Published online Dec 8, 2015. doi: 10.4254/wjh.v7.i28.2841
Peer-review started: August 18, 2015
First decision: October 14, 2015
Revised: October 22, 2015
Accepted: November 10, 2015
Article in press: November 11, 2015
Published online: December 8, 2015
Processing time: 109 Days and 17.5 Hours
AIM: To compare efficacy of telaprevir (TVR) and simeprevir (SMV) combined with pegylated interferon (PEG-IFN) and ribavirin (RBV) while treating chronic hepatitis C (CHC).
METHODS: In all, 306 CHC patients were included in this study. There were 159 patients in the TVR combination therapy group and 147 patients in the SMV combination therapy group. To evaluate pretreatment factors contributing to sustained virological response at 12 wk (SVR12), univariate and multivariate analyses were performed in TVR and SMV groups. To adjust for patient background between TVR and SMV groups, propensity score matching was performed. Virological response during treatment and SVR12 were evaluated.
RESULTS: Overall rates of SVR12 [undetectable serum hepatitis C virus (HCV) RNA levels] were 79.2% and 69.4% in TVR and SMV groups, respectively. Patients in the SMV group were older, had higher serum HCV RNA levels, lower hemoglobin, higher prevalence of unfavorable interleukin-28B (IL28B) genotype (rs8099917), and poorer response to previous PEG-IFN and RBV treatment. Propensity score matching was performed to adjust for backgrounds (n = 104) and demonstrated SVR12 rates of 74.0% and 73.1% in the TVR and SMV groups, respectively. In the TVR group, discontinuation rates were higher because of adverse events; however, breakthrough and nonresponse was more frequent in the in SMV group. Multivariate analysis revealed IL28B genotype (rs8099917) as the only independent predictive factor of SVR12 in both groups.
CONCLUSION: SVR12 rates were almost identical following propensity score matching.
Core tip: We evaluated and compared the efficacy of telaprevir (TVR) and simeprevir (SMV) in combination with pegylated interferon and ribavirin in the treatment of chronic hepatitis C. patients in real-world clinical settings. In the TVR group, the proportion of patients achieving a virological response was higher than that in the SMV group according to the original data. After propensity score matching, the proportion of patients achieving a virological response during treatment and after 12 wk was almost identical between the two groups with no significant difference observed.
-
Citation: Fujii H, Nishimura T, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Sumida Y, Mitsuyoshi H, Yokomizo C, Tanaka S, Ishikawa H, Nishioji K, Kimura H, Takami S, Nagao Y, Takeuchi T, Shima T, Sawa Y, Minami M, Yasui K, Itoh Y. Comparison of peg-interferon, ribavirin plus telaprevir
vs simeprevir by propensity score matching. World J Hepatol 2015; 7(28): 2841-2848 - URL: https://www.wjgnet.com/1948-5182/full/v7/i28/2841.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i28.2841
Chronic hepatitis C (CHC) infection is associated with a greatly increased risk of liver cirrhosis and hepatocellular carcinoma. There are an estimated 130-170 million people infected with hepatitis C virus (HCV) worldwide[1] and approximately 1.5-2 million in Japan[2]. The combination of pegylated interferon (PEG-IFN) and ribavirin (RBV) dual therapy was previously the standard care for CHC, and was administered for 48-72 wk in patients with genotype 1 and for 24 wk in genotype 2. Sustained virological response (SVR) rates are approximately 40%-50% in former group treated for 48 wk and approximately 80% in the latter treated for 24 wk[3-5].
Novel drug classes, including inhibitors of the NS3/NS4 protease of HCV polyprotein (protease inhibitors), have recently become available[6-8]. Of these, telaprevir (TVR) was the first to be approved in Japan for the treatment of CHC. In a clinical trial of TVR triple combination therapy (TVR, PEG-IFN, and RBV) for 24 wk in Japan, rapid reductions in serum HCV RNA levels were observed with a SVR rate of approximately 70%[9,10]. However, treatment discontinuation because of adverse events, including skin rash, anemia, and thrombocytopenia, occurred in up to 21% patients[11]. Thus, the TVR triple combination therapy is no longer recommended[12].
Simeprevir (SMV) is a second generation NS3/NS4 protease inhibitor[13]. The QUEST 1 and QUEST 2 phase 3 clinical trials demonstrated SVRs of 80% and 81% in patients treated with SMV triple combination therapy (SMV, PEG-IFN, and RBV), respectively. Similar results have been reported in phase 3 clinical trials conducted in Japan[14-16]. TVR and SMV were approved for use in clinical practice in Japan in December 2011 and December 2013, respectively. We previously treated patients with CHC using TVR or SMV as PEG-IFN and RBV-based triple combination therapy with an NS3/NS4 protease inhibitor; however, “drug lag” between TVR and SMV, causing a difference in clinical backgrounds between the two regimens prior to treatment initiation, prevented fair comparison of the efficacy of TVR and SMV in real-world clinical practice. The aim of this study was to evaluate and compare the efficacy of TVR or SMV for the treatment of CHC patients in Japan.
Patients were enrolled at Kyoto Prefectural University of Medicine and 8 affiliated hospitals in Kinki area of Japan (Kyoto, Osaka, Nara, Shiga Prefecture) from 2012 to 2014. Study protocols were approved by the ethics committee of each institution and conformed to the provisions of the Declaration of Helsinki. Patients enrolled in this study were diagnosed with CHC by board-certified hepatologists. Eligible patients were 20-80 years of age and had chronic HCV genotype 1 infection with HCV RNA levels of 5.0 log10 IU/mL or higher at screening.
Patients with decompensated liver disease, chronic hepatitis B, co-infection with human immunodeficiency virus, autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, or Wilson’s disease were excluded. Patients with uncontrollable hypertension or diabetes mellitus, and those with a history of alcohol abuse, were also excluded. Patients were followed-up monthly for the assessment of liver function and virological markers during treatment and until 12 wk after the completion of triple therapy. All patients gave informed consent to participate in this study.
In the TVR group, three patients were lost to follow-up and extreme protocol deviation (e.g., extended PEG-IFN and RBV therapy for up to 48 wk) occurred in seven patients. In the SMV group, two patients were lost to follow-up and extreme protocol deviation (e.g., extended PEG-IFN and RBV therapy for up to 48 wk) occurred in nine patients. Patients lost to follow-up or with extreme protocol deviation were excluded from the analysis.
The therapeutic outcomes of previous PEG-IFN and RBV therapy were classified into the following two groups: Undetectable serum HCV RNA levels at the end of the treatment period with quantifiable HCV RNA levels during follow-up (relapse group); and detectable HCV RNA levels at the end of the treatment period (other group).
Patients received per os telaprevir (Telavic; Mitsubishi Tanabe Pharma, Osaka, Japan) 2250 mg/d or simeprevir (Sovriad; Janssen Pharmaceutical K.K., Tokyo, Japan) 100 mg/d, combined with weekly subcutaneous injections of PEG-IFN alpha 2b (Peg-Intron; MSD, Tokyo, Japan) of 1.5 μg/kg and per os administration of RBV (Rebetol; MSD, Tokyo, Japan) of 600-1000 mg/d in accordance with prescribing information for 12 wk followed by PEG-IFN alpha 2b and RBV between weeks 12 and 24. In the TVR group, patients with lower serum hemoglobin levels began therapy at a reduced dose of TVR 1500 mg/d according to the judgment of treating physicians (2250 mg/d, 66 patients; 1500 mg/d, 93 patients). In the SMV group, patients began therapy at a dose of 100 mg/d. Dose reductions or discontinuation of TVR, SMV, PEG-IFN, and RBV were according to the judgment of treating physicians. Patients were followed-up for at least 12 wk after final treatment administration to assess SVR.
HCV RNA responses during therapy were classified into the following groups: Detectable HCV RNA levels at the end of the treatment period (nonresponse group); reappearance of HCV RNA during treatment (breakthrough group); and undetectable serum HCV RNA levels at the end of the treatment period with quantifiable HCV RNA levels during follow-up (relapse group). SVR12 was defined as undetectable serum HCV RNA levels at 12 wk after the end of treatment. Therapeutic effects were evaluated using intention-to-treat analysis.
Blood samples were obtained for routine biochemical and hematological assessments at treatment initiation, on treatment weeks 2, 4, 8, 12, 16, 20, 24, at the end of treatment (EOT), and at 12 wk after EOT. The antiviral effects were assessed by measuring serum HCV RNA levels using the COBAS TaqMan HCV test (Roche Molecular Diagnostics, Tokyo, Japan) with a lower limit of quantitation of 15 IU/mL. Interleukin 28B (IL28B; rs8099917) genotyping was accordingly performed in the majority of patients. In brief, DNA was extracted from peripheral whole blood (100 μL) with DNeasy Blood and Tissue Kits (QIAGEN, Valencia, CA) according to the manufacturer’s instructions. Genotypes were determined using a Light Cycler (Roche, Osaka, Japan). Subsequent gene sequencing was performed to validate amplified polymerase chain reaction (PCR) products. Primers and probes used for PCR were as follows: Forward primer, 5’-CAACATGGAGAGTTAAAGTAAGTCTTG-3’; reverse primer, 5’-TGCTGGGCCCTAACTGAT-3’; probe 1, LC Red 640-TTGGGTGACATTGCTCACAGAAAGG-Phosphate; and probe 2, CCAGCTACCAAACTGTATACAGCATGGTTCCA-Fluorescein.
Baseline continuous data were expressed as median with interquartile ranges in parentheses, and categorical variables were expressed as numbers. Univariate analyses were performed using chi-squared or Mann-Whitney U-tests as appropriate. All P-values of < 0.05 of two-tailed tests were considered significant. Multivariate logistic regression was used to identify significant independent predictive factors of SVR12. Results were expressed as Odds ratios and 95%CI. All statistical analyses were performed using the SPSS 22.0 statistical package (SPSS Incorporated, Chicago, Illinois, United States).
To adjust for patient background between TVR and SMV groups, propensity score matching was performed. Propensity score models were estimated using a logistic regression model that adjusts for patient characteristics (age, gender, body mass index, HCV RNA level, leukocyte count, hemoglobin, platelet count, and IL28 SNPs) listed in Table 1. Confounders were selected according to their potential association with the outcome on the basis of clinical knowledge and previous studies[17]. The propensity score matching model was validated by the Hosmer and Lemeshow goodness-of-fit test (P = 0.638) and by the value of the area under the curve (0.66, 95%CI: 0.594-0.724). One SMV patient was matched to one TVR patient using nearest neighbor matching without replacement. Propensity scores were matched using a caliper width 0.25 logit of the SD. The standardized difference was used to assess the covariate balance. McNemarr’s tests were performed after matching.
| Unmatched patients | Standardized | Propensity score matched patients | Standardized | |||||
| Telaprevir | Simeprevir | P value | difference | Telaprevir | Simeprevir | P value | difference | |
| No. of patients | n = 159 | n = 147 | n = 104 | n = 104 | ||||
| Age (yr) | 60 (51.0-65.0) | 63 (54.5-70.0) | 0.002 | 0.348 | 61.5 (53.0-65.8) | 60.5 (52.0-67.0) | NS | 0.0154 |
| Gender (male/female) | 77/82 | 67/80 | NS | 0.057 | 45/59 | 49/55 | NS | 0.0773 |
| Body mass index (kg/m2) | 23.9 (21.7-25.7) | 23.2 (21.1-25.0) | NS | 0.202 | 23.6 (21.1-25.3) | 23.4 (21.2-25.2) | NS | 0.0747 |
| Laboratory data | ||||||||
| Level of viremia (log IU/mL) | 6.7 (6.3-7.0) | 6.8 (6.3-7.2) | NS | 0.210 | 6.7 (6.3-7.0) | 6.6 (6.2-7.1) | NS | 0.0158 |
| Leukocyte count (/mm3) | 5060 (4200-5800) | 4920 (4100-5800) | NS | 0.094 | 5000 (4200-5700) | 5020 (4150-5800) | NS | 0.0272 |
| Hemoglobin (g/dL) | 14.1 (13.1-15.0) | 13.8 (12.9-14.7) | NS | 0.175 | 14 (13.0-14.8) | 13.9 (12.9-15.0) | NS | 0.0264 |
| Platelet count (× 104/mm3) | 15 (12.5-19.8) | 15.1 (11.7-20.1) | NS | 0.053 | 15 (12.9-20.0) | 15.1 (11.8-20.1) | NS | 0.0032 |
| SNP of IL28B (TT/non-TT/unknown) | 99/34/26 | 89/43/15 | NS | 0.155 | 74/30 | 73/31 | NS | 0.0211 |
| Other data | ||||||||
| Prior treatment response relapse/other | 43/30 | 31/32 | NS | 0.196 | 31/22 | 23/20 | NS | 0.100 |
The baseline patient characteristics in the TVR group (n = 159) and SMV group (n = 147) are shown in Table 1 as “unmatched patients”. Patients in the SMV group were significantly older than patients in the TVR group. High viral load, low hemoglobin levels, the non-TT IL28B genotype, and relapse following previous PEG-IFN and RBV treatment were more commonly observed in the SMV group compared with the TVR group.
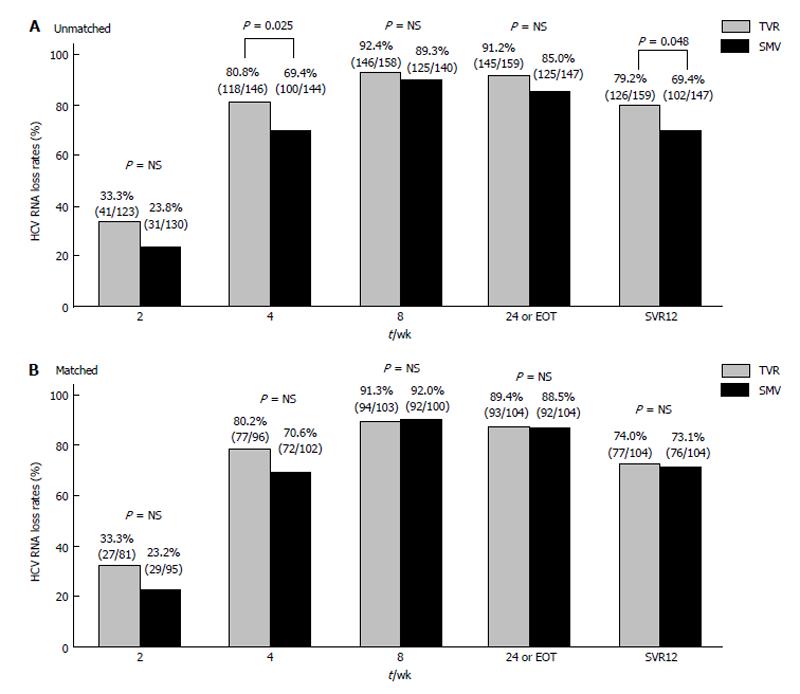
In the TVR group, the overall SVR12 was 79.2% (126 of 159 patients). Undetectable HCV RNA levels were achieved during treatment in 33.3% (41 of 123), 80.8% (118 of 146), 92.4% (146 of 158), and 91.2% (145 of 159) of patients at 2, 4, 8 wk, and EOT or 24 wk, respectively. In the SMV group, the overall SVR12 rate was 69.4% (102 of 147 patients). Undetectable HCV RNA levels were achieved during treatment in 23.8% (31 of 130), 69.4% (100 of 144), 89.3% (125 of 140), and 85.0% (125 of 147) of patients at 2, 4, 8 and EOT or 24 wk, respectively (Figure 1A).
In the TVR group, 10 patients demonstrated nonresponse, and breakthrough occurred in 4 patients. Relapse occurred in 19 patients. In patients with nonresponse, 8 patients discontinued TVR because of adverse events within the first 4 wk of treatment (four skin rash, one renal dysfunction, two appetite loss, one unknown). In the SMV group, 15 patients demonstrated nonresponse, and breakthrough occurred in eight patients. Relapse occurred in 22 patients. In patients with nonresponse, one patient discontinued within the first 4 wk of treatment (transient visual field defect). There was a trend toward greater rates of treatment discontinuation because of adverse events in the TVR group and nonresponse and breakthrough in the SMV group.
To evaluate pretreatment factors contributing to SVR12, univariate and multivariate analyses were performed in TVR and SMV groups including the following variables: Age, gender, body mass index, IL28B (rs8099917) genotype, viral load, leukocyte count, hemoglobin, and platelet counts (Table 2). In the TVR group, IL28B genotypes significantly correlated with SVR12 according to univariate analysis. In multivariable logistic regression analysis, IL28B genotype was found to be a significant independent predictor of SVR12 (OR = 4.316; 95%CI: 1.804-10.327, P = 0.001). In the SMV group, age and IL28B genotype significantly correlated with SVR12 according to univariate analysis. In multivariable logistic regression analysis, significant independent predictors of SVR were IL28B genotype (OR = 8.598; 95%CI: 3.388-21.817; P < 0.001), age (OR = 0.933; 95%CI: 0.889-0.980; P = 0.006), and viral load (OR = 0.335; 95%CI: 0.157-0.715, P = 0.005). Propensity score matching analysis was subsequently performed to reduce bias caused by differing baseline patient characteristics between TVR and SMV groups (Tables 1 and 2, propensity score matched patients). Following one-to-one matching of the two groups according to propensity score, 104 patients from the TVR group and 104 patients from the SMV group were matched according to baseline characteristics (Tables 1 and 2). Majority of covariates were statistically similar between the two groups (Table 1, Propensity score matched patients). Multivariable logistic regression analysis demonstrated that IL28B genotype significantly associated with SVR12 in both groups (TVR: OR = 7.739; 95%CI: 2.111-28.375, P = 0.002; and SMV: OR = 8.594; 95%CI: 2.777-26.598, P < 0.001; Table 2).
| Parameters | Telaprevir | Simeprevir | ||||
| SVR | Non-SVR | P value | SVR | Non-SVR | P value | |
| Unmatched patients | ||||||
| Univariate analysis | n = 126 | n = 33 | n = 102 | n = 45 | ||
| Age (yr) | 58.0 (51.0-65.0) | 62.0 (59.6-65.5) | NS | 61.0 (52.8-67.3) | 66.0 (56.5-71.0) | 0.016 |
| Gender (male/female) | 62/64 | 15/18 | NS | 48/54 | 19/26 | NS |
| Body mass index (kg/m2) | 23.8 (21.7-25.7) | 24.6 (21.7-26.0) | NS | 23.2 (21.0-25.1) | 23.5 (21.3-26.0) | NS |
| Level of viremia (log IU/mL) | 6.7 (6.3-7.0) | 6.6 (6.3-7.0) | NS | 6.8 (6.2-7.1) | 6.9 (6.4-7.3) | NS |
| Leukocyte count (/mm3) | 5100 (4200-5700) | 5100 (4400-6600) | NS | 5000 (4300-5800) | 4800 (3800-5800) | NS |
| Hemoglobin (g/dL) | 14.1 (13.2-15.1) | 14.1 (12.8-14.8) | NS | 13.9 (13.1-14.7) | 13.7 (12.7-14.8) | NS |
| Platelet count (× 104/mm3) | 15.0 (12.8-19.8) | 15.0 (12.5-20.1) | NS | 15.3 (11.9-20.5) | 15.0 (11.1-18.3) | NS |
| SNP of IL28B (TT/non-TT) | 84/19 | 15/15 | < 0.001 | 72/19 | 17/24 | < 0.001 |
| Multivariate analysis | Odds ratio (95%CI) | Odds ratio (95%CI) | ||||
| SNP of IL28B (TT/non-TT) | 4.316 (1.804-10.327) | 0.001 | 8.598 (3.388-21.817) | < 0.001 | ||
| Age (yr) | 0.933 (0.889-0.980) | 0.006 | ||||
| Level of viremia (log IU/mL) | 0.335 (0.157-0.715) | 0.005 | ||||
| Propensity score matched patients | ||||||
| Univariate analysis | n = 77 | n = 27 | n = 76 | n = 28 | ||
| Age (yr) | 60.0 (52.5-66.5) | 64.0 (60.0-65.0) | NS | 59.0 (51.0-66.0) | 65.0 (56.0-71.0) | 0.021 |
| Gender (male/female) | 33/44 | 12/15 | NS | 37/39 | 12/16 | NS |
| Body mass index (kg/m2) | 23.1 (20.6-25.0) | 24.0 (21.4-26.6) | NS | 23.2 (21.3-25.1) | 23.6 (21.1-26.1) | NS |
| Level of viremia (log IU/mL) | 6.7 (6.3-7.0) | 6.7 (6.4-6.9) | NS | 6.7 (6.1-7.0) | 6.6 (6.3-7.2) | NS |
| Leukocyte count (/mm3) | 5000 (4100-5700) | 5100 (4400-6700) | NS | 5100 (4400-5900) | 4600 (3500-5700) | NS |
| Hemoglobin (g/dL) | 14.0 (13.1-14.8) | 13.9 (13.0-14.9) | NS | 14.0 (12.9-15.0) | 13.8 (12.9-14.7) | NS |
| Platelet count (× 104/mm3) | 15.0 (12.3-19.7) | 15.4 (12.9-20.3) | NS | 15.1 (11.9-20.4) | 15.0 (11.0-17.2) | NS |
| SNP of IL28B (TT/non-TT) | 61/16 | 13/14 | 0.002 | 61/15 | 12/16 | < 0.001 |
| Multivariate analysis | Odds ratio (95%CI) | Odds ratio (95%CI) | ||||
| SNP of IL28B (TT/non-TT) | 7.739 (2.111-28.375) | 0.002 | 8.594 (2.777-26.598) | < 0.001 | ||
Before adjustment, the proportion of patients achieving virological responses at 4 wk and after 12 wk treatment significantly differed between the TVR group and SMV group. In general, a greater proportion of patients in the TVR group had a virological response than that in the SMV group (Figure 1A). After one-to-one propensity score matching, the proportions of patients achieving a virological response during treatment and after 12 wk treatment were similar between the two groups (SVR12: TVR, 74.0%; SMV, 73.1%; Figure 1B).
In the present study, we evaluated and compared the efficacy of TVR and SMV in combination with PEG-IFN and RBV in the treatment of CHC patients in real-world clinical settings in Japan. Both regimens achieved higher SVR rates compared with that using the dual combination therapy with PEG-IFN and RBV[6-10,14-16]. In the TVR group, the proportion of patients achieving a virological response was higher than in the SMV group according to the original data. A number of patients discontinued TVR therapy because of adverse events at the beginning of treatment. After propensity score matching, the proportion of patients achieving a virological response during treatment and after 12 wk was almost identical between the two groups with no significantly difference observed (Figure 1B).
Patients in the SMV group appeared to have a greater prevalence of unfavorable baseline characteristics. Patients in SMV group were statistically older, had higher viral loads, lower hemoglobin levels, and a higher prevalence of unfavorable IL28 genotypes (rs809997) compared with that in the TVR group. These pretreatment factors are known to influence the efficacy of IFN-based therapies[17]. As previously reported, Japanese patients infected with HCV genotype 1b are substantially older than Western patients[18]. A large proportion of patients able to tolerate IFN-based therapies were cured with previous therapies. Patients with unfavorable baseline characteristics remain untreated. In addition, according to academic guidelines[19], TVR therapy should be avoided in older patients with low hemoglobin levels in anticipation of future therapeutic options. As a result, a greater prevalence of unfavorable baseline characteristics were observed in patients in the SMV group.
In the present study, a greater proportion of patients in the TVR group discontinued treatment because of adverse events. Previously reported adverse events associated with TVR treatment include anemia, skin rash, and severe fatigue[11]. Cutaneous adverse effects caused by TVR have been frequently reported and are rare but are characterized by rapid development of lethal severe skin complications, such as Stevens-Johnson syndrome and drug-induced hypersensitivity syndrome[20,21]. Patients with these skin complications may have stopped the TVR treatment earlier. We administrated an initial dose of TVR 1500 mg/d in majority of patients to prevent treatment-induced anemia[22]. In contrast, the incidence of severe adverse events was low in the SMV group. Therefore, a smaller number of patients discontinued therapy in the SMV group.
Viral dynamics during treatment were similar to previous reports in both groups[16,23]. However, breakthrough and nonresponse was more frequent in the SMV group. Before matching, the TVR group had a higher SVR12 rate than that of the SMV group. After propensity score matching, this difference diminished and SVR12 rates were similar between the two groups. Reddy et al[24] reported a randomized control study between SMV and TVR for previous null or partial responders. Although the differences were observed in dosage, race, approved combined interferon, and treatment duration in their report, viral breakthrough was more frequent with SMV therapy than with TVR therapy similar to the present report.
The SVR rate in the SMV group in the present study was lower than in the CONCERTO-4 study[16]. As our study was in a real-world clinical setting, patients were generally older (proportion of patients aged > 65 years, 42.3% vs CONCERTO-4, 22.8%) and had lower platelet counts (platelet counts < 15 × 104/mm3: 47.7% vs CONCERTO-4, 31.6%) in our study. Baseline patient characteristics in our study may have resulted in a lower SVR12 rate.
The major limitation of the present study was the inability to evaluate several factors known to influence treatment efficacy. We did not examine amino acid substitutions of the HCV core region 70 and 91[23], NS5A interferon sensitivity determining region[25], interferon/ribavirin resistance determining region[26], or resistance-associated mutations of HCV NS3/NS4 proteases[27-29].
Treatment approaches to CHC are rapidly changing worldwide[30,31]. At present, direct-acting antiviral agent (DAA) combination therapy (daclatasvir and asunaprevir) is available for patients with HCV genotype 1 in Japan. Interferon-free DAA combination therapy has demonstrated an overall SVR12 rate of 85%[32]. Although the majority of patients with HCV infection may be treated with DAAs combination regimens, PEG-IFN and RBV-based treatment may still have utility in a small number of patients that do not respond to DAAs therapies.
In conclusion, both TVR and SMV regimens achieved high SVR12 rates. In the original analysis, TVR appeared to demonstrate an increased anti-viral efficacy compared with that of SMV. After propensity score matching, the proportion of patients achieving a virological response during treatment and after 12 wk treatment was almost identical between the two groups. Treatment discontinuation was more frequent in the TVR group because of adverse events at the beginning of treatment; however, nonresponse and breakthrough were more frequently observed in the SMV group.
The combination of pegylated interferon (PEG-IFN) and ribavirin (RBV) dual therapy was previously the standard care for chronic hepatitis C (CHC). Novel drug classes, including inhibitors of the NS3/NS4 protease of hepatitis C virus (HCV) polyprotein (protease inhibitors), have recently become available. Of these, telaprevir (TVR) triple combination therapy (TVR, PEG-IFN, and RBV) was the first to be approved in Japan in December 2011. Simeprevir (SMV), second generation protease inhibitor, was approved in Japan in December 2013. “Drug lag” between TVR and SMV, causing a difference in clinical backgrounds between the two regimens prior to treatment initiation, prevented fair comparison of the efficacy of TVR and SMV in real-world clinical practice.
The authors’ group evaluated and compared the efficacy of TVR or SMV for the treatment of CHC patients in Japan with propensity score matching to adjust for patient background between two groups.
Before adjustment, the proportion of patients achieving virological responses significantly differed between the TVR group and SMV group. In general, a greater proportion of patients in the TVR group had a virological response than that in the SMV group. After one-to-one propensity score matching, the proportions of patients achieving a virological response during treatment and after 12 wk treatment were similar between the two groups.
In the TVR group, the proportion of patients achieving a virological response was higher than in the SMV group according to the original data. A number of patients discontinued TVR therapy because of adverse events at the beginning of treatment. Breakthrough and nonresponse was more frequent in the SMV group. After propensity score matching, this difference diminished and sustained virological response 12 rates were similar between the two groups.
TVR is the first inhibitor of the NS3/NS4 protease of HCV polyprotein (protease inhibitors) in Japan. SMV is a second generation NS3/NS4 protease inhibitor. Propensity score matching attempt is used to reduce the background difference between TVR and SMV groups.
The article is well written. It’s clear and can help the authors’ to understand the new drug treatment efficiency in a big cohort of HCV patience.
P- Reviewer: Conti B S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1847] [Article Influence: 153.9] [Reference Citation Analysis (3)] |
| 2. | Namiki I, Nishiguchi S, Hino K, Suzuki F, Kumada H, Itoh Y, Asahina Y, Tamori A, Hiramatsu N, Hayashi N. Management of hepatitis C; Report of the Consensus Meeting at the 45th Annual Meeting of the Japan Society of Hepatology (2009). Hepatol Res. 2010;40:347-368. [PubMed] |
| 3. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] [DOI] [Full Text] |
| 4. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] |
| 5. | Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350-1359. [PubMed] |
| 6. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 7. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 8. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 9. | Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology. 2010;52:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;56:78-84. [PubMed] |
| 11. | Sherman KE. Managing adverse effects and complications in completing treatment for hepatitis C virus infection. Top Antivir Med. 2012;20:125-128. [PubMed] |
| 12. | Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. JSH Guidelines for the Management of Hepatitis C Virus Infection: A 2014 Update for Genotype 1. Hepatol Res. 2014;44 Suppl S1:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Welch NM, Jensen DM. Pegylated interferon based therapy with second-wave direct-acting antivirals in genotype 1 chronic hepatitis C. Liver Int. 2015;35 Suppl 1:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Simeprevir with peginterferon/ribavirin for treatment-naïve hepatitis C genotype 1 patients in Japan: CONCERTO-1, a phase III trial. J Hepatol. 2014;61:219-227. [PubMed] |
| 15. | Izumi N, Hayashi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Ki R, Komada Y, Seto C. Once-daily simeprevir with peginterferon and ribavirin for treatment-experienced HCV genotype 1-infected patients in Japan: the CONCERTO-2 and CONCERTO-3 studies. J Gastroenterol. 2014;49:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Kumada H, Hayashi N, Izumi N, Okanoue T, Tsubouchi H, Yatsuhashi H, Kato M, Rito K, Komada Y, Seto C. Simeprevir (TMC435) once daily with peginterferon-α-2b and ribavirin in patients with genotype 1 hepatitis C virus infection: The CONCERTO-4 study. Hepatol Res. 2015;45:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol. 2008;49:634-651. [PubMed] |
| 18. | Yoshizawa H, Tanaka J, Miyakawa Y. National prevention of hepatocellular carcinoma in Japan based on epidemiology of hepatitis C virus infection in the general population. Intervirology. 2006;49:7-17. [PubMed] |
| 19. | Editors of the Drafting Committee for Hepatitis Management Guidelines: The Japan Society of Hepatology. Guidelines for the Management of Hepatitis C Virus Infection: First edition, May 2012, The Japan Society of Hepatology. Hepatol Res. 2013;43:1-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Shuster M, Do D, Nambudiri V. Severe cutaneous adverse reaction to telaprevir. Dermatol Online J. 2015;21:pii: 13030/qt2zq8z9zt. [PubMed] |
| 21. | Federico A, Sgambato D, Cotticelli G, Gravina AG, Dallio M, Beneduce F, Ruocco E, Romano M, Loguercio C. Skin Adverse Events During Dual and Triple Therapy for HCV-Related Cirrhosis. Hepat Mon. 2014;14:e16632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Sezaki H, Suzuki F, Hosaka T, Akuta N, Fukushima T, Hara T, Kawamura Y, Kobayashi M, Suzuki Y, Saitoh S. Effectiveness and safety of reduced-dose telaprevir-based triple therapy in chronic hepatitis C patients. Hepatol Res. 2014;44:E163-E171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M. Amino acid substitution in HCV core region and genetic variation near the IL28B gene affect viral dynamics during telaprevir, peginterferon and ribavirin treatment. Intervirology. 2012;55:417-425. [PubMed] |
| 24. | Reddy KR, Zeuzem S, Zoulim F, Weiland O, Horban A, Stanciu C, Villamil FG, Andreone P, George J, Dammers E. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis. 2015;15:27-35. [PubMed] |
| 25. | Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, Ogura Y, Izumi N, Marumo F, Sato C. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;334:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 734] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 26. | El-Shamy A, Nagano-Fujii M, Sasase N, Imoto S, Kim SR, Hotta H. Sequence variation in hepatitis C virus nonstructural protein 5A predicts clinical outcome of pegylated interferon/ribavirin combination therapy. Hepatology. 2008;48:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Lin C, Gates CA, Rao BG, Brennan DL, Fulghum JR, Luong YP, Frantz JD, Lin K, Ma S, Wei YY. In vitro studies of cross-resistance mutations against two hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061. J Biol Chem. 2005;280:36784-36791. [PubMed] |
| 28. | Halfon P, Locarnini S. Hepatitis C virus resistance to protease inhibitors. J Hepatol. 2011;55:192-206. [PubMed] |
| 29. | Sarrazin C, Zeuzem S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology. 2010;138:447-462. [PubMed] |
| 30. | Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19:837-849. [PubMed] |
| 32. | Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 453] [Article Influence: 41.2] [Reference Citation Analysis (0)] |