Published online Apr 27, 2022. doi: 10.4254/wjh.v14.i4.791
Peer-review started: October 20, 2021
First decision: November 17, 2021
Revised: December 13, 2021
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: April 27, 2022
Processing time: 184 Days and 0.9 Hours
Nonalcoholic fatty liver disease (NAFLD) is characterized by hypertriglyceridemia, increased low-density lipoprotein cholesterol levels, and reduced high-density lipoprotein cholesterol (HDL-C) particles. Previous studies have shown that the total cholesterol to high-density lipoprotein cholesterol ratio (TC/HDL-C) was superior to other lipid metabolism biomarkers for predicting NAFLD risk and could be a new indicator of NAFLD. However, the association between TC/HDL-C and NAFLD in patients with hepatitis B virus (HBV) has not yet been determined.
To investigate the association between TC/HDL-C and NAFLD in a population with chronic hepatitis B (CHB).
In this study, 183 HBV-infected patients were enrolled. All participants underwent blood chemistry examinations and abdominal ultrasound. Univariate and multivariate logistic regression models, curve fitting analysis, and threshold calculation were used to assess the relationship between TC/HDL-C and NAFLD.
The overall prevalence of NAFLD was 17.49% (n = 32) in the 183 CHB par
This study demonstrated a non-linear correlation between TC/HDL-C and NAFLD in a population with CHB. TC/HDL-C was positively associated with NAFLD when TC/HDL-C was less than 4.9 but not when TC/HDL-C was more than 4.9.
Core Tip: Nonalcoholic fatty liver disease (NAFLD) and chronic hepatitis B (CHB) are both common chronic liver diseases. In this observational cross-sectional study, we explored the association between NAFLD and a lipid metabolism biomarker [the total cholesterol to high-density lipoprotein cholesterol ratio, total cholesterol to high-density lipoprotein cholesterol ratio (TC/HDL-C)] in a population with CHB. Our findings showed a non-linear correlation between TC/HDL-C and NAFLD. TC/HDL-C was positively associated with NAFLD when TC/HDL-C was less than 4.9 but not when TC/HDL-C was more than 4.9.
- Citation: Zhou YG, Tian N, Xie WN. Total cholesterol to high-density lipoprotein ratio and nonalcoholic fatty liver disease in a population with chronic hepatitis B. World J Hepatol 2022; 14(4): 791-801
- URL: https://www.wjgnet.com/1948-5182/full/v14/i4/791.htm
- DOI: https://dx.doi.org/10.4254/wjh.v14.i4.791
Chronic hepatitis B (CHB) is a common disease threatening public health and is a leading cause of multitudinous liver-related morbidity and mortality[1]. In 2016, about 257 million people were affected by hepatitis B virus (HBV) infection worldwide, with an estimated prevalence of 3.5%[2]. Over the past decades, with the implementation of nucleoside analogs (NAs) and hepatitis B vaccine, the risk of liver cirrhosis complications and hepatocellular carcinoma (HCC) have been substantially reduced in CHB patients[3]. However, since approximately 25% of the CHB population with nonalcoholic fatty liver disease (NAFLD) have hepatic steatosis, the effects of CHB on hepatosteatosis have recently been garnering attention[4].
NAFLD is a common chronic hepatic disease worldwide that is closely associated with cardiovascular disease, metabolic disorders, and end-stage liver diseases such as cirrhosis and HCC[5]. The prevalence of NAFLD has been increasing, and in the past few years it has reached alarming proportions (29.1%) in China due to changes in lifestyle habits and rapid socio-economic growth[6]; therefore, increased awareness to recognize NAFLD as a chronic liver disease is urgently needed.
Due to the growing prevalence of NAFLD, the coexistence of HBV infection and NAFLD is commonly observed around the world. However, a clear association between these two diseases remains questionable. Previous studies indicated that NAFLD could be inversely associated with the levels of HBV seromarkers[7,8], but interestingly, there is substantial evidence indicating an association between HBV infection and reduced incidence of hyperlipidemia or NAFLD[9,10].
Alterations in lipid metabolism are central drivers of disease progression, for instance, the progression of hepatic steatosis to nonalcoholic steatohepatitis (NASH) and hepatic fibrosis[11]. Therefore, deciphering the lipid metabolism characteristic of NAFLD is the crucial for disease treatment and prevention. NAFLD is characterized by hypertriglyceridemia, increased low-density lipoprotein cholesterol (LDL-C) levels, and reduced high-density lipoprotein cholesterol (HDL-C) particles[12,13]. A recent study showed that the total cholesterol to high-density lipoprotein cholesterol ratio (TC/HDL-C) was better at predicting NAFLD risk than other markers such as total cholesterol (TC), HDL-C, and the ratio of apolipoprotein B (ApoB) to apolipoprotein A1 (ApoA1) and might be a new indicator of NAFLD[14]. However, the association between TC/HDL-C and NAFLD in an HBV-infected population has not yet been investigated. Therefore, the objective of this study was to assess the correlation between TC/HDL-C and NAFLD in a population with CHB.
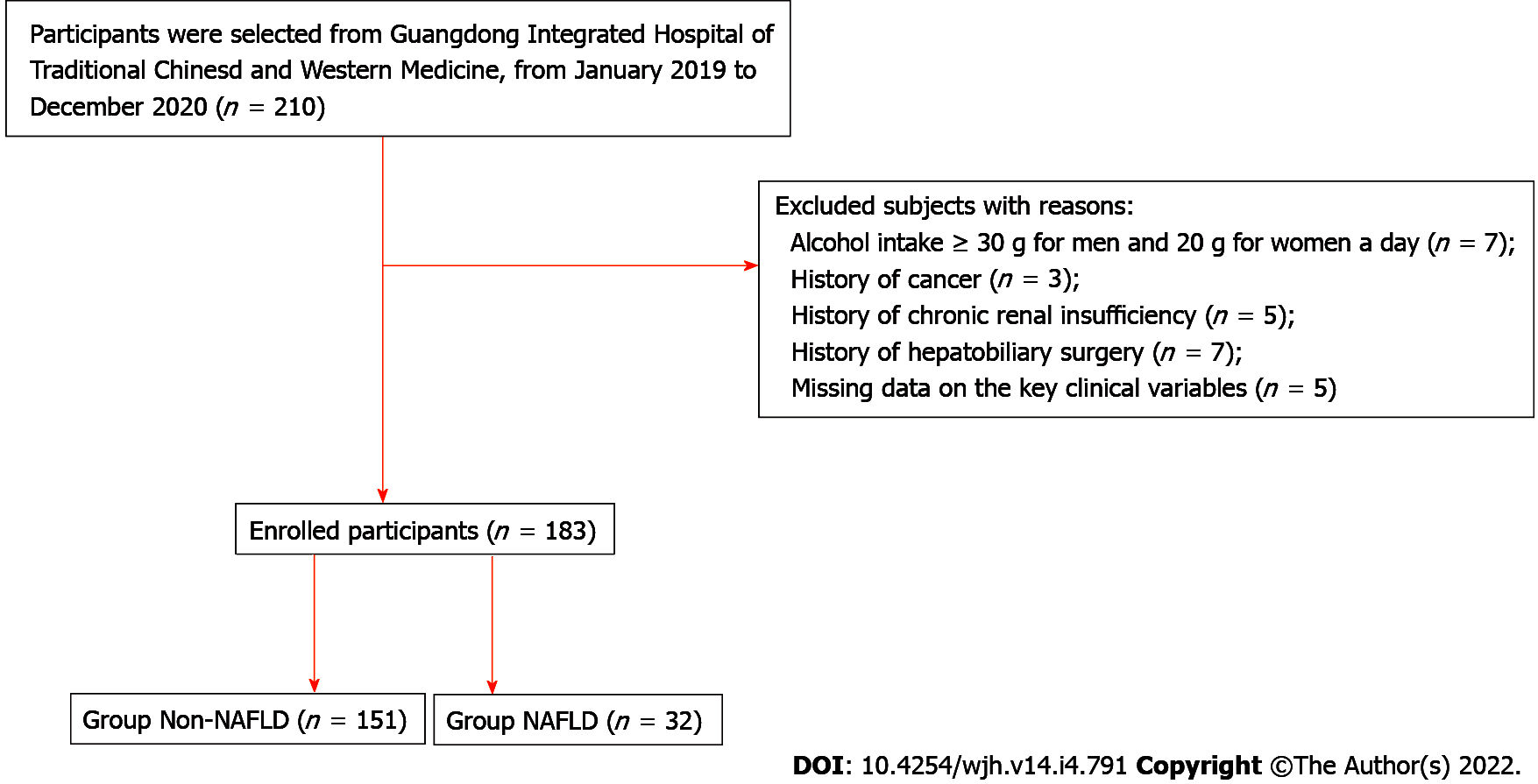
This was a retrospective, observational study comprising of HBV-infected patients who were treated at the Integrated Traditional Chinese and Western Medicine Hospital of Foshan (Guangdong, China) from January 2019 to December 2020. The study flow chart is illustrated in Figure 1. Chronic HBV infection was defined by hepatitis B surface antigen (HBsAg) positive for more than 6 mo[1]. All participants underwent abdominal ultrasonography for NAFLD and blood tests for assessing lipid metabolism and hepatic and renal function. Each participant completed a detailed questionnaire concerning information on their sex, age, alcohol consumption history, disease history, and medication history. Patients were excluded if they had (1) a daily alcohol intake ≥ 30g (for men) or 20 g (for women); (2) history of cancer; (3) history of chronic renal insufficiency; (4) history of hepatobiliary surgery; and (5) missing data on the key clinical variables required for study analysis.
Blood samples were collected from all patients after an overnight fasting of at least 8 h. Peripheral venous blood was drawn from their cubital vein. Blood test parameters, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GT), creatinine (CRE), uric acid (UA), triglyceride (TG), TC, HDL-C, LDL-C, ApoA1, and ApoB, were measured using an Olympus AU-640 autoanalyzer (Olympus, Tokyo, Japan). Platelets (PLT) were measured using the Sysmex 2100 whole blood cell analyzer (Sysmex, Kobe, Japan). Hepatitis B serum examinations included the detection of HBV-DNA level, HBsAg, and hepatitis B e antigen (HBeAg) using polymerase chain reaction. HBV-DNA(+) was defined as a level of serum HBV-DNA over 100 IU/mL. TC/HDL-C was defined as TC divided by HDL-C. The patient's height and weight were measured while wearing light clothing without shoes. Body mass index (BMI) was calculated by dividing a person’s weight in kilograms by the square of their height in meters. Abdominal ultrasound was used to detect the presence of NAFLD. NAs therapy was defined as the use of oral NAs antiviral drugs for more than 3 mo. All the above data were obtained from the Clinical Laboratory of the Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine.
The research project was submitted to and approved by the Ethics Committee of Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine (Approval number: 2018-1254). No informed consent was required because this was a retrospective observational study.
Ultrasonography was the most commonly used examination for fatty liver screening due to its noninvasiveness, low cost, and easy operability[15]. Abdominal ultrasonography was performed on all enrollees by two trained ultrasound physicians using the ACUSON X150 ultrasound system (Siemens, Munich, Germany). The presence of at least two of the following criteria was required for considering fatty liver: (1) More than 5% of hepatocytes had excessive hepatic fat accumulation and steatosis[16]; (2) Diffuse echo enhancement of the liver relative to the kidney; (3) Occurrence of ultrasonic beam attenuation; and (4) Poor visualization of intrahepatic structures. After excluding alcohol abuse and other hepatic diseases, NAFLD was then formally diagnosed by abdominal ultrasonography[17].
All statistical analyses were conducted using SPSS (version 23.0, IBM, Armonk, NY, United States) and EmpowerStats (http://www.empowerstats.com, X&Y solutions, Inc. Boston, MA, United States) software. Normal distribution continuous variables are expressed as mean ± standard deviation, and t-test was used for group comparison. Non-normal distribution variables are described as median [interquartile range (IQR)], and the Mann-Whitney U test was used to compare the groups. Categorical variables were presented as their corresponding number and percentage (n, %) and compared using the chi-squared test. All enrollees were stratified into two groups based on the presence or absence of NAFLD on ultrasonography. Then, the demographic characteristics of the study participants of the two groups were assessed. Univariate analyses of all variables were conducted using a binary logistic regression analysis model. Based on their TC/HDL-C, the patients were also divided into three groups according to TC/HDL-C tertiles: TC/HDL-C ≤ 3.5, 3.5 < TC/HDL-C ≤ 5, and TC/HDL-C > 5. Multivariable models were constructed as follows: Model 1 was not adjusted for other pertinent clinical variables; Model 2 was adjusted for sex and age; Model 3 was adjusted for sex, age, BMI, AST, ALT, γ-GT, PLT, HBsAg, CRE, UA, TG, TC, HDL-C, LDL-C, ApoA1, ApoB, HBV-DNA (+), HBeAg(+), and NAs. Lastly, a non-linear relationship between TC/HDL-C and NAFLD was investigated, and smooth curve fitting was also used. P values (two-tailed) less than 0.05 were considered statistically significant.
The demographic characteristics of the study participants with and without NAFLD are shown in Table 1. In the whole study population, the overall prevalence of NAFLD was 17.49% (n = 32). A total of 183 patients (70.5% males) were included in this study. Their mean age was 45.41 ± 11.59 years, and their average BMI was 23.14 ± 2.63 kg/m2. The TC/HDL-C of the non-NAFLD and NAFLD groups were 3.83 ± 0.75 and 4.44 ± 0.77, respectively (P < 0.01). Compared with the non-NAFLD group, patients from the NAFLD group had higher levels of BMI, ALT, γ-GT, PLT, UA, TG, TC, LDL-C, ApoB, and TC/HDL-C (P < 0.05). Conversely, age, HBV-DNA (+) levels, and usage of NAs were significantly lower in the NAFLD group than in the non-NAFLD group (P < 0.01). However, there was no statistically significant difference between the two groups in terms of sex and levels of AST, HBsAg, CRE, HDL-C, ApoA1, and HBeAg (+) (P > 0.05).
| Parameters | Total, n = 183 | Non-NAFLD, n = 151 | NAFLD, n = 32 | P value |
| Sex (male) | 129 (70.5%) | 103 (68.2%) | 26 (81.3%) | 0.14 |
| Age (yr) | 45.41 ± 11.59 | 46.29 ± 12.01 | 41.25 ± 8.30 | < 0.01 |
| BMI (kg/m2) | 23.14 ± 2.63 | 22.73 ± 2.40 | 25.05 ± 2.82 | < 0.01 |
| AST (U/L) | 25 (21-32) | 25 (21-31) | 25.5 (19.75-34.53) | 0.75 |
| ALT (U/L) | 28 (19-41) | 26 (19-38) | 39.5 (27-59.5) | < 0.01 |
| γ-GT (U/L) | 25 (19-37) | 24 (18-32) | 39 (25-55.5) | < 0.01 |
| PLT (× 109/L) | 208.37 ± 61.13 | 203.76 ± 63.49 | 230.09 ± 42.90 | 0.03 |
| HBsAg (IU/mL) | 0.09 | |||
| ≤ 1500 | 105 (57.4%) | 91 (60.3%) | 14 (43.8%) | |
| > 1500 | 78 (42.6%) | 60 (39.7%) | 18 (56.3%) | |
| CRE (μmol/L) | 77.73 ± 17.96 | 77.14 ± 18.09 | 80.49 ± 17.28 | 0.34 |
| UA (μmol/L) | 345.20 ± 92.53 | 332.61 ± 85.18 | 404.56 ± 103.65 | < 0.01 |
| TG (μmol/L) | 1.22 ± 0.63 | 1.08 ± 0.42 | 1.85 ± 0.99 | < 0.01 |
| TC (μmol/L) | 4.59 ± 0.98 | 4.52 ± 0.99 | 4.89 ± 0.82 | 0.05 |
| HDL-C (μmol/l) | 1.18 ± 0.25 | 1.19 ± 0.26 | 1.11 ± 0.14 | 0.08 |
| LDL-C (μmol/L) | 2.72 ± 0.68 | 2.66 ± 0.65 | 2.98 ± 0.73 | 0.02 |
| ApoA1 (g/L) | 1.36 ± 0.19 | 1.36 ± 0.19 | 1.35 ± 0.15 | 0.85 |
| ApoB (g/L) | 0.91 ± 0.18 | 0.89 ± 0.17 | 0.97 ± 0.18 | 0.02 |
| TC/HDL-C | 3.94 ± 0.79 | 3.83 ± 0.75 | 4.44 ± 0.77 | < 0.01 |
| HBV-DNA (+) | 131 (71.6%) | 116 (76.8%) | 15 (46.9%) | < 0.01 |
| HBeAg (+) | 49 (26.8%) | 39 (25.8%) | 10 (31.3%) | 0.52 |
| NAs | 168 (91.8%) | 144 (95.4%) | 24 (75%) | < 0.01 |
Binary logistic regression of independent risk factors of NAFLD is shown in Table 2. Univariate analysis indicated that age, BMI, ALT, γ-GT, PLT, UA, TG, LDL-C, ApoB, TC/HDL-C, and HBV-DNA (+) were significantly positively correlated with NAFLD (P < 0.05), whereas age and NAs were negatively correlated with NAFLD. However, no significant association between NAFLD and sex, AST, HBsAg, CRE, TC, HDL-C, ApoA1, and HBeAg(+) were observed (P > 0.05).
| Parameters | Statistics | OR | (95%CI) | P value |
| Gender (male) | 129 (70.5%) | 2.02 | (0.78-5.23) | 0.15 |
| Age | 45.41 ± 11.59 | 0.96 | (0.92-1.00) | 0.03 |
| BMI | 23.14 ± 2.63 | 1.40 | (1.20-1.63) | < 0.01 |
| AST | 25 (21–32) | 0.99 | (0.98-1.02) | 0.99 |
| ALT | 28 (19–41) | 1.01 | (1.00-1.02) | 0.04 |
| γ-GT | 25 (19–37) | 1.02 | (1.01-1.04) | < 0.01 |
| PLT | 208.37 ± 61.13 | 1.01 | (1.00-1.01) | 0.03 |
| HBsAg (IU/mL) | 0.09 | |||
| ≤ 1500 | 105 (57.4%) | 0.51 | (0.24-1.11) | |
| > 1500 | 78 (42.6%) | 1.95 | (0.90-4.22) | |
| CRE | 77.73 ± 17.96 | 1.01 | (0.99-1.03) | 0.34 |
| UA | 345.20 ± 92.53 | 1.01 | (1.00-1.01) | < 0.01 |
| TG | 1.22 ± 0.63 | 6.72 | (3.07-14.71) | < 0.01 |
| TC | 4.59 ± 0.98 | 1.46 | (1.00-2.15) | 0.05 |
| HDL-C | 1.18 ± 0.25 | 0.19 | (0.03-1.16) | 0.07 |
| LDL-C | 2.72 ± 0.68 | 1.94 | (1.11-3.39) | 0.02 |
| ApoA1 | 1.36 ± 0.19 | 0.81 | (0.10-6.44) | 0.84 |
| ApoB | 0.91 ± 0.18 | 11.86 | (1.37-102.86) | 0.03 |
| TC/HDL-C | 3.94 ± 0.79 | 2.55 | (1.55-4.19) | < 0.01 |
| HBV-DNA (+) | 131 (71.6%) | 3.74 | (1.70-8.28) | < 0.01 |
| HBeAg (+) | 49 (26.8%) | 1.31 | (0.57-2.00) | 0.53 |
| NAs | 168 (91.8%) | 0.15 | (0.05-0.44) | < 0.01 |
Logistic regression model was used to evaluate the association between TC/HDL-C and NAFLD. The unadjusted and adjusted models are shown in Table 3. In model 1, TC/HDL-C was positively correlated with NAFLD [odds ratio (OR) = 0.94, 95% confidence interval (CI): 1.55-4.19, P < 0.01). In model 2 (adjusted for sex and age), the relationship between TC/HDL-C and NAFLD were significant (OR = 0.96, 95%CI: 1.52-4.51, P < 0.01). However, this association was not detected in model 3 (OR = -2.27, 95%CI: 0.0001-79.91, P = 0.50). The same trend was observed for TC/HDL-C from 3.5 to 5 in model 1 and model 2 (P < 0.05).
| Variable | Model 1 | Model 2 | Model 3 | ||||||
| OR | 95%CI | P value | OR | 95%CI | P value | OR | 95%CI | P value | |
| TC/HDL-C | 0.94 | 1.55-4.19) | < 0.01 | 0.96 | (1.52-4.51) | < 0.01 | -2.27 | (0.01-79.91) | 0.5 |
| TC/HDL-C | |||||||||
| ≤ 3.5 | Reference | Reference | Reference | ||||||
| 3.5-5 | -2.53 | (0.01-0.48) | 0.01 | 1.59 | (1.10-21.94) | 0.04 | -0.64 | (0.04-6.77) | 0.63 |
| > 5 | -0.77 | (0.14-1.50) | 0.2 | 2.29 | (1.60-61.40) | 0.01 | -1.74 | (0.01-14.45) | 0.44 |
| P for trend | 0.02 | 0.05 | 0.73 | ||||||
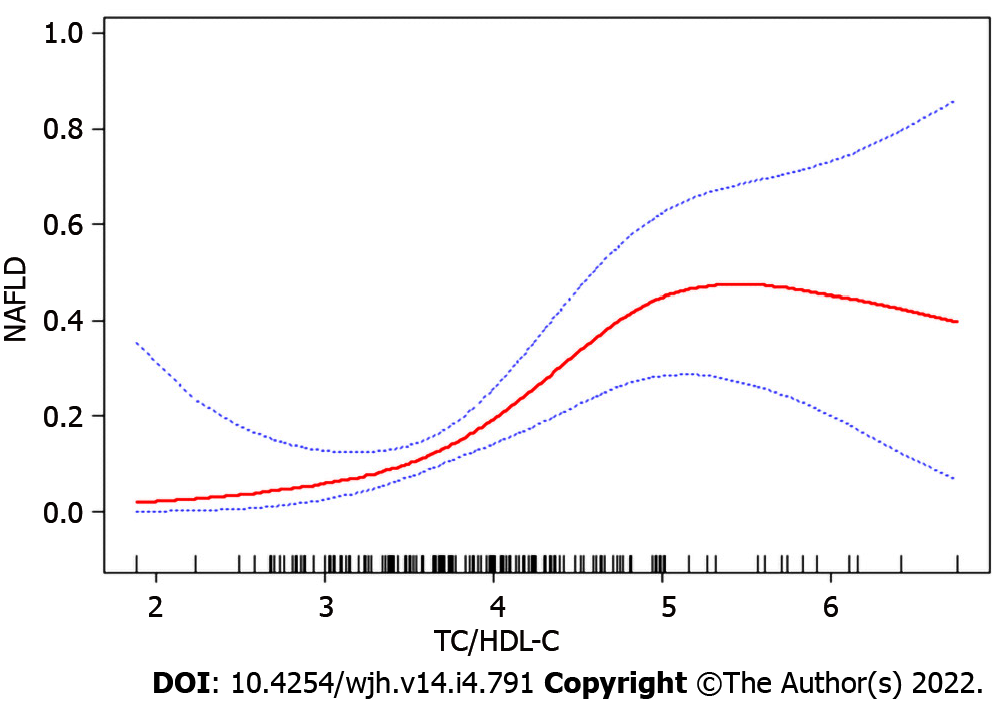
Since TC/HDL-C was a continuous variable, we analyzed the non-linear relationship with NAFLD. After adjusting for all variables and conducting smooth curve fitting, we found that the relationship between TC/HDL-C and NAFLD was non-linear (Figure 2). The inflection point was calculated as 4.9 by a piecewise linear regression model. On the left side of the inflection point, TC/HDL-C was found to be positively correlated with NAFLD (β = 5.4, 95%CI: 2.3-12.6, P < 0.01). However, no significant correlation was observed between TC/HDL-C and NAFLD on the right side of the inflection point (β = 0.5 95%CI: 0.1-2.2, P = 0.39) (Table 4).
| TC/HDL-C | β | 95%CI | P value |
| < 4.9 | 5.4 | (2.3-12.6) | < 0.01 |
| ≥ 4.9 | 0.5 | (0.1-2.2) | 0.39 |
In epidemiologic studies, the highest incidences of NAFLD were reported in the Middle East (32%) and South America (31%), followed by Asia (27%) and the United States (24%)[18]. However, in a study involving 810 northern Japanese children, the prevalence of fatty liver was observed to be only 2.6% based on ultrasonographic criteria[19], but the prevalence of NAFLD was increased to 77% among obese children[20]. In our previous study, we found that the incidence rate of NAFLD in the general population was 35.92%[21]. The above studies only focused on the prevalence of NAFLD in the general population. In this present study, the overall prevalence of NAFLD in the investigated CHB population was 17.49%. Consistent with our findings, previous studies indicated that the prevalence of NAFLD with and without HBsAg positivity was 14.3% and 28.6%, respectively (P < 0.01)[22]. A prior study observed a low incidence of NAFLD in their investigated CHB population and hypothesized that such could be mainly because HBV infection influences the secretion of a variety of adipokines and alterations in lipid profiles[23].
Substantial evidence indicated an association between HBV infection and reduced incidence of hyperlipidemia or NAFLD risk[9,10]. In a large cross-sectional study, the researchers observed that HBsAg-positive subjects had a significantly lower risk of NAFLD (OR = 0.42)[22]. Adiponectin may be central to this observed association. Adipokine may attenuate hepatic steatosis and the degree of its decline was shown to correlate with the severity of NAFLD[24]. Moreover, adiponectin levels were also shown to be positively correlated with HBV-DNA viral load in CHB patients[24,25].
However, the cross-talk between CHB and NAFLD remained controversial. There are studies indicating that NAFLD was inversely associated with the levels of HBV seromarkers[7,8]. In this present study, the proportion of HBV-DNA positivity in the NAFLD group (n = 15, 46.9%) was significantly lower than that in the non-NAFLD group (n = 116, 76.8%). Further, a large cohort study demonstrated that HBsAg clearance was significantly higher in CHB patients with hepatic steatosis than in those without[26], and these results were in agreement with animal experiments[27,28].
In regards to treatment, long-term oral use of NAs drugs such as entecavir and tenofovir were the main anti-HBV treatment as they are simple and safe to use, which is recognized all over the world[1]. Thus, oral NAs therapy alone was the first option for patients with CHB. However, CHB patients with NAFLD needed additional treatment besides antiviral drugs. Lifestyle intervention was a basic method for losing weight. For severe cases, pharmacological treatment was required to regulate the patients’ lipid metabolism disorders[29].
Metabolic alterations in NAFLD may directly or indirectly affect the HBV-DNA levels of CHB patients[30]. Due to the common immune pathways of NAFLD and CHB, NAFLD-related metabolic stress may activate the suppressed innate immunity to restore the production of antiviral substances, which ultimately accelerates the clearance of HBV-DNA and HBsAg[31,32].
Metabolic syndrome is a highly prevalent concern in patients with NAFLD[17,33]. The typical characteristics of NAFLD are abnormal lipid accumulation in hepatocytes, hypertriglyceridemia, increased LDL-C levels, and reduced HDL-C particles. Metabolic perturbations promote liver injury and inflammation, which can lead to increased risk for hepatic fibrosis[34]. A cohort study of Chinese people with normal lipid metabolism indicated that a low-density lipoprotein to high-density lipoprotein (LDL/HDL) ratio was superior to other lipoproteins in identifying people at risk of NAFLD[35]. Studies from the Framingham Cardiovascular Institute also showed that a ratio of TC/HDL-C greater than 4 was a major risk factor for cardiovascular thrombosis[36]. In this present study, our results showed that TG, TC, LDL-C, ApoB, and TC/HDL-C had a significant increment in CHB patients combined with NAFLD. Concordant with the results of previous studies, we observed that although the levels of HDL-C and ApoA1 were decreased, no significant statistical difference was observed[12].
In our study, TC/HDL-C was a positive risk factor for NAFLD (P < 0.01) in univariate analysis. Previous studies suggested that there was a linear relationship between TC/HDL-C and NAFLD in the general population[14]. However, in this study, curve fitting analysis model showed that the association between TC/HDL-C and NAFLD was non-linear in the CHB population for an inflection point of 4.9. Thus, we speculated that TC/HDL-C was positively associated with NAFLD when the ratio of TC/HDL-C was less than 4.9 in the CHB population.
There were some limitations observed in this study. First, the investigated population was relatively small and therefore, large-scale studies are needed to validate our findings. Second, the assessment of NAFLD was based on hepatic ultrasonography rather than liver biopsy, which was the traditional gold standard for the assessment of NAFLD[37]. Patients could be reluctant to undergo liver biopsy because of its high cost, invasiveness and risk of complications[38]. Furthermore, fibrosis indices such as hyaluronic acid, laminin, procollagen III peptide, collagen type IV, and transient elastography were not included in the analyses due to missing data on fibrosis indices and could have been conducive to evaluating the relationship between NAFLD and different stages of CHB. In future studies, we will assess the relationship between fibrosis indices and TC/HDL-C. Lastly, this cross-sectional study only explored the relationship between the TC/HDL-C and NAFLD and was unable to reveal the causal and effect relationship between them.
In conclusion, the study demonstrated that the relationship between TC/HDL-C and NAFLD was non-linear in the CHB population. TC/HDL-C was positively correlated with NAFLD when TC/HDL-C was less than 4.9, but no such trend could be observed when the ratio of TC/HDL-C was more than 4.9.
Due to the growing prevalence of nonalcoholic fatty liver disease (NAFLD), the coexistence of hepatitis B virus (HBV) infection and NAFLD is commonly observed around the world. However, the cross-talk between these two diseases remained questionable.
Previous studies showed that the total cholesterol to high-density lipoprotein cholesterol ratio (TC/HDL-C) was a better predictor of NAFLD than other lipid metabolism biomarkers and might be a new indicator of NAFLD. However, the association between TC/HDL-C and NAFLD in an HBV-infected population has not been previously investigated.
To investigate the association between TC/HDL-C and NAFLD in a CHB population.
Univariate and multivariate logistic regression models, curve fitting analysis and threshold calculations were used to assess the relationship between TC/HDL-C and NAFLD.
A non-linear association was detected between TC/HDL-C and NAFLD in the CHB population at an inflection point of 4.9. The effect size on the left and right sides of inflection point were 5.4 (95%CI: 2.3-12.6, P < 0.01) and 0.5 (95%CI: 0.1-2.2, P = 0.39), respectively.
In the CHB population, the relationship between TC/HDL-C and NAFLD was non-linear. TC/HDL-C was positively correlated with NAFLD when TC/HDL-C was less than 4.9.
Further large-scale cohort studies are needed to validate whether TC/HDL-C is indeed a better predictor of NAFLD than other lipid metabolism biomarkers in the CHB population.
The authors appreciate all participants for their contribution to this study and the laboratory staff of the Guangdong Provincial Hospital of Integrated Traditional Chinese and Western Medicine.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kvit K, Ukraine Tziomalos K, Greece S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 368] [Article Influence: 52.6] [Reference Citation Analysis (2)] |
| 2. | Hutin Y, Nasrullah M, Easterbrook P, Nguimfack BD, Burrone E, Averhoff F, Bulterys M. Access to Treatment for Hepatitis B Virus Infection - Worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:773-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 3. | Yuen MF, Seto WK, Chow DH, Tsui K, Wong DK, Ngai VW, Wong BC, Fung J, Yuen JC, Lai CL. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antivir Ther. 2007;12:1295-1303. [PubMed] |
| 4. | Spradling PR, Bulkow L, Teshale EH, Negus S, Homan C, Simons B, McMahon BJ. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis B virus infection. J Hepatol. 2014;61:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Cai J, Zhang XJ, Li H. Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev. 2019;39:328-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 6. | Ding C, Fu X, Zhou Y, Liu X, Wu J, Huang C, Deng M, Li Y, Li L, Yang S. Disease burden of liver cancer in China from 1997 to 2016: an observational study based on the Global Burden of Diseases. BMJ Open. 2019;9:e025613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Kim GA, Lim YS, An J, Lee D, Shim JH, Kim KM, Lee HC, Chung YH, Lee YS, Suh DJ. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63:1325-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 329] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 8. | Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond). 2007;31:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Chen JY, Wang JH, Lin CY, Chen PF, Tseng PL, Chen CH, Chang KC, Tsai LS, Chen SC, Lu SN. Lower prevalence of hypercholesterolemia and hyperglyceridemia found in subjects with seropositivity for both hepatitis B and C strains independently. J Gastroenterol Hepatol. 2010;25:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Huang CY, Lu CW, Liu YL, Chiang CH, Lee LT, Huang KC. Relationship between chronic hepatitis B and metabolic syndrome: A structural equation modeling approach. Obesity (Silver Spring). 2016;24:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, She ZG, Zhu L, Cai J, Li H. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71:1851-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 449] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 12. | Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metab. 2020;42:101092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 322] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 13. | Chatrath H, Vuppalanchi R, Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin Liver Dis. 2012;32:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 14. | Ren XY, Shi D, Ding J, Cheng ZY, Li HY, Li JS, Pu HQ, Yang AM, He CL, Zhang JP, Ma YB, Zhang YW, Zheng TZ, Bai YN, Cheng N. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. 2019;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Castera L, Friedrich-Rust M, Loomba R. Noninvasive Assessment of Liver Disease in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1264-1281.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 1035] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 16. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (2)] |
| 17. | Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 892] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 18. | Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4054] [Cited by in RCA: 3785] [Article Influence: 540.7] [Reference Citation Analysis (2)] |
| 19. | Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 274] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, Chan IH, Yin J, Lam CW, Fok TF, Nelson EA. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Xie W, Chen S. A nomogram for estimating the probability of nonalcoholic fatty liver disease in a Chinese population: A retrospective cohort study. Medicine (Baltimore). 2020;99:e23049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Wong VW, Wong GL, Chu WC, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Chan HY, Woo J, Chan FK, Chan HL. Hepatitis B virus infection and fatty liver in the general population. J Hepatol. 2012;56:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Wong VW, Wong GL, Yu J, Choi PC, Chan AW, Chan HY, Chu ES, Cheng AS, Chim AM, Chan FK, Sung JJ, Chan HL. Interaction of adipokines and hepatitis B virus on histological liver injury in the Chinese. Am J Gastroenterol. 2010;105:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Chiang CH, Lai JS, Hung SH, Lee LT, Sheu JC, Huang KC. Serum adiponectin levels are associated with hepatitis B viral load in overweight to obese hepatitis B virus carriers. Obesity (Silver Spring). 2013;21:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Hsu CS, Liu WL, Chao YC, Lin HH, Tseng TC, Wang CC, Chen DS, Kao JH. Adipocytokines and liver fibrosis stages in patients with chronic hepatitis B virus infection. Hepatol Int. 2015;9:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology. 2009;49:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 27. | Zhang Z, Pan Q, Duan XY, Liu Q, Mo GY, Rao GR, Fan JG. Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model. J Gastroenterol Hepatol. 2012;27:1858-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Hu D, Wang H, Wang Y, Wan X, Yan W, Luo X, Ning Q. Non-alcoholic hepatic steatosis attenuates hepatitis B virus replication in an HBV-immunocompetent mouse model. Hepatol Int. 2018;12:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 29. | Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 800] [Article Influence: 100.0] [Reference Citation Analysis (2)] |
| 30. | Zhang J, Lin S, Jiang D, Li M, Chen Y, Li J, Fan J. Chronic hepatitis B and non-alcoholic fatty liver disease: Conspirators or competitors? Liver Int. 2020;40:496-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 389] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 32. | Zhang RN, Pan Q, Zhang Z, Cao HX, Shen F, Fan JG. Saturated Fatty Acid inhibits viral replication in chronic hepatitis B virus infection with nonalcoholic Fatty liver disease by toll-like receptor 4-mediated innate immune response. Hepat Mon. 2015;15:e27909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 34. | Nascimbeni F, Ballestri S, Machado MV, Mantovani A, Cortez-Pinto H, Targher G, Lonardo A. Clinical relevance of liver histopathology and different histological classifications of NASH in adults. Expert Rev Gastroenterol Hepatol. 2018;12:351-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Zou Y, Zhong L, Hu C, Zhong M, Peng N, Sheng G. LDL/HDL cholesterol ratio is associated with new-onset NAFLD in Chinese non-obese people with normal lipids: a 5-year longitudinal cohort study. Lipids Health Dis. 2021;20:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 36. | Castelli WP. Cardiovascular disease: pathogenesis, epidemiology, and risk among users of oral contraceptives who smoke. Am J Obstet Gynecol. 1999;180:S349-S356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V; LIDO Study Group. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 38. | Ballestri S, Nascimbeni F, Lugari S, Lonardo A, Francica G. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert Rev Gastroenterol Hepatol. 2019;13:667-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |