;77:374. [DOI] [Full Text] |
| 16. | Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 17. | Collins BT, Murad FM, Wang JF, Bernadt CT. Rapid on-site evaluation for endoscopic ultrasound-guided fine-needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol. 2013;121:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 270] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 19. | Matynia AP, Schmidt RL, Barraza G, Layfield LJ, Siddiqui AA, Adler DG. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Schmidt RL, Witt BL, Matynia AP, Barraza G, Layfield LJ, Adler DG. Rapid on-site evaluation increases endoscopic ultrasound-guided fine-needle aspiration adequacy for pancreatic lesions. Dig Dis Sci. 2013;58:872-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 22. | Arena M, Eusebi LH, Pellicano R, Palamara MA, Iabichino G, Consolo P, Fagoonee S, Opocher E, Barabino M, Luigiano C. Endoscopic ultrasound core needle for diagnosing of solid pancreatic lesions: is rapid on-site evaluation really necessary? Minerva Med. 2017;108:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 509] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 24. | Lee LS, Nieto J, Watson RR, Hwang AL, Muthusamy VR, Walter L, Jajoo K, Ryou MK, Saltzman JR, Saunders MD, Suleiman S, Kadiyala V. Randomized Noninferiority Trial Comparing Diagnostic Yield of Cytopathologist-guided versus 7 passes for EUS-FNA of Pancreatic Masses. Dig Endosc. 2016;28:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Cleveland P, Gill KR, Coe SG, Woodward TA, Raimondo M, Jamil L, Gross SA, Heckman MG, Crook JE, Wallace MB. An evaluation of risk factors for inadequate cytology in EUS-guided FNA of pancreatic tumors and lymph nodes. Gastrointest Endosc. 2010;71:1194-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, Domínguez-Muñoz JE. Rapid on-site evaluation of endoscopic-ultrasound-guided fine-needle aspiration diagnosis of pancreatic masses. World J Gastroenterol. 2014;20:9451-9457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 27. | Kong F, Zhu J, Kong X, Sun T, Deng X, Du Y, Li Z. Rapid On-Site Evaluation Does Not Improve Endoscopic Ultrasound-Guided Fine Needle Aspiration Adequacy in Pancreatic Masses: A Meta-Analysis and Systematic Review. PLoS One. 2016;11:e0163056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | van Riet PA, Cahen DL, Poley JW, Bruno MJ. Mapping international practice patterns in EUS-guided tissue sampling: outcome of a global survey. Endosc Int Open. 2016;4:E360-E370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Khan MA, Grimm IS, Ali B, Nollan R, Tombazzi C, Ismail MK, Baron TH. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363-E375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 30. | Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 255] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 31. | Binmoeller KF, Brand B, Thul R, Rathod V, Soehendra N. EUS-guided, fine-needle aspiration biopsy using a new mechanical scanning puncture echoendoscope. Gastrointest Endosc. 1998;47:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Muthusamy VR. Endoscopic Ultrasound-Guided Fine-Needle Aspiration Vs Fine-Needle Biopsy. Gastroenterol Hepatol (N Y). 2017;13:496-499. [PubMed] |
| 33. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K, Rindi G, Bories E, Dogloni C, Bruno M, Dominguez-Muñoz JE. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 34. | Larghi A, Iglesias-Garcia J, Poley JW, Monges G, Petrone MC, Rindi G, Abdulkader I, Arcidiacono PG, Costamagna G, Biermann K, Bories E, Doglioni C, Dominguez-Muñoz JE, Hassan C, Bruno M, Giovannini M. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: a multicenter prospective cohort study. Surg Endosc. 2013;27:3733-3738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Iwashita T, Nakai Y, Samarasena JB, Park DH, Zhang Z, Gu M, Lee JG, Chang KJ. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | DiMaio CJ, Kolb JM, Benias PC, Shah H, Shah S, Haluszka O, Maranki J, Sharzehi K, Lam E, Gordon SR, Hyder SM, Kaimakliotis PZ, Allaparthi SB, Gress FG, Sethi A, Shah AR, Nieto J, Kaul V, Kothari S, Kothari TH, Ho S, Izzy MJ, Sharma NR, Watson RR, Muthusamy VR, Pleskow DK, Berzin TM, Sawhney M, Aljahdi E, Ryou M, Wong CK, Gupta P, Yang D, Gonzalez S, Adler DG. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): results of a large North American multicenter study. Endosc Int Open. 2016;4:E974-E979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 37. | Adler DG, Muthusamy VR, Ehrlich DS, Parasher G, Thosani NC, Chen A, Buscaglia JM, Appannagari A, Quintero E, Aslanian H, Taylor LJ, Siddiqui A. A multicenter evaluation of a new EUS core biopsy needle: Experience in 200 patients. Endosc Ultrasound. 2019;8:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 38. | Saxena P, El Zein M, Stevens T, Abdelgelil A, Besharati S, Messallam A, Kumbhari V, Azola A, Brainard J, Shin EJ, Lennon AM, Canto MI, Singh VK, Khashab MA. Stylet slow-pull versus standard suction for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic lesions: a multicenter randomized trial. Endoscopy. 2018;50:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Attam R, Arain MA, Bloechl SJ, Trikudanathan G, Munigala S, Bakman Y, Singh M, Wallace T, Henderson JB, Catalano MF, Guda NM. "Wet suction technique (WEST)": a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 40. | Lee KY, Cho HD, Hwangbo Y, Yang JK, Han SJ, Choi HJ, Lee YN, Cha SW, Moon JH, Cho YD, Park SH, Lee TH. Efficacy of 3 fine-needle biopsy techniques for suspected pancreatic malignancies in the absence of an on-site cytopathologist. Gastrointest Endosc. 2019;89:825-831.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Kim JH, Park SW, Kim MK, Lee J, Kae SH, Jang HJ, Koh DH, Choi MH. Meta-Analysis for Cyto-Pathological Outcomes in Endoscopic Ultrasonography-Guided Fine-Needle Aspiration With and Without the Stylet. Dig Dis Sci. 2016;61:2175-2184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Lee JK, Lee KT, Choi ER, Jang TH, Jang KT, Lee JK, Lee KH. A prospective, randomized trial comparing 25-gauge and 22-gauge needles for endoscopic ultrasound-guided fine needle aspiration of pancreatic masses. Scand J Gastroenterol. 2013;48:752-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Fabbri C, Polifemo AM, Luigiano C, Cennamo V, Baccarini P, Collina G, Fornelli A, Macchia S, Zanini N, Jovine E, Fiscaletti M, Alibrandi A, D'Imperio N. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: a prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Facciorusso A, Stasi E, Di Maso M, Serviddio G, Ali Hussein MS, Muscatiello N. Endoscopic ultrasound-guided fine needle aspiration of pancreatic lesions with 22 versus 25 Gauge needles: A meta-analysis. United European Gastroenterol J. 2017;5:846-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Xu MM, Jia HY, Yan LL, Li SS, Zheng Y. Comparison of two different size needles in endoscopic ultrasound-guided fine-needle aspiration for diagnosing solid pancreatic lesions: A meta-analysis of prospective controlled trials. Medicine (Baltimore). 2017;96:e5802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Guedes HG, Moura DTH, Duarte RB, Cordero MAC, Santos MELD, Cheng S, Matuguma SE, Chaves DM, Bernardo WM, Moura EGH. A comparison of the efficiency of 22G versus 25G needles in EUS-FNA for solid pancreatic mass assessment: A systematic review and meta-analysis. Clinics (Sao Paulo). 2018;73:e261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Ang TL, Kwek AB, Seo DW, Paik WH, Cheng TY, Wang HP, Lau J. A prospective randomized study of the difference in diagnostic yield between endoscopic ultrasound-guided fine-needle aspiration (EUSFNA) needles with and without a side port in pancreatic masses. Endosc Int Open. 2015;3:E329-E333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park DY, Seo DW, Lee SK, Jang SJ, Yun SC, Kim MH. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 49. | Attili F, Fabbri C, Yasuda I, Fuccio L, Palazzo L, Tarantino I, Dewitt J, Frazzoni L, Rimbaş M, Larghi A. Low diagnostic yield of transduodenal endoscopic ultrasound-guided fine needle biopsy using the 19-gauge Flex needle: A large multicenter prospective study. Endosc Ultrasound. 2017;6:402-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Alatawi A, Beuvon F, Grabar S, Leblanc S, Chaussade S, Terris B, Barret M, Prat F. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015;3:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Cheng B, Zhang Y, Chen Q, Sun B, Deng Z, Shan H, Dou L, Wang J, Li Y, Yang X, Jiang T, Xu G, Wang G. Analysis of Fine-Needle Biopsy vs Fine-Needle Aspiration in Diagnosis of Pancreatic and Abdominal Masses: A Prospective, Multicenter, Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2018;16:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 52. | Tian L, Tang AL, Zhang L, Liu XW, Li JB, Wang F, Shen SR, Wang XY. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: a prospective comparison study. Surg Endosc. 2018;32:3533-3539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Wang J, Zhao S, Chen Y, Jia R, Zhang X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: A meta-analysis. Medicine (Baltimore). 2017;96:e7452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Rodrigues-Pinto E, Jalaj S, Grimm IS, Baron TH. Impact of EUS-guided fine-needle biopsy sampling with a new core needle on the need for onsite cytopathologic assessment: a preliminary study. Gastrointest Endosc. 2016;84:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 55. | Ishikawa T, Kawashima H, Ohno E, Tanaka H, Sakai D, Iida T, Nishio R, Yamamura T, Furukawa K, Nakamura M, Miyahara R, Hashimoto S, Ishigami M, Hirooka Y. Clinical Impact of EUS-Guided Fine Needle Biopsy Using a Novel Franseen Needle for Histological Assessment of Pancreatic Diseases. Can J Gastroenterol Hepatol. 2019;2019:8581743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Yang MJ, Yim H, Hwang JC, Lee D, Kim YB, Lim SG, Kim SS, Kang JK, Yoo BM, Kim JH. Endoscopic ultrasound-guided sampling of solid pancreatic masses: 22-gauge aspiration versus 25-gauge biopsy needles. BMC Gastroenterol. 2015;15:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 57. | Kamata K, Kitano M, Yasukawa S, Kudo M, Chiba Y, Ogura T, Higuchi K, Fukutake N, Ashida R, Yamasaki T, Nebiki H, Hirose S, Hoki N, Asada M, Yazumi S, Takaoka M, Okazaki K, Matsuda F, Okabe Y, Yanagisawa A. Histologic diagnosis of pancreatic masses using 25-gauge endoscopic ultrasound needles with and without a core trap: a multicenter randomized trial. Endoscopy. 2016;48:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Lee YN, Moon JH, Kim HK, Choi HJ, Choi MH, Kim DC, Lee TH, Cha SW, Cho YD, Park SH. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014;46:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Oh HC, Kang H, Lee JY, Choi GJ, Choi JS. Diagnostic accuracy of 22/25-gauge core needle in endoscopic ultrasound-guided sampling: systematic review and meta-analysis. Korean J Intern Med. 2016;31:1073-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Aadam AA, Wani S, Amick A, Shah JN, Bhat YM, Hamerski CM, Klapman JB, Muthusamy VR, Watson RR, Rademaker AW, Keswani RN, Keefer L, Das A, Komanduri S. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016;4:E497-E505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Hucl T, Wee E, Anuradha S, Gupta R, Ramchandani M, Rakesh K, Shrestha R, Reddy DN, Lakhtakia S. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy. 2013;45:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Lee BS, Cho CM, Jung MK, Jang JS, Bae HI. Comparison of Histologic Core Portions Acquired from a Core Biopsy Needle and a Conventional Needle in Solid Mass Lesions: A Prospective Randomized Trial. Gut Liver. 2017;11:559-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | van Riet PA, Larghi A, Attili F, Rindi G, Nguyen NQ, Ruszkiewicz A, Kitano M, Chikugo T, Aslanian H, Farrell J, Robert M, Adeniran A, Van Der Merwe S, Roskams T, Chang K, Lin F, Lee JG, Arcidiacono PG, Petrone M, Doglioni C, Iglesias-Garcia J, Abdulkader I, Giovannini M, Bories E, Poizat F, Santo E, Scapa E, Marmor S, Bucobo JC, Buscaglia JM, Heimann A, Wu M, Baldaque-Silva F, Moro CF, Erler NS, Biermann K, Poley JW, Cahen DL, Bruno MJ. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc. 2019;89:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 64. | Nayar MK, Paranandi B, Dawwas MF, Leeds JS, Darne A, Haugk B, Majumdar D, Ahmed MM, Oppong KW. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest Endosc. 2017;85:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Abdelfatah MM, Grimm IS, Gangarosa LM, Baron TH. Cohort study comparing the diagnostic yields of 2 different EUS fine-needle biopsy needles. Gastrointest Endosc. 2018;87:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 67. | Park SW, Chung MJ, Lee SH, Lee HS, Lee HJ, Park JY, Park SW, Song SY, Kim H, Chung JB, Bang S. Prospective Study for Comparison of Endoscopic Ultrasound-Guided Tissue Acquisition Using 25- and 22-Gauge Core Biopsy Needles in Solid Pancreatic Masses. PLoS One. 2016;11:e0154401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 68. | Karadsheh Z, Al-Haddad M. Endoscopic ultrasound-guided fine-needle aspiration needles: which one and in what situation? Gastrointest Endosc Clin N Am. 2014;24:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Vilmann P, Seicean A, Săftoiu A. Tips to overcome technical challenges in EUS-guided tissue acquisition. Gastrointest Endosc Clin N Am. 2014;24:109-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 70. | Dietrich CF, Sahai AV, D'Onofrio M, Will U, Arcidiacono PG, Petrone MC, Hocke M, Braden B, Burmester E, Möller K, Săftoiu A, Ignee A, Cui XW, Iordache S, Potthoff A, Iglesias-Garcia J, Fusaroli P, Dong Y, Jenssen C. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016;84:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 71. | Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 221] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Hwang CY, Lee SS, Song TJ, Moon SH, Lee D, Park DH, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound guided fine needle aspiration biopsy in diagnosis of pancreatic and peripancreatic lesions: a single center experience in Korea. Gut Liver. 2009;3:116-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Fabbri C, Luigiano C, Maimone A, Tarantino I, Baccarini P, Fornelli A, Liotta R, Polifemo A, Barresi L, Traina M, Virgilio C, Cennamo V. Endoscopic ultrasound-guided fine-needle biopsy of small solid pancreatic lesions using a 22-gauge needle with side fenestration. Surg Endosc. 2015;29:1586-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Muniraj T, Sah RP, Chari ST, Adams DB, Cotton PB, Zyromski NJ. Autoimmune pancreatitis: an update. Adams DB, Cotton PB, Zyromski NJ. Pancreatitis: Medical and surgical management. Chichester, UK: John Wiley & Sons Ltd; 2017; . [DOI] [Full Text] |
| 75. | Eloubeidi MA, Mehra M, Bean SM. EUS-guided 19-gauge trucut needle biopsy for diagnosis of lymphoma missed by EUS-guided FNA. Gastrointest Endosc. 2007;65:937-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Hijioka S, Hara K, Mizuno N, Imaoka H, Bhatia V, Mekky MA, Yoshimura K, Yoshida T, Okuno N, Hieda N, Tajika M, Tanaka T, Ishihara M, Yatabe Y, Shimizu Y, Niwa Y, Yamao K. Diagnostic performance and factors influencing the accuracy of EUS-FNA of pancreatic neuroendocrine neoplasms. J Gastroenterol. 2016;51:923-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 77. | Chen L, Nassar A, Kommineni VT, Zarka MA, Zhang J, Faigel D, Nguyen C, Halfdanarson TR, Pannala R. Endoscopic ultrasonography-guided fine-needle aspiration cytology of surgically confirmed cystic pancreatic neuroendocrine tumors: a Mayo Clinic experience. J Am Soc Cytopathol. 2015;4:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Beal HL, Shea JE, Witt BL, Adler DG, Mulvihill SJ, Downs-Kelly E, Firpo MA, Scaife CL. Accuracy of diagnosing PDA, neuroendocrine, and IPMN by EUS-FNA at a single institution. J of GHR. 2015;4:1844-1849. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 79. | Witt BL, Factor RE, Chadwick BE, Caron J, Siddiqui AA, Adler DG. Evaluation of the SharkCore® needle for EUS-guided core biopsy of pancreatic neuroendocrine tumors. Endosc Ultrasound. 2018;7:323-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 80. | Muniraj T, Aslanian HR. New Developments in Endoscopic Ultrasound Tissue Acquisition. Gastrointest Endosc Clin N Am. 2017;27:585-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, Thonke F, de Werth A, Soehendra N. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-36; quiz 751, 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 83. | Kulesza P, Eltoum IA. Endoscopic ultrasound-guided fine-needle aspiration: sampling, pitfalls, and quality management. Clin Gastroenterol Hepatol. 2007;5:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 84. | Bang JY, Varadarajulu S. Neoplasia in chronic pancreatitis: how to maximize the yield of endoscopic ultrasound-guided fine needle aspiration. Clin Endosc. 2014;47:420-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Naveed M, Siddiqui AA, Kowalski TE, Loren DE, Khalid A, Soomro A, Mazhar SM, Yoo J, Hasan R, Yalamanchili S, Tarangelo N, Taylor LJ, Adler DG. A Multicenter comparative trial of a novel EUS-guided core biopsy needle (SharkCore™) with the 22-gauge needle in patients with solid pancreatic mass lesions. Endosc Ultrasound. 2018;7:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 86. | Larsen MH, Fristrup CW, Detlefsen S, Mortensen MB. Prospective evaluation of EUS-guided fine needle biopsy in pancreatic mass lesions. Endosc Int Open. 2018;6:E242-E248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 87. | Raymond SLT, Yugawa D, Chang KHF, Ena B, Tauchi-Nishi PS. Metastatic neoplasms to the pancreas diagnosed by fine-needle aspiration/biopsy cytology: A 15-year retrospective analysis. Diagn Cytopathol. 2017;45:771-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Lachter J. Basic technique in endoscopic ultrasound-guided fine needle aspiration for solid lesions: What needle is the best? Endosc Ultrasound. 2014;3:46-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |