Published online Nov 26, 2016. doi: 10.4252/wjsc.v8.i11.384
Peer-review started: June 3, 2016
First decision: July 5, 2016
Revised: July 22, 2016
Accepted: September 21, 2016
Article in press: September 22, 2016
Published online: November 26, 2016
Processing time: 174 Days and 21.6 Hours
To investigate β-catenin (CTNNB1) signaling in cancer and stem cells, the gene expression and pathway were analyzed using bioinformatics.
The expression of the catenin β 1 (CTNNB1) gene, which codes for β-catenin, was analyzed in mesenchymal stem cells (MSCs) and gastric cancer (GC) cells. Beta-catenin signaling and the mutation of related proteins were also analyzed using the cBioPortal for Cancer Genomics and HOMology modeling of Complex Structure (HOMCOS) databases.
The expression of the CTNNB1 gene was up-regulated in GC cells compared to MSCs. The expression of EPH receptor A8 (EPHA8), synovial sarcoma translocation chromosome 18 (SS18), interactor of little elongation complex ELL subunit 1 (ICE1), patched 1 (PTCH1), mutS homolog 3 (MSH3) and caspase recruitment domain family member 11 (CARD11) were also shown to be altered in GC cells in the cBioPortal for Cancer Genomics analysis. 3D complex structures were reported for E-cadherin 1 (CDH1), lymphoid enhancer binding factor 1 (LEF1), transcription factor 7 like 2 (TCF7L2) and adenomatous polyposis coli protein (APC) with β-catenin.
The results indicate that the epithelial-mesenchymal transition (EMT)-related gene CTNNB1 plays an important role in the regulation of stem cell pluripotency and cancer signaling.
Core tip: β-catenin signaling consists of several pathway cascades, such as those that are involved in pluripotent stem cell generation and cancer. Several genes, including EPHA8, SS18, ICE1, PTCH1, MSH3 and CARD11, are mutated along with CTNNB1. The expression of the CTNNB1, CDH1, MYC, LEF1 and TCF7L2 genes, which are related to the CTNNB1 network, is up-regulated in diffuse-type GC cells compared to MSCs. 3D complex structures for β-catenin (CTNB1_HUMAN) with LEF_MOUSE and TF7L2_HUMAN were found using the HOMCOS database. The EMT-related gene CTNNB1 plays an important role in pluripotent stem cell signaling and cancer signaling.
- Citation: Tanabe S, Kawabata T, Aoyagi K, Yokozaki H, Sasaki H. Gene expression and pathway analysis of CTNNB1 in cancer and stem cells. World J Stem Cells 2016; 8(11): 384-395
- URL: https://www.wjgnet.com/1948-0210/full/v8/i11/384.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v8.i11.384
Changes in the phenotypes of cancer and stem cells are related to changes in gene expression and protein signaling. This study aims to reveal the β-catenin (CTNNB1) regulation in diffuse-type gastric cancer (GC) cells and mesenchymal stem cells (MSCs). Wnt/β-catenin signaling is necessary for epithelial-mesenchymal transitions (EMT)[1]. Stem cell division is strongly correlated with cancer risk, and this highlights the importance of molecular signaling in stem cells and cancer cells[2]. Epigenetics and stem cell functions are regulated by several exogenous stimuli, including cell-cell and cell-matrix interactions[3]. To ensure the safety of therapeutic stem cell applications in terms of stem cell modification, an understanding of the regulation of the stem cells and their niche is necessary[4]. In the case of bone metastasis, the tissue-specific stromal response for prostate cancer can be identified by a molecular signature for which a novel mechanism has been revealed in hematopoietic and prostate epithelial stem cell niches[5].
Cancer stem cell (CSC) maintenance requires hypoxia-inducible factor (HIF)-α transcription factors and the inhibitor of DNA binding 2 (ID2)[6]. The down-regulated expression of ID2 is associated with a poor prognosis in hepatocellular carcinoma[7].
Because the compendium of gene expression, chromosomal copy number and sequencing data from human cancer cell lines, which is called the Cancer Cell Line Encyclopedia (CCLE), has revealed that genomic data are capable of predicting anti-cancer drug sensitivity, molecular and network analyses should be carried out[8]. It has been reported that cadherin 1 (CDH1) is up-regulated in diffuse-type GC cells compared to MSCs[9]. However, CDH2 was down-regulated in diffuse-type GC cells compared to MSCs; this provides a useful indicator - the ratio of CDH2 to CDH1 expression - to distinguish the mesenchymal and epithelial phenotypes of the cells[9]. It has been reported that catenin β 1 (CTNNB1) is mutated in hepatocellular carcinoma[10,11]. To further elucidate the EMT phenotype and the molecules that are involved in β-catenin signaling in cancer, the CTNNB1 network and the β-catenin binding partners have been investigated in this report using bioinformatics tools such as microarray analysis and databases.
Gene expression in MSCs (n = 12) and diffuse-type GC cells (n = 5) was analyzed using Human Genome U133 Plus 2.0 microarrays, as previously described[9,12]. In brief, total RNA was purified from the cells, biotinylated and hybridized to microarrays. The signal intensity of each gene transcript was analyzed and compared between MSCs and diffuse-type GC cells. The microarray data for MSCs and diffuse-type GC cells are available to the public in NCBI’s Gene Expression Omnibus (GEO) database and are accessible via GEO Series accession number GSE7888 (https://http://http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE7888) and GSE42252 (https://http://http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE42252), respectively[9,12].
Diffuse-type GC tissues were originally provided by the National Cancer Center Hospital after obtaining written informed consent from each patient and approval by National Cancer Center Institutional Review Board. All cancer specimens were reviewed and classified histopathologically according to the Japanese Classification of Gastric Cancer. Tissue specimens were immediately frozen with liquid nitrogen after surgical extraction, and stored at -80 °C until microarray analysis[9,13]. The existing data already available to the public were analyzed in the article.
The cancer genomics data analysis was performed relative to CTNNB1 using the cBioPortal for Cancer Genomics (http://www.cbioportal.org)[14,15]. The term “CTNNB1” was searched in the cBioPortal for Cancer Genomics database, and a cross-cancer alteration summary was obtained for CTNNB1. A study on stomach adenocarcinoma was further analyzed for enrichments[16]. Genes with mutations that were enriched in samples that contained altered CTNNB1 were selected in the cBioPortal for cancer genomics for further study.
3D complex structures were searched in the HOMology modeling of COmplex Structure (HOMCOS) database (http://homcos.pdbj.org) using the search engine that was provided by the VaProS server (http://pford.info/vapros)[17]. The UniProtID “CTNB_HUMAN” was input as the query for the “searching contact molecule” field of the HOMCOS. Only close homologues (sequence identity > 95%) were selected. The complex structures that were found were superimposed using the MATRAS program[18].
The data were expressed as the mean ± SE. For the statistics, Student’s t test was used. P < 0.01 was considered as statistically significant.
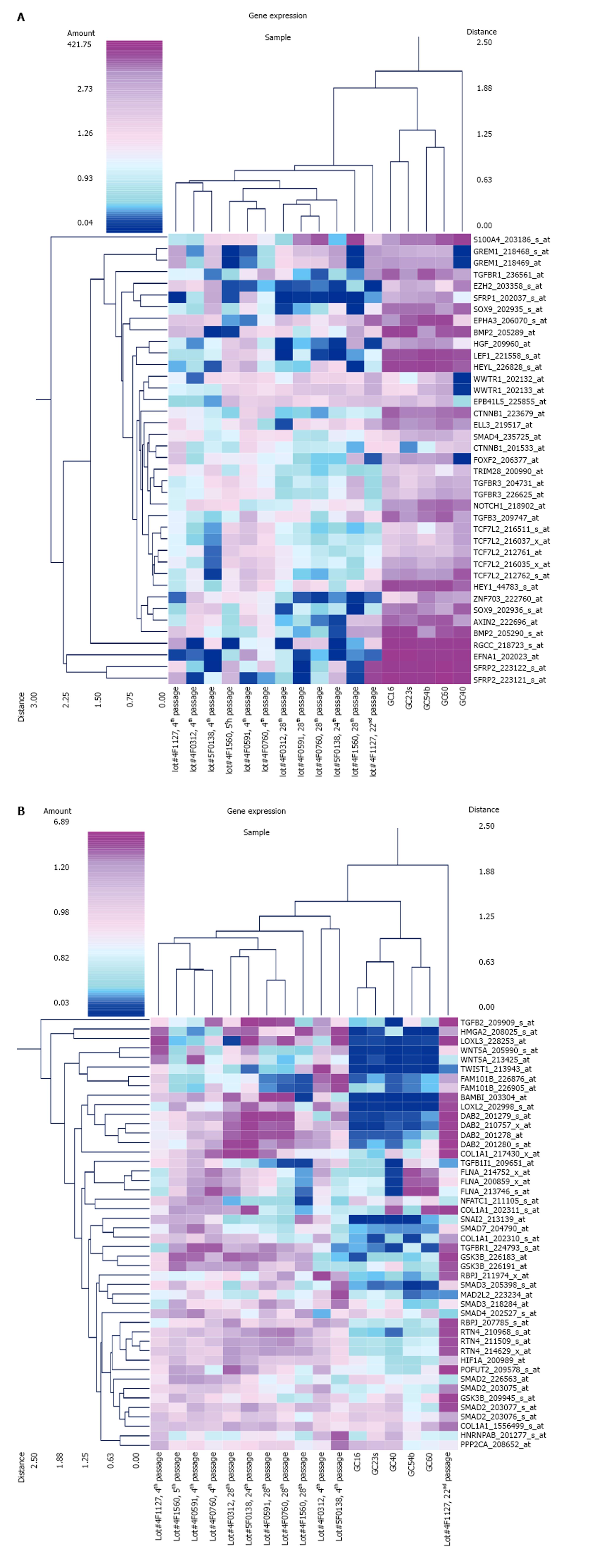
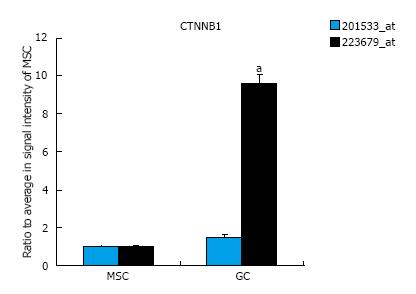
The expression of EMT-related genes in MSCs and diffuse-type GC cells is shown in Figure 1. The genes for which probe sets included the “EMT” term in the Gene Ontology (GO) Biological Process field were selected as EMT-related genes. The average signal intensity for early-stage MSCs, late-stage MSCs, or GC cells was greater than 500. Panel A shows the results of a cluster analysis of 39 probe sets that were up-regulated in diffuse-type GC cells compared to early-stage MSCs (n = 6 in early-stage MSCs, n = 6 in late-stage MSCs, n = 5 in GC). Panel B shows the results of a cluster analysis of 46 probe sets that were down-regulated in diffuse-type GC cells compared to early-stage MSCs (n = 6 in early-stage MSCs, n = 6 in late-stage MSCs, n = 5 in GC). To evaluate CTNNB1 expression in cancer and stem cells, the expression of the CTNNB1 gene was compared in MSCs and diffuse-type GC cells, and the results indicate that CTNNB1 is up-regulated in GC cells (Figure 2). One of the probe sets was up-regulated more than 8-fold over its expression level in MSCs, whereas the other probe sets showed no increases in expression in GC cells compared to MSCs.
3D complex structures of β-catenin
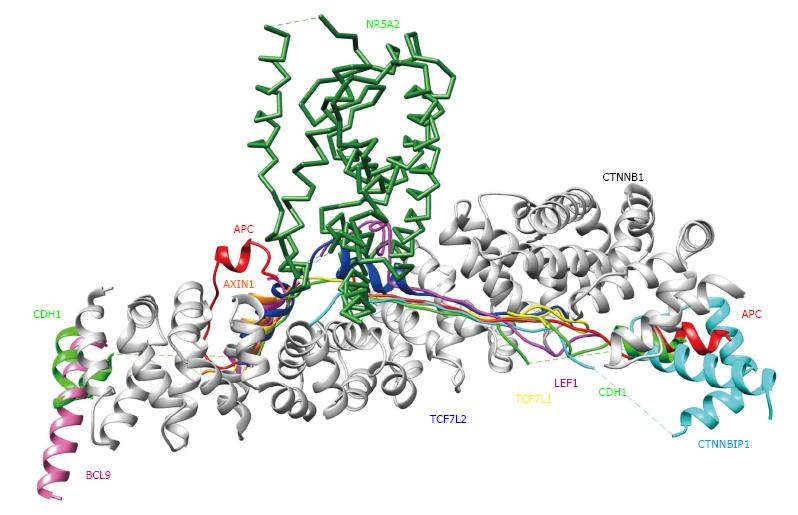
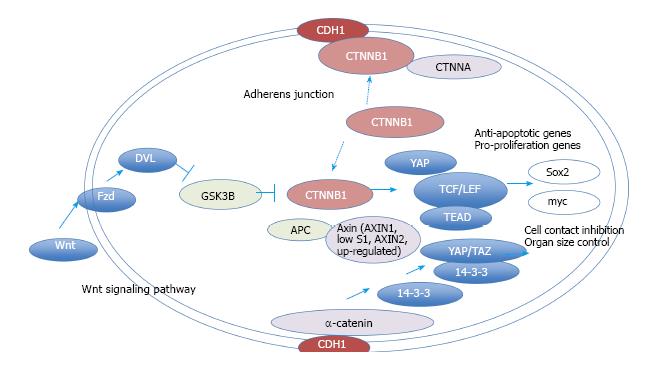
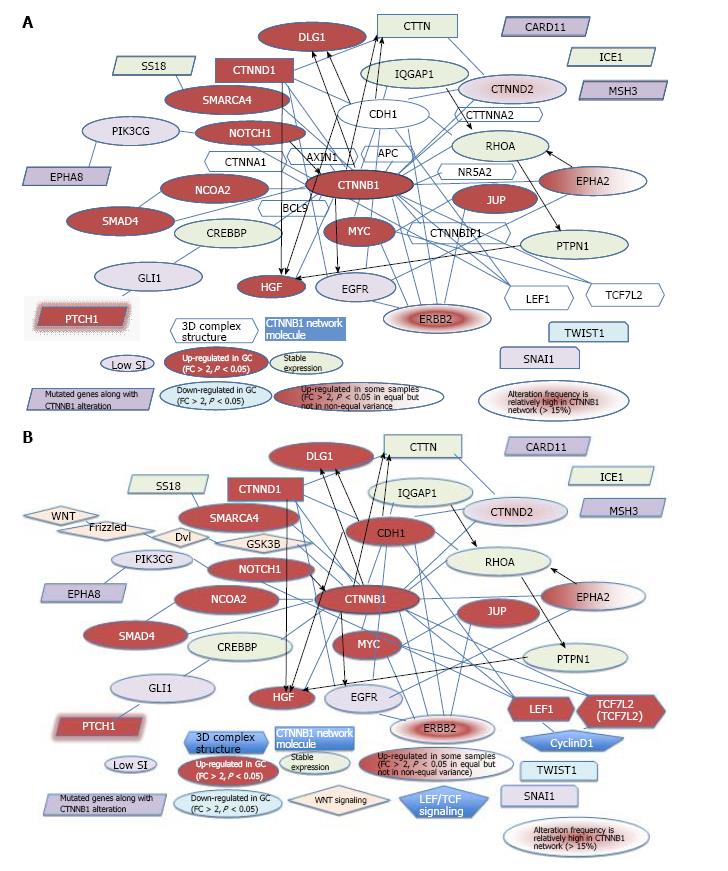
To verify and explore protein-protein interactions with β-catenin, 3D complex structures of β-catenin were found using the HOMCOS database (http://homcos.pdbj.org)[17] and are summarized in Table 1. Figure 3 shows the superimposed 3D structure of the complex. Most of the proteins bind to the inner concave surface of the armadillo repeat region of β-catenin by using their 40-60 residue length extended peptides [adenomatous polyposis coli protein (APC), E-cadherin 1 (CDH1), catenin beta interacting protein 1 (CTNNBIP1), lymphoid enhancer binding factor 1 (LEF1), transcription factor 7 like 1 (TCF7L1) and transcription factor 7 like 2 (TCF7L2)]. CDH1 and B-cell CLL/lymphoma 9 (BCL9) bind to the N-terminal region of the repeat that has the alpha-helical peptides. The nuclear receptor subfamily 5 group A member 2 (NR5A2) ligand binding domain binds to the middle of the armadillo repeat region. Of these binding factors, the transcription of the CDH1, LEF1 and TCF7L2 genes was up-regulated in GC cells (Table 1). It has been reported that a small molecule antagonist of the β-catenin/T-cell transcription factor 4 [TCF4; official name is transcription factor 7 like 2 (TCF7L2)] interaction inhibits self-renewal of CSCs and suppresses tumorigenesis[19]. The 3D complex structures of β-catenin and TCF7L2 are available[20,21]. The complex structure of NR5A2 has also been reported[22]. NR5A2 (or liver receptor homolog-1; LRH1) is a member of the nuclear hormone receptor family of transcription factors that play essential roles in development, metabolism, and cancer and are implicated in Wnt/β-catenin signaling[22]. NR5A2 is essential for the early development and maintenance of pluripotent mouse embryonic stem (ES) cells[22,23]. Network models for CTNNB1, the Wnt signaling pathway, Hippo signaling pathway and adherens junction signaling in cancer are shown in Figure 4. CTNNB1 binds to CDH1 near the cellular membrane or to TCF to transcribe anti-apoptotic or pro-proliferation genes, such as SRY-box 2 (SOX2) or v-myc avian myelocytomatosis viral oncogene homolog (MYC) (Figure 4). Wnt stimulation prevents glycogen synthase kinase 3 beta (GSK3β) from phosphorylating CTNNB1 and leads to CTNNB1 translocation into the nucleus to induce transcription. The 3D complex structure (PDB code: 1m1e) clearly shows how CDH1 binds to CTNNB1 in the mouse model.
| pdb_id | β-catenin (CTNNB1) | Proteins that interact with β-catenin | |||||||
| ChainID | Length | UniProtID | Molecule | ChainID | Length | UniProtID | Contact protein name | Regulation of gene expression in GC cells compared to MSCs | |
| 1th1 | B | 513 | CTNB1_HUMAN | APC | D | 54 | APC_HUMAN | Adenomatous polyposis coli protein | Not changed/- |
| 1qz7 | A | 524 | CTNB1_HUMAN | AXIN1 | B | 17 | AXN_XENLA | Axin-1 | - |
| 3sl9 | B | 165 | CTNB1_HUMAN | BCL9 | D | 23 | BCL9_HUMAN | B-cell CLL/lymphoma 9 protein | - |
| 1i7w | C | 509 | CTNB1_MOUSE | CDH1 | D | 60 | CADH1_MOUSE | Cadherin-1 | Up-regulated |
| 1m1e | A | 512 | CTNB1_MOUSE | CTNNBIP1 | B | 65 | CNBP1_HUMAN | Beta-catenin-interacting protein 1 | - |
| 3oux | A | 503 | CTNB1_MOUSE | LEF1 | B | 47 | LEF1_MOUSE | Lymphoid enhancer-binding factor 1 | Up-regulated |
| 3tx7 | A | 504 | CTNB1_HUMAN | NR5A2 | B | 218 | NR5A2_HUMAN | Nuclear receptor subfamily 5 group A member 2 | - |
| 1g3j | A | 439 | CTNB1_HUMAN | TCF7L1 | B | 34 | T7L1A_XENLA | Transcription factor 7-like 1-A | - |
| 1jdh | A | 508 | CTNB1_HUMAN | TCF7L2 | B | 38 | TF7L2_HUMAN | Transcription factor 7-like 2 | Up-regulated |
| 1dow | B | 32 | CTNB1_MOUSE | CTNNA1 | A | 205 | CTNA1_MOUSE | Catenin alpha-1 | Not changed/- |
| 4ons | D | 56 | CTNB1_MOUSE | CTNNA2 | C | 230 | CTNA2_MOUSE | Catenin alpha-2 | - |
CTNNB1 is listed in 21 pathways in Kyoto Encyclopedia of Genes and Genomes (KEGG), including the Rap1 signaling pathway, Wnt signaling pathway, Hippo signaling pathway, focal adhesion regulation, adherens junction regulation, tight junction regulation, signaling pathways that regulate the pluripotency of stem cells, leukocyte transendothelial migration, melanogenesis, the thyroid hormone signaling pathway; bacterial invasion of epithelial cells, pathogenic Escherichia coli infection, HTLV-I infection, and various cancer pathways. The following conditions use the aforementioned pathways and are also thus implicated: Proteoglycans in cancer, colorectal cancer, endometrial cancer, prostate cancer, thyroid cancer, basal cell carcinoma, and arrhythmogenic right ventricular cardiomyopathy (ARVC) (http://www.genome.jp/dbget-bin/www_bget?hsa:1499). The inhibition of GSK3β kinase activates β-catenin, which stimulates endoderm induction via the degradation of Tcf7l1 and forkhead box A2 (FoxA2) expression[24]. Wnt signaling induces intracellular β-catenin signaling via GSK3β kinase inhibition and dephosphorylation of β-catenin[25-28]. The inhibition of β-catenin decreases proliferation and induces apoptosis in the mantle cell lymphoma cell line[29]. Noncanonical Wnt signaling is activated in circulating tumor cells from the prostate that are anti-androgen-resistant[30].
The Cancer Genome Atlas Research Network project has indicated that there is a characteristic molecular signature for ras homolog family member A (RHOA) mutations in diffuse type stomach adenocarcinoma[16]. Two-hundred and ninety-five primary gastric adenocarcinomas have been investigated, and mutations in RHOA have been enriched in genomically stable subtype, diffuse-type GC cells[16]. The analysis with cBioPortal showed that CTNNB1 was altered in 24 (8%) of 287 cases/patients in stomach adenocarcinoma: 4 amplifications, 2 deep deletions, 12 missense mutations, 5 truncating mutations and 1 inframe mutation. Several gene mutations occurred concurrently with CTNNB1 alterations in stomach adenocarcinoma (Table 2). The development of mutations in EPH receptor A8 (EPHA8), synovial sarcoma translocation chromosome 18 (SS18), interactor of little elongator complex ELL subunit 1 (ICE1), patched 1 (PTCH1), mutS homolog 3 (MSH3) and caspase recruitment domain family member 11 (CARD11) occurred alongside the CTNNB1 alterations (Table 2). Of the mutated genes, PTCH1 expression was up-regulated in GC cells compared to MSCs (Table 2). The GO of the mutated genes is shown in Table 3. EPHA8 possesses kinase activity, SS18 is involved in cell morphogenesis, ICE1 may play a role in positive regulation of intracellular protein transport, PTCH1 is involved in morphogenesis and cell growth, MSH3 is involved in mismatch repair, and CARD11 regulates B cell proliferation, apoptosis and NF-κB signaling, according to GO biological process (Table 3). GO biological process terms in Table 3 are based on Affymetrix annotation (http://www.affymetrix.com/estore/) and gene information in NCBI (http://www.ncbi.nlm.nih.gov/).
| Gene symbol | Gene title | Cytoband | Mutation percentage | Log ratio | P-value | Ratio of GC cells to MSCs | |
| In altered group | In unaltered group | ||||||
| EPHA8 | EPH receptor A8 | 1p36.12 | 29.17% | 2.28% | 3.68 | 1.45E-05 | Signal intensity is low |
| SS18 | Synovial sarcoma translocation Chromosome 18 | 18q11.2 | 16.67% | 0.00% | > 10 | 3.84E-05 | 0.6 |
| 1.4 | |||||||
| ICE1 | Interactor of little elongator complex ELL subunit 1 | 5p15.32 | 33.33% | 4.56% | 2.87 | 4.74E-05 | 1.5 |
| PTCH1 | Patched 1 | 9q22.3 | 29.17% | 3.42% | 3.09 | 8.16E-05 | 16.6 |
| MSH3 | MutS homolog 3 | 5q14.1 | 20.83% | 1.14% | 4.19 | 1.28E-04 | Signal intensity is low |
| CARD11 | Caspase recruitment domain family, member 11 | 7p22 | 29.17% | 4.18% | 2.8 | 2.03E-04 | Signal intensity is low |
| Gene symbol | Gene ontology biological process |
| EPHA8 | Protein phosphorylation // substrate-dependent cell migration // cell adhesion // transmembrane receptor protein tyrosine kinase signaling pathway // multicellular organismal development // nervous system development // axon guidance // phosphorylation // neuron remodeling // peptidyl-tyrosine phosphorylation // regulation of cell adhesion // neuron projection development // regulation of cell adhesion mediated by integrin // positive regulation of MAPK cascade // positive regulation of phosphatidylinositol 3-kinase activity // protein autophosphorylation // ephrin receptor signaling pathway |
| SS18 | Microtubule cytoskeleton organization // cell morphogenesis // transcription, DNA-templated // regulation of transcription, DNA-templated // cytoskeleton organization // response to drug // positive regulation of transcription from RNA polymerase II promoter // ephrin receptor signaling pathway |
| ICE1 | Positive regulation of intracellular protein transport // positive regulation of protein complex assembly // positive regulation of transcription from RNA polymerase III promoter // snRNA transcription from RNA polymerase II promoter // snRNA transcription from RNA polymerase III promoter |
| PTCH1 | Negative regulation of transcription from RNA polymerase II promoter // branching involved in ureteric bud morphogenesis // neural tube formation // neural tube closure // heart morphogenesis // signal transduction // smoothened signaling pathway // smoothened signaling pathway // regulation of mitotic cell cycle // pattern specification process // brain development // negative regulation of cell proliferation // epidermis development // regulation of smoothened signaling pathway // response to mechanical stimulus // organ morphogenesis // dorsal/ventral pattern formation // response to chlorate // positive regulation of cholesterol efflux // response to organic cyclic compound // protein processing // spinal cord motor neuron differentiation // neural tube patterning // dorsal/ventral neural tube patterning // neural plate axis specification // embryonic limb morphogenesis // mammary gland development // response to estradiol // response to retinoic acid // regulation of protein localization // limb morphogenesis // hindlimb morphogenesis // regulation of growth // negative regulation of multicellular organism growth // regulation of cell proliferation // response to drug // glucose homeostasis // negative regulation of sequence-specific DNA binding transcription factor activity // keratinocyte proliferation // negative regulation of osteoblast differentiation // negative regulation of smoothened signaling pathway // negative regulation of smoothened signaling pathway // negative regulation of epithelial cell proliferation // negative regulation of cell division // pharyngeal system development // mammary gland duct morphogenesis // mammary gland epithelial cell differentiation // smoothened signaling pathway involved in dorsal/ventral neural tube patterning // cell differentiation involved in kidney development // somite development // cellular response to cholesterol // cellular response to cholesterol // renal system development // cell proliferation involved in metanephros development // protein targeting to plasma membrane |
| MSH3 | Meiotic mismatch repair // ATP catabolic process // DNA repair // mismatch repair // cellular response to DNA damage stimulus // reciprocal meiotic recombination // somatic recombination of immunoglobulin gene segments // maintenance of DNA repeat elements // negative regulation of DNA recombination // positive regulation of helicase activity |
| CARD11 | Positive regulation of cytokine production // signal transduction // positive regulation of B cell proliferation // T cell costimulation // Fc-epsilon receptor signaling pathway // positive regulation of T cell proliferation // regulation of apoptotic process // positive regulation of I-kappaB kinase/NF-kappaB signaling // thymic T cell selection // positive regulation of interleukin-2 biosynthetic process // innate immune response // regulation of B cell differentiation // regulation of T cell differentiation // nucleotide phosphorylation // regulation of immune response // T cell receptor signaling pathway // positive regulation of T cell activation // positive regulation of NF-kappaB transcription factor activity |
β-catenin signaling model
Several β-catenin-binding proteins, such as LEF1 or TCF7L2, share high mobility group (HMG)-box domains, which suggests that β-catenin signaling switches mechanisms with the binding of different transcription factors. 3D complex structures show that CDH1, LEF1 and TCF7L2 bind to β-catenin. The role of β-catenin signaling in the pluripotentcy pathway should be investigated to reveal its mechanism in cancer and stem cells. The Wnt pathway is located upstream, and TCF, downstream of CTNNB1 in the cascade[31]. The merged network model of the β-catenin signaling network and CDH1, together with molecules in the 3D complex structures and genes mutated along with the CTNNB1 alteration is shown in Figure 5A. The merged network model of the CTNNB1, Wnt, and TCF signaling networks and CDH1, together with molecules in the 3D complex structures and genes mutated along with the CTNNB1 alteration is shown in Figure 5B. Of the common genes, EPHA8, SS18 and PTCH1 interact with phosphatidylinositol-4,5-bisphosphate-3-kinase catalytic subunit gamma (PIK3CG), SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4 (SMARCA4), and GLI family zinc finger 1 (GLI1), respectively, whereas CARD11, ICE1, MSH3 have no known interactions with molecules in the CTNNB1 network. The networks for stomach adenocarcinoma that were generated using cBioPortal for Cancer Genomics for CTNNB1 alone and for CTNNB1 with the 6 genes that are mutated along with the CTNNB1 alteration have been partially merged in Figure 5. Catenin delta 2 (CTNND2) and erb-b2 receptor tyrosine kinase 2 (ERBB2) showed a relatively high frequency of mutation (> 15% in 287 tumor samples) in the analysis using cBioPortal for Cancer Genomics of the CTNNB1 network in stomach adenocarcinoma (TCGA, Nature 2014)[16]. The genes that were up-regulated in GC cells compared to MSCs are shown in red, whereas the down-regulated genes are shown in light blue (Fold change > 2, P < 0.05, n = 12 in MSCs, n = 5 in GC; the average signal intensity of MSCs or GC cells is greater than 500). The expression of the CTNNB1, CDH1, notch1 (NOTCH1), hepatocyte growth factor (HGF), PTCH1, discs large homolog 1, scribble cell polarity complex component (DLG1), LEF1, CTNND1, SMARCA4, nuclear receptor coactivator 2 (NCOA2), SMAD family member 4 (SMAD4), MYC, junction plakoglobin (JUP), TCF7L2 and ERBB2 genes was up-regulated in GC cells compared to MSCs, whereas the expression of twist family bHLH transcription factor 1 (TWIST1) was down-regulated in GC cells compared to MSCs. The expression of EPHA2 was up-regulated in some GC samples. The expression of the IQ motif-containing GTPase activating protein 1 (IQGAP1), SS18, ICE1, cortactin (CTTN), RHOA, CREB binding protein (CREBBP) and protein tyrosine phosphatase, non-receptor (PTPN1) genes was not altered in MSCs and GC cells. The expression of the EPHA8, PIK3CG, CARD11, MSH3, GLI1, epidermal growth factor receptor (EGFR), snail family zinc finger 1 (SNAI1) and CTNND2 genes was not examined due to a low signal intensity. The alteration frequencies of CTNND2 and ERBB2 are relatively high in the CTNNB1 network (> 15%), according to the cBioPortal for Cancer Genomics. Interestingly, IQGAP2 was up-regulated in GC cells compared to MSCs.
In summary, the CTNNB1 gene expression was up-regulated in diffuse-type GC compared to MSC. The various molecules are regulated with CTNNB1, which suggests the CTNNB1 signaling network in cancer and stem cells. EMT-related genes have been reported to be induced by transforming growth factor (TGF)-β or epidermal growth factor (EGF), and genes in the Wnt signaling pathway are mutated in non-small cell lung cancer[32-34]. The expression of β-catenin was up-regulated in the TGF-β1-induced EMT model and was inhibited by cucurbitacin B treatment[32]. Solid tumors induce hypoxia, leading to HIF-1α protein regulation of molecules that are involved in angiogenesis, erythropoiesis, metabolism, cell survival and cell proliferation[35]. SNAI2 and TWIST1 were down-regulated in GC cells compared to MSCs, whereas SNAI1 expression was not detected because of low signal intensity[9,36,37]. Because SNAI and TWIST are associated with EMT, the regulation of their expression is important for understanding EMT mechanisms. Although 3D complex structures of SNAI2 and TWIST1 with β-catenin are not available, some indirect β-catenin signaling cascade may be involved in the SNAI2 and TWIST1 pathway[38,39]. TGFβ is also an important factor in EMT[40]. TGFβ regulates osteoblast differentiation, whereas calycosin-7-O-β-D-glucopyranoside-induced osteoblast differentiation is regulated via the bone morphogenetic protein (BMP) and Wnt/β-catenin-signaling pathway[41]. The TGFβ-induced nuclear translocation of β-catenin has been reported to be one of the key factors that activates the EMT program[42-45]. Wnt/β-catenin is regulated in stem cells, and Wnt target genes are controlled by the TCF/β-catenin complex[46].
In gastrointestinal cancer, somatic mutations that provoke an immune response have been found in tumor-infiltrating lymphocytes, which may be very specific to the individual and are targets for cancer immunotherapy[47]. KRAS-mutation-specific T cells, as well as personalized mutation-specific T cells, have been identified, and these may be useful in the future for individual cancer immunotherapeutics[47]. It has been reported that Helicobacter pylori up-regulates Nanog and Oct4 expression via Wnt/β-catenin signaling[48]. Wnt/β-catenin signaling and the phosphorylation of β-catenin may be involved in stemness in gastric cancer[48].
In conclusion, CTNNB1 plays an important role in the regulation of stem cell pluripotency and cancer signaling. For future direction, precise analyses of Wnt signaling, Notch signaling, and Ephrin signaling are needed to reveal the entire picture of β-catenin signaling in cancer and stem cells. RHO mutations, and regulator of G-protein signaling, with network analysis tools, such as Cytoscape, must be investigated for a greater understanding of this process.
The authors would like to thank Professor Kei Yura for introducing the VaProS database. This study used VaProS, a data-cloud developed by the Information Core of the Platform Project for Supporting Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science) from the Japan Agency for Medical Research and Development (AMED).
β-catenin signaling is essential in pluripotent stem cells and cancer. It is also involved in the epithelial-mesenchymal transitions (EMT). CTNNB1 is activated by Wnt, and the binding of CTNNB1 to transcription factors leads to pluripotent gene regulation.
The regulation of pluripotency and proliferation is important for elucidating the mechanism of cell phenotype transitions. The EMT mechanism should be investigated to better understand cancer resistance to therapeutics.
The 3D complex structures of β-catenin and related molecules were studied using molecular networks, which is an innovation in the field. The mutated genes that were altered along with CTNNB1 in stomach adenocarcinoma samples were also investigated.
These results may affect the study of the pluripotency mechanism and potential therapeutic predictions of gastric cancer. The genes in the molecular network that are related to CTNNB1 may be the targets of predictive medicine for cancer and disease using pluripotent cells.
EMT is a cellular phenotype of a transition from an epithelial to a mesenchymal cell type. EMT is regulated in cancer metastasis and malignancy, and it is related to the acquisition of resistance in cancer cells to therapeutics. It is important to understand the EMT mechanism to understand the mechanisms of cancer resistance.
In general, the manuscript is interesting not only for scientific reasons, but also due to its potential clinical relevance, since it provides some light about the relationships between stem and cancer cells.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dawe G, Politi L S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Shan S, Lv Q, Zhao Y, Liu C, Sun Y, Xi K, Xiao J, Li C. Wnt/β-catenin pathway is required for epithelial to mesenchymal transition in CXCL12 over expressed breast cancer cells. Int J Clin Exp Pathol. 2015;8:12357-12367. [PubMed] |
| 2. | Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 442] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 3. | Crowder SW, Leonardo V, Whittaker T, Papathanasiou P, Stevens MM. Material Cues as Potent Regulators of Epigenetics and Stem Cell Function. Cell Stem Cell. 2016;18:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 4. | Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2599] [Cited by in RCA: 2505] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 5. | Özdemir BC, Hensel J, Secondini C, Wetterwald A, Schwaninger R, Fleischmann A, Raffelsberger W, Poch O, Delorenzi M, Temanni R. The molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights expansion of hematopoietic and prostate epithelial stem cell niches. PLoS One. 2014;9:e114530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Lee SB, Frattini V, Bansal M, Castano AM, Sherman D, Hutchinson K, Bruce JN, Califano A, Liu G, Cardozo T. An ID2-dependent mechanism for VHL inactivation in cancer. Nature. 2016;529:172-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Tsunedomi R, Iizuka N, Harada S, Oka M. Susceptibility of hepatoma-derived cells to histone deacetylase inhibitors is associated with ID2 expression. Int J Oncol. 2013;42:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5947] [Cited by in RCA: 5820] [Article Influence: 447.7] [Reference Citation Analysis (0)] |
| 9. | Tanabe S, Aoyagi K, Yokozaki H, Sasaki H. Gene expression signatures for identifying diffuse-type gastric cancer associated with epithelial-mesenchymal transition. Int J Oncol. 2014;44:1955-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, Tsuji S, Donehower LA, Slagle BL, Nakamura H. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 611] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 11. | Chang MT, Asthana S, Gao SP, Lee BH, Chapman JS, Kandoth C, Gao J, Socci ND, Solit DB, Olshen AB. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat Biotechnol. 2016;34:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 603] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 12. | Tanabe S, Sato Y, Suzuki T, Suzuki K, Nagao T, Yamaguchi T. Gene expression profiling of human mesenchymal stem cells for identification of novel markers in early- and late-stage cell culture. J Biochem. 2008;144:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Aoyagi K, Minashi K, Igaki H, Tachimori Y, Nishimura T, Hokamura N, Ashida A, Daiko H, Ochiai A, Muto M. Artificially induced epithelial-mesenchymal transition in surgical subjects: its implications in clinical and basic cancer research. PLoS One. 2011;6:e18196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8187] [Cited by in RCA: 11261] [Article Influence: 938.4] [Reference Citation Analysis (0)] |
| 15. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9144] [Cited by in RCA: 12814] [Article Influence: 985.7] [Reference Citation Analysis (0)] |
| 16. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4859] [Article Influence: 441.7] [Reference Citation Analysis (2)] |
| 17. | Kawabata T. HOMCOS: an update server to search and model complex 3D structures. J Struct Funct Gennomics. 2016;In press. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Kawabata T, Nishikawa K. Protein structure comparison using the markov transition model of evolution. Proteins. 2000;41:108-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Fang L, Zhu Q, Neuenschwander M, Specker E, Wulf-Goldenberg A, Weis WI, von Kries JP, Birchmeier W. A Small-Molecule Antagonist of the β-Catenin/TCF4 Interaction Blocks the Self-Renewal of Cancer Stem Cells and Suppresses Tumorigenesis. Cancer Res. 2016;76:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Graham TA, Ferkey DM, Mao F, Kimelman D, Xu W. Tcf4 can specifically recognize beta-catenin using alternative conformations. Nat Struct Biol. 2001;8:1048-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a beta-catenin/Tcf complex. Cell. 2000;103:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 332] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Yumoto F, Nguyen P, Sablin EP, Baxter JD, Webb P, Fletterick RJ. Structural basis of coactivation of liver receptor homolog-1 by β-catenin. Proc Natl Acad Sci USA. 2012;109:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Gu P, Goodwin B, Chung AC, Xu X, Wheeler DA, Price RR, Galardi C, Peng L, Latour AM, Koller BH. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005;25:3492-3505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Morrison G, Scognamiglio R, Trumpp A, Smith A. Convergence of cMyc and β-catenin on Tcf7l1 enables endoderm specification. EMBO J. 2016;35:356-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Gao C, Chen G, Kuan SF, Zhang DH, Schlaepfer DD, Hu J. FAK/PYK2 promotes the Wnt/β-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3β. Elife. 2015;4:e10072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 26. | Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 677] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 27. | Hernández AR, Klein AM, Kirschner MW. Kinetic responses of β-catenin specify the sites of Wnt control. Science. 2012;338:1337-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Zhang X, Garcia Abreu J, Peng L. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 29. | He J, Huang Y, Weng J, Xiao L, Weng K, Ma X. Specific Inhibition of β-Catenin in Jeko-1 Mantle Cell Lymphoma Cell Line Decreases Proliferation and Induces Apoptosis. Med Sci Monit. 2015;21:2218-2224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 568] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 31. | Freeman J, Smith D, Latinkic B, Ewan K, Samuel L, Zollo M, Marino N, Tyas L, Jones N, Dale TC. A functional connectome: regulation of Wnt/TCF-dependent transcription by pairs of pathway activators. Mol Cancer. 2015;14:206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Shukla S, Sinha S, Khan S, Kumar S, Singh K, Mitra K, Maurya R, Meeran SM. Cucurbitacin B inhibits the stemness and metastatic abilities of NSCLC via downregulation of canonical Wnt/β-catenin signaling axis. Sci Rep. 2016;6:21860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Stewart DJ, Chang DW, Ye Y, Spitz M, Lu C, Shu X, Wampfler JA, Marks RS, Garces YI, Yang P. Wnt signaling pathway pharmacogenetics in non-small cell lung cancer. Pharmacogenomics J. 2014;14:509-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Hubaux R, Thu KL, Lam WL. Re: the Wnt signaling pathway in non-small cell lung cancer. J Natl Cancer Inst. 2014;106:pii: dju188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Masoud GN, Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1342] [Cited by in RCA: 1400] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 36. | Tanabe S, Aoyagi K, Yokozaki H, Sasaki H. Regulated genes in mesenchymal stem cells and gastric cancer. World J Stem Cells. 2015;7:208-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Tanabe S. Signaling involved in stem cell reprogramming and differentiation. World J Stem Cells. 2015;7:992-998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 38. | Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1357] [Cited by in RCA: 1638] [Article Influence: 163.8] [Reference Citation Analysis (0)] |
| 39. | Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1435] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 40. | Kusunose M, Hashimoto N, Kimura M, Ogata R, Aoyama D, Sakamoto K, Miyazaki S, Ando A, Omote N, Imaizumi K. Direct regulation of transforming growth factor β-induced epithelial-mesenchymal transition by the protein phosphatase activity of unphosphorylated PTEN in lung cancer cells. Cancer Sci. 2015;106:1693-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Jian J, Sun L, Cheng X, Hu X, Liang J, Chen Y. Calycosin-7-O-β-d-glucopyranoside stimulates osteoblast differentiation through regulating the BMP/WNT signaling pathways. Acta Pharm Sin B. 2015;5:454-460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Zhao L, Li W, Zang W, Liu Z, Xu X, Yu H, Yang Q, Jia J. JMJD2B promotes epithelial-mesenchymal transition by cooperating with β-catenin and enhances gastric cancer metastasis. Clin Cancer Res. 2013;19:6419-6429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875-4887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 391] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 44. | Roussos ET, Keckesova Z, Haley JD, Epstein DM, Weinberg RA, Condeelis JS. AACR special conference on epithelial-mesenchymal transition and cancer progression and treatment. Cancer Res. 2010;70:7360-7364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H, Mikulits W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5:1169-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 46. | Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3740] [Cited by in RCA: 4400] [Article Influence: 338.5] [Reference Citation Analysis (0)] |
| 47. | Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, Gartner JJ, Zheng Z, Li YF, Ray S. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 605] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 48. | Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, Hu CJ, Dong H, Yang SM. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/β-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |