Published online Aug 26, 2016. doi: 10.4252/wjsc.v8.i8.260
Peer-review started: March 22, 2016
First decision: April 20, 2016
Revised: April 25, 2016
Accepted: June 27, 2016
Article in press: June 29, 2016
Published online: August 26, 2016
Processing time: 153 Days and 16.1 Hours
Urothelial carcinoma (UC) of the bladder is characterized by high recurrence rate where a subset of these cells undergoes transition to deadly muscle invasive disease and later metastasizes. Urothelial cancer stem cells (UroCSCs), a tumor subpopulation derived from transformation of urothelial stem cells, are responsible for heterogeneous tumor formation and resistance to systemic treatment in UC of the bladder. Although the precise reason for pathophysiologic spread of tumor is not clear, transcriptome analysis of microdissected cancer cells expressing multiple progenitor/stem cell markers validates the upregulation of genes that derive epithelial-to-mesenchymal transition. Experimental studies on human bladder cancer xenografts describe the mechanistic functions and regulation of epithelial plasticity for its cancer-restraining effects. It has been further examined to be associated with the recruitment of a pool of UroCSCs into cell division in response to damages induced by adjuvant therapies. This paper also discusses the various probable therapeutic approaches to attenuate the progressive manifestation of chemoresistance by co-administration of inhibitors of epithelial plasticity and chemotherapeutic drugs by abrogating the early tumor repopulation as well as killing differentiated cancer cells.
Core tip: A subset of bladder cancer cells, known as urothelial cancer stem cells, have abilities to self-renew, generate tumor heterogeneity via differentiation, and are actually responsible for tumor relapse and metastasis formation. Delineating the mechanistic complexity between epithelial plasticity and cancer stemness in malignant transformation of urothelial carcinoma provides the basis for designing rational therapies. Differentiation and elimination therapies targeting the potential biomarkers could prove to be clinically beneficial by suppressing the cancer stemness and inhibiting epithelial-to-mesenchymal transition phenotype and would provide novel opportunities for targeted therapeutic approaches in the clinical management of patients.
- Citation: Garg M. Epithelial plasticity in urothelial carcinoma: Current advancements and future challenges. World J Stem Cells 2016; 8(8): 260-267
- URL: https://www.wjgnet.com/1948-0210/full/v8/i8/260.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i8.260
Urothelial carcinoma (UC) of the bladder, also known as transitional cell carcinoma of the bladder, is the sixth most common cause of cancer-related deaths worldwide[1]. It is the second most frequent cancer of the genitourinary tract where men are at four times greater risk than women. It is caused by the accumulation of genetic or epigenetic changes in the urothelium due to its exposure to multiple risk factors including tobacco and occupational/environmental carcinogens (polycyclic aromatic hydrocarbons). People working in leather, dye, rubber industries, painters, pesticide applicators or those having chronic urinary tract infections are more prone to develop urothelial carcinoma.
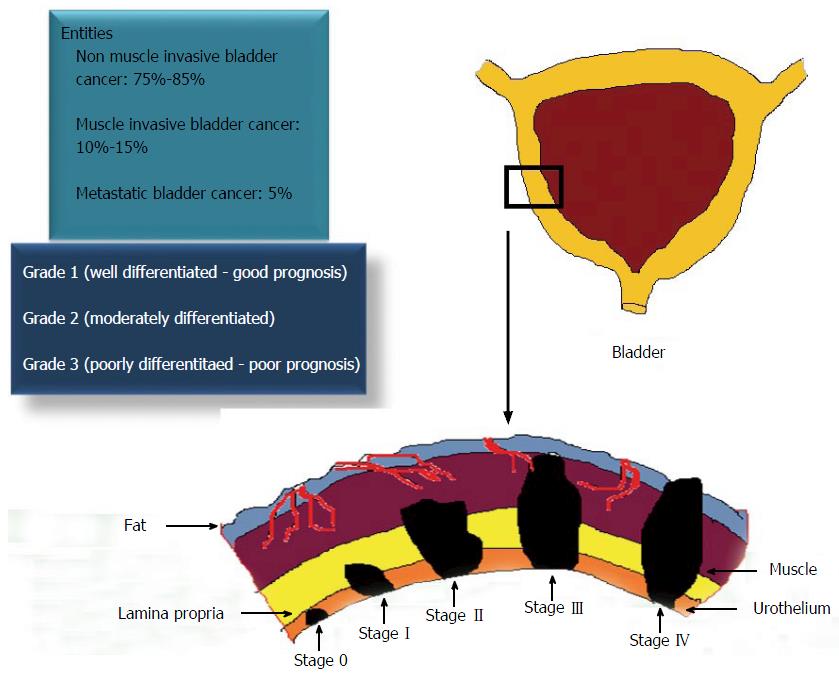
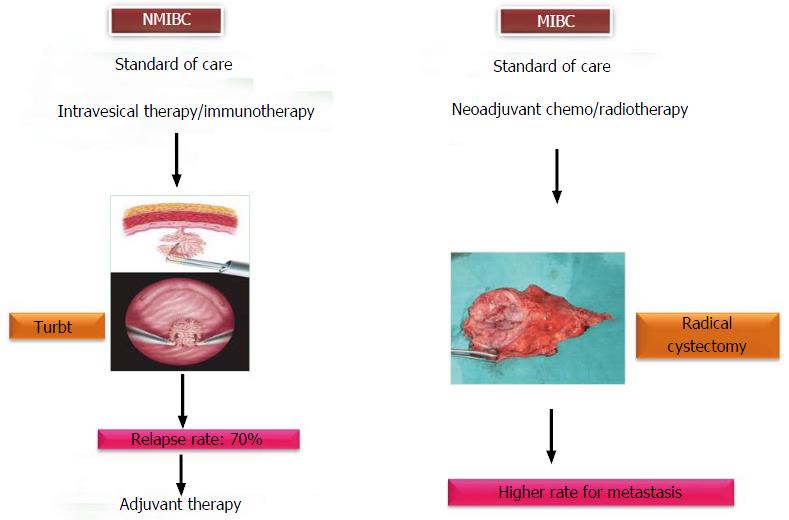
UC of the bladder is a heterogeneous disease, which can arise through two different pathways - non-invasive papillary pathway and invasive pathway. It represents a spectrum of neoplasms, including non-muscle invasive bladder cancer (NMIBC), muscle invasive bladder cancer (MIBC) and metastatic lesions. Tumor staging and grading (Tumor Node and Metastasis classification by World Health Organization/International Society of Urology Pathologists, 2004) are the gold standard prognosticators for defining the various entities of UC of the bladder (Figure 1)[2]. Despite the successful treatment of NMIBC through transurethral resection of bladder tumor (TURBT), 70% to 80% of them have a tendency to recur. Hence, there is a need for regular cystoscopy and examination of cytologic and molecular markers in urine, blood or tumor tissues in bladder cancer patients. This intense surveillance after treatment makes this cancer, one of the most costliest cancers to manage. Although in the majority of the cases, these papillary bladder tumors are not lethal, however, 20%-30% of them can progress to more aggressive, invasive and metastatic bladder tumors with an overall survival rate of 5% (Figure 2).
Characterization of molecular and biological mechanisms responsible for distinct bladder tumor phenotypes would facilitate personalization of more effective treatment decisions. Multiple genetic and epigenetic abnormalities are known to be associated with diverse types of urological malignancies. Cancer stem cell theory sheds further light on understanding the biology of the origin of distinct oncological pathways and heterogeneous nature of this disease.
This paper discusses the current concepts on the aberrant activation of epithelial-to-mesenchymal transition (EMT), also known as epithelial plasticity, as one of the primary causes of transformation of urothelial stem cells (UroSCs). Further, recent advancements on the functions of urothelial cancer stem cells (UroCSCs), a tumor subpopulation derived from transformation of UroSCs, in the pathophysiology and its clinical implications in the treatment of UC of the bladder are reviewed.
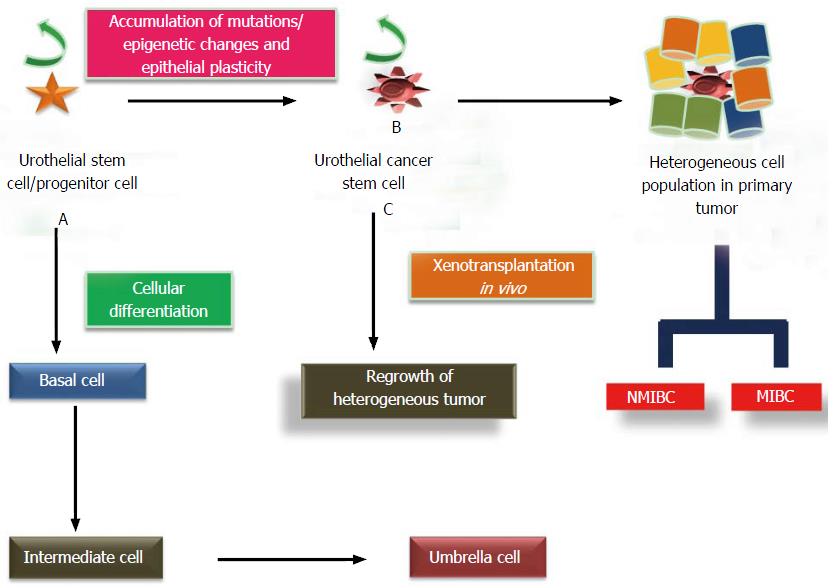
The stratified epithelial lining of the urinary bladder wall, also known as urothelium, consists of unilayered polygonal basal cells which are in direct contact with the basement membrane, intermediate cells and umbrella cells. Many recent studies report the existence of a self-renewing unipotent population of slow cycling, label-retaining cells with long life span and high integrin subunit beta 4 expression, also known as urothelial stem cells, as clonal patches among basal cell layer. High nuclear-cytoplasmic ratio and expression of CD44, laminin receptor, cytokeratins (CK-5/14, CK17), β1 and β4 integrins are some of the characteristic features of UroSCs[3]. These cells confer increased regenerative and proliferative potential, lower apoptosis rate and multilineage differentiation at the edge of the basement membrane as compared to other cell types. These cells undergo cellular differentiation to give rise to transit-amplifying cells of intermediate cell layers and later umbrella cells. However, an alternative hypothesis suggests that adult stem cells can give rise to two cell lineages and hence, umbrella cells are formed separately from intermediate/basal cells (Figure 3). Lineage tracing experiments in the murine model of carcinogenesis provide a cellular and genetic basis for the diversity in bladder cancer lesions which could be responsible for their clinical and morphological differences. According to the experimental results of this study, the low grade, non-invasive papillary lesions arise from intermediate cells whereas Keratin 5 expressing basal cells are likely the progenitors of flat carcinoma in situ, a flat aggressive lesion, as well as of muscle-invasive lesions depending on the genetic background[1]. A study by Dancik et al[4] screened 874 bladder cancer patients in five cohorts for the identification of UroCSCs in muscle invasive tumors and validated the hypothesis of differential origin of non-muscle invasive and muscle invasive tumors from distinct progenitor cells. These results provide a paradigm shift in better understanding the biology of urothelial carcinoma for significant diagnostic and therapeutic implications.
Mutational insults in adult UroSCs and differentiated progenies, help them in acquiring tumorigenic properties and result in the origin of a subpopulation of high tumor-initiating potential cells called UroCSCs. Characterization studies on these cells describe their self-renew ability, clonogenic and proliferative potential. In addition, their capability to conserve cellular heterogeneity via differentiation can be explained by the research studies on the regrowth of heterogeneous tumor after in vivo xenotransplantation of a small number of UroCSCs in immunodeficient mice (Figure 3). These characteristic features of UroCSCs document their large amount of functional resemblance with the normal adult stem cells.
UroCSCs have been examined for the upregulation of various oncogenes which help them to acquire self-renewability. Beta-catenin (β-catenin), signal transducer and activator of transcription 3, glioma associated oncogene 1, B lymphoma Mo-MLV insertion region 1 homolog (BMI1), POU domain, class 5, transcription factor 1/octamer-binding transcription factor 4 (POU5F1/Oct4), sex determining region Y-box 2 (SOX2), Kruppel-like factor 4, v-myc myelocytomatosis viral oncogene homolog (avian) (MYC, formerly C-MYC) and NANOG are the oncogenes/transcription factors that have been observed to be responsible for maintaining the pluripotent properties of stem cells and aggressiveness of tumor invasion[5-7].
Studies on the identification of co-expression of keratin 5 and CD44 markers on UroCSCs distinguish them from differentiated tumor cells and support their basal-like phenotype. Binding of CD47, a marker of tumor-initiating cells, to signal-regulatory protein alpha on macrophages and subsequent inhibition of phagocytosis of tumor cells make it a suitable drug target[8]. Increased expression of POU5F1, an embryonic stem cell marker, and high aldehyde dehydrogenase activity in a fraction of CD44+ tumors correlate with increased clonogenic capacity of UroCSCs, and poor prognosis in UCs[9]. Identification of an extracellular marker, prominin 1 (PROM1+) (CD133+) and intracellular markers POU5F1+, and nestin (NES+) on putative UroCSCs confer them self-renewal ability and proliferative advantages in clonogenic assays. However, in due course of time, they allow these UroCSCs to lose stem cell phenotype as well as proliferative capacity and initiate the process of differentiation[10]. Differentially expressed cancer stem cell markers CD24/CD44/CD47 in the urothelial cancer cells of bladder cancer patients undergoing radical cystectomy could be of therapeutic value as their presence influenced cancer-specific survival of patients[11]. Many cell surface markers, intracellular proteins and their activities are examined to identify and characterize the putative UroCSCs, however, due to the lack of consensus on these markers, functional assays have been studied to confirm the stem cell phenotype of these tumor cells.
Pumping of DNA-binding dyes, Hoechst 33342 and DyeCycle violet, out of the cells due to overexpression of ABC (ATP-binding cassette) transporters/multidrug resistance (MDR) pumps are considered important features of a side population of urothelial cancer cells, enriched for CSCs. Co-localization of ABC transporters, ABCG2 and ABCB1 (MDR1) and other stem cell markers including POU5F1 and BMI1 further validates their identity and existence[12]. Initiation of tumor formation upon subcutaneous injection of a small number of SP of urothelial cancer cells into immunocompromised mice has been examined by clonogenic assays, and these cells showed rapid cell growth, chemo and radioresistance.
Accumulating evidence suggests that UroCSCs/progenitor cells exhibiting epithelial plasticity are quiescent, show increased DNA damage response, pump drugs out of the cells, reside in difficult-to-reach CSC protective niches and are less affected by antiproliferative therapies.
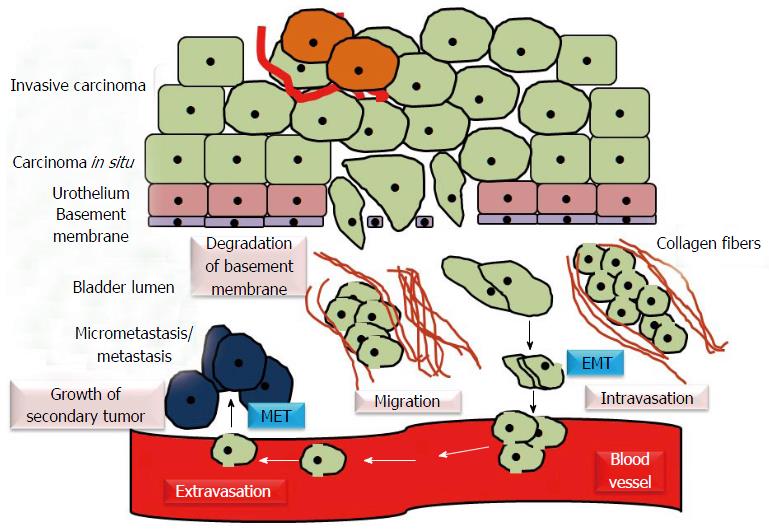
During the analysis of transcriptome of microdissected muscle invasive urothelial carcinoma of bladder/MIBC, the cancer cells expressing multiple progenitor/stem cell markers were found to be enriched with elevated levels of genes that derive and regulate EMT[13]. The process of EMT is characterized by the loss of cell polarity and cell-cell adhesion by sessile, epithelial cells and their transition to motile, mesenchymal stem cells with increased migratory and invasive potential. Cells acquire phenotypic or epithelial plasticity when they gain the ability to dynamically switch over between different phenotypic states[14]. EMT helps to establish metastasis by allowing the motile cells to invade the surrounding tissues, intravasate, move to distant sites through bloodstream, extravasate and colonize the target organs. Re-establishment of cancer cells with more epithelial phenotype at metastatic sites can be induced through mesenchymal-to-epithelial transition (MET) (Figure 4).
A study by Franzen et al[15] demonstrates the increased expression of several mesenchymal markers, including α-smooth muscle actin, S100A4 and snail, in urothelial cells treated with muscle invasive bladder cancer exosomes (small secreted vesicles that contain proteins, mRNAs and miRNAs and can potentially modulate signaling cascades in recipient cells) as compared with phosphate-buffered saline-treated cells. Moreover, these treated urothelial cells showed loss of epithelial markers, E-cadherin and β-catenin in association with increased migratory and invasive properties.
Loss of E-cadherin, a tumor suppressor gene, and abnormal expression of N and P-cadherin (cadherin switching) have been shown to be key mediators in invasive and malignant phenotype of cancer. In addition, activation of WNT signaling cascade by tumor cells owing to decreased E-cadherin levels, loss of β-catenin expression, its nuclear translocation and increased transcriptional activity have been examined to be associated with epithelial plasticity of tumor cells, disease aggression and metastasis formation. One of the serious implications of cadherin switching include the development of cancer stem cell phenotype and this makes the cadherin cell adhesion molecules and associated pathways, the probable target candidates for inhibition of cancer progression[16].
Tumor stroma/microenvironment has been shown to regulate tumor behavior by maintaining UroCSC population, its properties and EMT. Although the exact mechanism is not known, secretion of stroma-modulating growth factors including basic fibroblast growth factor 2, vascular endothelial growth factor, platelet-derived growth factor, epidermal growth factor receptor (EGFR) ligands, colony stimulating factors, and transforming growth factor-beta; extracellular matrix-degrading proteins, such as matrix metalloproteinases; and chemoattractants result in activation of fibroblasts, inflammatory cells, mesenchymal stem cells, smooth muscle cells, and adipocytes[17,18]. This contributes to angiogenesis, tumor growth, invasion and metastasis formation.
Intravesical instillations of drugs or adjuvant therapies following TURBT are the standard of care for non-muscle invasive cancer. Similarly neoadjuvant therapies with radiotherapeutic or chemotherapeutic drugs and in some cases radical cystectomy are the standard treatment options for more aggressive muscle invasive disease[19].
Cytotoxic effects of these drugs can potentially debulk tumor masses initially but tumors progressively develop between or after multiple treatment cycles in due course of time. The SP of tumor cells was found to be enriched for UroCSCs which can possibly contribute to progressive development of therapeutic resistance through enhanced survival. A number of experimental studies on human bladder cancer xenografts provide the probable mechanistic explanation for unexpected proliferative response to repopulate residual tumor cells between chemotherapy cycles. Urothelial carcinoma cell lines were examined for enriching CSCs with CD90 and CK14 expression and the effects of short- and long-term treatment with cisplatin on tumor initiating potential of these separated cells were studied. Substantial phenotypic plasticity as evident by increased expression of EMT markers, an altered pattern of CKs, and WNT-pathway target genes were observed in these sublines and instead of inducing apoptosis, it promoted neighboring CSC repopulation and subsequently the development of clinical resistance to cisplatin[20]. A strong correlation between the existence of CSC-like cells in the population of cisplatin-resistant bladder cancer cells, levels of Bmi1 and Nanog expression and the degree of malignancy of urothelial carcinoma tissues has been observed. This may play a role in the progression and drug resistance of bladder cancer[21].
Recruitment of a quiescent pool of UroCSCs into cell division in response to the cytotoxic effects of clinical drugs, similar to the mobilization of UroSCs during wound repair, reduces the efficacy of existing drugs and dramatically accelerates the pathophysiological spread of more aggressive type of bladder cancer. Combinatorial approaches based on in vivo administration of inhibitors of epithelial plasticity could be the probable therapeutic strategy for enhancing chemotherapeutic drug-induced damages by abrogating early tumor repopulation (source of cancer) and killing a bulk of bladder cancer cells, thereby customizing a new method to counter CSC-driven resistance, prevent relapse and improve the survival outcome in the patients with UC of the bladder.
Sox4, a biomarker of UroCSCs and one of the important candidate oncogenes, results in advanced cancer stages and poor survival rate. The results of its knockdown include reduced sphere formation and enriched cell population with high levels of aldehyde dehydrogenase [ALDH (high)]; inhibition of cell migration, colony formation as well as MET; and decreased tumor formation potential of urothelial cancer cells[22]. The essential role of αv integrins has been shown in migration, EMT and maintenance of ALDH activity, tumor growth and metastasis. Therefore, targeting of αv integrins could be a promising therapeutic approach for prevention of metastatic bladder cancer. Treatment with an αv integrin antagonist and its knockdown in the bladder carcinoma cell lines resulted in reduced expression levels of EMT-inducing transcription factors including SNAI2 and self-renewal genes NANOG and BMI1; low ALDH activity; and decreased CDH1 (E-cadherin)/CDH2 (N-cadherin), indicative of a shift towards epithelial phenotype and decreased proliferative, migratory, clonogenic capacity and metastatic growth[23]. Overexpression of EGFR has been examined to be associated with poor prognosis in epithelial cancers. Hence, targeting cancer cells with an EGFR inhibitor (anti-EGFR antibody, cetuximab) has been shown to increase the expression of CDH1 and confer cancer cells with epithelial phenotypic property[24]. Implications of miRNAs (a class of small non-coding RNA molecules of 21-23 nucleotides in length) in the maintenance of epithelial plasticity, cancer stemness and mediating drug sensitivities make it a potential therapeutic system towards eradication of tumor recurrence and metastasis[25,26]. Forced expression of miR-200 family (miR-200a, miR-200b, miR-200c, miR-141, and miR-429) has been associated with induction of MET in mesenchymal bladder cancer cell lines, which thereby restored EGFR inhibitor sensitivity to attenuate tumor aggressiveness in bladder cancer[25]. Re-expression of miR-23b may be a beneficial therapeutic strategy for the treatment of human bladder cancer by targeting Zeb1, a crucial regulator of EMT, inhibiting cell proliferation and migration and inducing apoptosis[27].
Direct suppression of epithelial plasticity with the use of inhibitors or knocking down EMT markers can also potentially reduce migration, invasion, and survival of cancer cells. Inhibitory effects of prostate-derived E-twenty six (Ets) factor (PDEF), an epithelium-specific member of the Ets family of transcription factors, on the proliferation, invasion, and tumorigenesis have been studied. Its ectopic overexpression in bladder carcinoma cells has been examined to modulate EMT by upregulating E-cadherin expression and downregulating the expression of N-cadherin, SNAIL, SLUG, and vimentin, thereby resulting in lower migration and invasion abilities of cancer cells[28]. Molecular mechanisms for ERK1/2 inhibitor to exert its antiproliferative effects in bladder cancer have been investigated. Treatment of SV-HUC-1 cells with ERK1/2 inhibitor (U0126)significantly reduced the expression of EMT markers including Snail, β-catenin, Vimentin, and MMP-2[29].
Besides inhibiting epithelial plasticity which can check dissemination and migration of invasive cells, it is also important to attenuate the reestablishment of cancer cells at distant sites through MET mechanism. In addition, elimination therapies are required to modulate the properties of UroCSCs, hence facilitate their chemosensitivity and apoptosis. This can be achieved by the application of inhibitors to target ABC transporters and drug-detoxifying enzymes. Cracking the difficult-to-reach protective niche of UroCSCs and creating an inhospitable microenvironment for them as well as for heterogeneous cancer cells at primary and distant sites may provide a basis for developing improved and effective therapeutic strategies for selective elimination of tumor cells. One of the recent studies identify the possible role of connexins, gap junction proteins found in the smooth muscles of detrusor muscle, in bladder tumorigenesis. Preliminary assessment detects the upregulation of connexin 43 in human urothelial carcinomas. Its functions in enhancing the adherence of tumor cells to stroma, increased migration potential as well as dissemination of cancer cells make it a promising target for genetic therapeutic approaches[30].
Long-term follow-up of patients and definite prediction of the biomarkers for patient survival or disease progression are the most important requirements in designing suitable therapies. High-throughput drug screening for its anticancer effects, reliable methods for detecting the population of UroCSCs, their characterization and validation in appropriate disease models are some of the additional challenges for successful therapies.
Understanding the mechanisms and biology of UroCSCs that can control their proliferation and differentiation allows the possibility of developing effective anti-cancer drugs. Deciphering the connection between epithelial plasticity and cancer stemness paves the way to design rationale therapies for its anti-tumor effects in the clinical management of bladder cancer.
Depending upon the genomic integrity and its background, UroCSCs in basal urothelium aggressively colonize a significant region of stratified urothelium to generate histologically different tumor lesions, identical to muscle invasive bladder cancer and carcinoma in situ. However, intermediate cells derived from the cellular differentiation of UroSCs can give rise to non-muscle invasive papillary lesions, suggestive of dual pathways of urothelial carcinogenesis. Basal-cell specific markers are examined to be good candidates for enriching UroCSCs in the SP of tumor cells. These cells are characterized by remarkable plasticity, contribute to tumor heterogeneity, relapse, and metastasis, and thereby carry significant information in the clinical management of bladder cancer. Therapeutic applications of EMT inhibitors to reverse the epithelial plasticity may account for inhibitory functions of UroCSCs, reduced migratory and invasive properties of cancer cells and can improve therapeutic planning for better patient management.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Simone G, Tschernig T S- Editor: Qiu S L- Editor: Wang TQ E- Editor: Li D
| 1. | Van Batavia J, Yamany T, Molotkov A, Dan H, Mansukhani M, Batourina E, Schneider K, Oyon D, Dunlop M, Wu XR. Bladder cancers arise from distinct urothelial sub-populations. Nat Cell Biol. 2014;16:982-991, 1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22 Suppl 2:S96-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Shuto M, Warigaya K, Watanabe H, Shimizu M, Fukuda T, Murata S. Correlation analysis of nuclear morphology, cytokeratin and Ki-67 expression of urothelial carcinoma cells. Pathol Int. 2013;63:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Dancik GM, Owens CR, Iczkowski KA, Theodorescu D. A cell of origin gene signature indicates human bladder cancer has distinct cellular progenitors. Stem Cells. 2014;32:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Moad M, Pal D, Hepburn AC, Williamson SC, Wilson L, Lako M, Armstrong L, Hayward SW, Franco OE, Cates JM. A novel model of urinary tract differentiation, tissue regeneration, and disease: reprogramming human prostate and bladder cells into induced pluripotent stem cells. Eur Urol. 2013;64:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Jóźwicki W, Brożyna AA, Siekiera J. Expression of OCT4A: the first step to the next stage of urothelial bladder cancer progression. Int J Mol Sci. 2014;15:16069-16082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Garg M. Urothelial cancer stem cells and epithelial plasticity: current concepts and therapeutic implications in bladder cancer. Cancer Metastasis Rev. 2015;34:691-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J, Chang HY, van de Rijn M. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:14016-14021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 494] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 9. | Keymoosi H, Gheytanchi E, Asgari M, Shariftabrizi A, Madjd Z. ALDH1 in combination with CD44 as putative cancer stem cell markers are correlated with poor prognosis in urothelial carcinoma of the urinary bladder. Asian Pac J Cancer Prev. 2014;15:2013-2020. [PubMed] |
| 10. | Bentivegna A, Conconi D, Panzeri E, Sala E, Bovo G, Viganò P, Brunelli S, Bossi M, Tredici G, Strada G. Biological heterogeneity of putative bladder cancer stem-like cell populations from human bladder transitional cell carcinoma samples. Cancer Sci. 2010;101:416-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Hofner T, Macher-Goeppinger S, Klein C, Schillert A, Eisen C, Wagner S, Rigo-Watermeier T, Baccelli I, Vogel V, Trumpp A. Expression and prognostic significance of cancer stem cell markers CD24 and CD44 in urothelial bladder cancer xenografts and patients undergoing radical cystectomy. Urol Oncol. 2014;32:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Zhu D, Wan X, Huang H, Chen X, Liang W, Zhao F, Lin T, Han J, Xie W. Knockdown of Bmi1 inhibits the stemness properties and tumorigenicity of human bladder cancer stem cell-like side population cells. Oncol Rep. 2014;31:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | He F, Melamed J, Tang MS, Huang C, Wu XR. Oncogenic HRAS Activates Epithelial-to-Mesenchymal Transition and Confers Stemness to p53-Deficient Urothelial Cells to Drive Muscle Invasion of Basal Subtype Carcinomas. Cancer Res. 2015;75:2017-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Garg M. Epithelial-mesenchymal transition - activating transcription factors - multifunctional regulators in cancer. World J Stem Cells. 2013;5:188-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 15. | Franzen CA, Blackwell RH, Todorovic V, Greco KA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis. 2015;4:e163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 16. | Bryan RT. Cell adhesion and urothelial bladder cancer: the role of cadherin switching and related phenomena. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271-11278. [PubMed] |
| 18. | Enkelmann A, Heinzelmann J, von Eggeling F, Walter M, Berndt A, Wunderlich H, Junker K. Specific protein and miRNA patterns characterise tumour-associated fibroblasts in bladder cancer. J Cancer Res Clin Oncol. 2011;137:751-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Garg M. Prognostic and therapeutic applications of the molecular events in clinical management of urothelial carcinoma of bladder. J Exp Ther Oncol. 2014;10:301-316. [PubMed] |
| 20. | Skowron MA, Niegisch G, Fritz G, Arent T, van Roermund JG, Romano A, Albers P, Schulz WA, Hoffmann MJ. Phenotype plasticity rather than repopulation from CD90/CK14+ cancer stem cells leads to cisplatin resistance of urothelial carcinoma cell lines. J Exp Clin Cancer Res. 2015;34:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Zhang Y, Wang Z, Yu J, Shi Jz, Wang C, Fu Wh, Chen Zw, Yang J. Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett. 2012;322:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Shen H, Blijlevens M, Yang N, Frangou C, Wilson KE, Xu B, Zhang Y, Zhang L, Morrison CD, Shepherd L. Sox4 Expression Confers Bladder Cancer Stem Cell Properties and Predicts for Poor Patient Outcome. Int J Biol Sci. 2015;11:1363-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | van der Horst G, Bos L, van der Mark M, Cheung H, Heckmann B, Clément-Lacroix P, Lorenzon G, Pelger RC, Bevers RF, van der Pluijm G. Targeting of alpha-v integrins reduces malignancy of bladder carcinoma. PLoS One. 2014;9:e108464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Black PC, Brown GA, Inamoto T, Shrader M, Arora A, Siefker-Radtke AO, Adam L, Theodorescu D, Wu X, Munsell MF. Sensitivity to epidermal growth factor receptor inhibitor requires E-cadherin expression in urothelial carcinoma cells. Clin Cancer Res. 2008;14:1478-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Garg M. Targeting microRNAs in epithelial-to-mesenchymal transition-induced cancer stem cells: therapeutic approaches in cancer. Expert Opin Ther Targets. 2015;19:285-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 26. | Zhang K, Shi B, Chen J, Zhang D, Zhu Y, Zhou C, Zhao H, Jiang X, Xu Z. Bone marrow mesenchymal stem cells induce angiogenesis and promote bladder cancer growth in a rabbit model. Urol Int. 2010;84:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Majid S, Dar AA, Saini S, Deng G, Chang I, Greene K, Tanaka Y, Dahiya R, Yamamura S. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS One. 2013;8:e67686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Tsui KH, Lin YH, Chung LC, Chuang ST, Feng TH, Chiang KC, Chang PL, Yeh CJ, Juang HH. Prostate-derived ets factor represses tumorigenesis and modulates epithelial-to-mesenchymal transition in bladder carcinoma cells. Cancer Lett. 2016;375:142-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Zhao L, Geng H, Liang ZF, Zhang ZQ, Zhang T, Yu DX, Zhong CY. Benzidine induces epithelial-mesenchymal transition in human uroepithelial cells through ERK1/2 pathway. Biochem Biophys Res Commun. 2015;459:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Comberg D, Gauer A, Tschernig T. First findings of gap junction proteins in human urothelial carcinoma. World J Urol. 2016;34:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |