TO THE EDITOR
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as nonalcoholic fatty liver disease, represents a spectrum of conditions ranging from simple hepatic steatosis to more advanced stages, such as metabolic steatohepatitis and liver fibrosis[1]. MASLD is the leading cause of chronic liver disease worldwide and affects nearly a quarter of the adult population, highlighting its significant public health impact[2,3]. This disease represents a growing public health burden, driven by the increasing prevalence of obesity and type 2 diabetes mellitus, which are closely associated with its pathogenesis. The interplay of metabolic dysfunctions, including insulin resistance (IR) and lipotoxicity, exacerbates hepatic inflammation and fibrosis, significantly increasing morbidity and mortality[4,5]. Given these metabolic bases, effective treatment of MASLD requires interventions targeting both hepatic and systemic metabolic dysfunctions while promoting sustained lifestyle modifications.
Lifestyle factors such as poor dietary habits, physical inactivity, and excessive calorie intake are central to the progression of nonalcoholic fatty liver disease/MASLD[6,7]. High consumption of refined sugars and saturated fats promotes IR and lipotoxicity, whereas sedentary behaviour reduces energy expenditure and metabolic flexibility, paving the way for hepatic fat accumulation, inflammation and fibrosis[8-11]. These interconnected effects are compounded by gut microbiota dysbiosis, which facilitates systemic inflammation and perpetuates liver injury[12]. Evidence suggests that weight loss exceeding 7%-10% of total body weight can result in histological improvements, including fibrosis regression and steatosis resolution, underscoring the importance of effective lifestyle interventions[13,14]. Nevertheless, achieving and maintaining significant weight loss remains a challenge for many people, requiring the development of comprehensive and personalized lifestyle intervention programs[15]. Conventional therapeutic approaches often focus on dietary counselling, structured exercise regimens, and behavioral modifications aimed at promoting long-term healthy habits[16,17]. Although these interventions can be effective, limitations such as unequal access, follow-up difficulties and adherence problems persist[18-20]. To overcome these barriers, emerging technologies, including mobile health (mHealth) apps, wearable fitness tracking devices and telemedicine platforms, offer promising avenues to improve patient engagement, provide personalized guidance and enhance the scalability and sustainability of lifestyle-based interventions[21].
Digital health platforms integrating personalized care through internet-enabled devices have emerged as a promising solution to overcome these challenges[22,23]. One such intervention is the RESET care program, a digital health platform that integrates dietary guidance, structured exercise and cognitive behavioral therapy (CBT), all via a mobile app equipped with Internet of Things (IoT) devices, such as body composition analyzers and smartwatches. This program was specifically designed to overcome common barriers to lifestyle interventions by providing real-time monitoring, personalized feedback, and behavioral reinforcement, all of which are essential for long-term adherence and sustainable metabolic improvements. In their recent study, Soni et al[23] evaluated the effectiveness of RESET care in managing MASLD, demonstrating significant weight loss and metabolic improvements in participants undergoing the comprehensive intervention. This letter to the editor explores the transformative role of digital health in reshaping MASLD care, critically analyzing the findings of RESET care within the broader context of emerging digital innovations in metabolic disease management. Specifically, we discuss how technology-enhanced interventions can close existing gaps in MASLD treatment, improve patient adherence and engagement, and provide scalable and cost-effective solutions for chronic disease management.
THE ROLE OF DIGITAL HEALTH
Digital health technologies have radically transformed chronic disease management, offering innovative solutions to address the inherent limitations of traditional health care systems[24]. Chronic diseases, such as type 2 diabetes mellitus, cardiovascular conditions, hypertension and obesity, require continuous monitoring, long-term lifestyle modifications and personalized interventions[25]. Traditional care models, which often rely on episodic in-person visits, are insufficient to meet the dynamic needs of these diseases. Digital health tools fill this gap by enabling real-time data collection, improving patient engagement, and facilitating more proactive and personalized care[26,27]. One of the most impactful developments in digital health is the integration of wearable devices and IoT technologies. Wearables, such as smartwatches, fitness trackers and biosensors, continuously monitor vital parameters such as physical activity, heart rate, blood glucose levels and sleep patterns[28,29].
Furthermore, mHealth apps complement wearable and IoT technologies by offering personalized recommendations, medication reminders and behavioral interventions[30]. These mHealth apps often use evidence-based behavioral strategies, such as gamification, goal setting and motivational messaging, to improve compliance with lifestyle modifications and treatment plans. By enabling users to log meals, exercise routines and symptoms, these apps allow individuals to track their progress and actively participate in their health care[31]. In addition, the integration of artificial intelligence into mHealth care platforms provides personalized information, predict potential health risks and offer tailored solutions to mitigate them[30].
Finally, telemedicine platforms further strengthen the role of digital health by bridging the gap between patients and health care professionals, especially in underserved or remote areas[32]. These platforms facilitate virtual consultations, enabling timely follow-up, medication adjustments, and provide immediate information, thus reducing the need for in-person visits. This continuity of care is especially crucial for chronic disease management, where frequent monitoring and adjustments are often necessary to achieve optimal outcomes[33].
CLINICAL BENEFITS OF DIGITAL HEALTH IN MASLD
The RESET care program, presented by Soni et al[23], exemplifies how digital health technologies can revolutionize the management of MASLD. This 12-week intervention leverages a combination of tailored diet plans, structured exercise routines and CBT through a mobile app supported by IoT devices, including a body composition analyzer and smartwatch. The program provides continuous monitoring and real-time feedback, addressing both the behavioral and biological dimensions of MASLD while offering a scalable, patient-centred approach to care.
The RESET care program demonstrated notable differences in the outcomes of its three intervention groups, underscoring the value of a multimodal approach. Group C (n = 6), which integrated dietary counselling, structured exercise routines, and CBT, achieved the most significant improvements, including a mean weight reduction of 6.99 kg (101.10 ± 17.85 vs 94.11 ± 17.38), a body mass index (BMI) reduction of 2.18 kg/m² (32.90 ± 3.02 vs 30.72 ± 3.41) and notable reductions in subcutaneous (1.26%, 23.68 ± 2.06 vs 22.42 ± 2.97) and visceral fat (2.16%, 15.30 ± 2.65 vs 13.14 ± 3.17). These results exceeded the critical threshold of 7% weight loss associated with significant improvements in MASLD. Group B (n = 9), which combined diet and exercise, presented moderate reductions in weight (82.61 ± 9.29 vs 79.90 ± 8.64) and BMI (30.14 ± 2.26 vs 29.14 ± 1.93) but lacked the added benefits of CBT. Group A (n = 7), which focused solely on dietary counselling, achieved the smallest changes, highlighting the limitations of single-modality interventions. Differences between groups were statistically significant (weight P = 0.00050, BMI P = 0.00003, subcutaneous fat P = 0.00198) and visceral fat P = 0.00015).
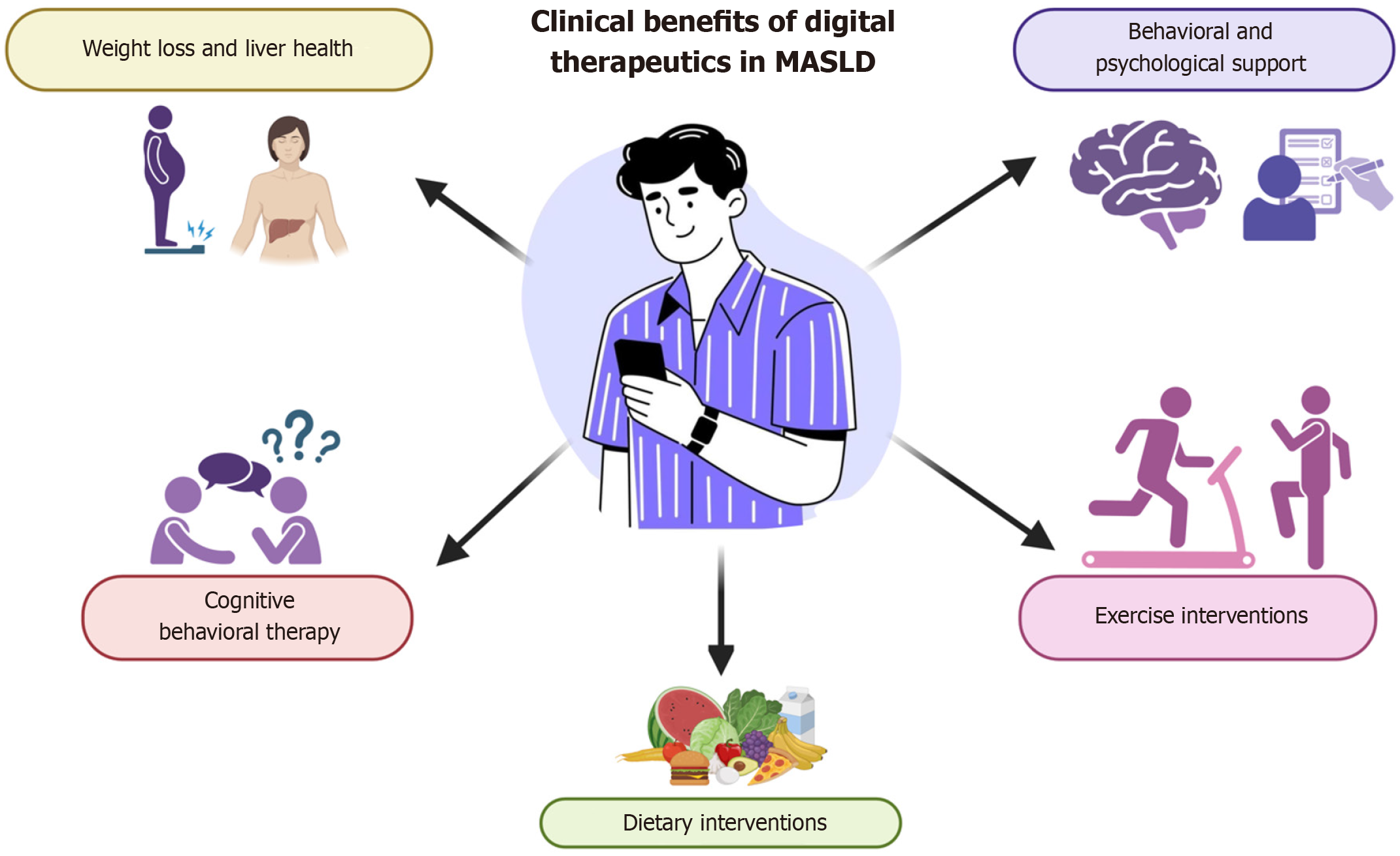
Emerging evidence underscores the scalability of digital therapeutics (DTx) in the treatment of MASLD[26,34]. Zhou et al[34] highlighted the ability of DTx to bridge health gaps through remote monitoring, personalized lifestyle interventions, and adherence support through interactive platforms. These platforms combine health education, peer-to-peer support, and clinical follow-up to optimize outcomes, positioning DTx as a promising tool for chronic disease management. Programs such as RESET demonstrate the clinical potential of DTx, achieving significant results such as reductions in weight, triglyceride levels and liver stiffness (Figure 1). For example, a United States study of a mobile technology intervention for MASLD reported that, after six months, 50% of participants experienced improvements in these markers, and 75% showed improvements in controlled attenuation parameters and physical function[34,35].
Figure 1 Clinical benefits of digital therapeutics in metabolic dysfunction-associated liver disease.
The clinical advantages of digital therapeutics in the management of metabolic dysfunction-associated liver disease are substantial. Essential components encompass personalized interventions aimed at weight reduction and the enhancement of liver health, alongside behavioral and psychological support. Additionally, cognitive behavioral therapy, exercise interventions, and dietary modifications are crucial aspects of this approach. These elements are seamlessly integrated through digital platforms, thus providing continuous and tailored support to foster patient engagement and improve health outcomes. MASLD: Metabolic dysfunction-associated liver disease.
DTx improves user engagement and motivation by incorporating gamification, reminders and personalized feedback. Zhou et al[34] highlighted how these tools can transform passive patient behavior into active disease management, significantly improving treatment adherence and clinical outcomes. The inclusion of CBT in programs such as RESET further addresses psychological barriers to sustained behaviour change. CBT fosters self-efficacy, goal-setting, and problem-solving skills, which are critical for maintaining long-term lifestyle modifications[35]. For patients with MASLD, mitigating the emotional and psychological stress associated with obesity and metabolic syndrome through CBT indirectly improves adherence to dietary and physical activity recommendations. These insights underscore the importance of holistic care models that address both the physical and the psychological dimensions of chronic diseases such as MASLD[36,37].
Dietary and exercise interventions via digital platforms have also been shown to be effective. A German study using web-based exercise programs reported improvements in peak oxygen consumption and weight reduction after only eight weeks, with participants citing interactive and personalized information as key motivators[34]. Similarly, platforms such as Fitbit have demonstrated success in tracking and adapting diet and physical activity plans, highlighting how mobile apps can sustain lifestyle modifications over the long term.
CLINICAL IMPLICATIONS AND FUTURE DIRECTIONS
Programmes such as RESET demonstrate the feasibility and effectiveness of digital health interventions in clinical settings. By combining structured lifestyle modifications with digital tools, such interventions overcome traditional barriers, such as limited access to health care providers or the logistical challenges of frequent in-person visits[23,26,36-38]. These tools enhance patient autonomy and optimize health care resource utilization. Furthermore, emerging data suggest that integrating digital health platforms into routine care could significantly reduce the long-term health care burden of MASLD by preventing disease progression[26,34]. For example, interventions focusing on visceral fat reduction, a key contributor to liver inflammation, have shown promise when combined with structured dietary and exercise programs[14,36-39].
Visceral fat reduction plays a crucial role in improving MASLD, as excess visceral adiposity drives hepatic fat accumulation through increased free fatty acid flux to the liver, exacerbating IR and inflammation[1]. Digital interventions such as RESET facilitate structured caloric restriction and macronutrient balancing, promoting lipolysis and visceral fat mobilization. In addition, exercise interventions, particularly moderate- to high-intensity training, improve mitochondrial oxidative capacity and fatty acid oxidation, reducing hepatic triglyceride accumulation and improving insulin sensitivity[40,41]. The observed reductions in visceral and subcutaneous fat suggest that digital platforms effectively drive metabolic improvements through sustained lifestyle modifications, rather than mere short-term weight loss.
Another key advantage of digital health interventions is their ability to improve long-term adherence to lifestyle modifications, addressing one of the main barriers in the management of MASLD. Traditional lifestyle interventions often face high dropout rates and poor adherence, limiting their effectiveness. By integrating self-management features, personalized information and behavioral reinforcement strategies, digital health platforms can maintain patient motivation and engagement. Studies suggest that gamification elements and real-time progress tracking improve adherence to dietary and physical activity recommendations, leading to greater weight loss maintenance and metabolic benefits[42,43]. Additionally, CBT and behavioral reinforcement strategies can modulate reward processing circuitry, shifting eating behaviors from impulsive consumption to goal-oriented decision making[44]. These mechanisms may explain why participants in the comprehensive intervention group (diet + exercise + CBT) show higher adherence rates and more pronounced metabolic improvements than those receiving diet-only or diet plus exercise interventions.
Challenges and future directions
While digital interventions such as RESET show considerable promise, they are not without limitations. One primary challenge is the generalizability of the results, as many studies rely on small, homogeneous populations. For example, Hannah et al[27] demonstrated variability in weight loss and adherence rates across different demographic groups. Future research should prioritize larger, more diverse cohorts to validate these findings and explore the individual contributions of dietary changes, exercise, and CBT to treatment outcomes[35]. Additionally, the cost and accessibility of digital health platforms remain significant barriers in low-resource settings. Efforts must be made to develop affordable, culturally tailored solutions to maximize their global impact[26,34,35]. Finally, long-term studies are needed to evaluate the sustainability of benefits derived from DTx and to assess their role in preventing complications such as NASH or cirrhosis.
Finally, data security and privacy remain critical challenges to the widespread adoption of digital health platforms. With the increasing reliance on real-time monitoring and data sharing, ensuring the confidentiality and protection of patient information is paramount[42]. Robust encryption protocols, secure data storage and compliance with international data protection standards are essential to safeguard sensitive health care information. In addition, clear communication with users about how their data will be used and protected is crucial to build trust and encourage participation in digital health programs[43-47].
CONCLUSION
Digital health interventions offer a scalable, patient-centered approach to MASLD treatment by integrating structured lifestyle modifications, real-time monitoring, and behavioral reinforcement. Programs such as reset demonstrate their potential to improve adherence, reduce visceral fat, and improve metabolic outcomes, but their long-term effects on fibrosis progression and MASLD complications require further study. For clinical integration, digital tools must complement existing pharmacological and lifestyle therapies, and trained healthcare professionals must leverage patient-generated data for personalized interventions. Cost-effectiveness remains a key factor, requiring economic evaluations to justify reimbursement and political support. To ensure scalability, strategies must address disparities in digital access, affordability and health literacy, with culturally tailored, low-cost solutions integrated into primary care and telemedicine networks. Future research should focus on long-term adherence, personalized interventions and healthcare integration to maximize impact. By overcoming these challenges, digital health technologies can become a key component of MASLD prevention and treatment, improving outcomes on a global scale.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Mexico
Peer-review report’s classification
Scientific Quality: Grade A, Grade B, Grade C, Grade D
Novelty: Grade A, Grade B, Grade C, Grade C
Creativity or Innovation: Grade A, Grade C, Grade C, Grade C
Scientific Significance: Grade A, Grade B, Grade C
P-Reviewer: Adnyana IMDM; Pandya A; Wang W S-Editor: Bai Y L-Editor: A P-Editor: Zhao YQ