Published online May 27, 2024. doi: 10.4254/wjh.v16.i5.766
Revised: January 31, 2024
Accepted: April 9, 2024
Published online: May 27, 2024
Processing time: 169 Days and 23.4 Hours
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary liver cancer associated with an appalling prognosis. The diagnosis and manage
Core Tip: Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) represents a poorly understood rare primary liver tumor with a gruesome prognosis. Molecular and genetic characterization of this disease is vital for exploring newer treatment modalities to improve the survival of patients afflicted with this rare entity. In this review, we give an account of the recent developments in the pathology, diagnostic approach, and management of cHCC-CCA.
- Citation: Vij M, Veerankutty FH, Rammohan A, Rela M. Combined hepatocellular cholangiocarcinoma: A clinicopathological update. World J Hepatol 2024; 16(5): 766-775
- URL: https://www.wjgnet.com/1948-5182/full/v16/i5/766.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i5.766
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary liver cancer with morphological features of both hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA). This unique cancer is drawing increasing clinical and pathological consideration due to its heterogenous morphological features, molecular characteristics, aggressive clinical nature, and diagnostic difficulties. The incidence of cHCC-CCA has been rising, probably because of its increased recognition in surgical specimens[1]. The natural history of this rare entity is still not fully understood. The ambiguous and complex nature of this cancer makes early diagnosis difficult for primary physicians and radiologists. Recently, the World Health Organization (WHO) updated its histological classification system for cHCC-CCA. Over the last decade there has been increasing interest and enthusiasm to explore the diagnostic and prognostic value of the immunohistochemical expression of a protein called ‘nestin’ in patients with cHCC-CCA[2,3]. This minireview focuses on the recent developments in the histological and genetic features of cHCC-CCA.
cHCC-CCA accounts for 0.4%-14.2% of all primary liver cancers depending on geographical location, risk factors, and criteria used for inclusion[4-9]. In the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute database, cHCC-CCA accounted for 0.77% of the cases[5]. The true incidence of cHCC-CCA is, however, likely to be underestimated, as most patients do not undergo liver resection or transplantation and may have thus been wrongly diagnosed with either hepatocellular or biliary cancer clinically. cHCC-CCA carries a poorer prognosis overall than HCC and iCCA alone[10]. Risk factors for cHCC-CCA include hepatitis B virus infection, hepatitis C virus infection, alcohol consumption, and primary sclerosing cholangitis[11]. Reports of cHCC-CCA are available in both cirrhotic and noncirrhotic livers, in contrast to HCC which is more common in cirrhosis, and iCCA, which is more common in patients with noncirrhotic liver[12]. Asian studies have shown male predominance. However, studies from Western countries have shown no gender predilection[13]. The median age at diagnosis of cHCC-CCA is sixth to seventh decade. Clinical signs and symptoms of cHCC-CCA are most often associated with the advanced stage of the cancer and may include weakness, jaundice, loss of weight, or abdominal discomfort. Few patients may present with increased levels of both alpha-fetoprotein (AFP) and CA 19-9 serum levels, suggesting the ambiguous nature of cHCC-CCA[14].
Although the cell of origin of cHCC-CCA remains elusive, various hypotheses have been proposed, such as: (1) Incidental coexistence of HCC and iCCA within the same cancer; (2) malignant transformation of a hepatic stem cell; and (3) dedifferentiation of an HCC or an iCCA.
The definition of cHCC-CCA has evolved over time. Wells et al described the first case of cHCC-CCA more than 100 years ago and suggested the common embryological development of both hepatocytes and cholangiocytes as the origin of cHCC-CCA[14]. Fifty years later, ALLEN and LISA[15] reported 5 cases of mixed liver cancers and classified them into three types: (1) Separate nodules of hepatocellular and cholangiocarcinoma (CCA) (double); (2) contiguity with intermingling (combined); and (3) intimately associated due to origin from the same focus (mixed). Calderaro et al[2] in 1954 reported that 4% of primary liver cancer show both hepatocellular and biliary differentiation. In 1985, Goodman et al[16] reviewed 24 cases of cHCC-CCAs and modified cHCC-CCA classification into Type I or “collision tumors,” displaying the occurrence of both HCC and CCA separately, Type II or “transitional tumors,” in which there were areas of intermediate differentiation and an identifiable transition between HCC and iCCA and Type III or “fibrolamellar tumors” containing mucin-producing pseudoacini[16]. Currently, only the subtype with an intimate intermixing of HCC and CCA elements, labeled as type 3 tumor by ALLEN and LISA[15] and type II (transitional) tumor by Goodman et al[16] are considered as cHCC-CCA.
The WHO 2000 classification defined cHCC-CCA as a tumor containing both hepatocellular and distinct or separate CCA[17,18]. The tumor should show the presence of both bile and mucin. Immunohistochemical expression of polyclonal CEA and Hep Par was suggested for the hepatocellular component and demonstration of neutral mucin by the PAS-diastase reaction for the biliary component. The WHO 2010 Classification redefined this cancer, which ran into controversy for various reasons. They proposed a classical type of cHCC-CCA (tumor containing unequivocal, intimately mixed elements of both HCC and iCCA), and cHCC-CCA with stem/progenitor cell features having 3 subtypes: Typical, intermediate cell, and cholangiocellular[19]. The consensus classification published in 2019 by WHO put forwarded the definition of cHCC-CCA as a primary hepatic cancer with unequivocal existence of both hepatocytic and cholangiocytic differentiation within a single tumor and recommended that the diagnosis should be based on morphology only[17]. Pathologists can perform immunostaining to confirm both histologic components, but the diagnosis should not be designated on immunostaining alone. There was no further subcategorization as progenitor cells can be seen in all forms of cHCC-CCA and there is no clinical relevance of subclassification. This was also suggested in a recently published consensus paper[20]. If stem/progenitor cells are identified in a tumor, the pathologist can mention it in the comments section of the pathology report. A separate category of intermediate carcinoma is added in the 2019 WHO classification. Cholangiocellular carcinoma is a biliary-derived tumor and is now classified as iCCA[21].
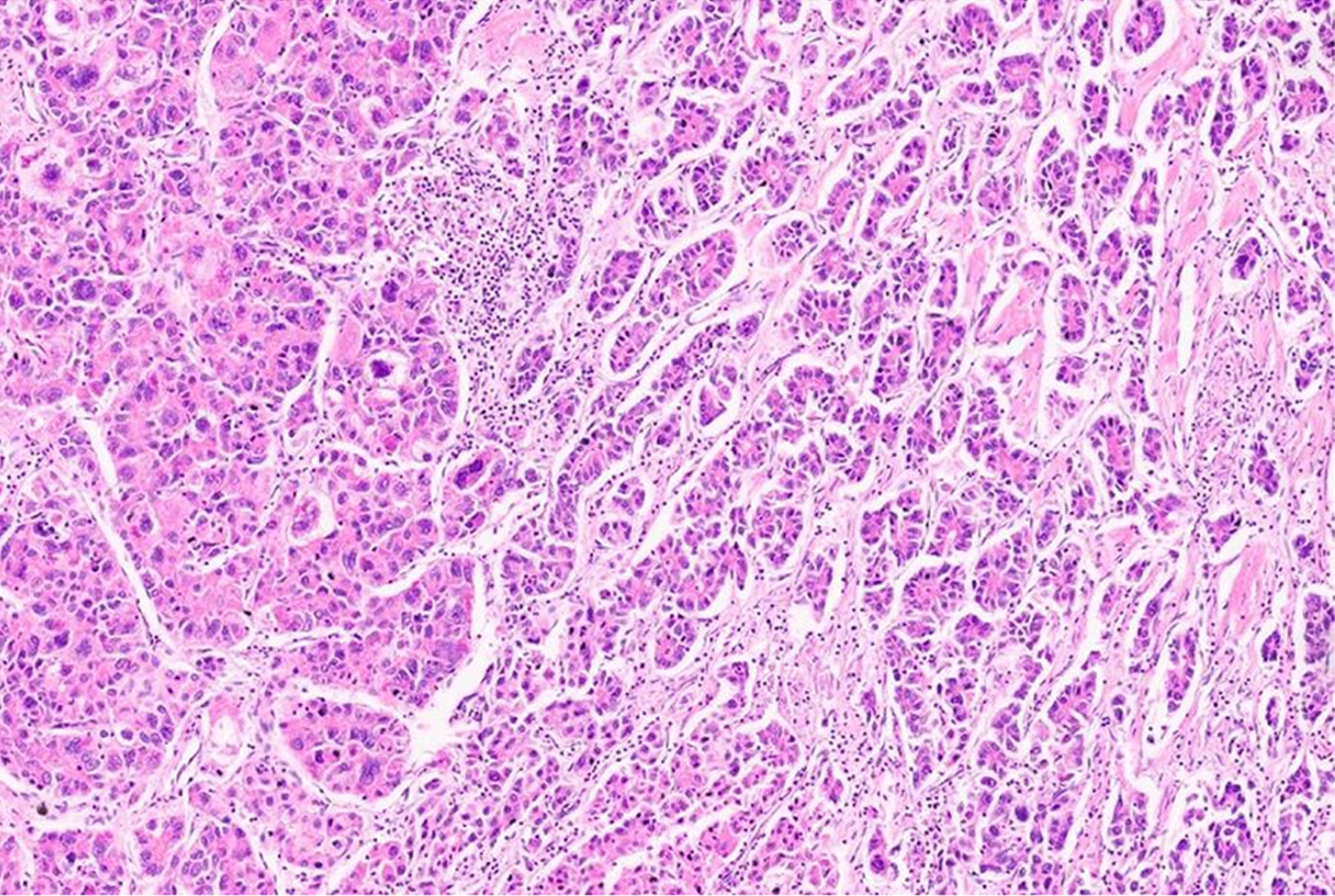
Careful grossing of cHCC-CCA is recommended and all areas with any area showing change in tumor color, texture, and firmness should be adequately sampled[20]. The hepatocellular component in cHCC-CCA shows all growth patterns described for HCC including variable-sized trabeculae, pseudoglands, and arrangement in sheets (Figure 1). Similarly, all forms of cytological features and cellular differentiation are also reported. The cholangiocytic component shows malignant acini embedded in desmoplastic stroma and can be well or poorly differentiated. There is no minimum amount of hepatocytic and cholangiocytic components that certifies cHCC-CCA diagnosis. One can identify reactive ductular reaction at the edge of HCC and this should not be considered as a malignant cholangiocytic component. Both malignant components may be intermingled, or lie in separate areas of a tumor, though focal areas of merging can often be discerned[17]. The two components may show abrupt or gradual transition. It is important to keep in mind that clear histological distinction of both components may be difficult in areas of merging on HE stains as neoplastic cells of either hepatic or cholangiocytic morphologic type may contain intracytoplasmic inclusions such as hyaline material and/or steatosis and are often negative for mucin stains[20]. Rarely other histological components, such as squamous, endocrine or sarcomatous have been described in cHCC-CCA.
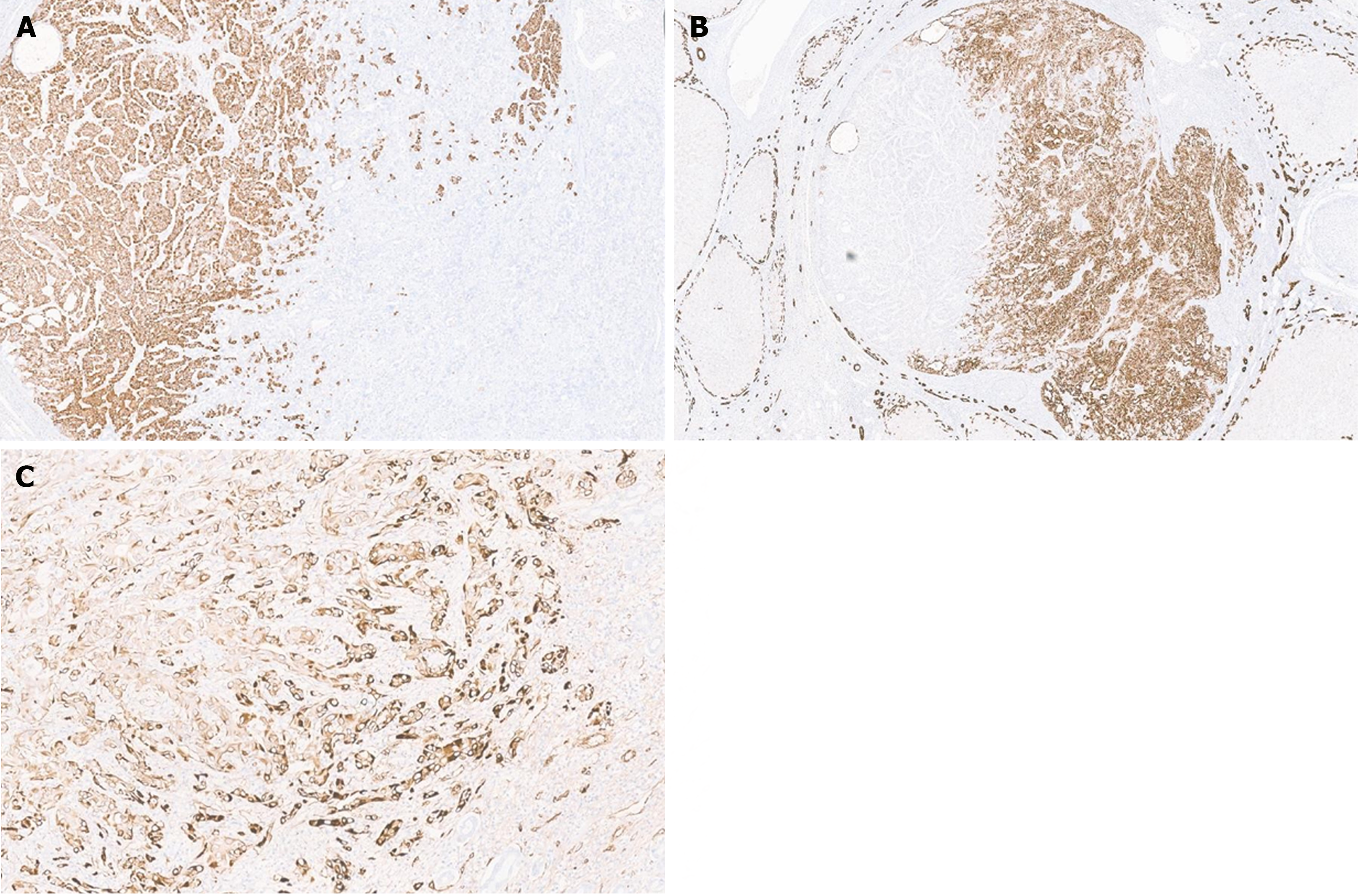
Stem/progenitor cell features consist of small cells with a high nucleo-cytoplasmic ratio, hyperchromatic nuclei, and scant cytoplasm, and are usually identified at the stromal epithelial interface[22]. One does not see mitosis in these stem cells. Intermediate carcinoma is characterized by trabeculae or strands of small, monotonous neoplastic cells with hyperchromatic round to oval nuclei and scant cytoplasm embedded in the desmoplastic stroma and shows simultaneous immunostaining by both hepatocytic and biliary markers, suggesting stem cell origin[23]. Pathological diagnosis of cHCC-CCA, stem/progenitor cell features, and intermediate carcinoma are also based on assessment on HE stains; immunostaining is supplementary to the morphological diagnosis. For hepatocellular differentiation, one can utilize Arginase-1, Hep-par1 (Figure 2A), Glypican 3, pCEA (canalicular expression), and CD10 (canalicular expression), and for CCA EMA (Figure 2B), CK7, CK19, EpCAM can be utilized. Awareness of positive expression of CK and CK19 in the hepatocellular component is important and this should not be mislabeled as cholangiocytic differentiation. Stem cells show overlap with immunohistochemical markers of cholangiocytic differentiation including CK19, CD56 and EpCAM; so, interpretation as cholangiocytes or stem cells should depend on morphological features. Positive immunostaining of nestin has been suggested to identify the subset of cHCC-CCA associated with the worst clinical outcome[2]. A few markers like CD117, CD133, Sox-9 can be considered stem cell markers. Recent studies have shown that intermediate cells stain with nestin[23] (Figure 2C). However, our team have shown that nestin can be negative in intermediate cell carcinoma[24].
Considering the rarity, molecular studies of cHCC-CCA are limited, and like pathological features, reports describing the genomic character of these tumors have shown marked heterogeneity (Table 1)[8,10,11,21,25-33]. This also demonstrates that correct pathological diagnosis of cHCC-CCA is challenging, and these molecular studies might have included HCC or iCCA tumors for analysis[34]. Previously published studies demonstrated that these tumors have a distinct mutational profile with chromosomal instability, which is closer to iCCA[8]. Few molecular studies supported the concept of a stem/progenitor cell origin with enrichment in stem/progenitor-like signatures[11,26]. Some studies have showed that that the genetics of classical cHCC-CCA are recognizably different from iCCA but almost identical to typical HCC[27]. The most frequent somatic genetic alterations reported in HCC are TERT promoter, CTNNB1, AXIN1 and TP53 mutations. Studies on genomic landscape of CCA have reported mutations in IDH, FGFR2, BAP1, ARID1A, TP53, KRAS, and PBRM1.
| Ref. | Technology | Molecular features/conclusion |
| Fujii et al[30] | Loss of heterozygosity, polymerase chain reaction | 4q, 17p, 8p, 16q; common clonal evolution |
| Cazals-Hatem et al[8] | Loss of heterozygosity, Genome wide allelotyping | TP53, chromosome instability |
| Coulouarn et al[26] | Genome-wide transcriptional analysis | TGFβ and Wnt/β-catenin; Stem/progenitor features |
| Fujimoto et al[31] | Whole genome sequencing | TERT/IDH1/2, KRAS; Closer to HCC if hepatitis background and to; CCA in absence of hepatitis |
| Moeini et al[21] | Whole exome sequencing | TP53, TERT, IDH1/2, Chromosomal instability, MYC, IGF2, mTOR, Stem/progenitor features |
| Chen et al[54] | Whole genome sequencing | IDH1/2; Stem/progenitor features |
| Sasaki et al[29] | Whole genome sequencing | KRAS, IDH1/2, ARID1A, TERT; Variable association of HCC and/or CCA mutations |
| Jeon et al[32] | Multiplexed polymerase chain reaction-based sequencing | TP53, PTEN, MET, c-MYC, CDK6, CTNNB1, CCND1; Clonal heterogeneity |
| Wang et al[11] | Whole exome sequencing | VCAN, ACVR21, FCGBP; Stemness nature, monoclonal origin, intratumoral heterogeneity |
| Liu et al[10] | Whole exome sequencing, RNAseq | TP53, CTNNB1 |
| Joseph et al[27] | Capture-based next generation sequencing | TERT, TP53, cell cycle genes (CCND1, CCNE1, CDKN2A), receptor tyrosine kinase/Ras/PI3-kinase pathway genes (MET, ERBB2, KRAS, PTEN), chromatin regulators (ARID1A, ARID2) and Wnt pathway genes (CTNNB1, AXIN, APC) |
| Sasaki et al[33] | Direct sequence | TERT, ARID1A, PBRM1, ARID2, BAP1, p53, KRAS, IDH1/2; Cholangiocellular carcinoma is different from cHCC-CCA |
| Xue et al[25] | Whole genome sequencing | TP53, TERT, AXIN1, KMT2D; Monoclonal origin in combined and mixed type; Both mono- and multiclonal origins in the separate type |
Xue et al[25] performed genomic and transcriptomic landscaping of 133 cHCC-CCA cases which included separate, combined, and mixed subtypes and were classified according to Alan and Lisa classification. An integrative comparison of cHCC-CCA with HCC and intrahepatic CCA revealed that combined and mixed types of cHCC-CCAs are distinct subtypes with different clinical and molecular features. Combined type cHCC-CCA showed strong ICC-like features, such as higher expression of EPCAM, KRT19, and PRDM5, as well as enrichment of KRAS mutations and higher expression of KRAS. In contrast, mixed type cHCC-CCA showed Hoshida-S2-like HCC features, such as higher expression levels of AFP, GPC3, APOE, and SALL4, as well as a higher level of serum AFP. The most frequently mutated driver genes in their study were TP53, TERT promoter, AXIN1, and KMT2D mutations that may be associated with either HCC or iCCA. They also revealed both monoclonal and multiclonal origins in the separate type cHCC-CCAs, whereas combined and mixed type cHCC-CCA were all monoclonal origin. Notably, cHCC-CCAs showed significantly higher expression of nestin, suggesting nestin may serve as a biomarker for diagnosing cHCC-CCA.
Moeini et al[21] described the different genomic status of cHCC-CCA in 18 patients with mixed HCC-CCA encompassing all subclasses described in WHO 2010 classification. Progenitor/stem-cell cancers were characterized by enrichment of progenitor-like signatures, and specific activation of MYC, IGF, Notch, and mTOR oncogenic pathways and chromosomal instability. Classical cHCC-CCA showed a significant correlation in the copy number aberrations of the iCCA and HCC components, suggesting a clonal origin. Pure cholangiocellular carcinoma cases in their series showed significant upregulation of transforming growth factor beta signaling and enrichment for inflammation-related and immune response signatures, suggesting that they represent a form of iCCA and should not be included under cHCC-CCA. The reported molecular features of cHCC-CCA parallel its biphenotypic morphological appearance. However, the number of investigated cases remains low, and further validation in larger studies is needed to establish a robust pathomolecular classification of cHCC-CCA[34].
RNA-sequencing revealed enrichment of the interleukin 6 (IL-6) signaling pathway in cHCC-CCA tumors compared to HCC tumors. Single-cell RNA-sequencing analysis revealed that IL-6 is expressed by immune and parenchymal cells during senescence, and that IL-6 is part of the senescence-associated secretory phenotype. These results could be used for the development of novel treatments for these aggressive tumors. Recently may studies have reported several significantly mutated genes with different prevalence in HCC, iCCA and cHCC-CCA[35-37] (Table 2). Artificial intelligence (AI) is widely used for the analysis of images in pathology. Calderaro et al[38] demonstrated that AI-based recategorization of cHCC-CCA into one of the “pure” HCC or iCCA groups is feasible, and perhaps refine the prognostication, which is crucial given the therapeutic implications, and also enable us to find or whether a given cHCC-CCA tumor is genetically more identical to HCC or iCCA.
| Cholangiocarcinoma | Hepatocellular carcinoma | Combined hepatocellular-cholangiocarcinoma |
| TP53 | TERT promoter | TERT promoter |
| KRAS | TP53 | TP53 |
| IDH1 | CTNNB1 | MET |
| PTEN | ERBB2 | |
| ARID1A | KRAS | |
| EPPK1 | PTEN | |
| ECE2 | ||
| FYN |
Most cases of cHCC-CCA are detected on surveillance ultrasound in the cirrhotic population. Sonography often reveals a hypoechoic mass with a hyperechoic central area or a heterogenous hypoechoic mass reflecting the histological diversity of the tumor. The lesion often demonstrates a combination of typical enhancement patterns of both HCC and iCCA on contrast-enhanced computed tomography and is frequently categorized as ‘LI-RADS metastasis (LR-M)’[39]. On magnetic resonance imaging (MRI), the cHCC-CC can be hypointense on T1W images and demonstrates intermediate to high signal intensity with or without the presence of a central hypointense focus on T2W images that corresponds to a fibrotic component or central CCA. A study by Hwang et al[40] evaluating features to differentiate cHCC-CCA from iCCA on gadoxetic acid-enhanced MRI demonstrated that a lobular shape with a complete target appearance and weak rim enhancement supported the diagnosis of CC, while strong peripheral enhancement with an irregular shape and the lack of the target sign was in favor of cHCC-CC, particularly the HCC predominant type.
The role of tru-cut biopsies for the diagnosis of cHCC-CCA is controversial, considering the histological heterogeneity in these cancers. Also, how many biopsies are to be done shows both components are represented for an accurate pathological diagnosis. It has also been suggested that owing to the lack of biopsies for primary liver cancers, as most management decisions are taken just on radiological findings, multiple cases of cHCC-CCA are currently misdiagnosed as HCC[34]. In a series of 21 cHCC-CCA, one recent study evaluated a two-step strategy, combining imaging using computed tomography and/or MRI as the first step and then biopsy as the second step. This improved the diagnostic performance of cHCC-CCA with a sensitivity of 60% and a specificity of 82%, as compared to the performance of radiology or pathology alone[41]. Tumor biopsy can also be evaluated for molecular features and specific oncogenic pathways involved in tumor biology which might help in patient management[34].
Like HCC and CC, surgery remains the cornerstone of the management of cHCC-CC. In the case of localized disease with good liver reserve, the surgery is the best available treatment option for cHCC-iCCA at present and can provide the maximum overall survival (OS) (median OS of 25.7 months). Nevertheless, the majority of patients affected with this ailment are often not fit for a resection[42]. Liver transplantation (LT) is a reasonable treatment option in selected cHCC-CC patients with cirrhosis. A systematic review of retrospective studies has reported a median disease-free survival of 14.2 months and a median OS of 37.1 months with LT[43]. Recently, a group of Chinese researchers has come forward with a scoring system known as prognostic estimation of cholangiocellular-HCC after resection (PECAR) score to assess the recurrence risk after resection of cHCC-CC. Increased gamma-glutamyl transferase, macrovascular invasion, male sex, and hilar lymphoid metastasis are found to be independent predictors of recurrence[44]. The PECAR scoring system needs to be externally validated before it can be universally accepted.
cHCC-CC tumors are less vascularized compared to HCC, and the response to locoregional therapies like trans arterial chemoembolisation is suboptimal. However, they can be considered in selected inoperable cases and for downstaging larger lesions to potentially resectable ones. There is no optimal systemic therapy regimen for cHCC-CC patients yet. Though many researchers have tried a combination of gemcitabine, and platinum drugs with or without a tyrosine kinase inhibitor (sorafenib) or a vascular endothelial growth factor antibody (Bevacizumab), but all these studies were retrospective and heterogenous in nature[45-47].
In recent years, immunotherapy has made significant progress, opening up a new treatment alternative for HCC and CC[48-50]. A multicenter study from France comprising 96 patients with cHCC-CCA identified a subgroup of cHCC-CCA that exhibits features of an ongoing intratumor immune response, along with an activation of gene signatures predictive of response to immunotherapy in HCC[51]. This subclass may benefit from immunotherapy, with improved survival. Satake et al[52] reported promising results with atezolizumab plus bevacizumab in patients with advanced cHCC-CC. Recently, a retrospective, multicenter cohort study reported an overall response rate of 29% with immune checkpoint inhibitors in patients with cHCC-CC[53]. Recently published studies on the role of immunotherapy have been discussed in Table 3[52-60].
| Ref. | Study type | Drug | Number of patients | Outcome |
| Tahover et al[55] | Case report | Ipilimumab and nivolumab, followed by nivolumab | 1 | ECOG performance status improved from 3 to 0. Repeated PET-CT showed near complete response, ca125 decreased by 90% and liver function tests normalized |
| Rizell et al[56] | Case report | Pembrolizumab | 1 (Post resection, pulmonary metastasis) | Complete remission of the pulmonary metastases. There was no sign of cancer recurrence neither in the liver nor in the lungs at 33 months after the start of the checkpoint inhibition treatment |
| Diab et al[57] | Retrospective case series | Of the six patients 4 (66%) received PD-1 inhibitor alone and 2 (34%) received combination therapy with CTLA-4 inhibitor | 6 | Objective response rate was 83.3%. One patient achieved complete response and had a treatment holiday after receiving treatment for 2 yr, and restarted immunotherapy upon relapse. Four patients had a partial response, of which two passed away after disease progression. One patient had stable disease on 2 different lines of immunotherapy then progressed |
| Saito et al[58] | Case report | Atezolizumab plus bevacizumab | 1 (3 months after resection, multiple lymph node metastases) | 7.5-month progression-free survival |
| Satake et al[52] | Case series | Atezolizumab plus bevacizumab | 6 | Three partial responses and one stable disease as the best responses |
| Saint et al[59] | Case report | Pembrolizumab | 1 | Complete response which was maintained over time along with toxicity-free tumor control after 18 months treatment |
| Pomej et al[53] | Retrospective, multicenter cohort | Three (43%) patients received atezolizumab plus bevacizumab, two (29%) patients were treated with nivolumab alone, and one (14%) patient each received pembrolizumab alone and nivolumab in combination with TACE, respectively | 7 | Overall response rate was 29% with a disease control rate of 43% |
| Zhou et al[60] | Case report | Sintilimab, lenvatinib, and nab-paclitaxel | 1 |
cHCC-CC represents a distinctive primary hepatic malignancy with ambiguous morphological and genomic characteristics mimicking both hepatocytic and cholangiocytic differentiation. Owing to the rarity of cHCC-CC, the pathological and molecular characteristics of this neoplasm remain poorly characterized. There is a lack of standardization in the treatment of cHCC-CC, especially for those patients with unresectable tumors. Further studies with international collaborations are essential to better understand the disease biology and improve the management of patients with this rare neoplasm.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Jiang W, China S-Editor: Qu XL L-Editor: A P-Editor: Cai YX
| 1. | Spolverato G, Bagante F, Tsilimigras D, Ejaz A, Cloyd J, Pawlik TM. Management and outcomes among patients with mixed hepatocholangiocellular carcinoma: A population-based analysis. J Surg Oncol. 2019;119:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Calderaro J, Di Tommaso L, Maillé P, Beaufrère A, Nguyen CT, Heij L, Gnemmi V, Graham RP, Charlotte F, Chartier S, Wendum D, Vij M, Allende D, Diaz A, Fuster C, Rivière B, Herrero A, Augustin J, Evert K, Calvisi DF, Leow WQ, Leung HHW, Bednarsch J, Boleslawski E, Rela M, Chan AW, Forner A, Reig M, Pujals A, Favre L, Allaire M, Scatton O, Uguen A, Trépo E, Sanchez LO, Chatelain D, Remmelink M, Boulagnon-Rombi C, Bazille C, Sturm N, Menahem B, Frouin E, Tougeron D, Tournigand C, Kempf E, Kim H, Ningarhari M, Michalak-Provost S, Kather JN, Gouw ASH, Gopal P, Brustia R, Vibert E, Schulze K, Rüther DF, Weidemann SA, Rhaiem R, Nault JC, Laurent A, Amaddeo G, Regnault H, de Martin E, Sempoux C, Navale P, Shinde J, Bacchuwar K, Westerhoff M, Lo RC, Sebbagh M, Guettier C, Lequoy M, Komuta M, Ziol M, Paradis V, Shen J, Caruso S. Nestin as a diagnostic and prognostic marker for combined hepatocellular-cholangiocarcinoma. J Hepatol. 2022;77:1586-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Sasaki M, Sato Y, Nakanuma Y. Is nestin a diagnostic marker for combined hepatocellular-cholangiocarcinoma? Histopathology. 2022;80:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Schizas D, Mastoraki A, Routsi E, Papapanou M, Tsapralis D, Vassiliu P, Toutouzas K, Felekouras E. Combined hepatocellular-cholangiocarcinoma: An update on epidemiology, classification, diagnosis and management. Hepatobiliary Pancreat Dis Int. 2020;19:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Garancini M, Goffredo P, Pagni F, Romano F, Roman S, Sosa JA, Giardini V. Combined hepatocellular-cholangiocarcinoma: a population-level analysis of an uncommon primary liver tumor. Liver Transpl. 2014;20:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, Joh JW. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Wang AQ, Zheng YC, Du J, Zhu CP, Huang HC, Wang SS, Wu LC, Wan XS, Zhang HH, Miao RY, Sang XT, Zhao HT. Combined hepatocellular cholangiocarcinoma: Controversies to be addressed. World J Gastroenterol. 2016;22:4459-4465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Cazals-Hatem D, Rebouissou S, Bioulac-Sage P, Bluteau O, Blanché H, Franco D, Monges G, Belghiti J, Sa Cunha A, Laurent-Puig P, Degott C, Zucman-Rossi J. Clinical and molecular analysis of combined hepatocellular-cholangiocarcinomas. J Hepatol. 2004;41:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Wakizaka K, Yokoo H, Kamiyama T, Ohira M, Kato K, Fujii Y, Sugiyama K, Okada N, Ohata T, Nagatsu A, Shimada S, Orimo T, Kamachi H, Taketomi A. Clinical and pathological features of combined hepatocellular-cholangiocarcinoma compared with other liver cancers. J Gastroenterol Hepatol. 2019;34:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Liu ZH, Lian BF, Dong QZ, Sun H, Wei JW, Sheng YY, Li W, Li YX, Xie L, Liu L, Qin LX. Whole-exome mutational and transcriptional landscapes of combined hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma reveal molecular diversity. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2360-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Wang A, Wu L, Lin J, Han L, Bian J, Wu Y, Robson SC, Xue L, Ge Y, Sang X, Wang W, Zhao H. Whole-exome sequencing reveals the origin and evolution of hepato-cholangiocarcinoma. Nat Commun. 2018;9:894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Raevskaya O, Appelman H, Razumilava N. A Contemporary Approach to Diagnosis and Treatment of Combined Hepatocellular-Cholangiocarcinoma. Curr Hepatol Rep. 2020;19:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Stavraka C, Rush H, Ross P. Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. J Hepatocell Carcinoma. 2019;6:11-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 16. | Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma. A histologic and immunohistochemical study. Cancer. 1985;55:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Overi D, Carpino G, Cardinale V, Franchitto A, Safarikia S, Onori P, Alvaro D, Gaudio E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 417] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 19. | Akiba J, Nakashima O, Hattori S, Tanikawa K, Takenaka M, Nakayama M, Kondo R, Nomura Y, Koura K, Ueda K, Sanada S, Naito Y, Yamaguchi R, Yano H. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol. 2013;37:496-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 260] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 21. | Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, Montal R, Torrens L, Martinez-Quetglas I, Fiel MI, Hao K, Villanueva A, Thung SN, Schwartz ME, Llovet JM. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66:952-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology. 2003;43:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Malvi D, de Biase D, Fittipaldi S, Grillini M, Visani M, Pession A, D'Errico A, Vasuri F. Immunomorphology and molecular biology of mixed primary liver cancers: is Nestin a marker of intermediate-cell carcinoma? Histopathology. 2020;76:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Radhakrishnan S, Martin CA, Vij M, Raju LP, Gowripriya G, Jana K, Rammohan A, Jothimani D, Kaliamoorthy I, Veldore VH, Rela M. Biphenotypic Immunohistochemical Features and NTRK1 Amplification in Intermediate Cell Carcinoma of the Liver. Int J Surg Pathol. 2023;31:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 25. | Xue R, Chen L, Zhang C, Fujita M, Li R, Yan SM, Ong CK, Liao X, Gao Q, Sasagawa S, Li Y, Wang J, Guo H, Huang QT, Zhong Q, Tan J, Qi L, Gong W, Hong Z, Li M, Zhao J, Peng T, Lu Y, Lim KHT, Boot A, Ono A, Chayama K, Zhang Z, Rozen SG, Teh BT, Wang XW, Nakagawa H, Zeng MS, Bai F, Zhang N. Genomic and Transcriptomic Profiling of Combined Hepatocellular and Intrahepatic Cholangiocarcinoma Reveals Distinct Molecular Subtypes. Cancer Cell. 2019;35:932-947.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 26. | Coulouarn C, Cavard C, Rubbia-Brandt L, Audebourg A, Dumont F, Jacques S, Just PA, Clément B, Gilgenkrantz H, Perret C, Terris B. Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis. 2012;33:1791-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Joseph NM, Tsokos CG, Umetsu SE, Shain AH, Kelley RK, Onodera C, Bowman S, Talevich E, Ferrell LD, Kakar S, Krings G. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol. 2019;248:164-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Sasaki M, Sato H, Kakuda Y, Sato Y, Choi JH, Nakanuma Y. Clinicopathological significance of 'subtypes with stem-cell feature' in combined hepatocellular-cholangiocarcinoma. Liver Int. 2015;35:1024-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Sasaki M, Sato Y, Nakanuma Y. Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology. 2017;70:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Fujii H, Zhu XG, Matsumoto T, Inagaki M, Tokusashi Y, Miyokawa N, Fukusato T, Uekusa T, Takagaki T, Kadowaki N, Shirai T. Genetic classification of combined hepatocellular-cholangiocarcinoma. Hum Pathol. 2000;31:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Fujimoto A, Furuta M, Shiraishi Y, Gotoh K, Kawakami Y, Arihiro K, Nakamura T, Ueno M, Ariizumi S, Nguyen HH, Shigemizu D, Abe T, Boroevich KA, Nakano K, Sasaki A, Kitada R, Maejima K, Yamamoto Y, Tanaka H, Shibuya T, Shibata T, Ojima H, Shimada K, Hayami S, Shigekawa Y, Aikata H, Ohdan H, Marubashi S, Yamada T, Kubo M, Hirano S, Ishikawa O, Yamamoto M, Yamaue H, Chayama K, Miyano S, Tsunoda T, Nakagawa H. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun. 2015;6:6120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 32. | Jeon J, Maeng LS, Bae YJ, Lee EJ, Yoon YC, Yoon N. Comparing Clonality Between Components of Combined Hepatocellular Carcinoma and Cholangiocarcinoma by Targeted Sequencing. Cancer Genomics Proteomics. 2018;15:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Sasaki M, Sato Y, Nakanuma Y. Cholangiolocellular Carcinoma With "Ductal Plate Malformation" Pattern May Be Characterized by ARID1A Genetic Alterations. Am J Surg Pathol. 2019;43:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: An update. J Hepatol. 2021;74:1212-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 35. | Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, Calatayud AL, Pinyol R, Pelletier L, Balabaud C, Laurent A, Blanc JF, Mazzaferro V, Calvo F, Villanueva A, Nault JC, Bioulac-Sage P, Stratton MR, Llovet JM, Zucman-Rossi J. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1340] [Article Influence: 134.0] [Reference Citation Analysis (0)] |
| 36. | Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JS, Zhao X, Li Y, Li Q, Wang H, Hu J, Kong G, Wu M, Ding C, Chen N, Hu H. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 37. | Roßner F, Sinn BV, Horst D. Pathology of Combined Hepatocellular Carcinoma-Cholangiocarcinoma: An Update. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 38. | Calderaro J, Ghaffari Laleh N, Zeng Q, Maille P, Favre L, Pujals A, Klein C, Bazille C, Heij LR, Uguen A, Luedde T, Di Tommaso L, Beaufrère A, Chatain A, Gastineau D, Nguyen CT, Nguyen-Canh H, Thi KN, Gnemmi V, Graham RP, Charlotte F, Wendum D, Vij M, Allende DS, Aucejo F, Diaz A, Rivière B, Herrero A, Evert K, Calvisi DF, Augustin J, Leow WQ, Leung HHW, Boleslawski E, Rela M, François A, Cha AW, Forner A, Reig M, Allaire M, Scatton O, Chatelain D, Boulagnon-Rombi C, Sturm N, Menahem B, Frouin E, Tougeron D, Tournigand C, Kempf E, Kim H, Ningarhari M, Michalak-Provost S, Gopal P, Brustia R, Vibert E, Schulze K, Rüther DF, Weidemann SA, Rhaiem R, Pawlotsky JM, Zhang X, Luciani A, Mulé S, Laurent A, Amaddeo G, Regnault H, De Martin E, Sempoux C, Navale P, Westerhoff M, Lo RC, Bednarsch J, Gouw A, Guettier C, Lequoy M, Harada K, Sripongpun P, Wetwittayaklang P, Loménie N, Tantipisit J, Kaewdech A, Shen J, Paradis V, Caruso S, Kather JN. Deep learning-based phenotyping reclassifies combined hepatocellular-cholangiocarcinoma. Nat Commun. 2023;14:8290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 39. | Sanada Y, Shiozaki S, Aoki H, Takakura N, Yoshida K, Yamaguchi Y. A clinical study of 11 cases of combined hepatocellular-cholangiocarcinoma Assessment of enhancement patterns on dynamics computed tomography before resection. Hepatol Res. 2005;32:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Hwang J, Kim YK, Park MJ, Lee MH, Kim SH, Lee WJ, Rhim HC. Differentiating combined hepatocellular and cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma using gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2012;36:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 41. | Gigante E, Ronot M, Bertin C, Ciolina M, Bouattour M, Dondero F, Cauchy F, Soubrane O, Vilgrain V, Paradis V. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular-cholangiocarcinoma. Liver Int. 2019;39:2386-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Fowler K, Saad NE, Brunt E, Doyle MB, Amin M, Vachharajani N, Tan B, Chapman WC. Biphenotypic Primary Liver Carcinomas: Assessing Outcomes of Hepatic Directed Therapy. Ann Surg Oncol. 2015;22:4130-4137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Gentile D, Donadon M, Lleo A, Aghemo A, Roncalli M, di Tommaso L, Torzilli G. Surgical Treatment of Hepatocholangiocarcinoma: A Systematic Review. Liver Cancer. 2020;9:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 44. | Tian MX, Luo LP, Liu WR, Deng W, Yin JC, Jin L, Jiang XF, Zhou YF, Qu WF, Tang Z, Wang H, Tao CY, Fang Y, Qiu SJ, Zhou J, Liu JF, Fan J, Shi YH. Development and validation of a prognostic score predicting recurrence in resected combined hepatocellular cholangiocarcinoma. Cancer Manag Res. 2019;11:5187-5195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, Horiguchi S, Takahashi H, Suzuki E, Moriguchi M, Tsuji K, Otsuka T, Asagi A, Kojima Y, Takada R, Morizane C, Mizuno N, Ikeda M, Ueno M, Furuse J. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018;109:2549-2557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 46. | Salimon M, Prieux-Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, Matysiak-Budnik T, Bennouna J, Tiako Meyo M, Lecomte T, Zaanan A, Touchefeu Y. Gemcitabine plus platinum-based chemotherapy for first-line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer. 2018;118:325-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 47. | Trikalinos NA, Zhou A, Doyle MBM, Fowler KJ, Morton A, Vachharajani N, Amin M, Keller JW, Chapman WC, Brunt EM, Tan BR. Systemic Therapy for Combined Hepatocellular-Cholangiocarcinoma: A Single-Institution Experience. J Natl Compr Canc Netw. 2018;16:1193-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 48. | Lei Q, Yan X, Zou H, Jiang Y, Lai Y, Ung COL, Hu H. Efficacy and safety of monotherapy and combination therapy of immune checkpoint inhibitors as first-line treatment for unresectable hepatocellular carcinoma: a systematic review, meta-analysis and network meta-analysis. Discov Oncol. 2022;13:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 49. | Yao WY, Gong W. Immunotherapy in cholangiocarcinoma: From concept to clinical trials. Surg Pract Sci. 2021;5:100028. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Gutiérrez-Larrañaga M, González-López E, Roa-Bautista A, Rodrigues PM, Díaz-González Á, Banales JM, López-Hoyos M, Santos-Laso A, Crespo J. Immune Checkpoint Inhibitors: The Emerging Cornerstone in Cholangiocarcinoma Therapy? Liver Cancer. 2021;10:545-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Nguyen CT, Caruso S, Maille P, Beaufrère A, Augustin J, Favre L, Pujals A, Boulagnon-Rombi C, Rhaiem R, Amaddeo G, di Tommaso L, Luciani A, Regnault H, Brustia R, Scatton O, Charlotte F, Brochériou I, Sommacale D, Soussan P, Leroy V, Laurent A, Le VK, Ta VT, Trinh HS, Tran TL, Gentien D, Rapinat A, Nault JC, Allaire M, Mulé S, Zucman-Rossi J, Pawlotsky JM, Tournigand C, Lafdil F, Paradis V, Calderaro J. Immune Profiling of Combined Hepatocellular- Cholangiocarcinoma Reveals Distinct Subtypes and Activation of Gene Signatures Predictive of Response to Immunotherapy. Clin Cancer Res. 2022;28:540-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Satake T, Shibuki T, Watanabe K, Sasaki M, Imaoka H, Mitsunaga S, Kojima M, Ikeda M. Case Report: Atezolizumab plus bevacizumab for combined hepatocellular-cholangiocarcinoma. Front Oncol. 2023;13:1234113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Pomej K, Balcar L, Shmanko K, Welland S, Himmelsbach V, Scheiner B, Mahyera A, Mozayani B, Trauner M, Finkelmeier F, Weinmann A, Vogel A, Pinter M. Clinical characteristics and outcome of patients with combined hepatocellular-cholangiocarcinoma-a European multicenter cohort. ESMO Open. 2023;8:100783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Chen J, He J, Deng M, Wu HY, Shi J, Mao L, Sun Q, Tang M, Fan XS, Qiu YD, Huang Q. Clinicopathological, radiologic, and molecular study of 23 combined hepatocellular-cholangiocarcinomas with stem cell features, cholangiolocellular type. Hum Pathol. 2017;64:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Tahover E. An exceptional response to immunotherapy doublet in combined hepatocellular carcinoma-cholangiocarcinoma. Ann Oncol. 2019;30:vii15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Rizell M, Åberg F, Perman M, Ny L, Stén L, Hashimi F, Svanvik J, Lindnér P. Checkpoint Inhibition Causing Complete Remission of Metastatic Combined Hepatocellular-Cholangiocarcinoma after Hepatic Resection. Case Rep Oncol. 2020;13:478-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Diab O, Khan M, Abbasi S, Saeed A, Kasi A, Baranda JC, Sun W, Al-Rajabi RMT. Efficacy of immunotherapy in hepatocholangiocarcinoma (HCC-CC): Proof of concept. J Clin Oncol. 2021;39:e16194-16194. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Saito N, Hatanaka T, Nakano S, Hazama Y, Yoshida S, Hachisu Y, Tanaka Y, Yoshinaga T, Kashiwabara K, Kubo N, Hosouchi Y, Tojima H, Kakizaki S, Uraoka T. A case of unresectable combined hepatocellular and cholangiocarcinoma treated with atezolizumab plus bevacizumab. Clin Case Rep. 2022;10:e6129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Saint A, Benchetrit M, Novellas S, Ouzan D, Falk AT, Leysalle A, Barriere J. Prolonged efficacy of pembrolizumab in a patient presenting a multi-treated metastatic hepatocholangiocarcinoma. Therap Adv Gastroenterol. 2020;13:1756284820935189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Zhou N, Lei CF, Tan SR, Huang QY, Zhang SY, Liang ZX, Gou HF. Case report: Remarkable response to sintilimab, lenvatinib, and nab-paclitaxel in postoperative metastatic chemotherapy-resistant combined hepatocellular-cholangiocarcinoma. Front Pharmacol. 2023;14:1190967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |