Copyright
©The Author(s) 2015.
World J Hepatol. Oct 28, 2015; 7(24): 2535-2542
Published online Oct 28, 2015. doi: 10.4254/wjh.v7.i24.2535
Published online Oct 28, 2015. doi: 10.4254/wjh.v7.i24.2535
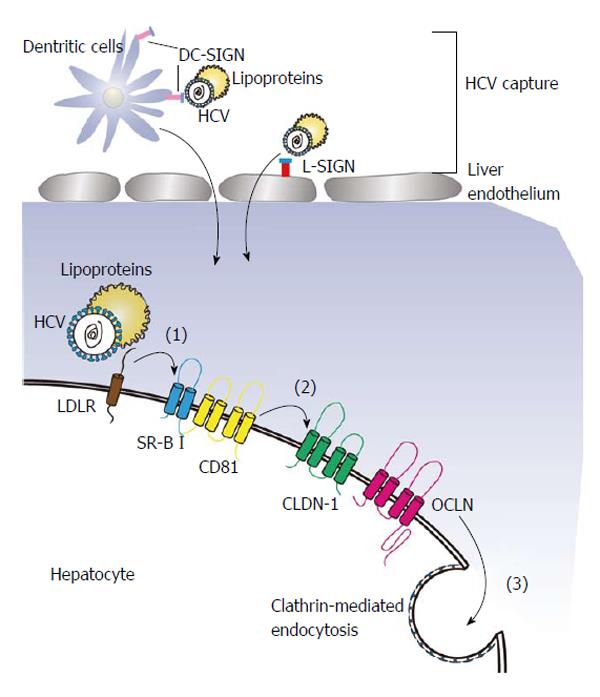
Figure 1 Process of hepatitis C virus cell entry.
After being captured by DC-SIGN and L-SIGN, virions with apolipoprotein may first attach a host cell by interacting with LDLR on the cell surface (1), following by binding to CD81 and SR-BI (2), and finally by a later utilization of the tight junction protein CLDN1 and OCLN (3). HCV: Hepatitis C virus; SR-BI: Scavenger receptor class B type I; LDLR: Low-density lipoprotein receptor; CLDN-1: Claudin-1; OCLN: Occluding.
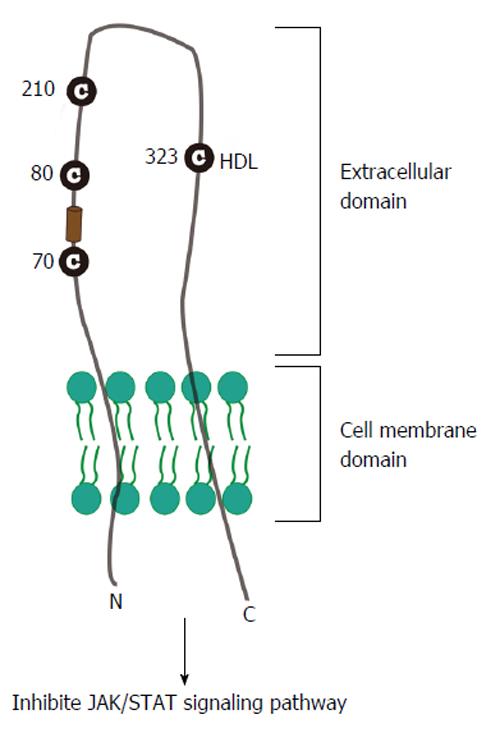
Figure 2 Model of scavenger receptor class B type I topology and its relevance for hepatitis C virus entry.
The SR-BI regions comprise cytoplasmic C-terminal and N-terminal domains separated by a large extracellular domain. Cholesterol uptake and HCV entry is mainly mediated by extracellular domain. Particularly, C323 is critical for SR-BI-mediated cholesterol ester uptake. Amino acids 70-87 and the single residue E210 of SR-BI are required for E2 recognition in HCV entry. SR-BI: Scavenger receptor class B type I; HCV: Hepatitis C virus; HDL: High-density lipoprotein; JAK/STAT: Janus kinase/signal transducer and activator of transcription.
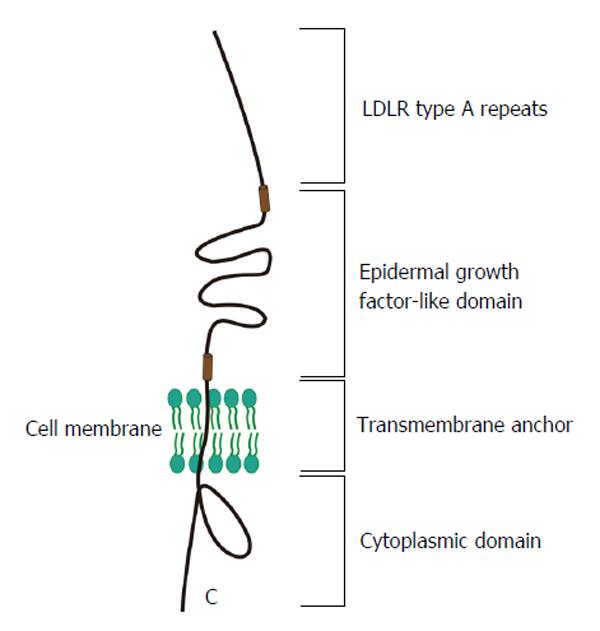
Figure 3 Structure of low-density lipoprotein receptor and its relevance for hepatitis C virus entry.
LDLR is structurally composed by four motifs: LDLR type A repeats, which is the main binding site for ligand; an epidermal growth factor-like domain, which is response to the change of pH to release ligand; a transmembrane anchor and a cytoplasmic domain, which mediates clustering of the receptors into the clathrin-coated pit. LDLR: Low-density lipoprotein receptor.
- Citation: Lyu J, Imachi H, Fukunaga K, Yoshimoto T, Zhang H, Murao K. Roles of lipoprotein receptors in the entry of hepatitis C virus. World J Hepatol 2015; 7(24): 2535-2542
- URL: https://www.wjgnet.com/1948-5182/full/v7/i24/2535.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i24.2535