Published online Nov 28, 2015. doi: 10.4254/wjh.v7.i27.2749
Peer-review started: July 6, 2015
First decision: September 8, 2015
Revised: September 20, 2015
Accepted: November 10, 2015
Article in press: November 11, 2015
Published online: November 28, 2015
Processing time: 147 Days and 6.6 Hours
AIM: To evaluate the efficacy of vitamin E treatment on liver stiffness in nonalcoholic fatty liver disease (NAFLD).
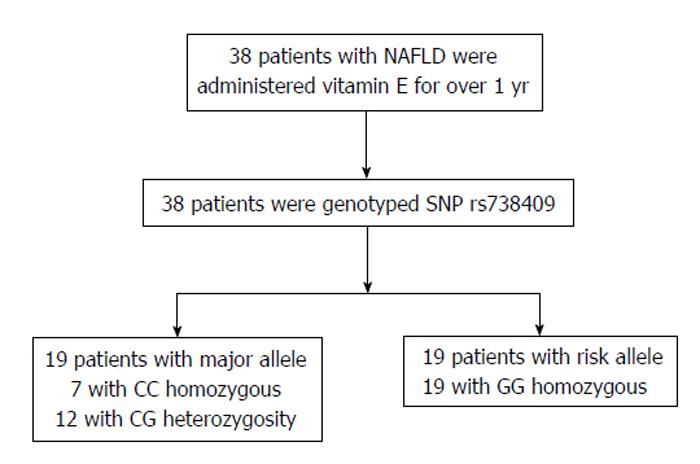
METHODS: Thirty-eight NAFLD patients were administered vitamin E for > 1 year. The doses of vitamin E were 150, 300, or 600 mg; three times per day after each meal. Responses were assessed by liver enzyme levels [aspartate aminotransferase (AST), alanine aminotranferease (ALT), and γ-glutamyl transpeptidase (γ-GTP)], noninvasive scoring systems of hepatic fibrosis-4 [FIB-4 index and aspartate aminotransferase-to-platelet index (APRI)], and liver stiffness [velocity of shear wave (Vs)] measured by acoustic radiation force impulse elastography. Vs measurements were performed at baseline and 12 mo after baseline. The patients were genotyped for the patatin-like phospholipase domain containing 3 (PNPLA3) polymorphisms and then divided into either the CC/CG or GG group to examine each group’s responses to vitamin E treatment.
RESULTS: We found marked differences in the platelet count, serum albumin levels, alkaline phosphatase levels, FIB-4 index, APRI, and Vs at baseline depending on the PNPLA3 polymorphism. AST, ALT, and γ-GTP levels (all P < 0.001); FIB-4 index (P = 0.035); APRI (P < 0.001); and Vs (P < 0.001) significantly decreased from baseline to 12 mo in the analysis of all patients. In the subset analyses of PNPLA3 genotypes, AST levels (P = 0.011), ALT levels (P < 0.001), γ-GTP levels (P = 0.005), APRI (P = 0.036), and Vs (P = 0.029) in genotype GG patients significantly improved, and AST and ALT levels (both P < 0.001), γ-GTP levels (P = 0.003), FIB-4 index (P = 0.017), and APRI (P < 0.001) in genotype CC/CG patients.
CONCLUSION: One year of vitamin E treatment improved noninvasive fibrosis scores and liver stiffness in NAFLD patients. The responses were similar between different PNPLA3 genotypes.
Core tip: Responses to vitamin E treatment in nonalcoholic fatty liver disease patients were assessed by noninvasive scoring systems of hepatic fibrosis, and liver stiffness (velocity of shear wave) was measured by acoustic radiation force impulse elastography. Vitamin E treatment for 1 year improved not only liver enzyme levels but also noninvasive fibrosis scores and liver stiffness. Subsequently, the patients were divided into two groups according to patatin-like phospholipase domain containing 3 (PNPLA3) genotype (CC/CG or GG) to examine whether either group responded differently to the treatment. The responses were similar between different PNPLA3 genotypes.
- Citation: Fukui A, Kawabe N, Hashimoto S, Murao M, Nakano T, Shimazaki H, Kan T, Nakaoka K, Ohki M, Takagawa Y, Takamura T, Kamei H, Yoshioka K. Vitamin E reduces liver stiffness in nonalcoholic fatty liver disease. World J Hepatol 2015; 7(27): 2749-2756
- URL: https://www.wjgnet.com/1948-5182/full/v7/i27/2749.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i27.2749
Nonalcoholic fatty liver disease (NAFLD) is a common liver disease[1], and nonalcoholic steatohepatitis (NASH) characterized by steatosis with necroinflammation and eventual fibrosis[2] can lead to end-stage cirrhosis and hepatocellular carcinoma[3].
Although there are no generally approved treatments for NASH, several treatment options have demonstrated efficacy in various clinical trials. Statins[4-7], insulin sensitizers such as thiazolidinediones[8,9], and metformin[10] are effective for the treatment of NAFLD[11]. Oxidative stress plays a central role in the transition from simple steatosis to NASH[12]. An effective therapeutic strategy is to target reduction in oxidative stress using, for example, administration of vitamin E. A recent trial has provided substantial evidence for the previously suggested efficacy of vitamin E in inducing histological improvement of NASH[13].
Evaluation of liver fibrosis is essential in chronic liver diseases because the prognosis of the diseases and the treatment decisions often depend on fibrosis. Liver biopsy is still considered the gold standard for liver fibrosis assessment, despite being an invasive method and not completely risk free[14]. In recent years, noninvasive methods, aimed at replacing liver biopsy, have been developed for the evaluation of liver fibrosis. Development of noninvasive tools will enable monitoring of the disease progression and response to therapy. Velocity of shear wave (Vs) measured by acoustic radiation force impulse (ARFI) has been reported to be a good method for assessing the stage of liver fibrosis[15,16]. Vs has been reported to be useful in diagnosing NAFLD[17-19].
The single-nucleotide polymorphism rs738409 (C > G) in the patatin-like phospholipase domain containing 3 (PNPLA3) was strongly associated with increased hepatic fat levels and with hepatic inflammation in NAFLD patients[20-23]. However, the effect of the PNPLA3 polymorphisms on the response to treatment has not been reported.
The aims of this study were to evaluate the efficacy of vitamin E treatment for NAFLD by noninvasive methods and to assess the association between the treatment response and the PNPLA3 polymorphism present.
Vitamin E was administered for > 1 year to 38 patients with NAFLD as treatments for atherosclerosis, diabetic retinopathy, or prevention of lipid peroxidation from January 2011 to July 2015. The patients showed no improvement in aminotransferase levels following lifestyle modification such as dietary modification and exercise before beginning vitamin E treatment. Their clinical data were retrospectively studied (Table 1). The diagnosis of NAFLD was confirmed by liver biopsy in 10 patients, by ultrasonic examination in 23 patients, and by presence of cirrhosis with no obvious etiology and with metabolic risk factors such as obesity and metabolic syndrome in 5 patients[11]. None of the patients consumed > 40 g of alcohol per day. The patients who increased the dose of or started other medicines for NAFLD, such as pioglitazone, metformin, ursodeoxycholic acid, statins, ezetimibe, or angiotensin 2 receptor antagonist, during the study period were excluded from this study.
| All patients | PNPLA3 genotype | P value | ||
| CC/CG | GG | PNPLA3 CC/CG vs GG | ||
| No. of subjects | 38 | 19 | 19 | |
| Sex (male/female) | 10/28 | 6/13 | 4/15 | 0.461 |
| Age (yr) | 62.0 ± 11.6 | 58.4 ± 11.9 | 65.6 ± 10.1 | 0.061 |
| Height (cm) | 156.6 ± 9.9 | 158.7 ± 11.3 | 154.4 ± 7.6 | 0.226 |
| Body weight (kg) | 68.2 ± 14.9 | 69.7 ± 16.1 | 66.7 ± 13.5 | 0.685 |
| Body mass index (kg/m2) | 27.9 ± 4.7 | 27.7 ± 4.0 | 28.0 ± 5.3 | 0.988 |
| Classification of NAFLD | ||||
| Fatty liver confirmed by an imaging examination | 23 | 13 | 10 | 0.347 |
| NASH confirmed by a liver biopsy | 9 | 5 | 4 | |
| Nonviral liver cirrhosis diagnosed clinically | 5 | 1 | 4 | |
| Burn-out NASH confirmed by a liver biopsy | 1 | 0 | 1 | |
| Child-Pugh grade of cirrhotic patients (A/B/C) | 4/2/0 | 1/0/0 | 3/2/0 | 0.439 |
| Concurrent diabetes mellitus (+/-) | 15/23 | 5/14 | 10/9 | 0.097 |
| Concurrent hepatocellular cancer (+/-) | 0/38 | 0/19 | 0/19 | - |
| Dosage of vitamin E (150 mg/300 mg/600 mg) | 2/4/32 | 2/1/16 | 0/3/16 | 0.223 |
| Concurrent medication | ||||
| Pioglitazone | 3 | 2 | 1 | 0.547 |
| Metformin | 2 | 2 | 0 | 0.146 |
| Ursodeoxycholic acid | 27 | 14 | 13 | 0.721 |
| HMG-CoA reductase inhibitor | 13 | 6 | 7 | 0.732 |
| Ezetimibe | 7 | 6 | 1 | 0.036a |
| Angiotensin receptor 2 antagonist | 9 | 6 | 3 | 0.252 |
| Serum biochemical tests | ||||
| Platelet count (× 104/μL) | 16.7 ± 7.5 | 19.3 ± 7.1 | 14.1 ± 6.9 | 0.046a |
| Prothrombin activity (%) | 98.5 ± 18.7 | 104.2 ± 17.6 | 93.2 ± 18.2 | 0.061 |
| Hemoglobin A1c (%) | 6.38 ± 1.06 | 6.26 ± 0.97 | 6.49 ± 1.14 | 0.394 |
| Total protein (g/dL) | 7.42 ± 0.52 | 7.47 ± 0.49 | 7.36 ± 0.54 | 0.435 |
| Serum albumin (g/dL) | 4.27 ± 0.39 | 4.41 ± 0.36 | 4.14 ± 0.37 | 0.032a |
| Total bilirubin (mg/dL) | 0.98 ± 0.50 | 0.84 ± 0.29 | 1.11 ± 0.61 | 0.172 |
| AST (IU/L) | 61.1 ± 29.9 | 58.2 ± 32.2 | 64.1 ± 27.2 | 0.191 |
| ALT (IU/L) | 68.5 ± 41.2 | 69.9 ± 36.0 | 67.1 ± 45.7 | 0.385 |
| Alkaline phosphatase (IU/L) | 312 ± 108 | 270 ± 85 | 355 ± 112 | 0.032a |
| γ-GTP (IU/L) | 87.4 ± 70.0 | 72.6 ± 59.1 | 101.5 ± 76.4 | 0.142 |
| Total cholesterol (mg/dL) | 185 ± 37 | 194 ± 43 | 176 ± 28 | 0.172 |
| Triglycerides (mg/dL) | 151 ± 85 | 147 ± 99 | 156 ± 64 | 0.351 |
| Cholinesterase (IU/L) | 333 ± 124 | 353 ± 91 | 313 ± 147 | 0.172 |
| Scoring systems of hepatic fibrosis | ||||
| FIB-4 index | 3.80 ± 2.78 | 2.61 ± 1.74 | 4.98 ± 3.10 | 0.006a |
| APRI | 1.50 ± 0.94 | 1.17 ± 0.75 | 1.84 ± 0.98 | 0.014a |
| Velocity of shear wave (m/s) | 2.20 ± 0.91 | 1.81 ± 0.76 | 2.56 ± 0.89 | 0.010a |
The total doses of 150, 300 or 600 mg vitamin E were orally given into 3 administrations per day after each meal for > 1 year: 150 mg for 2 patients, 300 mg for 4 patients, or 600 mg for 32 patients (100 mg is equivalent to 100 IU; Eisai Pharmaceutical Co., Tokyo, Japan). In the American Association for the Study of Liver Diseases practice guideline, vitamin E was recommended to be administered to nondiabetic adults with biopsy-proven NASH at a daily dose of 800 IU[11]. However, the dosage of vitamin E accepted by health insurance in Japan is 150-300 mg for atherosclerosis or diabetic retinopathy, and 300-600 mg for prevention of lipid peroxidation.
Laboratory data were collected at three time points: at baseline (beginning of vitamin E administration), 6 and 12 mo after baseline. Two noninvasive scoring systems of hepatic fibrosis, the fibrosis-4 (FIB-4) index and the aspartate aminotransferase-to-platelet index (APRI), were calculated from the measurements using the originally reported formulas[24,25]. The APRI formula was aspartate aminotransferase (AST) level (U/L)/AST (upper limit of normal; U/L)/platelet (109/L) × 100 and the FIB-4 score formula was age (years) × AST level (U/L)/platelet (109/L) × [alanine aminotranferease (ALT)]1/2 (U/L). These noninvasive scoring systems were used at each of the time points.
Vs measurement by ARFI elastography was performed at baseline and 12 mo after baseline using a Siemens ACUSON S2000 (Siemens Medical Systems Co., Ltd., Tokyo, Japan). The examination was performed on the right lobe of the liver. A measurement depth of 2-3 cm below the liver capsule was chosen. Ten successful acquisitions at different locations were performed on each patient, and the results are expressed as median values in m/s. Vs is considered to be proportional to the square root of tissue elasticity. Histological improvement was not examined because sequential liver biopsy was not performed.
Genomic DNA was extracted from whole blood samples using QIA amp DNA Mini Kits (Qiagen, Tokyo, Japan), according to the manufacturer’s protocol. The rs738409 PNPLA3 SNP was genotyped using TaqMan predesigned SNP genotyping assays (Applied Biosystems, Tokyo, Japan), according to the manufacturer’s protocol. The patients were divided into two groups by PNPLA3 genotype (CC/CG vs GG) to examine the different responses to vitamin E in each group (Figure 1).
Differences in the two groups in terms of clinical characteristics and laboratory values at baseline were analyzed using either the χ2 test or the Mann-Whitney U test. Differences between the laboratory values obtained at three time points were analyzed using the Freedman test. Differences between the laboratory values obtained at two time points were analyzed using Wilcoxon’s signed-rank test with Bonferroni’s correction. Differences were judged as significant if the P value was < 0.05 (two-tailed). All statistical analyses were conducted using SPSS software (SPSS Statistics Version 22; IBM Co., Armonk, NY).
Seven obese patients included in this study exceeded their ideal body weight (body mass index was > 32 kg/m2), and 39.5% of patients had type 2 diabetes (Table 1). Platelet counts and serum albumin levels in the GG group were significantly lower than those in the CC/CG group (P = 0.046, and P = 0.032, respectively). Alkaline phosphatase levels, FIB-4 index, APRI, and Vs in the GG group were significantly higher than those in the CC/CG group (P = 0.032, P = 0.006, P = 0.014, and P = 0.010, respectively).
Body weight in all patients did not change from baseline to 12 mo (68.2 ± 14.9 kg and 72.4 ± 16.8 kg, respectively). There were no patients who achieved > 7% weight loss during study periods.
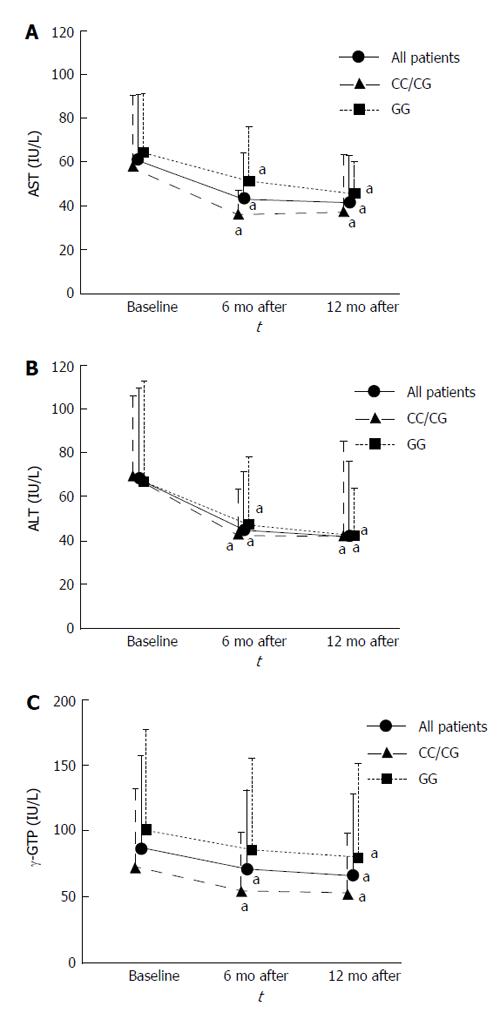
Serum AST, ALT and γ-glutamyl transpeptidase (γ-GTP) levels in all patients significantly decreased from baseline to 6 mo (P < 0.001, P < 0.001, and P = 0.019, respectively) and 12 mo (P < 0.001, P < 0.001, and P < 0.001, respectively). Those in the CC/CG group also significantly decreased from baseline to 6 mo (P = 0.004, P = 0.022, and P = 0.047, respectively) and 12 mo (P < 0.001, P < 0.001, and P = 0.003, respectively). Serum AST and ALT levels in the GG group significantly decreased from baseline to 6 mo (P = 0.045, and P = 0.004, respectively) and 12 mo (P = 0.011, and P < 0.001, respectively), and serum γ-GTP levels in the GG group significantly decreased from baseline to 12 mo (P = 0.005) (Figure 2).
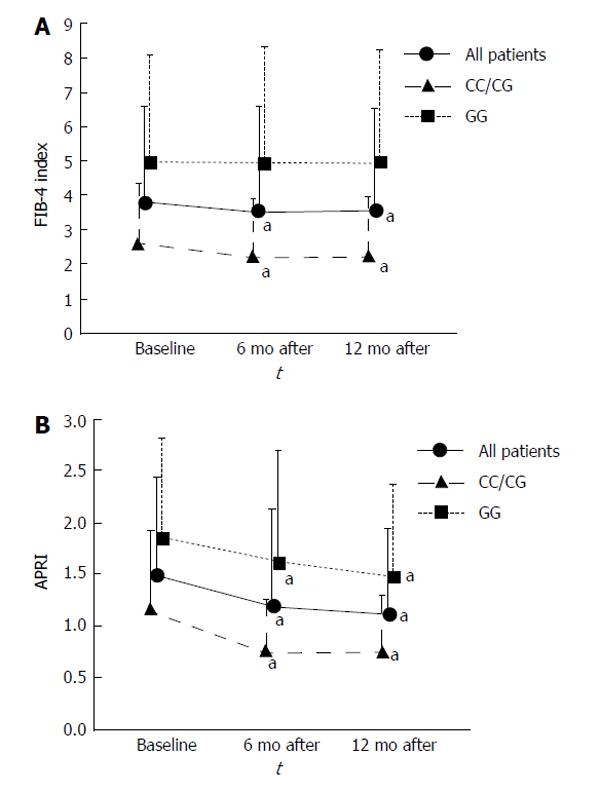
The FIB-4 index in all patients significantly decreased from baseline to 6 and 12 mo (P = 0.015 and P = 0.035, respectively). FIB-4 index in the CC/CG group also significantly decreased from baseline to 6 and 12 mo (P = 0.014 and P = 0.017, respectively). On the other hand, the FIB-4 index did not change in the GG group (Figure 3A).
APRI in all patients significantly decreased from baseline to 6 and 12 mo (P < 0.001 and P < 0.001, respectively). APRI in the CC/CG group significantly decreased from baseline to 6 and 12 mo (P = 0.004 and P < 0.001, respectively). APRI in the GG group also significantly decreased from baseline to 6 and 12 mo (P = 0.028 and P = 0.036, respectively; Figure 3B).
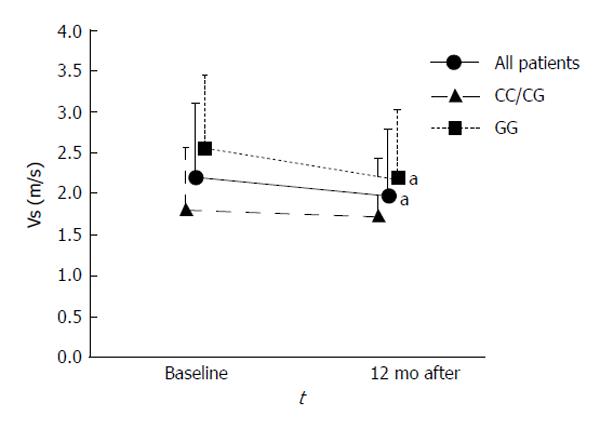
Vs in all patients decreased from baseline to 12 mo (P = 0.005). Vs in the GG group also decreased from baseline to 12 mo (P = 0.029), and Vs in the CC/CG group also tended to decrease; however, the decrease was not significant (Figure 4).
The present study showed that a 1-year treatment of vitamin E improved not only laboratory values but also the noninvasive scores related to hepatic fibrosis and liver stiffness in NAFLD patients. The treatment responses are similar between different PNPLA3 genotypes.
In general, lifestyle modification should be the first line of treatment in patients with NAFLD, and it was reported that weight reduction greater than 7% achieved through lifestyle intervention improves aminotransferase levels and liver histology[26]. Because the weight of patients in the present study did not change during the study period, it was assumed that the outcomes were not affected by weight loss.
Recently, a large, multicenter randomized controlled trial was conducted by the NASH Clinical Research Network to evaluate the efficacy of vitamin E treatment for amelioration of NASH in adults [pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis (PIVENS)][13]. This trial reported that serum AST, ALT, and γ-GTP levels in the vitamin E group decreased compared with the placebo group and that the changes occurred in the first 24 wk and were sustained throughout the study period. In our study, the changes occurred 6 mo after baseline, which was consistent with the PIVENS study.
Liver biopsy is regarded as the gold standard in the assessment of patients with NAFLD/NASH. However, liver biopsy is an invasive procedure with potential complications, and sampling error can result in substantial misdiagnosis and staging inaccuracies. Recently, several indices have been developed for noninvasive tests that help to diagnose advanced liver disease. The FIB-4 index and APRI can easily be used at the bedside or in an outpatient setting because of the simple calculation of only a few laboratory values. In our study, the FIB-4 index and APRI markedly decreased from baseline to 6 and 12 mo. These findings may indicate that the administration of vitamin E improved liver fibrosis. However, since these scoring systems are calculated using ALT, the reduction of the score may be attributed to the reduction of hepatic inflammation.
In recent years, several studies have reported the usefulness of ARFI elastography for the assessment of liver stiffness and a positive correlation between Vs and biopsy-proven fibrosis stage in patients with NAFLD[17-19]. ARFI elastography has mainly been used in diagnosis[27] and there are no reports of it being used for assessment of the efficacy of vitamin E treatment in NAFLD patients. In our study, Vs markedly decreased from baseline to 12 mo. In the subset analysis of 32 patients without six patients with daily doses of 150 mg and 300 mg of vitamin E, Vs markedly decreased from baseline to 12 mo (P = 0.004). The reduction of Vs probably indicates a reduction in liver fibrosis. The PIVENS trial reported that the vitamin E group had a reduction in steatosis, lobular inflammation, and activity score, whereas fibrosis scores did not markedly improve[13]. The fact that ARFI revealed a reduction in liver stiffness in the present study despite no demonstration of a significant reduction in fibrosis by liver biopsies in the PIVENS trial may indicate that ARFI is more sensitive than liver biopsies for detecting the reduction of fibrosis. There may be possibility that the reduction of Vs is attributed to factors other than reduction of fibrosis.
Yoneda et al[17] reported that Vs differed between groups with different inflammatory activity in 54 patients. Fierbinteanu Braticevici et al[19] reported that Vs had a positive correlation with inflammation in 64 patients. On the other hand, Palmeri et al[18] reported that Vs was not associated with inflammation scores in 172 patients. Thus, the association between Vs and inflammation is still unclear, and further studies are required in this field.
The association between the PNPLA3 polymorphisms with not only fatty liver and triglyceride content, but also with inflammation and fibrosis in NAFLD has been reported[22,28,29]. A meta-analysis reported that GG homozygous had a 3.24-fold greater risk of higher necroinflammatory scores and a 3.2-fold greater risk of developing fibrosis than CC homozygous[29]. In the present study, there were some differences at baseline depending on the PNPLA3 polymorphism. The platelet counts and serum albumin levels were lower, and alkaline phosphatase levels, the FIB-4 index, APRI, and Vs were higher in GG patients than in CC/CG patients. Our results were consistent with previous reports.
In the subset analyses of PNPLA3 genotypes, AST and ALT levels, APRI, and Vs in genotype GG patients and AST, ALT, and γ-GTP levels, FIB-4 index, and APRI in genotype CC/CG patients improved following vitamin E treatment. Vitamin E treatment for 1 year improved not only liver enzyme levels but also noninvasive fibrosis scores and liver stiffness in NAFLD patients. The responses are similar between different PNPLA3 genotypes.
The most effective dosage of vitamin E is unclear. In the PIVENS trial, 800 IU of vitamin E was administered per day. However, a previous study reported that patients with vascular disease or diabetes mellitus who received long-term supplementation with vitamin E (400 IU/d) had a higher risk of heart failure and hospitalization for heart failure[30]. These results may suggest that we have to avoid vitamin E treatment for patients with vascular disease or diabetes mellitus. In the present study, no patients had heart failure during the observation period.
The present study had several limitations: (1) it was a retrospective study; (2) there was no control group; (3) the sample size was insufficient to provide significant differences in some indicators; and (4) sequential liver biopsy was not performed for observing histological improvement. The preliminary findings of the present study need further verification via a well-controlled, prospective study with a sufficiently large sample size to confirm the efficacy of vitamin E by noninvasive scoring systems of hepatic fibrosis and Vs and differences of response according to PNPLA3 polymorphisms.
In conclusion, vitamin E treatment for 1 year improved not only laboratory values but also the noninvasive scores of hepatic fibrosis and liver stiffness in NAFLD patients. These responses were similar between different PNPLA3 genotypes.
The authors thank Dr. Hiroshi Takahashi of the Division of Medical Statistics, Fujita Health University for assisting with statistical analysis.
Nonalcoholic fatty liver disease (NAFLD) is a common liver disease, and eventual fibrosis can lead to end-stage cirrhosis and hepatocellular carcinoma. An effective therapeutic strategy is to target reduction in oxidative stress using, for example, administration of vitamin E. Liver biopsy is still considered the gold standard for liver fibrosis assessment, despite being an invasive method and not completely risk free. Velocity of shear wave (Vs) measured by acoustic radiation force impulse (ARFI) has been reported to be a good method for assessing the stage of liver fibrosis. The single-nucleotide polymorphism rs738409 (C > G) in the patatin-like phospholipase domain containing 3 (PNPLA3) was strongly associated with increased hepatic fat levels and with hepatic inflammation in NAFLD patients. In this study, the authors evaluated the efficacy of vitamin E treatment for NAFLD by noninvasive methods and to assess the association between the treatment response and the patatin-like phospholipase domain containing 3 (PNPLA3) polymorphism.
Few prior reports showed that vitamin E improved serum aspartate aminotransferase (AST), alanine aminotranferease (ALT), and γ-glutamyl transpeptidase (γ-GTP) levels and reduced steatosis, lobular inflammation, and activity score, but did not markedly improve fibrosis scores in nonalcoholic steatohepatitis (NASH). The results of the authors’ study contribute to non-invasive evaluation of the efficacy of vitamin E treatment for NAFLD/NASH.
The pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis trial reported that serum AST, ALT, and γ-GTP levels in the vitamin E group decreased compared with the placebo group, and that vitamin E group had a reduction in steatosis, lobular inflammation, and activity score, whereas fibrosis scores did not markedly improve. The present study showed that a 1-year treatment of vitamin E improved not only laboratory values but also the noninvasive scores related to hepatic fibrosis and liver stiffness in NAFLD patients, and that the treatment responses were similar between different PNPLA3 genotypes.
This study suggests that liver stiffness is useful for monitoring the efficacy of vitamin E treatment for NAFLD/NASH. The patients with NAFLD/NASH can be evaluated the therapeutic effect of vitamin E using noninvasive tools without liver biopsy.
ARFI: Vs measured by ARFI has been reported to be a good method for assessing the stage of liver fibrosis; PNPLA3: The single-nucleotide polymorphism rs738409 (C > G) in the PNPLA3 was strongly associated with increased hepatic fat levels and with hepatic inflammation in NAFLD patients.
The authors investigated the effect of vitamin E on NAFLD. They suggested that vitamin E treatment for 1 year reduced stiffness in NAFLD patients and the responses were similar between different PNPLA3 genotypes. The work was logically designed and nicely described.
P- Reviewer: Hassanain M, Lee MK, Montalto G, Streba LAM S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1619] [Article Influence: 115.6] [Reference Citation Analysis (1)] |
| 2. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2885] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 3. | Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 365] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 4. | Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: a histopathological follow-up study. J Hepatol. 2007;47:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 8. | Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review and meta analysis. J Hepatol. 2011;55:1383-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 10. | Shyangdan D, Clar C, Ghouri N, Henderson R, Gurung T, Preiss D, Sattar N, Fraser A, Waugh N. Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review. Health Technol Assess. 2011;15:1-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 12. | Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2010;38:2293-2301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 13. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2466] [Article Influence: 164.4] [Reference Citation Analysis (2)] |
| 14. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1580] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 15. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 16. | Nishikawa T, Hashimoto S, Kawabe N, Harata M, Nitta Y, Murao M, Nakano T, Mizuno Y, Shimazaki H, Kan T. Factors correlating with acoustic radiation force impulse elastography in chronic hepatitis C. World J Gastroenterol. 2014;20:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 269] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 18. | Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Fierbinteanu Braticevici C, Sporea I, Panaitescu E, Tribus L. Value of acoustic radiation force impulse imaging elastography for non-invasive evaluation of patients with nonalcoholic fatty liver disease. Ultrasound Med Biol. 2013;39:1942-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2592] [Article Influence: 152.5] [Reference Citation Analysis (0)] |
| 21. | Kawaguchi T, Sumida Y, Umemura A, Matsuo K, Takahashi M, Takamura T, Yasui K, Saibara T, Hashimoto E, Kawanaka M. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS One. 2012;7:e38322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Hotta K, Yoneda M, Hyogo H, Ochi H, Mizusawa S, Ueno T, Chayama K, Nakajima A, Nakao K, Sekine A. Association of the rs738409 polymorphism in PNPLA3 with liver damage and the development of nonalcoholic fatty liver disease. BMC Med Genet. 2010;11:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Zain SM, Mohamed R, Mahadeva S, Cheah PL, Rampal S, Basu RC, Mohamed Z. A multi-ethnic study of a PNPLA3 gene variant and its association with disease severity in non-alcoholic fatty liver disease. Hum Genet. 2012;131:1145-1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1163] [Article Influence: 72.7] [Reference Citation Analysis (1)] |
| 25. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3238] [Article Influence: 147.2] [Reference Citation Analysis (0)] |
| 26. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 971] [Article Influence: 64.7] [Reference Citation Analysis (1)] |
| 27. | Yoshioka K, Hashimoto S, Kawabe N. Measurement of liver stiffness as a non-invasive method for diagnosis of non-alcoholic fatty liver disease. Hepatol Res. 2015;45:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 28. | Sookoian S, Castaño GO, Burgueño AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009;50:2111-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 29. | Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 739] [Article Influence: 52.8] [Reference Citation Analysis (1)] |
| 30. | Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 813] [Article Influence: 40.7] [Reference Citation Analysis (0)] |