Published online Apr 26, 2017. doi: 10.4252/wjsc.v9.i4.68
Peer-review started: January 10, 2017
First decision: February 17, 2017
Revised: March 16, 2017
Accepted: April 18, 2017
Article in press: April 19, 2017
Published online: April 26, 2017
Processing time: 107 Days and 11.2 Hours
In spite of modern treatment, acute myocardial infarction (AMI) still carries significant morbidity and mortality worldwide. Even though standard of care therapy improves symptoms and also long-term prognosis of patients with AMI, it does not solve the critical issue, specifically the permanent damage of cardiomyocytes. As a result, a complex process occurs, namely cardiac remodeling, which leads to alterations in cardiac size, shape and function. This is what has driven the quest for unconventional therapeutic strategies aiming to regenerate the injured cardiac and vascular tissue. One of the latest breakthroughs in this regard is stem cell (SC) therapy. Based on favorable data obtained in experimental studies, therapeutic effectiveness of this innovative therapy has been investigated in clinical settings. Of various cell types used in the clinic, autologous bone marrow derived SCs were the first used to treat an AMI patient, 15 years ago. Since then, we have witnessed an increasing body of data as regards this cutting-edge therapy. Although feasibility and safety of SC transplant have been clearly proved, it’s efficacy is still under dispute. Conducted studies and meta-analysis reported conflicting results, but there is hope for conclusive answer to be provided by the largest ongoing trial designed to demonstrate whether this treatment saves lives. In the meantime, strategies to enhance the SCs regenerative potential have been applied and/or suggested, position papers and recommendations have been published. But what have we learned so far and how can we properly use the knowledge gained? This review will analytically discuss each of the above topics, summarizing the current state of knowledge in the field.
Core tip: Since the first successful bone marrow stem cells transplantation performed 15 years ago in a patient with acute myocardial infarction, we have witnessed a mounting body of data as regards this cutting-edge therapy. During the reporting period, conflicting results have been stated, scientific papers have been under investigation, strategies to enhance the stem cells regenerative potential have been applied and/or suggested, position papers and recommendations have been published. This review will analytically discuss each of the above topics, summarizing the current state of knowledge in the field.
- Citation: Micheu MM, Dorobantu M. Fifteen years of bone marrow mononuclear cell therapy in acute myocardial infarction. World J Stem Cells 2017; 9(4): 68-76
- URL: https://www.wjgnet.com/1948-0210/full/v9/i4/68.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v9.i4.68
The optimal management of acute myocardial infarction (AMI) still remains elusive, although it represents an illness with one of the highest morbi-mortality and one of the highest healthcare costs worldwide. The quick and efficient restoring of myocardial blood flow is the most appropriate strategy for reducing the size of the infarcted area. Even though standard-of-care therapy diminishes the area at risk to become necrotic, cardiac remodeling may occur in up to 60% of patients having suffered an AMI[1-3]. The conventional available treatments (whether pharmacological, interventional or surgical)[4] do not address the crucial issue of cell loss, thus being unable to completely prevent or reverse this pathological process which eventually leads to changes in size, shape, structure and function of the entire heart. One of the latest breakthroughs in this regard is stem cell (SC) therapy. By providing a potential source of new cells, heart function may be enhanced. Ideally, this process allows the replacement of non-functional cardiomyocytes and scar tissue with new fully functional contracting cells, as well as new blood vessels. Furthermore, transplanted SCs may secrete a variety of growth factors and cytokines, thereby enhancing myocyte survival and facilitating the migration of remote and/or resident cardiac SCs to the site of injury.
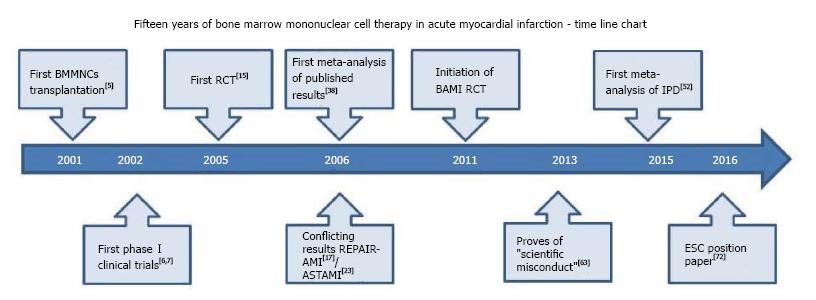
One of the first SC types which have been tested in clinical settings is autologous bone marrow SC. Since the first successful bone marrow SCs transplantation performed 15 years ago in a 46-year-old patient with AMI, we have witnessed a mounting body of data related to this effervescent domain: Conflicting results have been reported, scientific papers have been under investigation, strategies to enhance the SCs regenerative potential have been applied and/or suggested, position papers and recommendations have been published. A time line chart of accomplishments performed during the last fifteen years is depicted in Figure 1. But what have we learned so far and how can we properly use the knowledge gained? This review will analytically discuss each of the above topics, summarizing the current state of knowledge in the field.
Bone marrow is a very heterogeneous compartment with multiple SC populations with putative cardiac regenerative potential (e.g., hematopoietic SCs, mesenchymal SCs, endothelial progenitor cells, etc.).
The regenerative potential of adult autologous SCs after AMI was assessed for the first time in 2001 by a German group[5]. They used unfractionated bone marrow mononuclear stem cells (BMMNCs), which contained both hematopoietic and nonhematopoietic cells, a protocol that was extensively used subsequently. After selective catheterization of the infarct-related artery, the BMMNCs suspension has been intracoronary injected. Ten weeks later, the infarct area had been notably reduced (from 24.6% to 15.7%); in addition, cardiac function had improved by 20%-30%. Accordingly, the authors concluded that intracoronary administration of human autologous adult BMMNCs is feasibly in clinical settings and that it can promote myocardial regeneration after transmural infarction.
The following years were characterized by a series of small Phase I clinical trials whose primary achievement was demonstrating the feasibility and safety of this ground-breaking therapy[6-10].
Since most of these studies have been comprehensively discussed in previous reviews[11-14], we will briefly point out their main characteristics. What they have in common is the small number of patients enrolled (with or without a control group) and the assessment of left ventricular ejection fraction (LVEF) as a surrogate marker of cardiac function. Although not designed to evaluate the efficacy of the therapy, the early trials reported a beneficial effect on cardiac function as revealed by increased global or regional LVEF, reduced endsystolic LV volumes and enhanced perfusion within the infarcted area 4 to 6 mo after SC transplantation depending on study design.
The next logical step was the appearance of randomized clinical trials (RCT) designed to test whether this therapy works. A wide variety of RCT have been conducted in this regard, with number of patients ranging from 20[15,16] to 204[17], but not all studies successfully blinding the participants and/or caregivers[15,16]. Studies varied also in terms of baseline LVEF, as well as diagnostic tests and procedures used to evaluate cardiac volumes and function. The most utilized imagistic method was cardiac echocardiography followed by cardiac magnetic resonance (CMR) - the “gold” standard for noninvasively characterizing cardiac function and viability, while LV angiography and single-photon emission computed tomography (SPECT) being exploited less frequently. Noteworthy, the timing of cell delivery after AMI, the quantity and quality of transplanted cells, as well as cell handling varied greatly, so is no wonder why apparently similar studies had different results.
Some of the hallmark studies using unfractionated bone marrow mononuclear SCs were conducted more than 10 years ago (Table 1). The BOOST study tested the usefulness of autologous BMMNCs intracoronary transfer 4.8 ± 1.3 d after AMI[10]. At baseline (n = 60), the two groups of patients were homogeneous in terms of LV volumes and function; 6 mo later, a mean global LVEF improvement of 6.7% in the cell therapy group and 0.7% in the control group (P value for between-group comparison = 0.0026) was documented, enhanced LV systolic function being predominantly witnessed in myocardial segments bordering the infarcted area.
| Study name | Clinical trials (gov ID) | Principal investigator | No. of included patients |
| BOOST[10,18,19] | NCT00264316 | Stefan Janssens | Treated (n = 30) |
| Control (n = 30) | |||
| REPAIR-AMI[17,20,21] | NCT00279175 | Andreas Zeiher | Treated (n = 101) |
| Control (n = 103) | |||
| ASTAMI[23] | NCT00199823 | Ketil Lunde | Treated (n = 50) |
| Control (n = 50) | |||
| BALANCE[22] | - | Bodo-Eckehard Strauer | Treated (n = 62) |
| Control (n = 62) | |||
| SWISS-AMI[24] | NCT00355186 | Roberto Corti | Treated (n = 133) |
| Control (n = 67) | |||
| TIME[25] | NCT00684021 | Robert Simari | Treated (n = 79) |
| Control (n = 41) | |||
| Late TIME[26] | NCT00684060 | Robert Simari | Treated (n = 57) |
| Control (n = 29) |
Although significant augmentation of LV function after SCs transplant have been observed in the first months, this positive effect seems to be fading in time. Long-term benefit of SC therapy was assessed in BOOST surviving patients. Eighteen months after AMI (n = 59), there were no significant differences between groups as regards global LVEF (P = 0.27), although the speed to LVEF recovery was significantly higher in patients receiving SC transplant (P = 0.001)[18].
Moreover, 5 years after randomization (n = 56), statistical analysis of data revealed no difference between groups with reference to cardiac dimensions or function. Repetitive CMR examinations indicated an evident dilatation of LV volumes, whereas LV function decreased during 61 mo follow-up[19].
Reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction (REPAIR-AMI) - the largest study reported so far, also demonstrated the benefit of BMMNCs intracoronary infusion in patients with optimally treated AMI. From the 204 patients included, 103 were randomly assigned to placebo group and 101 to receive SC therapy. Both groups were well matched with respect to baseline characteristics, procedural characteristics of reperfusion therapy and associated pharmacological therapy during the study. Three to 7 d after successful stent implantation, cell suspension or placebo medium was injected in the infarct-related artery. Four months later, significant improvement in both global and regional LV function was documented in the cell treated group. Of note, the study led by Andreas Zeiher was the first trial to evaluate the interaction between the BMMNCs treatment effect and the timing of cell delivery. Subgroup analysis revealed superior recovery of contractile function when cell infusion was administered on day 5 or later after PCI, while earlier administration - within 4 d after reperfusion therapy - had only minimal effects as regards LVEF improvement. Furthermore, intracoronary administration of BMC abolished LV end-systolic volume enlargement after the infarction.
Even though REPAIR-AMI was not powered to detect significant differences in major adverse clinical events between the cell therapy and control group, a reduction in the combined outcome of death, recurrence of MI, or any revascularization procedure was noticed[17].
As opposed to BOOST trial - in which positive effects have faded in time, 2- and 5-year follow-up of REPAIR-AMI patients demonstrated a persistent reduction of the combined end point of death, recurrent MI and rehospitalization for heart failure in the BMMNCs group compared with placebo. In addition, 2 years after AMI, SC therapy was still associated with a significant improvement in regional left ventricular contractility of infarcted segments[20,21].
The significant and longstanding positive effects of SC therapy were further confirmed by a study conducted by Bodo-Eckehard Strauer’s group: The BALANCE Study (Clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with AMI) which randomized 124 patients to BMMNCs (62 patients) or control (62 patients) 7 ± 2 d after AMI[22]. The patients were followed-up at specific time intervals (i.e., 3, 12, and 60 mo) by a variety of examinations (e.g., coronary angiography, right heart catheterization, biplane left ventriculography, electrocardiogram at rest and exercise, echocardiography, late potential, heart rate variability and 24-h Holter electrocardiogram). The authors reported significant improvements as regards LV performance, quality of life and mortality in their 5-year data.
But there were also some studies (not few) that challenged these optimistic findings. In some cases, contradictory results of similar studies were revealed simultaneously to the scientific community; this is the case of two well-known studies - REPAIR-AMI and ASTAMI respectively, which were published in the same issue of The New England Journal of Medicine in 2006[17,23]. As opposed to REPAIR-AMI, the trial conducted by the Norwegian group reported no changes in LVEF, LV volumes or infarct size assessed at 6 mo by SPECT, echocardiography and CMR in 97 patients treated with intracoronary BMMNCs vs placebo a median of 6 d post AMI.
The pile of negative findings expanded based on the results of 3 other studies - namely SWISS-AMI[24], TIME[25] and Late TIME[26] - thoroughly analyzed by Simari and colleagues in a paper on behalf of Cardiovascular Cell Therapy Research Network[27]. The Cardiovascular Cell Therapy Research Network (CCTRN) was intended to enable cell based therapies in the United States[28]; in this regard, CCTRN sponsored the TIME and LateTIME trials which aimed to evaluate the influence of BMMNCs delivery timing on LV function. The 3 studies mentioned above shared some similar characteristics, but differed in some other aspects. All were prospective, randomized, controlled trials designed to identify moderate to large placebo-adjusted LVEF improvements (from 3.5% to 5%) as assessed by CMR 4 or 6 mo after PCI. Cell dose and delivery were the same in each of the 3 studies - that was the intracoronary stop-flow technique described in the early 2000s[7], but cell handling varied: It was manual Ficoll processing in SWISS-AMI, while the investigators of the CCTRN studies went for automated Ficoll processing. The authors reported no benefit of intracoronary administration of BMMNCs related to LV function irrespective of the timing of delivery. But why apparently similar studies led to contradictory results? These conflicting outcomes have been debated - and to some extent explained - by a series of experts in the field[14,29,30].
A direct comparative analysis of methodology used in REPAIR-AMI[17] and ASTAMI[23] trials have revealed that seemingly minor changes in BMMNCs isolation and preservation protocols may have a major impact on functional activity of isolated cells, consequently affecting the clinical outcome. Seeger et al[29] collected bone marrow from healthy volunteers or patients with angiographically confirmed coronary artery disease. Equal aliquots from the same bone marrow aspirate were manipulated accordingly to either REPAIR-AMI (density gradient centrifugation using Ficoll, followed by overnight incubation in ex-vivo 10 medium + 20% autologous serum at room temperature), or ASTAMI (density gradient centrifugation using Lymphoprep, followed by overnight incubation in 0.9% NaCl + 20% heparin-plasma at 4 °C) protocol. Obtained BMMNCs were subsequently tested for various parameters of phenotype and function, with quite divergent results. REPAIR-AMI isolation protocol generated a superior number of total BMMNCs, but also more haematopoietic and mesenchymal SCs as compared to ASTAMI. Furthermore, cells isolated and stored according to German study yielded better results in terms of proliferative capacity, ability to migrate to the chemoattractant SDF-1 and improvement in blood flow in a mouse model of hind-limb ischaemia.
Moreover, there is a substantial individual variability related to quantitative but also qualitative changes of adult bone marrow SCs with age, cardiovascular risk factors and associated comorbidities, decreasing the efficiency of cell therapy particularly in patients who need it the most[31-35]. Studies have shown that young age and a superior number of CD34+ cells were independent predictors for treatment response to cell therapy, demonstrating the importance of patient’s cell product[36,37].
Additionally, the natural history of AMI has an unpredictable course modulated by upregulation and downregulation of a wide array of cytokines, growth and inflammatory factors. In specific subgroups of patients this changeable biological milieu could blur and/or make it difficult to distinguish a cell-based specific efficacy signal.
Some other potentially incriminated factors associated to result variability could be related to different times between AMI and SCs delivery or to variability methods for the assessment of ventricular function and perfusion (ventriculography, echo-cardiography, CMR, SPECT).
Because of low sample size and small effects, individual studies were underpowered to identify significant differences in major adverse clinical events between SC therapy and control group. Therefore, new approaches were needed. In hope of obtaining clear answers regarding the effectiveness of SC therapy, several meta-analysis were carried-out since 2006, but the controversies continued[13,36,38-52]. Extensive or less-extensive analysis were completed on different number of RCT (5-43) including different number of patients (482-2732 patients)[38,53,54]. Subgroup analysis were performed based on different parameters such as baseline LVEF, timing of SCs infusion from onset of AMI, the dose of BMMNCs infused and patients age. Although earlier meta-analysis reported that intracoronary BMMNCs infusion is associated with significant improvements of LV function and remodeling particularly in younger patients and patients with a more severely depressed LVEF at baseline[13,36,45], recent analysis revealed that intracoronary cell therapy provided only modest[53,54] or no benefit in terms of clinical events or changes in LV function[52].
But then, why such discrepancy even between meta-analysis reports? This was the theme of 2 very recent reviews published by well-known experts in the field[55,56]. As one would expect, the first variation factor to point the finger to is related to differences in the methodology used in conducting systematic reviews. All meta-analyses except the one reporting negative findings relied on published summary results from multiple trials, while the latter was based on individual patient data (IPD) collected directly from the researchers responsible for each study and further centrally re-analyzed. The 2 methodologies varied in data collection, data checking and data analysis. Although ACCRUE (Meta-Analysis of Cell-Based Cardiac Studies; NCT01098591) database comprised a pool of 1252 IPDs from 12 randomized studies in AMI settings, it included only about 60% of the available published trials, as a result raising concern for potential bias. Of course, there are some other disparity factors involved, such as insufficient power of included studies, patients’ heterogeneity and statistical heterogeneity[55,56].
In view of presented data, one can only state that meta-analyses failed as well to clarify whether or not SC therapy improves heart function and/or mortality in AMI patients. What is more, meta-analyses are not surrogates for large phase III RCTs. Consequently, the scientific community is eagerly waiting for the ongoing BAMI trial to provide a more conclusive answer as regards the efficacy of bone marrow cell therapy in AMI settings. BAMI (the Effect of Intracoronary Reinfusion of Bone Marrow-Derived Mononuclear Cells on All Cause Mortality in AMI; NCT01569178) is the largest and most aspiring trial to date, funded by the European Commission Seventh Framework Programme. It currently involves 19 partners planning to include 3000 patients from 10 European countries. The study aims to standardize methods of bone marrow cell collection, handling and delivery, as well as to test if the product and delivery method can lead to a 25% reduction in mortality.
Since BMMNCs yielded only modest improvements regarding LV function recovery after AMI, selected bone marrow SCs populations have been tested in clinical settings. Trials involving CD34+/133+ progenitor cells[57-61] or mesenchymal stem cells (MSCs)[62-65] had encouraging results, but none of these tested cells haven’t been clearly demonstrated to yield superior outcomes.
Of course, strategies to increase the number and potency of low-abundance progenitor cells in bone marrow cells (e.g., MSCs, CD34+/CD133+ cells) are needed. While in animal models a variety of genetic and nongenetic approaches aiming to improve therapeutic efficacy of transplanted cells have been tested, there is still a long road till translation into clinical settings. Some of the genetic strategies include enhancement of survival, proliferation and differentation capacity, as well as boost of paracrine factors synthesis. Nongenetic procedures in essence comprise preconditioning with various factors (physical factors, drugs, cytokines and growth factors), 3D aggregate formation or hydrogel encapsulation and coculture with other types of SCs (e.g., cardiac SCs)[66,67].
In addition, unlike in chronic ischemic disease, strategies to improve bone marrow SCs regenerative potential in acute settings are limited by the relatively short window of opportunity.
Most important drawbacks and limitations of BMMNCs in AMI settings are related to reduced regenerative potential of transplanted cells; therefore, finding strategies to intensify their survival, proliferation and differentation potential is a perpetual quest. But aside from these methodological features discussed in previous chapter, we would like to bring your attention to another issue, namely scientific inaccuracy. Unfortunately, SC research has not been avoided by scandals related to “scientific misconduct” in the field. It is the case of studies conducted by the German scientist Bodo-Eckehard Strauer. His papers have been comprehensively analyzed by Francis et al[68] who identified and exposed a series of discrepancies and contradictions such as number of patients receiving cells, baseline EF comparability and cell preparation. Although none of Strauer’s studies have been retracted, their results cannot be trusted any more. Nevertheless, we chose to include them in our review in order to provide the reader with an accurate depiction of SC therapy development in AMI settings, since the German investigator conducted not only the pioneering research in this area, but also one of the largest and most promising trials in the field. Besides affecting the credibility of the researchers, these inconsistencies may negatively influence the patients’ decision when considering enrollment in a SC-based clinical trial.
Predicting who will benefit and who will not from SC therapy is not currently possible, although efforts are being made in this direction[37]. In the era of Precision Medicine Initiative[69,70], being able to discriminate responders from nonresponders could be the first step toward tailored cell therapy. Prediction models for responder identification based on individual’s characteristics are mandatory, in order that every single patient gets optimal treatment according to his individual variations in genes, environment and lifestyle.
Investigators of future trials should carefully choose hard clinically meaningful end points not limited to one effect, but rather reflecting different categories of consequences, such as structural evaluations of the heart, cardiovascular physiological measurements, biomarkers (including transcriptomic-based biomarkers), functional capacity and quality of life[71].
The European Society of Cardiology Working Group Cellular Biology of the Heart has recently provided a series of recommendations on how to improve the therapeutic application of cell-based therapies for cardiac regeneration and repair[72]. Accordingly, upcoming studies should be designed to address precise hypotheses on delivery types and mechanisms of efficiency, rather than safety and efficacy endpoints only; comparison of different cell types, or a combination of cell types in RCTs should be completed; in-depth cell characterization - including cell function should be done in every clinical trial; also, strategies to boost both cellular and paracrine effects should be developed.
A substantial knowledge has been gained in the past 15 years since the first bone marrow SCs transplantation have been performed in a patient with AMI, but there are a lot of challenges to be faced until this therapy will gain a definitive place in clinical arena.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Romania
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Lee T, Li SC, Sanchez-Mendoza A, Shetty PH S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161-1172. [PubMed] |
| 2. | Pfeffer MA. Left ventricular remodeling after acute myocardial infarction. Annu Rev Med. 1995;46:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 571] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 4. | Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3540] [Cited by in RCA: 3705] [Article Influence: 285.0] [Reference Citation Analysis (0)] |
| 5. | Strauer BE, Brehm M, Zeus T, Gattermann N, Hernandez A, Sorg RV, Kögler G, Wernet P. [Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction]. Dtsch Med Wochenschr. 2001;126:932-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation. 2002;106:3009-3017. [PubMed] |
| 7. | Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913-1918. [PubMed] |
| 8. | Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, Schümichen C, Nienaber CA, Freund M, Steinhoff G. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Fernández-Avilés F, San Román JA, García-Frade J, Fernández ME, Peñarrubia MJ, de la Fuente L, Gómez-Bueno M, Cantalapiedra A, Fernández J, Gutierrez O. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circ Res. 2004;95:742-748. [PubMed] [DOI] [Full Text] |
| 10. | Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1650] [Cited by in RCA: 1499] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 11. | Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 636] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 12. | Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807-1818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Strauer BE, Steinhoff G. 10 years of intracoronary and intramyocardial bone marrow stem cell therapy of the heart: from the methodological origin to clinical practice. J Am Coll Cardiol. 2011;58:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Ruan W, Pan CZ, Huang GQ, Li YL, Ge JB, Shu XH. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chin Med J (Engl). 2005;118:1175-1181. [PubMed] |
| 16. | Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y, Fan B, Liu X, Zhang S, Sun A. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT-STAMI). Heart. 2006;92:1764-1767. [PubMed] [DOI] [Full Text] |
| 17. | Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1481] [Cited by in RCA: 1368] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 18. | Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 687] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 19. | Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, Hahn A, Fichtner S, Schaefer A, Arseniev L. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled BOOST trial. Eur Heart J. 2009;30:2978-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey DG. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Assmus B, Leistner DM, Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Sedding D, Yu J, Corti R. Long-term clinical outcome after intracoronary application of bone marrow-derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event-free survival. Eur Heart J. 2014;35:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Yousef M, Schannwell CM, Köstering M, Zeus T, Brehm M, Strauer BE. The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 889] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 24. | Sürder D, Manka R, Lo Cicero V, Moccetti T, Rufibach K, Soncin S, Turchetto L, Radrizzani M, Astori G, Schwitter J. Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation. 2013;127:1968-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA. 2012;308:2380-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 26. | Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 311] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 27. | Simari RD, Pepine CJ, Traverse JH, Henry TD, Bolli R, Spoon DB, Yeh E, Hare JM, Schulman IH, Anderson RD. Bone marrow mononuclear cell therapy for acute myocardial infarction: a perspective from the cardiovascular cell therapy research network. Circ Res. 2014;114:1564-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Simari RD, Moyé LA, Skarlatos SI, Ellis SG, Zhao DX, Willerson JT, Henry TD, Pepine CJ. Development of a network to test strategies in cardiovascular cell delivery: the NHLBI-sponsored Cardiovascular Cell Therapy Research Network (CCTRN). J Cardiovasc Transl Res. 2010;3:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Henry TD, Moyé L, Traverse JH. Consistently Inconsistent-Bone Marrow Mononuclear Stem Cell Therapy Following Acute Myocardial Infarction: A Decade Later. Circ Res. 2016;119:404-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1-E7. [PubMed] |
| 32. | MacEneaney OJ, Kushner EJ, Westby CM, Cech JN, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity (Silver Spring). 2010;18:1677-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Drela E, Stankowska K, Kulwas A, Rość D. Endothelial progenitor cells in diabetic foot syndrome. Adv Clin Exp Med. 2012;21:249-254. [PubMed] |
| 34. | Efimenko AY, Kochegura TN, Akopyan ZA, Parfyonova YV. Autologous Stem Cell Therapy: How Aging and Chronic Diseases Affect Stem and Progenitor Cells. Biores Open Access. 2015;4:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Micheu M, Oprescu O, Dorobantu M. Cantitative determination of endothelial progenitor cells – a new marker for the occurrence of coronary acute syndrome? J Inter Med Soci. 2010;21-26. |
| 36. | Delewi R, Hirsch A, Tijssen JG, Schächinger V, Wojakowski W, Roncalli J, Aakhus S, Erbs S, Assmus B, Tendera M. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014;35:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Zwetsloot PP, Gremmels H, Assmus B, Koudstaal S, Sluijter JP, Zeiher AM, Chamuleau SA. Responder Definition in Clinical Stem Cell Trials in Cardiology: Will the Real Responder Please Stand Up? Circ Res. 2016;119:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Hristov M, Heussen N, Schober A, Weber C. Intracoronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: a meta-analysis. J Cell Mol Med. 2006;10:727-733. [PubMed] |
| 39. | Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 40. | Zhang S, Sun A, Xu D, Yao K, Huang Z, Jin H, Wang K, Zou Y, Ge J. Impact of timing on efficacy and safetyof intracoronary autologous bone marrow stem cells transplantation in acute myocardial infarction: a pooled subgroup analysis of randomized controlled trials. Clin Cardiol. 2009;32:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Zhang SN, Sun AJ, Ge JB, Yao K, Huang ZY, Wang KQ, Zou YZ. Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: a meta-analysis of randomised controlled trials. Int J Cardiol. 2009;136:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Bai Y, Sun T, Ye P. Age, gender and diabetic status are associated with effects of bone marrow cell therapy on recovery of left ventricular function after acute myocardial infarction: a systematic review and meta-analysis. Ageing Res Rev. 2010;9:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Kuswardhani RA, Soejitno A. Bone marrow-derived stem cells as an adjunctive treatment for acute myocardial infarction: a systematic review and meta-analysis. Acta Med Indones. 2011;43:168-177. [PubMed] |
| 44. | Takagi H, Umemoto T. Intracoronary stem cell injection improves left ventricular remodeling after acute myocardial infarction: an updated meta-analysis of randomized trials. Int J Cardiol. 2011;151:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;CD006536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Zimmet H, Porapakkham P, Porapakkham P, Sata Y, Haas SJ, Itescu S, Forbes A, Krum H. Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail. 2012;14:91-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Chen L, Tong JY, Jin H, Ren XM, Jin H, Wang QJ, Ma GS. Long-term effects of bone marrow-derived cells transplantation in patients with acute myocardial infarction: a meta-analysis. Chin Med J (Engl). 2013;126:353-360. [PubMed] |
| 48. | Jeong H, Yim HW, Cho Y, Park HJ, Jeong S, Kim HB, Hong W, Kim H. The effect of rigorous study design in the research of autologous bone marrow-derived mononuclear cell transfer in patients with acute myocardial infarction. Stem Cell Res Ther. 2013;4:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ Cardiovasc Interv. 2014;7:156-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 50. | Liu B, Duan CY, Luo CF, Ou CW, Sun K, Wu ZY, Huang H, Cheng CF, Li YP, Chen MS. Effectiveness and safety of selected bone marrow stem cells on left ventricular function in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. Int J Cardiol. 2014;177:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Cong XQ, Li Y, Zhao X, Dai YJ, Liu Y. Short-Term Effect of Autologous Bone Marrow Stem Cells to Treat Acute Myocardial Infarction: A Meta-Analysis of Randomized Controlled Clinical Trials. J Cardiovasc Transl Res. 2015;8:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH. Meta-Analysis of Cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res. 2015;116:1346-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 53. | Lee SH, Hong JH, Cho KH, Noh JW, Cho HJ. Discrepancy between short-term and long-term effects of bone marrow-derived cell therapy in acute myocardial infarction: a systematic review and meta-analysis. Stem Cell Res Ther. 2016;7:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015;CD006536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 55. | Martin-Rendon E. Meta-Analyses of Human Cell-Based Cardiac Regeneration Therapies: What Can Systematic Reviews Tell Us About Cell Therapies for Ischemic Heart Disease? Circ Res. 2016;118:1264-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Zhao J, Ghafghazi S, Khan AR, Farid TA, Moore JB. Recent Developments in Stem and Progenitor Cell Therapy for Cardiac Repair. Circ Res. 2016;119:e152-e159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, Van Haute I, Lootens N, Heyndrickx G, Wijns W. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178-I183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 58. | Lipiec P, Krzemińska-Pakuła M, Plewka M, Kuśmierek J, Płachcińska A, Szumiński R, Robak T, Korycka A, Kasprzak JD. Impact of intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction on left ventricular perfusion and function: a 6-month follow-up gated 99mTc-MIBI single-photon emission computed tomography study. Eur J Nucl Med Mol Imaging. 2009;36:587-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, Musiałek P, Piwowarska W, Nessler J, Buszman P. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 60. | Colombo A, Castellani M, Piccaluga E, Pusineri E, Palatresi S, Longari V, Canzi C, Sacchi E, Rossi E, Rech R. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: results from a randomized controlled trial. J Cardiovasc Med (Hagerstown). 2011;12:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 61. | Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, Lerakis S, Sher S, Vaughan D, Perin E. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 62. | Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 828] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 63. | Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 999] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 64. | Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, Wang ZG, Wang YF, Zhu ZM, Li TC. A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol. 2013;168:3191-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Kwon W, Hong IS, Lee K. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 66. | Sart S, Ma T, Li Y. Preconditioning stem cells for in vivo delivery. Biores Open Access. 2014;3:137-149. [PubMed] [DOI] [Full Text] |
| 67. | Karpov AA, Udalova DV, Pliss MG, Galagudza MM. Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif. 2017;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Francis DP, Mielewczik M, Zargaran D, Cole GD. Autologous bone marrow-derived stem cell therapy in heart disease: discrepancies and contradictions. Int J Cardiol. 2013;168:3381-3403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3287] [Cited by in RCA: 3227] [Article Influence: 322.7] [Reference Citation Analysis (0)] |
| 70. | Jaffe S. Planning for US Precision Medicine Initiative underway. Lancet. 2015;385:2448-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Hare JM, Bolli R, Cooke JP, Gordon DJ, Henry TD, Perin EC, March KL, Murphy MP, Pepine CJ, Simari RD. Phase II clinical research design in cardiology: learning the right lessons too well: observations and recommendations from the Cardiovascular Cell Therapy Research Network (CCTRN). Circulation. 2013;127:1630-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Madonna R, Van Laake LW, Davidson SM, Engel FB, Hausenloy DJ, Lecour S, Leor J, Perrino C, Schulz R, Ytrehus K. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J. 2016;37:1789-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |