Published online May 27, 2020. doi: 10.4254/wjh.v12.i5.220
Peer-review started: January 1, 2020
First decision: February 19, 2020
Revised: March 30, 2020
Accepted: April 7, 2020
Article in press: April 7, 2020
Published online: May 27, 2020
Processing time: 147 Days and 9.6 Hours
Early diagnosis is critical for successful intervention before liver disease progresses to cirrhosis and hepatocellular carcinoma.
To examine a novel biomarker for probing early liver disease quickly using an automated immunology system.
This was a cross-sectional study. 140 patients at various stages of liver disease were randomly selected. The cohort consisted of patients who were treatment naïve and currently undergoing therapy. We included patients with diverse liver disease etiologies. Mac-2 binding protein glycosylation isomer (M2BPGi) levels in addition to different clinical parameters, co-morbidities and transient elastography results were collected and compared.
M2BPGi levels were significantly correlated with transient elastography for liver fibrosis staging across all disease etiologies. Statistically significant differences were observed in patients with F0-1; F2 and > F3 liver fibrosis. Further examination showed that M2BPGi levels were two-fold higher in F4 than F3 hepatitis C (HCV) patients. M2BPGi was observed to be etiology-specific and HCV patients had higher mean M2BPGi levels. We also observed significant correlations with aspartate aminotransferase to platelet ratio index and fibrosis-4 index as well as HBV DNA levels. Mean M2BPGi levels for HBV patients with a viral load lower than 2000 IU/mL was 1.75-fold lower than those with a viral load greater than 2000 IU/mL.
M2BPGi was observed to be a good indicator of early liver disease in patients with different etiologies. Our results provide reference cut-offs for different causes of liver disease and demonstrated the utility of this marker for early disease monitoring. This is useful for remote regions in developing countries.
Core tip: Mac-2 binding protein glycosylation isomer levels can be used for non-invasive liver fibrosis staging in the Vietnamese population with mixed etiologies. In early evaluations, significantly higher levels of this marker were observed in cirrhotic patients and showed good correlations with viral load testing in hepatitis B. This marker is convenient and useful, especially in resource limited countries for fast turnaround to assess liver disease.
- Citation: Pham TTT, Ho DT, Nguyen T. Usefulness of Mac-2 binding protein glycosylation isomer in non-invasive probing liver disease in the Vietnamese population. World J Hepatol 2020; 12(5): 220-229
- URL: https://www.wjgnet.com/1948-5182/full/v12/i5/220.htm
- DOI: https://dx.doi.org/10.4254/wjh.v12.i5.220
Chronic liver disease resulted in a high global burden of 1.5 billion people in 2017, which was mostly due to non-alcoholic fatty liver disease (NAFLD, 60%), hepatitis B (HBV, 29%), hepatitis C (HCV, 9%), and alcoholic liver disease (ALD, 2%)[1]. Chronic liver disease progressively leads to liver fibrosis, cirrhosis, and finally hepatocellular carcinoma (HCC). Globally, liver disease accounts for 2 million deaths per year, of which 1 million are due to cirrhosis and 1 million are due to hepatocellular carcinoma[2]. Vietnam has a high prevalence of hepatitis B and C, with a high HBV surface antigen (HBsAg) rate of 8.8%-19.0% and a high anti-HCV rate of 1.0%-3.3%[3]. It is estimated that HBV-related liver cancer and HCC will increase to 9400 and 25000, respectively, and HBV-related mortality will increase to 40000 in 2025 in Vietnam[4]. Given the disease impact in Vietnam, efforts to provide early detection of liver disease are critical.
Early diagnosis and treatment are key to improve disease outcomes as it can halt further liver disease progression and save lives. At present, pan-genotypic drug combinations of direct acting antivirals such as sofosbuvir/velpatasvir and glecaprevir/pibrentasvir have demonstrated high efficacy against different HCV genotypes[5]. Long-term HBV antivirals have been effective in managing the disease[6]. Nonetheless, most patients are in a chronic state and different degrees of liver fibrosis have manifested. Management of these patients requires clear understanding of their liver fibrosis staging[7,8]. At present, liver biopsy is considered the gold standard for liver fibrosis staging. However, liver biopsy has its limitations such as sampling bias and inter-observer variations[9]. In addition, liver biopsy is invasive and it is not feasible to carry out repeated liver biopsies for regular monitoring. In advanced stage liver disease patients (> F3), a 3-6 monthly evaluation is required. In response to these limitations, non-invasive tests using imaging methods such as transient elastography (TE)[10], magnetic resonance elastography[11], ultrasound-based elastography[12], and acoustic radiation force impulse[13] have emerged. Serum biomarkers have also been explored. These included hyaluronic acid, type IV collagen, type III procollagen-N-peptide[14], soluble Axl[15], and lincRNA-p21[16] among others. Surrogate markers (aspartate aminotransferase to platelet ratio index (APRI)[17], and Fibrosis-4 index (FIB-4)[18] are also some of the commonly used tests to assess liver disease. A number of these tests require costly equipment, and patient results turnaround and waiting times may limit their usefulness. On the other hand, other tests lack sufficient clinical sensitivity and specificity.
In recent years, Mac-2 binding protein glycosylation isomer (M2BPGi) was identified in patients with liver fibrosis[19]. Serum M2BPGi levels were found to correlate with liver fibrosis stage[20], reflect impaired liver function or cirrhosis and hepatectomy-related complications after surgery[21], correlate with liver stiffness[22], and was negatively correlated with sustained virological response (SVR)[23]. In addition to liver fibrosis in HCV and HBV, M2BPGi can be used to assess liver fibrosis in primary biliary cirrhosis[24], biliary atresia[25], primary sclerosing cholangitis[26], and NAFLD[27]. In this study, we examined M2BPGi as a biomarker for early monitoring of liver disease in a Vietnamese population. We compared M2BPGi levels with different clinical parameters, co-morbidities and TE results. M2BPGi was measured from blood samples using a sandwich immunoassay. The aim of the current study was to examine this novel diagnostic biomarker for probing early liver disease quickly and easily using an automated immunology system.
A cross-sectional study design was employed in this study. Institutional review board approval was waived as residual blood samples were used in the evaluation. The results of this study did not alter the course of treatment in these patients. Other than patient history and current laboratory results, no other patient identifiers were used. We randomly selected patients with diverse liver disease etiologies who were treated in MEDIC Medical Center, Ho Chi Minh City, Vietnam from January to April 2019. Patients with confirmed liver cancer were excluded. Routine clinical investigations as part of standard of care included liver function tests, TE and other associated fibrosis measurements. A total of 140 patient samples were analyzed.
As part of standard of care, TE (Fibroscan, Echosens, France) was performed on all patients. Staging of liver fibrosis in patients was based on the METAVIR scoring system (F0 to F4, where F0: No fibrosis and F4: Cirrhosis) and cut-offs adopted from manufacturer’s recommendations. Plasma and serum blood samples were taken from each patient for complete blood count and clinical biochemistry investigations. Liver function tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST) alkaline phosphatase (ALP), albumin and bilirubin were measured using a routine laboratory chemistry analyzer (Roche, Switzerland). APRI was calculated using the following equation: AST (/upper limit of normal) × 100/platelet count (PLT) (× 109/L)[17]. FIB-4 was calculated using the following equation: FIB-4 = age (years) × AST (U/L)/[PLT (109/L) × ALT (U/L)1/2][18]. PLT was obtained using a hematology analyzer among other blood parameters (Sysmex, Japan). Serum M2BPGi levels were measured on the HISCL 5000 automated immunoassay analyzer (Sysmex, Japan). 10 μL of sample were used and M2BPGi levels were measured by a sandwich immunoassay. Each reaction took 17 min and was performed automatically alongside HBsAg and anti-HCV assays. M2BPGi was expressed as the cut-off index (COI) and calibrated using the manufacturer’s calibrators.
All categorical variables were expressed as mean ± SD. Student t tests were used to compare two categorical variables and one-way analysis of variance (ANOVA) was used to compare multiple groups of liver fibrosis stages. A difference of P < 0.05 was considered statistically significant. Analysis was computed using Prism version 7.0 (Graphpad Inc., United States).
Patient demographics are shown in Table 1. A total of 140 patients were enrolled. This was a cross-sectional study involving randomly selected patients with diverse liver disease etiologies to assess the versatility of the marker M2BPGi. All patients were treated in the MEDIC Medical Center and assessed by their attending physicians for liver disease stage. The median age of the study cohort was 52 years and all suffered from chronic hepatitis. Among the patient group, the number of male and female patients was almost equal (52.9% vs 47.1%, respectively). Most of the selected patients were HBV or HCV patients, of which around 60% were HBV patients, and 2 were patients co-infected with HBV and HCV. The HBV and HCV cohorts, 35% and 13%, respectively, had immediate family members with the same condition or had progressed to HCC. In Vietnam, HBV is the predominant cause of HCC and was critical in this study. The range of TE, APRI, FIB-4 and M2BPGi measurements were 3–46.4 kPa, 0.07–7.02, 0.23–9.97 and 0.07- > 20 COI, respectively. Among the 140 patients, 35% of patients had higher than normal AST, ALT or Gamma glutamyl transferase. Alpha fetoprotein (AFP) measurements were obtained in these patients as part of an early indication of HCC. Only 1 patient (112 ng/mL) in this cohort had higher than normal AFP level.
| Parameters | Parameter value | |
| Sample size (n) | 140 | |
| Age (yr) | Range (mean ± SD) | 26-81 (52 ± 16) |
| Gender, n (%) | Male | 74 (52.9%) |
| Female | 66 (47.1%) | |
| Etiology, n (%) | HBV | 54 |
| HBV + NAFLD | 31 | |
| HCV | 37 | |
| HCV + NAFLD | 13 | |
| HCV + HBV | 2 | |
| NAFLD | 1 | |
| Alcoholic | 2 | |
| M2BPGi (COI) | Minimum | 0.065 |
| Median | 0.863 | |
| Maximum | > 20 | |
| TE (kPa) | Minimum | 3 |
| Median | 6.4 | |
| Maximum | 46.4 | |
| APRI | Minimum | 0.0737 |
| Median | 0.2997 | |
| Maximum | 7.0192 | |
| FIB-4 | Minimum | 0.2328 |
| Median | 1.119 | |
| Maximum | 9.9664 | |
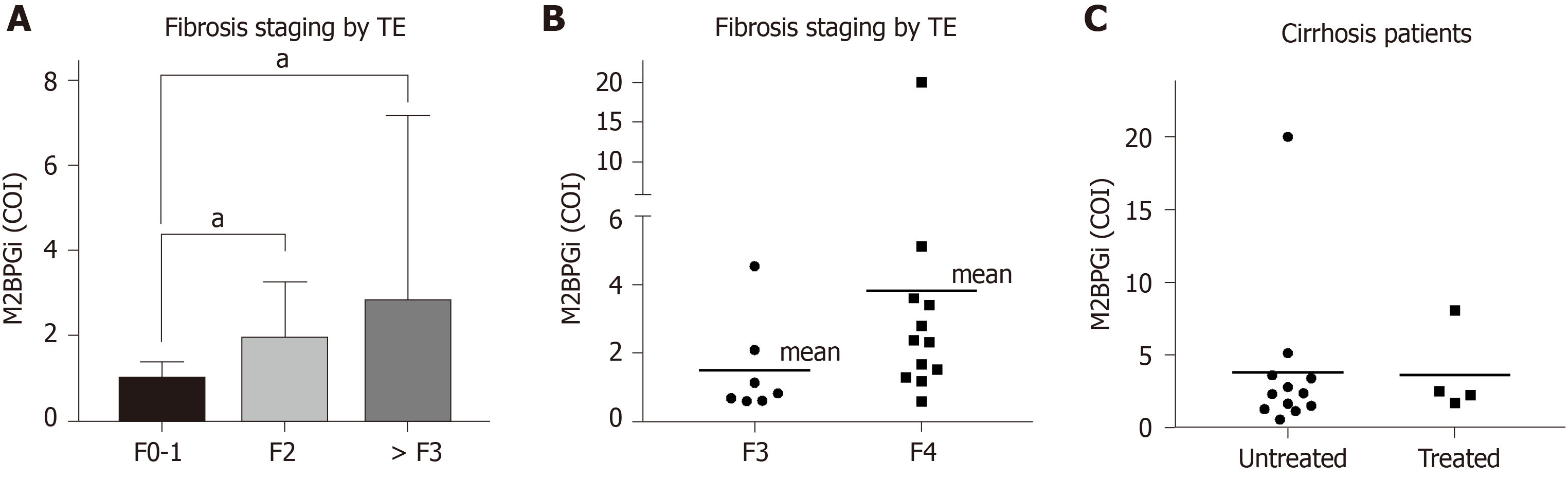
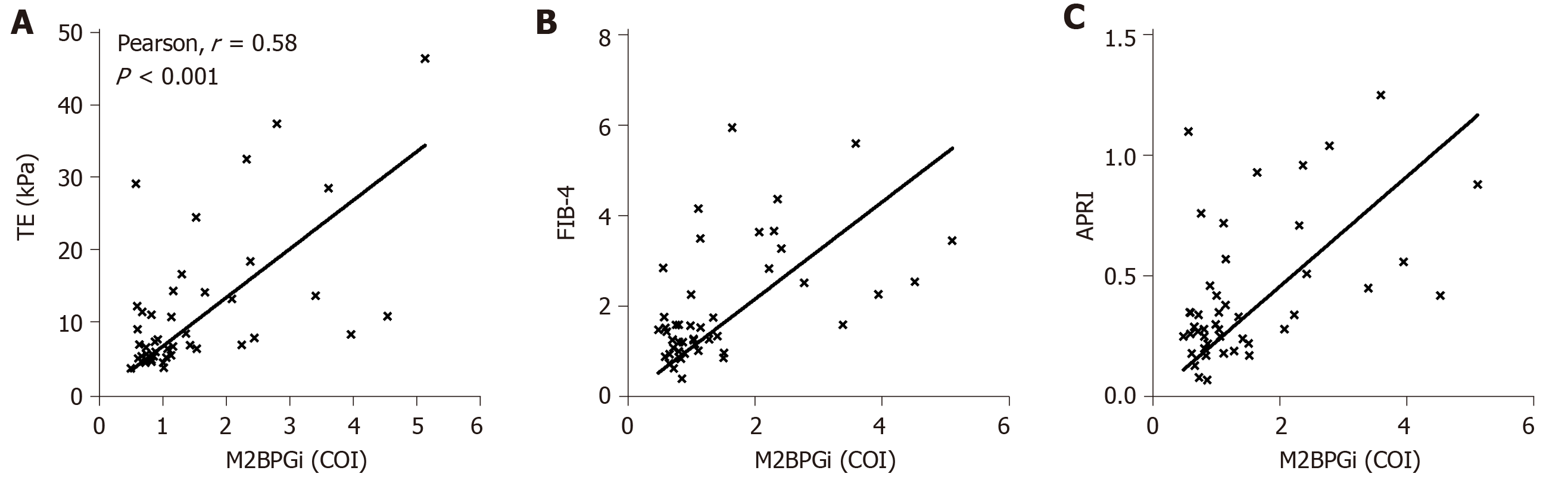
As shown in Figure 1A, M2BPGi levels increased with increasing fibrosis stages (as measured by TE) in HCV patients. The results were statistically significant when comparing the F2 cohort with F0-1 subjects. Patients with significant fibrosis require regular monitoring and this provides a quick method to stratify patient groups for clinicians. We also observed significantly more patients with severe fibrosis or cirrhosis than patients with normal liver in HCV positive individuals. In an attempt to further evaluate the discriminatory role of M2BPGi, we divided patients with > F3 fibrosis into the F3 and F4 groups (Figure 1B). The results showed a statistically significant difference in M2BPGi levels between F3 and F4 patients. The cirrhotic group (F4) had two-fold higher M2BPGi levels. These data supported the accurate staging of liver fibrosis using M2BPGi levels. More data are required to ensure statistical power for medical decisions; however, preliminary data demonstrated strong evidence of a clear discriminatory role. Within this cirrhotic patient group, some patients were currently undergoing treatment and some were treatment naïve. M2BPGi levels were observed to be insignificant between these two groups (Figure 1C). Previous reports have shown that M2BPGi may be affected by the treatment for viral hepatitis. In a comparison of fibrosis staging methods, we selected only treatment-naïve patients (Figure 2) to ensure an unbiased correlation. As shown in Figure 2, we found that M2BPGi levels showed good correlation with TE (A), FIB-4 (B), and APRI (C) in untreated patients (P < 0.001).
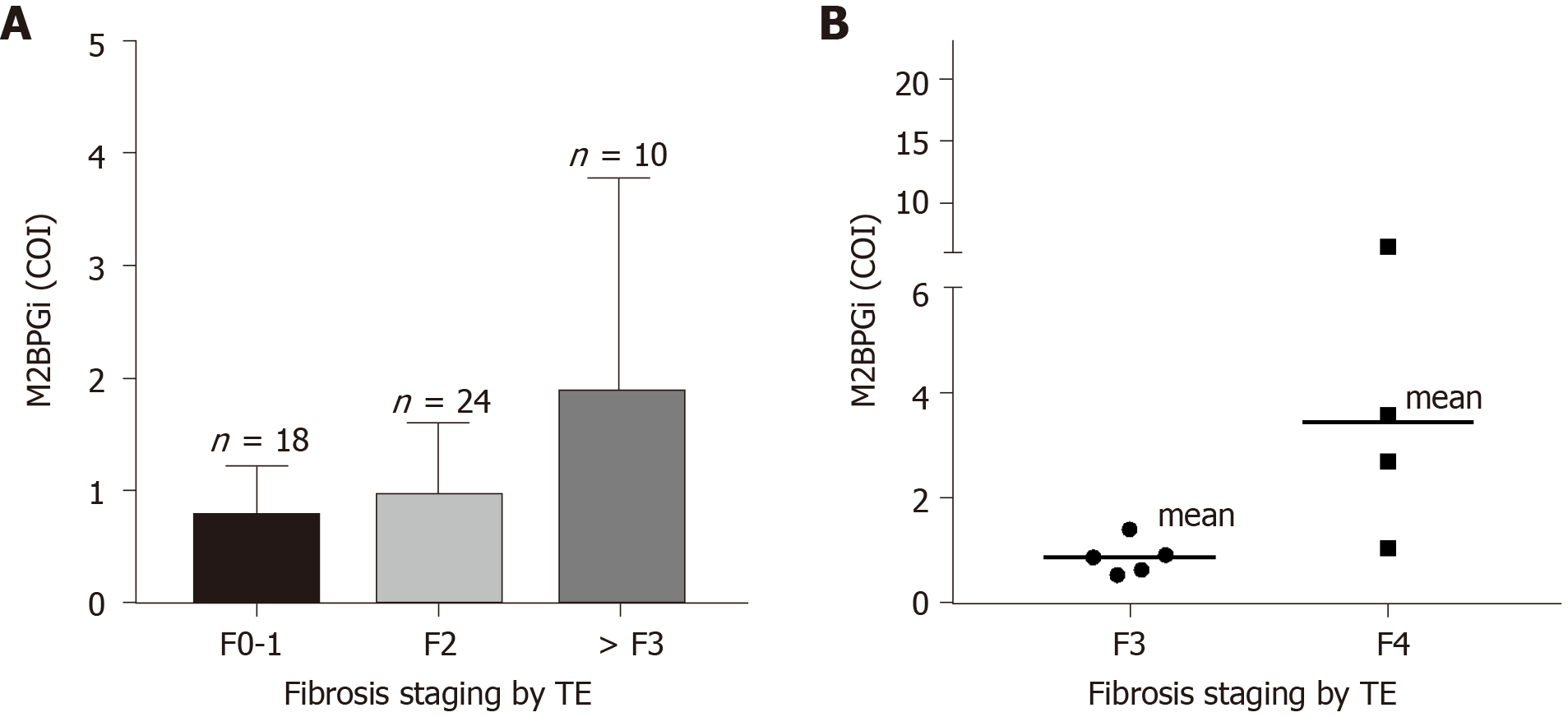
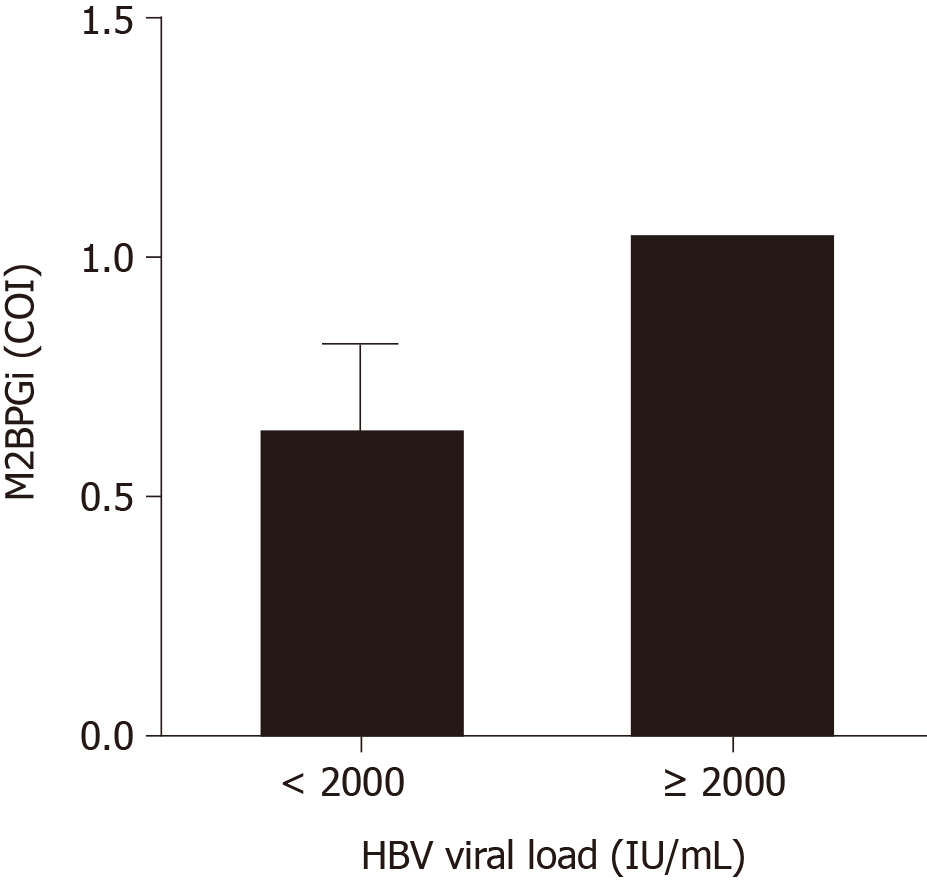
Similar to the results in the HCV group, we observed significant correlations in HBV patients. The limitation of the current study was that the distribution among this cohort leaned towards the early liver disease group with fewer severe fibrosis and cirrhosis patients. Nonetheless, from Figure 3A, it can be seen that M2BPGi levels increased with increasing fibrosis stage. The maximum detected M2BPGi level in patients with > F3 fibrosis staging was 4.0 COI with a mean value of 2.0 COI. This was more than 2-fold higher than early stage patients (F0-1) with a mean M2BPGi level of -0.8 COI. Compared with a similar early disease group in HCV patients, the mean levels were significantly lower in the HBV cohort indicating the need for etiology-specific cut-offs using M2BPGi. Further division of > F3 patients into F3 and F4 groups showed the same trend as previously seen in the HCV cohort (Figure 3B). More datapoints are required for better statistical comparison in advanced stage patients. In the same cohort, we attempted to understand if M2BPGi correlated with HBV DNA levels. HBV viral load is a required parameter during treatment monitoring especially in addressing treatment discontinuation and efficacy. From Figure 4, it can be seen that the mean M2BPGi level for hepatitis B patients with a viral load of < 2000 IU/mL was 1.75-fold lower than the group with a viral load greater or equal to 2000 IU/mL.
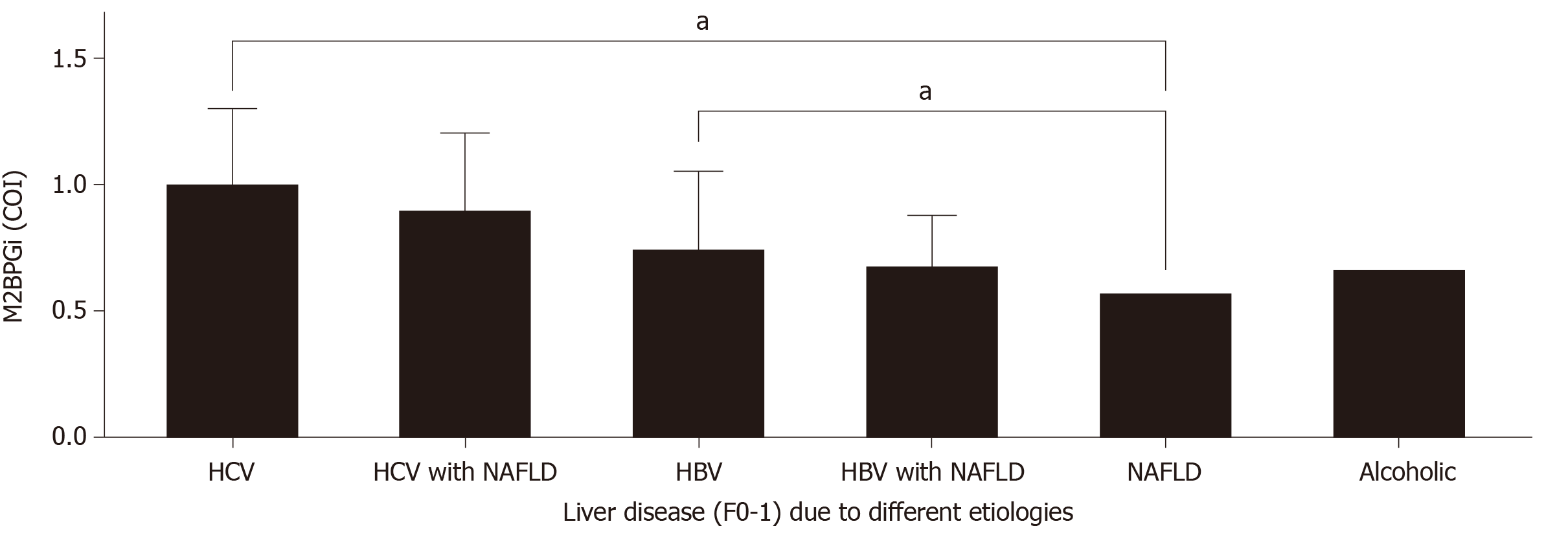
During early analysis, it was observed that M2BPGi levels were significantly different among the HBV and HCV cohorts within the same fibrosis stages. To address these differences further, the patient cohort was divided into HCV, HCV with NAFLD, HBV, HBV with NAFLD, NAFLD, and ALD as shown in Figure 5 for early disease patients (F0-1). ANOVA showed significant differences among these major groups. Viral hepatitis related liver disease alone resulted in higher M2BPGi levels compared with alcoholic liver disease (P < 0.01) and NAFLD (P < 0.01). Interestingly we did not observe any statistical significance in viral hepatitis patients with NAFLD comorbidity (HCV vs HCV and NAFLD; HBV vs HBV and NAFLD). These findings show that M2BPGi levels may be dominated by the presence of viral hepatitis in patients. Overall, we found that in early disease patients, M2BPGi levels were highest in HCV groups, followed by HBV and subsequently alcoholic liver disease and finally NAFLD cases.
Non-invasive tests in probing liver disease are gaining attention as liver biopsy is insufficient in addressing critical disease management roles. These methods are also more patient friendly and allow repeated sampling. However, equipment investment is not easy for medical facilities in remote regions of Vietnam in relation to advanced elastography methods. Hence, it is crucial to have a quick and straightforward method to detect and stratify liver disease. Therefore, serum biomarkers derived from automated analyzers which are easy to interpret without skilled operators are desirable.
The results of the present study showed that M2BPGi levels were correlated with fibrosis stage derived from TE measurements and positively correlated with other surrogate markers (FIB-4 and APRI). This concurs with other studies[28,29]. However, no correlation was found between M2BPGi levels in treatment naïve and treated cirrhotic HCV patients. Treatment failure is typically associated with cirrhotic cases and the current results may be representative of this phenomenon. Ura et al[30] found that SVR is negatively correlated with M2BPGi levels, highlighting that treatment response may be reflected in serial M2BPGi measurements. Cirrhotic patients remain a key target group for treatment and surveillance, of which M2BPGi may be useful. One limitation of our study is the small sample size of cirrhotic patients (n = 16), which may be insufficient to demonstrate the effects of anti-viral treatment on M2BPGi levels in cirrhotic patients. Future studies will examine the effects of cirrhosis regression on M2BPGi levels in hepatitis patients, and patients can be further stratified based on time of onset of cirrhosis[31], which affects regression.
In this study other than METAVIR-based staging, we found that HBV viral load was correlated with M2BPGi levels, showing that M2BPGi may be used as a surrogate marker for HBV viral load and disease severity. In fact, some studies have shown that M2BPGi level is a predictor of HCC and death[32], HBV-related HCC recurrence[33], and liver function and prognosis[34]. Hence, M2BPGi may complement HBV viral load testing to better understand disease severity and prediction of disease outcomes. Several studies have shown that other than liver fibrosis staging, M2BPGi level is a predictor of HCC after SVR[35], HCC survival[36], HCC recurrence[37], and cirrhosis survival[38].
M2BPGi is etiology-specific and our results showed that M2BPGi levels are higher in HCV than HBV patients, which is consistent with Nishikawa et al[39] and patients with viral hepatitis have higher M2BPGi levels than those with NAFLD and ALD. Therefore, different cut-off values should be assigned to different types of patients. The mechanism of this difference in M2BPGi level between HBV and HCV patients remains unclear, but our observational study showed that M2BPGi is dominated by the presence of viral hepatitis. With larger sample sizes in future investigations, we will be able to establish a clear disease-based cut-off to implement M2BPGi in routine clinical use.
The present study demonstrated the feasibility of using M2BPGi level as a surrogate marker for liver fibrosis in a Vietnamese population. This method is convenient, easy to use, and can guide prevention and treatment efforts for viral hepatitis in remote regions. At present, there is no national program against HBV and HCV in Vietnam[3] and mortality due to liver cancer is high in Vietnam[40]. In contrast, M2BPGi tests are reimbursable in South Korea and Japan. In future, national programs for treating HCV and HBV could be rolled out along with such tests to reduce the prevalence of liver fibrosis, cirrhosis, and HCC in Vietnam and thereby reduce mortality.
In conclusion, this study is the first to investigate mixed etiology testing of this marker and demonstrated a significant correlation with existing routine assays and treatment monitoring. Our results provide reference cut-offs for different causes of liver disease and demonstrated the utility of this marker for early disease monitoring. This is useful for remote regions in developing countries.
Non-invasive and rapid testing of liver disease for chronic hepatitis patients is crucial given its high prevalence in Vietnam.
Liver disease can be managed properly with timely treatment and control. Mac-2 binding protein glycosylation isomer (M2BPGi) offers the capability to stage fibrosis severity quickly and assess treatment response with longitudinal measurements.
This study aims to compare M2BPGi, a blood-based biomarker with existing methods of non-invasive testing and elastography in chronic hepatitis. In a preliminary assessment of treatment response, hepatitis B DNA concentrations were correlated with M2BPGi levels in respective patients.
In patients with liver disease of different etiologies, M2BPGi levels in residual blood samples were measured. Comparisons with transient elastography (TE) were made to establish preliminary clinical cut-offs. Pearson correlations were tested using different liver disease markers to establish any significant trends. M2BPGi levels in early disease patients were compared to show etiology specificity.
We established clear correlations between M2BPGi with TE and other non-invasive biomarkers. For fibrosis staging of both hepatitis B and C patients, we observed statistically significant correlations with M2BPGi. M2BPGi levels in early disease were higher in viral hepatitis patients indicating the need to establish different cut-offs. The results were also significantly correlated with hepatitis B viral load, which established the possibility of treatment assessment.
M2BPGi level is a useful addition to the current routine assessment of chronic hepatitis patients. This is performed by a routine immunoassay that enables fast turnaround time and quicker reporting for patients.
We envision this research to have clinical potential to improve treatment monitoring procedures and rapid assessment of liver disease. In a resource limited setting, this marker presents useful results to ensure patients are linked to care promptly.
We gratefully acknowledge the reagent support from Sysmex Vietnam.
Manuscript source: Invited conference manuscripts
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Viet Nam
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reshetnyak VI S-Editor: Wang YQ L-Editor: Webster JR E-Editor: Wu YXJ
| 1. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 710] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 2. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 2274] [Article Influence: 379.0] [Reference Citation Analysis (0)] |
| 3. | Huy Do S. Epidemiology of Hepatitis B and C Virus Infections and Liver Cancer in Vietnam. Euroasian J Hepatogastroenterol. 2015;5:49-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Nguyen VT, Law MG, Dore GJ. An enormous hepatitis B virus-related liver disease burden projected in Vietnam by 2025. Liver Int. 2008;28:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Sandmann L, Schulte B, Manns MP, Maasoumy B. Treatment of Chronic Hepatitis C: Efficacy, Side Effects and Complications. Visc Med. 2019;35:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1581] [Article Influence: 175.7] [Reference Citation Analysis (2)] |
| 7. | European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 655] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 8. | Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392:2313-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 367] [Article Influence: 52.4] [Reference Citation Analysis (2)] |
| 9. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1567] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 10. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, Beaugrand M, Palau R. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1933] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 11. | Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, Aryafar H, Sirlin CB, Loomba R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 12. | Şirli R, Sporea I, Popescu A, Dănilă M. Ultrasound-based elastography for the diagnosis of portal hypertension in cirrhotics. World J Gastroenterol. 2015;21:11542-11551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Chen CC, Hsu HT, Chen YL, Chen RC, Wu WP, Chou CT. Diagnostic Accuracy of Acoustic Radiation Force Impulse (ARFI) and Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein (WFA⁺-M2BP) in Patients with Chronic Liver Disease. Med Sci Monit. 2019;25:7169-7174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Tatsumi C, Kudo M, Ueshima K, Kitai S, Takahashi S, Inoue T, Minami Y, Chung H, Maekawa K, Fujimoto K, Akiko T, Takeshi M. Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology. 2008;51 Suppl 1:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Staufer K, Dengler M, Huber H, Marculescu R, Stauber R, Lackner C, Dienes HP, Kivaranovic D, Schachner C, Zeitlinger M, Wulkersdorfer B, Rauch P, Prager G, Trauner M, Mikulits W. The non-invasive serum biomarker soluble Axl accurately detects advanced liver fibrosis and cirrhosis. Cell Death Dis. 2017;8:e3135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Yu F, Zhou G, Huang K, Fan X, Li G, Chen B, Dong P, Zheng J. Serum lincRNA-p21 as a potential biomarker of liver fibrosis in chronic hepatitis B patients. J Viral Hepat. 2017;24:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3235] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 18. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1604] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 19. | Kuno A, Ikehara Y, Tanaka Y, Ito K, Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, Narimatsu H. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci Rep. 2013;3:1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 20. | Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, Gotoh M, Narimatsu H, Korenaga M, Mizokami M, Nishie A, Aishima S, Maehara Y. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 2015;50:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 21. | Hiyoshi M, Yano K, Nanashima A, Ikenoue M, Imamura N, Fujii Y, Hamada T, Nishida T. Usefulness of serum Mac-2 binding protein glycosylation isomer in patients undergoing hepatectomy: A case controlled study. Ann Med Surg (Lond). 2019;48:17-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Mak LY, Wong DK, Seto WK, Ning Q, Cheung KS, Fung J, Lai CL, Yuen MF. Correlation of serum Mac-2-binding protein glycosylation isomer (M2BPGi) and liver stiffness in chronic hepatitis B infection. Hepatol Int. 2019;13:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, Narimatsu H, Mizokami M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. 2018;53:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 137] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Ishii A, Iijima H, Nishiguchi S. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-γ-inducible protein-10 in primary biliary cirrhosis. Hepatol Res. 2016;46:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Ueno T, Kodama T, Noguchi Y, Nomura M, Saka R, Takama Y, Tazuke Y, Bessho K, Okuyama H. Serum Mac-2-binding protein (M2BPGi) as a marker of chronological liver fibrosis in biliary atresia patients with cirrhosis. Pediatr Surg Int. 2019;35:1065-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Umetsu S, Inui A, Sogo T, Komatsu H, Fujisawa T. Usefulness of serum Wisteria floribunda agglutinin-positive Mac-2 binding protein in children with primary sclerosing cholangitis. Hepatol Res. 2018;48:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Kamada Y, Ono M, Hyogo H, Fujii H, Sumida Y, Yamada M, Mori K, Tanaka S, Maekawa T, Ebisutani Y, Yamamoto A, Takamatsu S, Yoneda M, Kawada N, Chayama K, Saibara T, Takehara T, Miyoshi E; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG‐NAFLD). Use of Mac-2 binding protein as a biomarker for nonalcoholic fatty liver disease diagnosis. Hepatol Commun. 2017;1:780-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Fujita K, Kuroda N, Morishita A, Oura K, Tadokoro T, Nomura T, Yoneyama H, Arai T, Himoto T, Watanabe S, Masaki T. Fibrosis Staging Using Direct Serum Biomarkers is Influenced by Hepatitis Activity Grading in Hepatitis C Virus Infection. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Jekarl DW, Choi H, Lee S, Kwon JH, Lee SW, Yu H, Kim M, Kim Y, Sung PS, Yoon SK. Diagnosis of Liver Fibrosis With Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein (WFA-M2BP) Among Chronic Hepatitis B Patients. Ann Lab Med. 2018;38:348-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Ura K, Furusyo N, Ogawa E, Hayashi T, Mukae H, Shimizu M, Toyoda K, Murata M, Hayashi J. Serum WFA(+) -M2BP is a non-invasive liver fibrosis marker that can predict the efficacy of direct-acting anti-viral-based triple therapy for chronic hepatitis C. Aliment Pharmacol Ther. 2016;43:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Hytiroglou P, Theise ND. Regression of human cirrhosis: an update, 18 years after the pioneering article by Wanless et al. Virchows Arch. 2018;473:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 32. | Su TH, Peng CY, Tseng TC, Yang HC, Liu CJ, Liu CH, Chen PJ, Chen DS, Kao JH. Serum Mac-2-Binding Protein Glycosylation Isomer at Virological Remission Predicts Hepatocellular Carcinoma and Death in Chronic Hepatitis B-Related Cirrhosis. J Infect Dis. 2020;221:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Kim HS, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Park YN, Han DH, Kim KS, Choi JS, Choi GH, Kim HS. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein level predicts recurrence of hepatitis B virus-related hepatocellular carcinoma after curative resection. Clin Mol Hepatol. 2020;26:33-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Xu WP, Wang ZR, Zou X, Zhao C, Wang R, Shi PM, Yuan ZL, Yang F, Zeng X, Wang PQ, Sultan S, Zhang Y, Xie WF. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein evaluates liver function and predicts prognosis in liver cirrhosis. J Dig Dis. 2018;19:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Yasui Y, Kurosaki M, Komiyama Y, Takada H, Tamaki N, Watakabe K, Okada M, Wang W, Shimizu T, Kubota Y, Higuchi M, Takaura K, Tsuchiya K, Nakanishi H, Takahashi Y, Itakura J, Enomoto N, Izumi N. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts early occurrence of hepatocellular carcinoma after sustained virologic response by direct-acting antivirals for hepatitis C virus. Hepatol Res. 2018;48:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Inoue T, Tsuzuki Y, Iio E, Shinkai N, Matsunami K, Fujiwara K, Matsuura K, Nojiri S, Tanaka Y. Clinical Evaluation of Hepatocarcinogenesis and Outcome Using a Novel Glycobiomarker Wisteria floribunda Agglutinin-Positive Mac-2 Binding Protein (WFA+-M2BP) in Chronic Hepatitis C with Advanced Fibrosis. Jpn J Infect Dis. 2018;71:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, Murakawa M, Nitta S, Itsui Y, Azuma S, Kakinuma S, Nouchi T, Sakai H, Tomita M, Watanabe M; Ochanomizu Liver Conference Study Group. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 2017;67:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 38. | Hasegawa K, Takata R, Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, Yuri Y, Nakano C, Nishimura T, Yoh K, Aizawa N, Sakai Y, Ikeda N, Takashima T, Iijima H, Nishiguchi S. Impact of Wisteria floribunda Agglutinin-Positive Mac-2-Binding Protein in Patients with Hepatitis C Virus-Related Compensated Liver Cirrhosis. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Nishikawa H, Enomoto H, Iwata Y, Kishino K, Shimono Y, Hasegawa K, Nakano C, Takata R, Nishimura T, Yoh K, Ishii A, Aizawa N, Sakai Y, Ikeda N, Takashima T, Iijima H, Nishiguchi S. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein for patients with chronic hepatitis B and C: a comparative study. J Viral Hepat. 2016;23:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Ngoan le T, Lua NT, Hang LT. Cancer mortality pattern in Viet Nam. Asian Pac J Cancer Prev. 2007;8:535-538. [PubMed] |