Published online Apr 27, 2023. doi: 10.4254/wjh.v15.i4.577
Peer-review started: January 24, 2023
First decision: March 6, 2023
Revised: March 9, 2023
Accepted: March 29, 2023
Article in press: March 29, 2023
Published online: April 27, 2023
Processing time: 86 Days and 0 Hours
Only a few cases of chronic hepatitis B (CHB) with primary biliary cholangitis (PBC) have been reported based on histological evidence from liver biopsies.
To observe the clinicopathological features and outcomes of 11 patients with CHB infection complicated by PBC.
Eleven patients with CHB and PBC who underwent liver biopsy at the Zhenjiang Third Hospital, affiliated with Jiangsu University, and Wuxi Fifth People’s Hospital, from January 2005 to September 2020, were selected. All patients initia
Only five had elevated alkaline phosphatase levels, nine were positive for anti-mitochondrial antibody (AMA)-M2, and two were negative for AMA-M2. Two had jaundice and pruritus symptoms, 10 had mildly abnormal liver function, and one had severely elevated bilirubin and liver enzyme levels. The pathological characteristics of CHB complicated by PBC overlapped with those of PBC-autoimmune hepatitis (AIH). When necroinflammation of the portal area is not obvious, the pathological features of PBC are predominant, similar to the features of PBC alone. When the interface is severe, biliangitis will occur, with a large number of ductular reactions in zone 3. Unlike the PBC-AIH overlap pathology, this pathology is characterized by a small amount of plasma cell infiltration. Unlike PBC, lobulitis is often observed.
This is the first large case series to show that the rare pathological features of CHB with PBC are similar to those of PBC-AIH and small duct injury was observed.
Core Tip: We retrospectively observed the clinicopathological features and outcomes of 11 patients with chronic hepatitis B (CHB) infection complicated by primary biliary cholangitis (PBC). We found that CHB complicated with PBC had pathological characteristics overlapping with PBC-autoimmune hepatitis. When necroinflammation of the portal area is not obvious, the pathological features of PBC are superior, similar to the features of PBC alone, this pathology is characterized by a small amount of plasma cell infiltration. Unlike PBC alone, lobulitis is often present. All patients improved after antiviral and ursodeoxycholic acid treatment and stabilized after 1 year.
- Citation: Ye Y, Zhang Q, Lu ZH, Tan YW. Clinicopathological features of 11 cases of chronic hepatitis B infection complicated with primary biliary cholangitis. World J Hepatol 2023; 15(4): 577-584
- URL: https://www.wjgnet.com/1948-5182/full/v15/i4/577.htm
- DOI: https://dx.doi.org/10.4254/wjh.v15.i4.577
Chronic hepatitis B (CHB) remains the largest public health burden in China, with nearly 60 million people infected with the CHB virus and nearly 300000 deaths related to liver disease every year[1,2]. Primary biliary cholangitis (PBC) is a slowly progressing autoimmune disease that is prevalent in Northern Europe and North America. PBC remains uncommon compared with CHB in Asian populations. Large cohort studies have reported that, in Japan and Hong Kong, only 2.4% and 1.3% of PBC cases occur in patients with chronic liver disease, respectively[3]. A recent study from southern China reported that PBC occurred in 49.2 cases out of 100000 adults who underwent routine annual examinations[4]. Anti-mitochondrial antibody (AMA)-M2 was reported in 22 of the 325 patients (6.8%) with CHB[5]. A positive AMA result did not confirm the presence of PBC. Only a few cases of CHB with PBC based on histological evidence from liver biopsies have been reported. We retrospectively examined the clinicopathological features and outcomes of 11 patients with CHB complicated by PBC.
Eleven cases of CHB patients with PBC who underwent liver biopsy were all at the Third People’s Hospital of Zhenjiang City and the Fifth People’s Hospital of Wuxi City from January 2005 to September 2020. CHB infected persons were defined as hepatitis B surface antigen (HBsAg) lasting for more than half a year. PBC was diagnosed if two of the following three criteria were met: (1) Biochemical abnormalities reflecting cholestasis, such as elevated alkaline phosphatase (ALP) and γ-glutamyl transferase (GGT) levels given that extra- or intrahepatic bold tube obstruction is excluded by imaging examination; (2) positive serum AMA/AMA-M2 or other PBC-specific autoantibodies such as anti-GP210 and anti-SP100; and (3) histological evidence of chronic non-suppurative destructive cholangitis (CNSDC) and interlobular bile duct destruction in liver biopsy[6]. The exclusion criteria were a history of excessive alcohol consumption (defined as ≥ 20 g/d for men and ≥ 10 g/d for women)[7], drug-induced hepatitis, schistosomiasis liver disease, autoimmune hepatitis (AIH), or hepatitis A, C, or D. This study was approved by the Ethics Committee of The Third People’s Hospital of Zhenjiang City.
The following demographic data (sex and age) were collected: clinical history (medical history, drinking history, family history); antiviral treatment status; results of routine blood tests; biochemical indicators; hepatitis B pathogenic serological examination; and tumor indicators during liver biopsy, including total bilirubin, albumin, prealbumin, alanine aminotransferase, aspartate aminotransferase, GGT, ALP, platelet count, HBsAg, hepatitis B e antigen, hepatitis B virus (HBV) DNA level, alpha-fetoprotein, indicators of other types of viral hepatitis, human immunodeficiency virus antibody, and autoantibody examination results; antibodies (AMA) and subtypes (AMA-M2), antibodies against gp210, sp100, and immunoglobulins G (IgG) and M (IgM).
Liver biopsies were performed using a 16G puncture needle (Bard Peripheral Vascular Inc., United States) under ultrasound guidance by an experienced hepatologist. The length of the puncture tissue was greater than 1.5 cm and the tissue was fixed in formalin. The tissues were evaluated by two liver pathologists using the METAVIR and Ludwig staging systems[8,9].
There were 11 cases of CHB diagnosed with PBC, of which four were male and seven were female, with an average age of 42 years (range, 28-60 years); nine cases were positive for AMA-M2. Two AMA-M2-negative patients with elevated ALP and GGT levels but without other etiological explanations were diagnosed with PBC by liver biopsy. ALP levels were elevated in five of the 11 cases. Six patients had normal ALP levels, while four had elevated GGT levels and positive AMA-M2 results. Two patients attained a complete HBV response (HBV DNA < 10 U/L) before liver biopsy, and the remaining patients with CHB underwent primary antiviral treatment, with five receiving entecavir, three receiving tenofovir, and three receiving propofol tenofovir fumarate. Patient 7 was hospitalized for severe liver injury for approximately 2 mo. After control was achieved, patient 7 received outpatient treatment similar to that of the other patients (Table 1).
| No. | Sex | Age | TBIL | ALT | AST | ALP | GGT | TBA | Albumin (g/L) | AMA-M2 | gp120 | sp100 | ANA | IgM | IgG | HBV DNA (Log) | Platelet (1 × 109/L) | Fatigue | Pruritus |
| 1 | Woman | 42 | 22.2 | 87 | 56 | 47 | 67 | 17 | 32.2 | ++ | + | - | + | 4.25 | 21.15 | 7.21 | 9.21 | + | + |
| 2 | Man | 60 | 45.2 | 224 | 175 | 154 | 116 | 30 | 36.5 | + | - | - | - | 1.25 | 11.23 | 4.25 | 12.66 | - | - |
| 3 | Woman | 52 | 13.5 | 35 | 24 | 57 | 57 | 12 | 41.2 | + | - | - | - | 1.65 | 9.32 | 4.68 | 8.35 | - | - |
| 4 | Man | 37 | 15.5 | 22 | 16 | 46 | 47 | 8 | 38.2 | + | - | - | - | 1.42 | 8.65 | 5.32 | 16.35 | - | - |
| 5 | Man | 39 | 16.0 | 35 | 24 | 65 | 37 | 12 | 37.9 | + | - | - | - | 1.87 | 10.32 | - | 15.32 | - | - |
| 6 | Woman | 37 | 16.3 | 27 | 18 | 74 | 58 | 14 | 36.4 | ++ | - | - | - | 2.01 | 11.56 | 6.14 | 11.25 | - | - |
| 7 | Man | 57 | 422.4 | 243 | 215 | 767 | 837 | 147 | 32.2 | + | - | - | - | 4.36 | 24.32 | 4.35 | 13.65 | + | + |
| 8 | Woman | 52 | 20.4 | 156 | 87 | 167 | 224 | 25 | 40.2 | ++ | + | - | + | 0.98 | 10.48 | 3.57 | 14.25 | + | - |
| 9 | Woman | 32 | 11.3 | 25 | 11 | 37 | 26 | 37 | 41.6 | + | - | - | - | 1.14 | 9.65 | 4.25 | 24.22 | - | - |
| 10 | Woman | 28 | 32.7 | 18 | 15 | 166 | 89 | 18 | 39.2 | - | - | - | - | 1.58 | 13.64 | - | 21.03 | - | - |
| 11 | Woman | 51 | 25.6 | 87 | 26 | 185 | 98 | 9 | 38.1 | - | - | - | - | 1.67 | 10.35 | - | 18.21 | - | - |
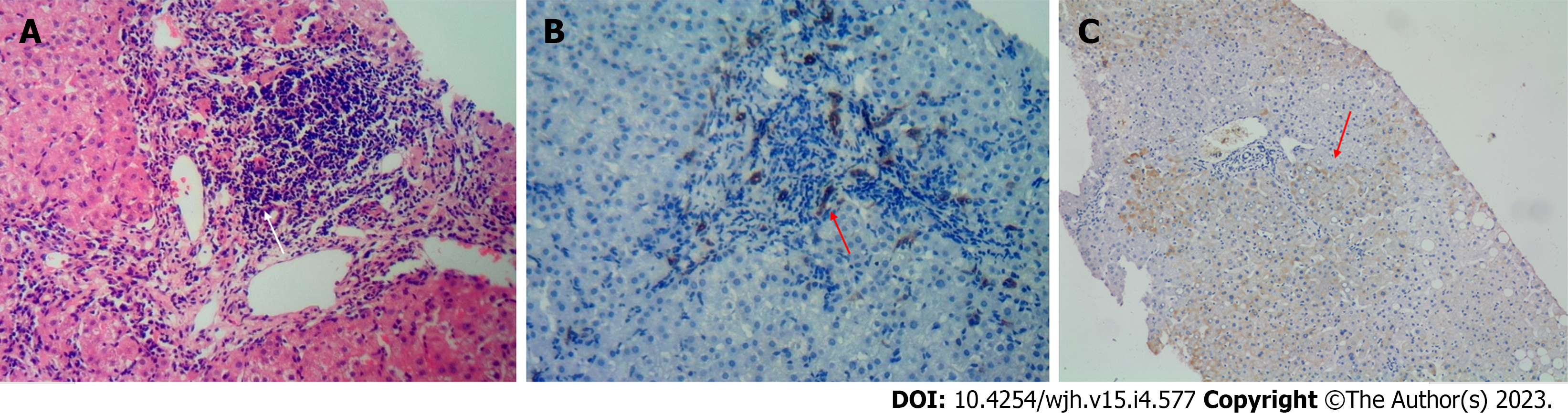
The lesions were primarily caused by necroinflammation of the portal tract. All cases showed bile duct injury; five showed a typical florid duct lesion (FDL) and three cases with severe interfacial necroinflammation showed PBC-AIH-like injury with fewer plasma cells. A ductular reaction was evident that extended deep into the hepatocyte plate, and bile duct interfacial necroinflammation was apparent. Six patients with normal ALP and AMA-M2 expression had bile duct injuries. Overall, the METAVIR staging was consistent with the Ludwig staging (Table 2, Figure 1).
| TBIL | ALT | AST | ALP | GGT | TBA | IgM | IgG | |
| Before treatment | 20.4 (11.3-422.4) | 35 (18-243) | 24 (11-215) | 66 (37-767) | 58 (17-837) | 17 (8-147) | 1.65 (0.98-4.37) | 12.7 (8.6-24.3) |
| After treatment | 17.7 (6.7-21.2) | 27 (12-63) | 21 (15-43) | 47 (38-107) | 25 (16-42) | 9 (6-20) | 1.4 (1.1-2.5) | 11.3 (6.8-15.3) |
| Statistical value | 1.223 | 2.351 | 1.656 | 1.326 | 2.369 | 1.632 | 2.435 | 0.931 |
| P value | 0.248 | 0.041 | 0.129 | 0.214 | 0.039 | 0.134 | 0.031 | 0.374 |
Three patients with CHB had previously received antiviral therapy, and eight patients with HBV DNA-positive liver biopsies received antiviral therapy. The antiviral drugs used were entecavir in five cases, tenofovir in three cases, and propofol tenofovir in three cases. Ten patients experienced viral remission (< 10 U/L) after six months, and one experienced viral remission after one year. ALP levels returned to normal in four of the five patients with elevated ALP levels, and one patient returned to normal levels. Of the eight patients with elevated GGT levels, seven returned to normal after six months and one returned to normal after one year (Table 3).
| No. | II | BN | LI | DI | FDL | BIH | DU | HBsAg staining | HBcAg staining | METAVIR activity score | METAVIR stage | Ludwig stage |
| 1 | 1 | - | 2 | + | + | - | - | + | + | 2 | F1 | I |
| 2 | 1 | - | 1 | + | - | - | - | - | - | 1 | F1 | II |
| 3 | 0 | - | 1 | + | + | - | - | + | - | 1 | F1 | I |
| 4 | 2 | + | 2 | + | - | + | - | - | + | 2 | F1 | II |
| 5 | 0 | - | 1 | + | + | - | - | + | - | 1 | F2 | I |
| 6 | 1 | - | 1 | + | - | + | - | + | - | 1 | F1 | I |
| 7 | 3 | + | 2 | + | + | + | - | + | + | 3 | F3 | II |
| 8 | 2 | + | 1 | + | - | + | - | - | - | 2 | F1 | I |
| 9 | 1 | - | 1 | + | - | + | + | + | - | 1 | F4 | III |
| 10 | 0 | - | 0 | + | + | - | - | - | - | 0 | F0 | I |
| 11 | 1 | - | 1 | + | - | - | - | + | - | 1 | F0 | I |
Hepatitis B is a lifelong immune-related disease. Raynaud’s phenomenon occurs in 2% of patients with CHB, arthralgia or arthritis in 3%, myalgia in 3%, Sjögren’s syndrome in 3%, glomerulonephritis in 3%, uveitis in 2%, and cryoglobulin in 2%[10]. In a Chinese study on the detection rate of autoantibodies against CHB, 58.2% of patients with CHB were found to have AMA-M2 (approximately 7%)[5]. The 11 patients in our study were selected from approximately 1500 CHB liver histology examiners at two centers. The AMA-M2 positivity rate was < 1%, which may be related to the fact that many patients did not undergo the AMA-M2 examination.
Logically, preclinical PBC refers to patients with positive AMA but normal enzymatic indicators (i.e., ALP and GGT) that reflect cholestasis and no PBC manifestation on histology who nonetheless progress to PBC during follow-up. A prospective multicenter cohort study in France[11] found a 5-year incidence of PBC of 16% in a population with positive AMA and normal ALP levels. A recent single-center study in Austria[12] reported that, after an average follow-up of 5.8 years, only 10.2% of 122 AMA-positive patients developed PBC. An earlier study also found that only one (4%) of 26 AMA-positive first-degree relatives with normal ALP levels developed PBC after an 8-9-year follow-up[13].
In most reported cases, the diagnosis of PBC in patients with CHB is delayed for many years[14]. All 11 patients initially visited the hospital because of CHB, and eight patients were found to be AMA-M2 positive during the course of treatment. Of these, two with CHB showed ALP elevation after antiviral treatment, and a bile duct injury was detected by liver puncture. Only two patients had jaundice and pruritus symptoms, ten had mild abnormal liver function, and one patient had very serious increases in bilirubin and liver enzyme levels that rapidly improved after antiviral and ursodeoxycholic acid (UDCA) treatment; liver histology showed severe necrosis and fibrosis.
In a recent single-center study in China, up to 80% of patients positive for AMA and with normal ALP were pathologically confirmed to have PBC[15], which is similar to the findings of a multicenter study in Switzerland[16]. Both studies[15,16] suggested that a high AMA titer and elevated IgM and ALP levels close to the upper limit of normal are predictive factors for PBC expression on histology. Notably, despite normal ALP levels, GGT levels were elevated in most patients in our study. In our 11 cases, only five had elevated ALP, and only three were AMA-M2 positive; nine cases were AMA-M2 positive, seven cases had elevated GGT, and only three cases had elevated ALP. For cases of CHB that are AMA-positive, even though ALP and GGT levels are normal, PBC is likely to be combined; therefore, it cannot be considered that CHB has autoantibodies at present. Changes in liver biochemical indicators in this population should be monitored every year, and for patients with elevated IgM and GGT levels, liver biopsy should be considered to determine the presence of PBC.
The pathological characteristics of CHB is primarily characterized by necroinflammation of the portal tract and lobular inflammation. Bridge-like multilobular necrosis can also occur in severe cases. Fibrosis usually occurs in the portal tract and gradually bridges the adjacent portal areas to form a package. The pathological characteristics of CHB are nonspecific and are difficult to differentiate from those of other chronic liver injuries. The main pathological feature of PBC is CNSDC, involving the interlobular bile duct (small bile duct). The characteristic lesion is the infiltration of lymphocytes around the bile duct and the formation of epithelioid granulomas, which is called incandescent cholangiopathy (FDL). When no small bile ducts were accompanied by a small artery in > 50% of the portal area, it was defined as a reduction or disappearance of the bile duct. Ludwig et al[8] divided PBC into four phases. We found that CHB combined with PBC had overlapping pathological characteristics similar to those of PBC-AIH. When necroinflammation in the portal area is not evident, the pathological characteristics of PBC are predominant over those of simple PBC. In contrast, when the interface inflammation is serious, cholangio interface inflammation occurs, and a large number of ductular reactions occur in zone 3. The overlapping pathological feature that differs from that of PBC-AIH is the small amount of plasma cell infiltration. Compared with simple PBC[8], lobular inflammation often occurs; 10 cases had lobular inflammation, and three cases were more serious. FDL are characteristic PBC lesions that do not always occur in patients with CHB or PBC. FDL was observed in five cases in Scheuer’s original report[17]. CNSDC has also been observed in the livers of patients with nodular cirrhosis. Additionally, the pathology of PBC is not always evenly distributed in the liver; therefore, sampling errors may occur when determining the stage of these systems.
Currently, there are no cohort observations of CHB overlapping with PBC. A Chinese study surveyed 379 HBsAg-negative patients with PBC, 52 of whom underwent a liver biopsy. The enrolled patients were divided into the anti-HBC-positive and anti-HBC-negative groups. Histological examination revealed that patients in the anti-HBC-positive group had more advanced PBC than those in the anti-HBC-negative group (P < 0.05)[18]. In a single-center retrospective review of all follow-up HBV (n = 1493) and hepatitis C virus (HCV; n = 526) patients[14], 17 were identified as having concurrent viral hepatitis and PBC, and most were found to have cirrhosis (10/17, 58.8%). The authors speculated that chronic viral hepatitis combined with PBC was a risk factor for cirrhosis; however, the diagnosis of PBC was mainly based on the presence of anti-mitochondrial antibodies and elevated cholesterol levels. Only one of our 11 patients was diagnosed with cirrhosis. After treatment, the disease was quickly controlled, and the liver transaminase levels stabilized and were normal. All patients achieved biochemical normality within one year. However, the aforementioned pathological characteristics of HBV infection combined with PBC suggest that, as observed in these cohort studies, HBV infection combined with PBC is more prone to disease progression.
The shortcomings of this cohort study are its small sample size and descriptive nature. Nevertheless, this is the first large case series to show that the rare pathological features of CHB with PBC are similar to those of PBC-AIH. Although ALP was normal in nearly 50% of patients, small duct injury was observed, and all patients responded effectively after antiviral and UDCA treatment. The lack of overlap between these two diseases is an aggravating phenomenon.
Chronic hepatitis B (CHB) and primary biliary cholangitis (PBC) are chronic liver diseases; however, CHB combined with PBC is uncommon.
There are few studies on the clinical and pathological characteristics of CHB combined with PBC, and current research is limited to case reports.
To explore the clinicopathological characteristics, diagnoses, and treatments of patients with CHB and PBC.
Eleven patients with chronic hepatitis B virus (HBV) infection and PBC who underwent liver biopsy at our hospital between January 2005 and September 2020 were selected. Demographic data, clinical biochemical indicators, autoantibodies, and virological indicators were also collected. The liver pathology was evaluated using the METAVIR and Ludwig staging systems.
Eleven patients with CHB were diagnosed with PBC, and nine were anti-mitochondrial antibody-M2 positive. Alkaline phosphatase (ALP) increased in five of the 11 cases. ALP levels were normal in six patients, but γ-glutamyl transferase levels were elevated in four patients. Pathological changes were primarily caused by inflammation of the portal area. All cases showed bile duct injury, five cases showed a typical florid duct region, and three cases with severe interfacial inflammation showed similar autoimmune hepatitis-PBC-like injury with few plasma cells. All the patients received antiviral and ursodeoxycholic acid treatment.
For the first time, the pathological characteristics of rare CHB complicated with PBC were observed in a large sample size. A normal ALP level cannot exclude the diagnosis of PBC. The pathological characteristics were similar to those of PBC-autoimmune hepatitis, but with fewer plasma cells.
Attention should be paid to the possibility of HBV combined with PBC. Pathology can provide important information, even if biochemical changes or specific antibodies are negative.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kishida Y, Japan; Rodrigues AT, Brazil S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Su S, Wong WC, Zou Z, Cheng DD, Ong JJ, Chan P, Ji F, Yuen MF, Zhuang G, Seto WK, Zhang L. Cost-effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health. 2022;10:e278-e287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 2. | Huang ZH, Lu GY, Qiu LX, Zhong GH, Huang Y, Yao XM, Liu XH, Huang SJ, Wu T, Yuan Q, Wang YB, Su YY, Zhang J, Xia NS. Risk of hepatocellular carcinoma in antiviral treatment-naïve chronic hepatitis B patients treated with entecavir or tenofovir disoproxil fumarate: a network meta-analysis. BMC Cancer. 2022;22:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Michitaka K, Nishiguchi S, Aoyagi Y, Hiasa Y, Tokumoto Y, Onji M; Japan Etiology of Liver Cirrhosis Study Group. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol. 2010;45:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Liu H, Liu Y, Wang L, Xu D, Lin B, Zhong R, Gong S, Podda M, Invernizzi P. Prevalence of primary biliary cirrhosis in adults referring hospital for annual health check-up in Southern China. BMC Gastroenterol. 2010;10:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Li BA, Liu J, Hou J, Tang J, Zhang J, Xu J, Song YJ, Liu AX, Zhao J, Guo JX, Chen L, Wang H, Yang LH, Lu J, Mao YL. Autoantibodies in Chinese patients with chronic hepatitis B: prevalence and clinical associations. World J Gastroenterol. 2015;21:283-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 906] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 7. | Wong VW, Wong GL, Woo J, Abrigo JM, Chan CK, Shu SS, Leung JK, Chim AM, Kong AP, Lui GC, Chan HL, Chu WC. Impact of the New Definition of Metabolic Associated Fatty Liver Disease on the Epidemiology of the Disease. Clin Gastroenterol Hepatol. 2021;19:2161-2171.e5. [PubMed] |
| 8. | Ludwig J. The nomenclature of chronic active hepatitis: an obituary. Gastroenterology. 1993;105:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Boozari B, Potthoff A, Mederacke I, Hahn A, Reising A, Rifai K, Wedemeyer H, Bahr M, Kubicka S, Manns M, Gebel M. Evaluation of sound speed for detection of liver fibrosis: prospective comparison with transient dynamic elastography and histology. J Ultrasound Med. 2010;29:1581-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Cacoub P, Saadoun D, Bourlière M, Khiri H, Martineau A, Benhamou Y, Varastet M, Pol S, Thibault V, Rotily M, Halfon P. Hepatitis B virus genotypes and extrahepatic manifestations. J Hepatol. 2005;43:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, Singh S, Grover M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology. 2017;152:1042-1054.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (2)] |
| 12. | Zandanell S, Strasser M, Feldman A, Tevini J, Strebinger G, Niederseer D, Pohla-Gubo G, Huber-Schönauer U, Ruhaltinger S, Paulweber B, Datz C, Felder TK, Aigner E. Low rate of new-onset primary biliary cholangitis in a cohort of anti-mitochondrial antibody-positive subjects over six years of follow-up. J Intern Med. 2020;287:395-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Gulamhusein AF, Juran BD, Atkinson EJ, McCauley B, Schlicht E, Lazaridis KN. Low incidence of primary biliary cirrhosis (PBC) in the first-degree relatives of PBC probands after 8 years of follow-up. Liver Int. 2016;36:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Rigopoulou EI, Zachou K, Gatselis NK, Papadamou G, Koukoulis GK, Dalekos GN. Primary biliary cirrhosis in HBV and HCV patients: Clinical characteristics and outcome. World J Hepatol. 2013;5:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Sun C, Xiao X, Yan L, Sheng L, Wang Q, Jiang P, Lian M, Li Y, Wei Y, Zhang J, Chen Y, Li B, Huang B, Peng Y, Chen X, Fang J, Qiu D, Hua J, Tang R, Leung P, Gershwin ME, Miao Q, Ma X. Histologically proven AMA positive primary biliary cholangitis but normal serum alkaline phosphatase: Is alkaline phosphatase truly a surrogate marker? J Autoimmun. 2019;99:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Terziroli Beretta-Piccoli B, Stirnimann G, Mertens J, Semela D, Zen Y, Mazzucchelli L, Voreck A, Kolbus N, Merlo E, Di Bartolomeo C, Messina P, Cerny A, Costantini S, Vergani D, Mieli-Vergani G; Swiss PBC Cohort Study Group. Primary biliary cholangitis with normal alkaline phosphatase: A neglected clinical entity challenging current guidelines. J Autoimmun. 2021;116:102578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Scheuer P. Primary biliary cirrhosis. Proc R Soc Med. 1967;60:1257-1260. [PubMed] |
| 18. | Zhang Y, Shi Y, Wu R, Wang X, Gao X, Niu J. Primary biliary cholangitis is more severe in previous hepatitis B virus infection patients. Eur J Gastroenterol Hepatol. 2018;30:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |