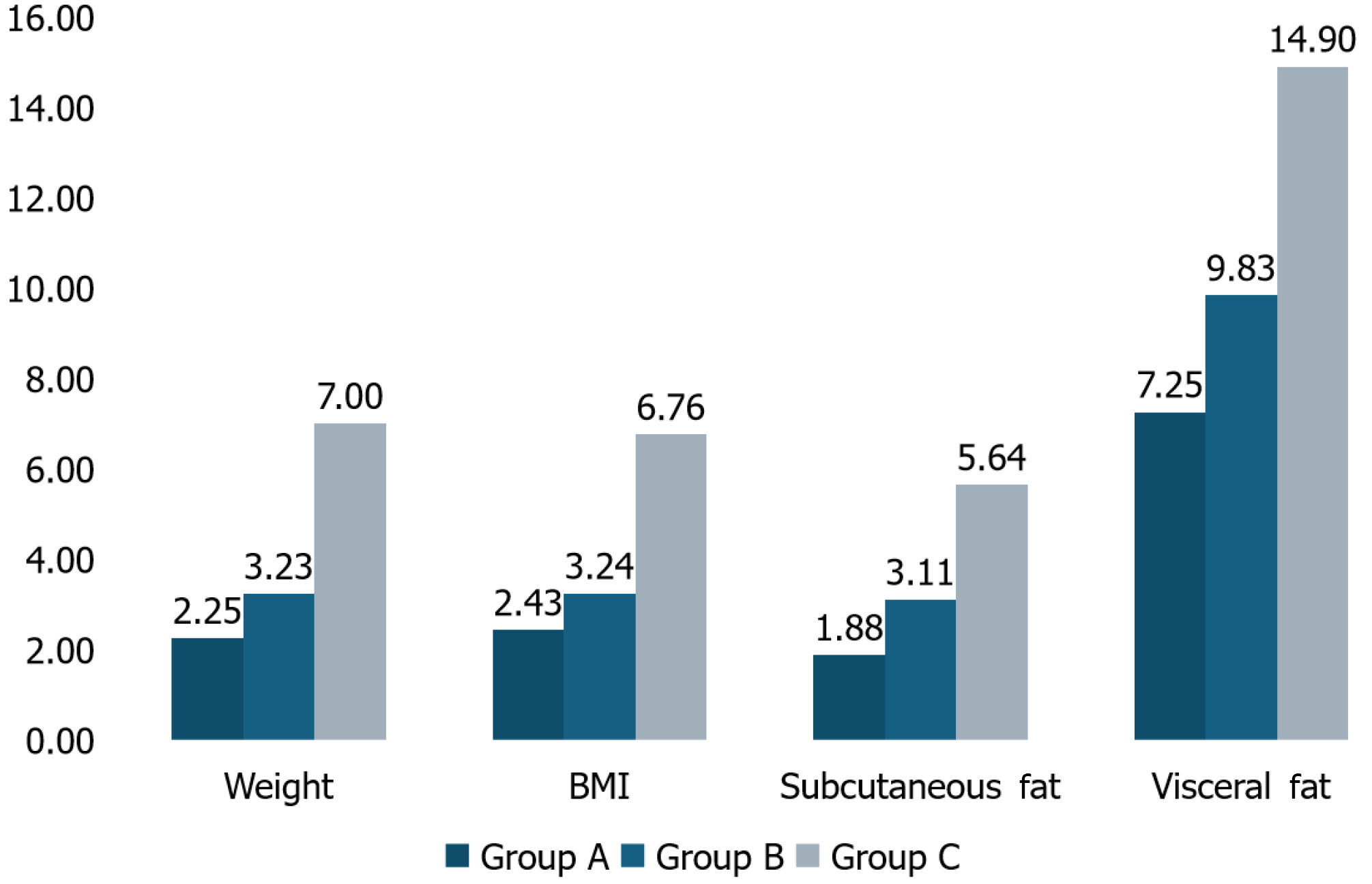Published online Jan 27, 2025. doi: 10.4254/wjh.v17.i1.101630
Revised: November 11, 2024
Accepted: December 2, 2024
Published online: January 27, 2025
Processing time: 106 Days and 19.9 Hours
Non-alcoholic fatty liver disease (NAFLD) management requires sustainable lifestyle modifications. This study aimed to evaluate the effectiveness of the RESET care plan, a comprehensive program that is an integrated personalized diet, exercise, and cognitive behavior therapy, delivered via MyTatva’s digital health application enabled through a body composition analyzer (BCA) and smartwatch.
To evaluates the effectiveness of the comprehensive program delivered via My
This retrospective observational study analyzed deidentified data from 22 par
All intervention groups showed significant improvement across all anthropometric parameters. Group C showed the most significant improvements, with mean weight reduction of 7% or more (6.99 ± 2.98 kg, 7.00% ± 3.39%; P = 0.002) from baseline, a benchmark associated with improved NAFLD conditions. Post-hoc analysis revealed that Group C had significantly greater improvements than Groups A and B. Weight reduction was observed in 85.7% of Group A participants, 77.8% of Group B participants, and 100% of Group C participants.
The comprehensive RESET care plan achieved a 7% weight reduction in 12 weeks, demonstrating its effectiveness in managing NAFLD. These results support adopting digitally supported, patient-centric approaches for NAFLD treatment.
Core Tip: This study evaluates the RESET care program, a comprehensive digital health intervention for managing non-alcoholic fatty liver disease (NAFLD). By integrating personalized diet, exercise, and cognitive behavioral therapy (CBT) through a mobile app supported by internet of things devices, the program offers continuous, tailored support for lifestyle modifications crucial to NAFLD management. Results demonstrate that participants receiving all three interventions (diet, exercise, CBT) achieved significant weight reduction and improved health outcomes. The RESET care program highlights the potential of digital tools in delivering accessible, scalable solutions for chronic disease management.
- Citation: Soni J, Pathak N, Gharia M, Aswal D, Parikh J, Sharma P, Mishra A, Lalan D, Maheshwari T. Effectiveness of RESET care program: A real-world-evidence on managing non-alcoholic fatty liver disease through digital health interventions. World J Hepatol 2025; 17(1): 101630
- URL: https://www.wjgnet.com/1948-5182/full/v17/i1/101630.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i1.101630
Non-alcoholic fatty liver disease (NAFLD) has emerged as a global health burden, affecting approximately 1 in 4 in
Effective NAFLD management requires both dietary and physical activity modifications. Healthy weight loss with sustained muscle mass plays a pivotal role, with a reduction of 3%-5% decreasing hepatic steatosis, 5%-7% improving NASH conditions, and 10% or more needed to reverse hepatic fibrosis[5]. Management also normalizes elevated liver enzymes (aspartate aminotransferase and alanine aminotransferase), enhances insulin sensitivity, and thereby reduces cardiovascular risk by improving endothelial function and increasing cardiorespiratory fitness[6]. However, diet or exercise alone is often not as effective as a combined approach. Integrating both balanced dietary changes and increased physical activity yields more sustainable improvements in NAFLD and overall metabolic health[1,7].
Traditional intervention methods usually involve in-person consultations, which often lack real-time and continuous patient monitoring. The recommendation of drastic changes in diet and exercise can also be overwhelming for patients, leading to low adherence rates. Many patients struggle to maintain these changes in the long-term due to a lack of con
The MyTatva digital health application offers the RESET plan, a novel comprehensive approach for NAFLD mana
The primary objective of this study was to compare the effectiveness of the RESET care program’s three intervention arms: Diet plan; diet + exercise plan; and diet + exercise + CBT plan.
This retrospective observational study utilized deidentified data from 22 patients, automatically retrieved through MyTatva’s app. Initially, physicians recommended MyTatva’s RESET care plan to 32 patients, of which 27 consented to participate. However, 4 patients did not meet the eligibility criteria, leaving 22 participants for the analysis.
The RESET care program is a 12-week digital health intervention aimed at managing NAFLD, focusing on diet, exercise, and CBT. The program integrates IoT devices such as a body composition analyzer (BCA) machine and a smart
Participants included adults aged 18-65 years with a BMI between 25 kg/m² and 40 kg/m², willing to use digital health technology (smartphone, BCA machine, and smartwatch), and able to provide informed consent during onboarding with the application. Participants needed to have stable internet access and basic technological knowledge. Participants with any secondary causes of hepatic steatosis (e.g., significant alcohol consumption, viral hepatitis), advanced liver disease
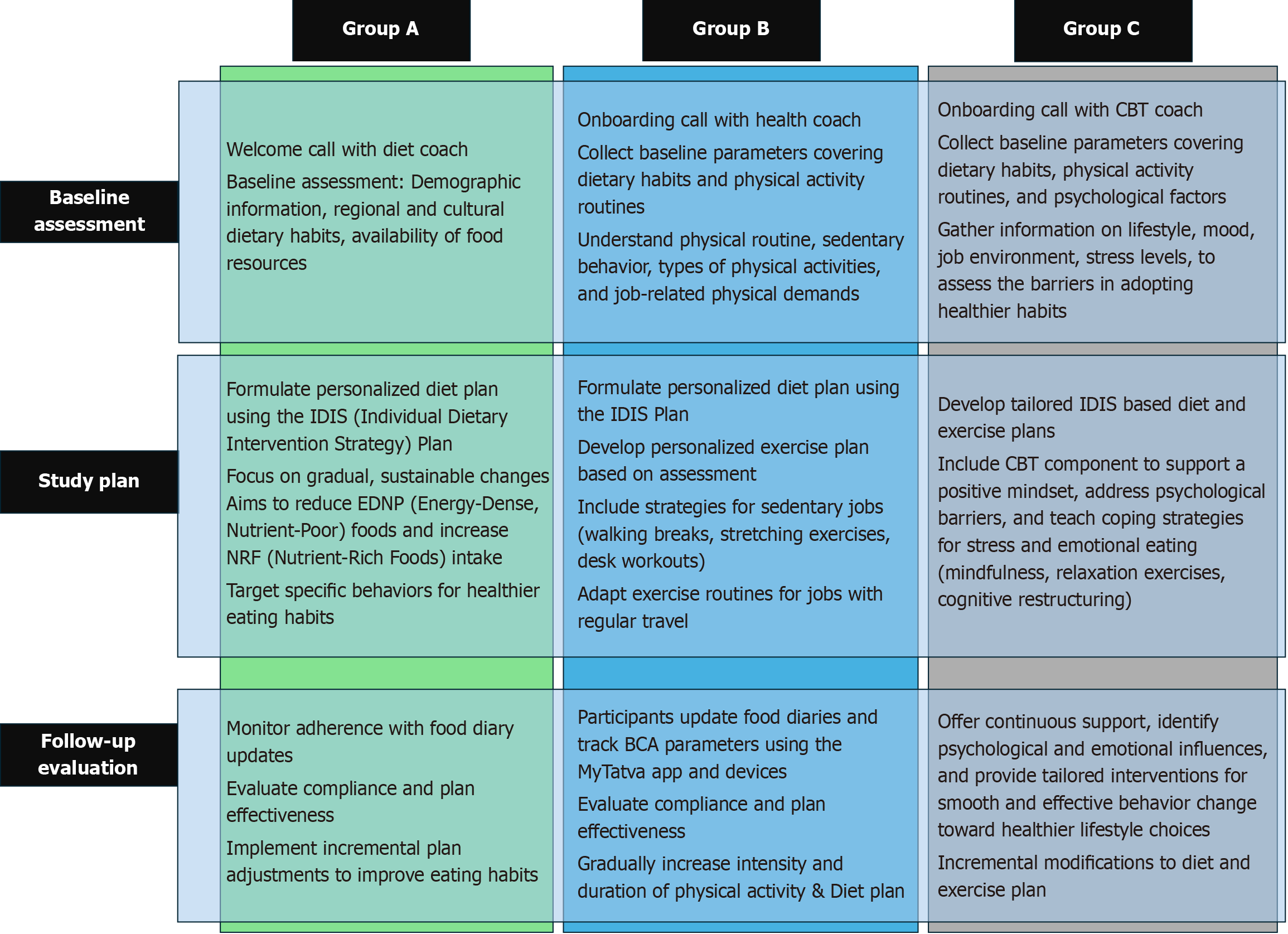
In this retrospective analysis, participants were categorized into three groups of the RESET care program offered through the digital health app. Group A consisted of participants who used the application for personalized dietary guidance. Group B participants received both the personalized diet plans and the structured exercise routines. Group C comprised participants who received the comprehensive RESET care program, including dietary plans, structured exercise routines, and CBT modules. This categorization enabled the evaluation of the effectiveness of varying levels of digital health intervention in improving health outcomes, allowing for a real-world assessment of the program’s impact.
Upon onboarding, the detailed baseline assessment included demographic information, ethnicity, medical history, family history, and current work status. Each intervention arm is designed to progressively enhance support and effectiveness, considering the unique needs of the participant (Figure 1).
Group A participants receive a personalized diet plan based on a unique Individual Dietary Intervention Strategy plan focusing on gradual and sustainable changes to their eating patterns. Participants in the diet plan option of the RESET care program received an introductory call from a dedicated diet coach to assess baseline parameters such as demo
Group B combined a personalized dietary plan with an exercise plan via the FITT approach tailored to individual physical activity levels and lifestyle requirements. This plan emphasizes incremental adjustments in both diet and exercise, supported by a 15-day follow-up by coaches. The personalized exercise plan in the RESET care program was developed based on participant baseline physical activity levels, job demands, and any physical limitations. For those with sedentary jobs, the plan included strategies like walking breaks, stretching exercises, and desk workouts. For par
Group C integrated CBT with diet and exercise interventions. This comprehensive approach addresses psychological barriers to lifestyle changes, providing continuous support through regular CBT sessions. These sessions help parti
After the 90-day intervention period, participants underwent a final assessment to evaluate changes in their anthropometric parameters.
To facilitate comprehensive data collection, patients were provided with a BCA machine. This device enabled auto-fetching of patients’ BCA measurements regularly, ensuring continuous real-time monitoring of their data. The collected data were organized in a Microsoft Excel spreadsheet. Analysis was conducted using SPSS Ver. 25.0 (IBM Corp., Armonk, NY, United States). Quantitative data were expressed as means and standard deviations. Statistical analysis was performed to establish significance at a P value < 0.05, adhering to a 5% significance level. A one-way ANOVA was performed to compare mean changes in anthropometric parameters among the three intervention groups. Post-hoc comparisons using Tukey’s Honest Significant Difference (HSD) test were conducted to further investigate significant differences between groups.
The study received approval from the ACEAS-Independent Ethics Committee, with protocol number: MTNAFLD120324. The study was conducted in accordance with the Declaration of Helsinki and its subsequent revisions.
The study cohort consisted of 22 participants, with a distribution of 31.8% in Group A, 40.9% in Group B, and 27.3% in Group C. The mean age of participants was 45.59 ± 11.02 years, with the majority being obese (72.7%). The cohort in
| Parameters | Total | Group A | Group B | Group C |
| Total | 22 (100) | 7 (31.8) | 9 (40.9) | 6 (27.3) |
| Male | 14 (63.6) | 5 (71.4) | 5 (55.5) | 4 (66.6) |
| Age in years | 45.59 ± 11.02 | 48.00 ± 12.03 | 45.89 ± 13.85 | 42.33 ± 2.94 |
| Overweight | 6 (27.3) | 1 (14.2) | 4 (44.4) | 1 (16.7) |
| Obese | 16 (72.7) | 6 (85.8) | 5 (55.6) | 5 (83.3) |
| Region | ||||
| North India | 4 (18.2) | 1 (25.0) | 2 (50.0) | 1 (25.0) |
| South India | 9 (40.9) | 1 (11.2) | 4 (44.4) | 4 (44.4) |
| West India | 6 (27.3) | 2 (33.3) | 3 (50.0) | 1 (16.7) |
| East India | 3 (13.6) | 3 (100.0) | 0 (0) | 0 (0) |
| Employment status | ||||
| Student | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Housewife | 4 (18.2) | 0 (0) | 2 (50.0) | 2 (50.0) |
| Employed | 12 (54.6) | 4 (33.3) | 6 (50.0) | 2 (16.7) |
| Self-employed | 3 (13.6) | 2 (66.7) | 1 (33.3) | 0 (0.00) |
| Retired | 3 (13.6) | 1 (33.3) | 0 (0) | 2 (66.7) |
| Residence | ||||
| Within metropolitan area | 22 (100) | 7 (31.8) | 9 (40.9) | 6 (27.3) |
| Dietary pattern | ||||
| Vegetarian | 7 (31.8) | 4 (57.1) | 2 (28.6) | 1 (14.3) |
| Non-vegetarian | 15 (68.2) | 3 (20) | 7 (46.7) | 5 (33.3) |
The overall cohort consisted of 31.8% vegetarians (n = 7) and 68.2% non-vegetarians (n = 15). Among the intervention groups, changes in participant dietary habits were evaluated by tracking the reduction in consumption of energy-dense, nutrient-poor (EDNP) foods and the increase in nutrient-rich foods (NRFs) over the course of the study. Participants also increased their consumption of fruits, vegetables, millet, eggs, chicken/fish, and pulses, as well as healthy fats like nuts and seeds (Table 2). Participants in all groups demonstrated a reduction in the consumption of refined and fried foods, sweets, high-salt snacks, meals from restaurants, and red meat (Table 3). At baseline, all groups had very low physical activity frequency; however, by the end of the study, there was a noticeable increase in physical activity across all groups, with Group C showing slightly better improvement.
| Parameters | Group A | Group B | Group C | |||
| Frequency (times per month) | Baseline | End of study | Baseline | End of study | Baseline | End of study |
| Fruit | 5.57 ± 1.17 | 10.71 ± 3.40 | 7.78 ± 3.40 | 10.89 ± 3.17 | 8.16 ± 2.78 | 12.16 ± 2.92 |
| Vegetables (salads/kachumber/homemade soups) | 12 ± 3.46 | 16.71 ± 3.54 | 11.33 ± 3.16 | 15.44 ± 2.69 | 6.17 ± 3.97 | 10.5 ± 4.37 |
| Millets (Bajra/Jowar/Nachni etc.) | 10.28 ± 3.03 | 15 ± 2.16 | 10.44 ± 3.84 | 13.55 ± 4.06 | 7.17 ± 3.06 | 11 ± 4.19 |
| Eggs | 5.57 ± 7.02 | 7.57 ± 9.47 | 8.11 ± 5.03 | 9.89 ± 6.27 | 9.16 ± 4.67 | 12 ± 6.06 |
| Chicken/fish | 4.57 ± 5.76 | 6 ± 7.83 | 8.44 ± 5.91 | 10.44 ± 6.83 | 7.67 ± 4.54 | 10.67 ± 5.89 |
| Dals/pulses/sprouts (moong, matki, chawli, chole, rajma) | 8.71 ± 4.34 | 11.42 ± 3.86 | 11.44 ± 4.63 | 13.89 ± 3.51 | 8.17 ± 3.19 | 15 ± 3.63 |
| Nuts and seeds (almonds, walnuts, flaxseeds, sunflower seeds, chia seeds) | 3 ± 2.16 | 7.28 ± 2.98 | 4.22 ± 3.56 | 7.88 ± 3.98 | 2.33 ± 1.75 | 6.33 ± 1.75 |
| Milk and milk products | 6.57 ± 2.57 | 9.71 ± 3.19 | 7.33 ± 3.04 | 9.78 ± 3.11 | 8.17 ± 2.56 | 12.67 ± 4.41 |
| Exercise (walk, jog, yoga, pranayama, dance, sports) | 3.71 ± 3.19 | 7.14 ± 3.33 | 2.33 ± 2.12 | 10.78 ± 2.38 | 4.33 ± 1.86 | 12.83 ± 1.94 |
| Parameters | Group A | Group B | Group C | |||
| Frequency (times per month) | Baseline | End of study | Baseline | End of study | Baseline | End of study |
| Refined food items (bread, pav, biscuits, cookies, rusk, toast, Khari, etc.) | 11.7 ± 3.9 | 8 ± 2.44 | 12.78 ± 6.06 | 8.33 ± 3.94 | 12.67 ± 7.11 | 8.33 ± 4.96 |
| Fried food (Puri, Kachori, Tikki, Bhature, Pakoras, Samosas, etc.) | 11 ± 3.31 | 7.28 ± 2.69 | 11.67 ± 6.12 | 7.22 ± 4.32 | 11.00 ± 2.60 | 6.67 ± 1.63 |
| Sweets (Laddu, Jalebi, Kulfi, Chocolate, Kheer, | 7.28 ± 4.34 | 5.85 ± 3.53 | 8.11 ± 5.32 | 5.22 ± 3.59 | 8.00 ± 3.22 | 6 ± 1.67 |
| High-salt snacks (Namkeen, Bhujia, Pickles, Papad, etc.) | 8.42 ± 3.25 | 5.28 ± 2.56 | 12.11 ± 8.22 | 7.33 ± 4.58 | 9.5 ± 6.15 | 7.5 ± 4.23 |
| Restaurant meals and/or takeouts | 12.28 ± 5.02 | 7.14 ± 2.6 | 8.44 ± 3.00 | 4.11 ± 1.69 | 14.83 ± 5.15 | 7.33 ± 3.67 |
| Red meat (mutton) | 3.42 ± 3.35 | 2 ± 1.91 | 3.88 ± 2.42 | 2.33 ± 0.86 | 5.16 ± 3.43 | 2.16 ± 1.32 |
Changes in anthropometric parameters were assessed across all three intervention groups over a 90-day period. Par
| Parameters | Change in anthropometric parameters | |||||
| Group A | Group B | Group C | ||||
| Baseline | 90 days | Baseline | 90 days | Baseline | 90 days | |
| Weight (kg) | 87.61 ± 16.06 | 85.61 ± 15.55 | 82.61 ± 9.29 | 79.90 ± 8.64 | 101.10 ± 17.85 | 94.11 ± 17.38 |
| BMI (kg/m2) | 33.08 ± 4.03 | 32.25 ± 3.60 | 30.14 ± 2.26 | 29.14 ± 1.93 | 32.90 ± 3.02 | 30.72 ± 3.41 |
| Muscle mass | 54.66 ± 11.25 | 53.94 ± 11.66 | 54.66 ± 11.25 | 53.94 ± 11.66 | 54.66 ± 11.25 | 53.94 ± 11.66 |
| Subcutaneous fat | 24.38 ± 1.82 | 23.92 ± 1.82 | 22.44 ± 2.16 | 21.74 ± 2.10 | 23.68 ± 2.06 | 22.42 ± 2.97 |
| Visceral fat | 15.05 ± 3.61 | 13.95 ± 3.32 | 12.34 ± 2.27 | 11.11 ± 1.97 | 15.30 ± 2.65 | 13.14 ± 3.17 |
A one-way ANOVA was performed to compare the mean changes in anthropometric parameters among the three in
| Parameter | P value | Significant pairwise comparisons1 |
| Weight change | 0.00050 | C > A, C > B |
| BMI change | 0.00003 | C > A, C > B |
| Subcutaneous fat change | 0.00198 | C > A, C > B |
| Visceral fat change | 0.00015 | C > A, C > B |
Participants in the comprehensive RESET care program (Group C) showed superior weight reduction and improvements in anthropometric parameters compared to Group A and Group B. The program’s effectiveness highlights the enhanced benefits of combining diet, exercise, and CBT for better NAFLD outcomes. Additionally, Group C had a slightly younger average age and a higher percentage of employed participants, which suggests that younger, employed individuals might be more motivated to integrate lifestyle changes effectively.
Diet plays a crucial role in the management of NAFLD, as excessive intake of EDNP foods can lead to fat accumulation in the liver through increased de novo lipogenesis, exacerbating hepatic steatosis, inflammation, and insulin resistance[13]. In contrast, NRFs, high in essential nutrients, fiber, healthy fats and antioxidants, help reduce hepatic fat accumulation, improve liver function, enhance insulin sensitivity, and combat inflammation[14,15]. The review findings by Perumpail et al[16] and Chai et al[17] support the significant roles of diet and exercise in managing NAFLD. Both reviews highlight that low-carbohydrate diets are more effective than low-fat diets in improving hepatic fat content, reducing BMI and triglycerides, and enhancing metabolic indicators[18]. The Mediterranean and Paleo diets emerged as promising regimens for NAFLD patients, leading to improved BMI and liver function[19]. In the present study, the RESET pro
Regular physical activity, such as 40-45-minute sessions three times a week, enhances metabolic health and reduces liver fat content[21]. Combining dietary modifications to compliment structured physical activity resulted in the most significant improvements in hepatic fat content, BMI, and other metabolic parameters. Chai et al[17] reported that participants in the diet-only group experienced an average BMI reduction of 1.5 kg/m² over 6 months. The diet combined with exercise group showed an even greater BMI decrease of 2.0 kg/m², highlighting the enhanced effectiveness of combining diet and exercise for comprehensive NAFLD management. Similarly, in the present study, we observed that the Group B participants reported more significant reductions in weight and BMI compared to Group A.
In the study by Wong et al[22], a 52-week lifestyle intervention involving diet and regular exercise group achieved a mean weight reduction of 5.6 kg and a BMI reduction of 1.5 kg/m², with 67% of non-obese and 61% of obese patients experiencing remission of NAFLD. Montemayor et al[23] reported that a combination of a customized hypocaloric diet and enhanced physical activity showed significant reductions in intrahepatic fat, BMI, and liver stiffness. Specifically, the mean weight reduction was 6.8 kg and BMI reduction was 2.6 kg/m² in the Mediterranean diet-physical activity group. Comparatively, in the present study, the reduction in BMI was 1 kg/m2, achieved in 12 weeks.
All intervention groups were effective in managing NAFLD; however, Group C showed a weight reduction of 7% (6.99 ± 2.98 kg) and a BMI reduction of 6.76% (2.18 ± 0.99 kg/m²). The enhanced effectiveness of the overall plan can be attributed to the combined benefits of diet and physical exercise, along with the psychological support provided by the CBT coach, which addresses behavioral barriers to adherence and promotes lasting lifestyle changes. Similarly, the study conducted by Moscatiello et al[24] demonstrates that participants who received CBT sessions experienced a significant weight loss of 5.6% over 2 years, compared to only 1.4% in the diet-only group. The study conducted by Montesi et al[25] demonstrated the effectiveness of CBT in the management of NAFLD with a significant reduction in BMI (-2.04 ± 1.42 kg/m²) in the CBT + exercise group compared to those who received CBT support only (-1.09 ± 1.68 kg/m²).
When combined with diet and exercise, CBT provides a comprehensive approach, resulting in significant weight and BMI reductions, and promoting long-term, sustainable improvements in anthropometric parameters. Our study’s intra-group analysis further supports this, showing significant reductions in anthropometric parameters across all intervention groups over the 12-week duration, with the highest reductions observed in Group C. The digitally delivered RESET care program offers a personalized approach based on root-cause analysis, tailoring interventions to individual needs and integrating CBT to ensure sustained lifestyle changes.
One of the main challenges in implementing the digital health intervention was ensuring participants’ consistent engagement with the application and monitoring tools, especially for those less familiar with technology. To address this, participants received thorough onboarding, including guidance on using the application, BCA, and smartwatch. Regular follow-up calls from health coaches helped maintain engagement, provide technical support, and encourage adherence to the program. For those who struggled with lifestyle changes, coaches emphasized gradual habit-building to foster long-term commitment to the intervention. The RESET care program offers potential economic benefits by reducing the need for frequent in-person visits, lowering travel costs, and saving time for participants. While initial setup costs for digital tools and training are necessary, the program’s scalability and potential to improve long-term health outcomes, particularly by preventing NAFLD complications, suggest that it may offer significant cost savings in managing chronic liver conditions. The small sample size restricts the generalizability of the findings and highlights the need for larger, long-term studies to assess the program’s effectiveness on a broader scale. Additionally, the reliance on self-reported dietary data may introduce reporting biases, and the study lacks insights into the individual contributions of each component, such as exercise-only or CBT-only interventions. Long-term follow-up is necessary to confirm the sustainability of the results.
The RESET care program is effective in NAFLD management, through promoting healthy weight loss and significant improvements in other anthropometric parameters. This addresses a critical need for a scalable, multifaceted approach to NAFLD management, by providing personalized, real-time monitoring through tailored interventions. This program could serve as a valuable tool for clinicians in remotely monitoring patients with chronic conditions like NAFLD, enhancing overall management and care.
We would like to thank all those who contributed to this research. We also appreciate the insights and contributions of our biostatistical consultant, whose expertise was instrumental in the analysis and interpretation of the data.
| 1. | Hydes TJ, Ravi S, Loomba R, E Gray M. Evidence-based clinical advice for nutrition and dietary weight loss strategies for the management of NAFLD and NASH. Clin Mol Hepatol. 2020;26:383-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, Kassir R, Singhal R, Mahawar K, Ramnarain D. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 455] [Article Influence: 151.7] [Reference Citation Analysis (1)] |
| 3. | Seaw KM, Henry CJ, Bi X. Relationship between Non-Alcoholic Fatty Liver Disease and Visceral Fat Measured by Imaging-Based Body Composition Analysis: A Systematic Review. Livers. 2023;3:463-493. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Loomis AK, Kabadi S, Preiss D, Hyde C, Bonato V, St Louis M, Desai J, Gill JM, Welsh P, Waterworth D, Sattar N. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J Clin Endocrinol Metab. 2016;101:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 5. | Hannah WN Jr, Harrison SA. Lifestyle and Dietary Interventions in the Management of Nonalcoholic Fatty Liver Disease. Dig Dis Sci. 2016;61:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (2)] |
| 6. | Keating SE, Sabag A, Hallsworth K, Hickman IJ, Macdonald GA, Stine JG, George J, Johnson NA. Exercise in the Management of Metabolic-Associated Fatty Liver Disease (MAFLD) in Adults: A Position Statement from Exercise and Sport Science Australia. Sports Med. 2023;53:2347-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Zhai H, Chen C, Wang N, Chen Y, Nie X, Han B, Li Q, Xia F, Lu Y. Blood lead level is associated with non-alcoholic fatty liver disease in the Yangtze River Delta region of China in the context of rapid urbanization. Environ Health. 2017;16:93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Ahmed MH, Abu EO, Byrne CD. Non-Alcoholic Fatty Liver Disease (NAFLD): new challenge for general practitioners and important burden for health authorities? Prim Care Diabetes. 2010;4:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Zelber-Sagi S, Moore JB. Practical Lifestyle Management of Nonalcoholic Fatty Liver Disease for Busy Clinicians. Diabetes Spectr. 2024;37:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 10. | Zhou R, Gu Y, Zhang B, Kong T, Zhang W, Li J, Shi J. Digital Therapeutics: Emerging New Therapy for Nonalcoholic Fatty Liver Disease. Clin Transl Gastroenterol. 2023;14:e00575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 11. | Motz V, Faust A, Dahmus J, Stern B, Soriano C, Stine JG. Utilization of a Directly Supervised Telehealth-Based Exercise Training Program in Patients With Nonalcoholic Steatohepatitis: Feasibility Study. JMIR Form Res. 2021;5:e30239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Akbar FN, Choirida SR, Muttaqin AZ, Ekayanti F, Hendarto H. Telemedicine as an Option of Healthcare Services in monitoring Non Alcoholic Fatty Liver Disease (NAFLD) Patients Facing COVID-19 Pandemic. [DOI] [Full Text] |
| 13. | Aboubakr A, Stroud A, Kumar S, Newberry C. Dietary Approaches for Management of Non-Alcoholic Fatty Liver Disease: A Clinician's Guide. Curr Gastroenterol Rep. 2021;23:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Kořínková L, Pražienková V, Černá L, Karnošová A, Železná B, Kuneš J, Maletínská L. Pathophysiology of NAFLD and NASH in Experimental Models: The Role of Food Intake Regulating Peptides. Front Endocrinol (Lausanne). 2020;11:597583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Dehghanseresht N, Jafarirad S, Alavinejad SP, Mansoori A. Association of the dietary patterns with the risk of non-alcoholic fatty liver disease among Iranian population: a case-control study. Nutr J. 2020;19:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Perumpail BJ, Cholankeril R, Yoo ER, Kim D, Ahmed A. An Overview of Dietary Interventions and Strategies to Optimize the Management of Non-Alcoholic Fatty Liver Disease. Diseases. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Chai XN, Zhou BQ, Ning N, Pan T, Xu F, He SH, Chen NN, Sun M. Effects of lifestyle intervention on adults with metabolic associated fatty liver disease: A systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14:1081096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 18. | Henney AE, Gillespie CS, Alam U, Hydes TJ, Cuthbertson DJ. Ultra-Processed Food Intake Is Associated with Non-Alcoholic Fatty Liver Disease in Adults: A Systematic Review and Meta-Analysis. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 19. | Abenavoli L, Di Renzo L, Boccuto L, Alwardat N, Gratteri S, De Lorenzo A. Health benefits of Mediterranean diet in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2018;12:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss Isakov N, Webb M, Orenstein D, Shibolet O, Kariv R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J Hepatol. 2018;68:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 21. | van der Windt DJ, Sud V, Zhang H, Tsung A, Huang H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018;18:89-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 22. | Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, Chim AM, Chan CK, Leung JK, Chu WC, Woo J, Chan HL. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (34)] |
| 23. | Montemayor S, Bouzas C, Mascaró CM, Casares M, Llompart I, Abete I, Angullo-Martinez E, Zulet MÁ, Martínez JA, Tur JA. Effect of Dietary and Lifestyle Interventions on the Amelioration of NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Moscatiello S, Di Luzio R, Bugianesi E, Suppini A, Hickman IJ, Di Domizio S, Dalle Grave R, Marchesini G. Cognitive-behavioral treatment of nonalcoholic Fatty liver disease: a propensity score-adjusted observational study. Obesity (Silver Spring). 2011;19:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Montesi L, Caselli C, Centis E, Nuccitelli C, Moscatiello S, Suppini A, Marchesini G. Physical activity support or weight loss counseling for nonalcoholic fatty liver disease? World J Gastroenterol. 2014;20:10128-10136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |