INTRODUCTION
The incidence of thyroid cancer is rapidly rising in the US accounting for 62980 cases with 1890 deaths every year[1]. It is the seventh most common cancer diagnosed in women and peaks earlier than in men. Despite its high prevalence, death rate from thyroid cancer is fairly stable from past many years. In general, thyroid cancer offers a good prognosis with an overall survival rate of approximately 90%[2]. Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) termed as differentiated thyroid cancer (DTC) contribute to majority of thyroid cancers sharing a superior prognosis. Medullary thyroid carcinoma (MTC), mostly acquired as a part of familial syndromes, display only modest cure rates. While surgical resection followed by radioiodine therapy remains the treatment of choice for localized thyroid cancer, it fails to eradicate tumors with aggressive behavior. In marked contrast to DTC, anaplastic carcinoma (ATC), an undifferentiated sub-type of thyroid cancer, has a higher propensity to locally invade nearby structures and metastasize rapidly. It approaches to almost 50% of all thyroid cancer-related deaths, the median survival being only six months[3]. The grim prognosis of ATC is due to the fact that it is diagnosed at an advanced stage which offers palliative treatment as the only option for patients suffering from the disease. Because of the chemo- and radio-resistant nature observed in aggressive thyroid cancers, many researchers have been continuously attempting to create new treatment strategies that are aimed at eradication of cancer cells. These trials led to a phenomenal breakthrough that the acquired resistance of thyroid cancer cells which initially were responding to conventional therapies may harbor heterogeneous cell types. Interestingly, these cells were hypothesized to be acquired with stem cell-like properties and were labelled a distinct entity called as cancer stem cells (CSCs)[3-5]. Recent advances in the stem cell technology have made it possible to understand diverse biological and molecular mechanisms that control the disease process; however, the validity of the origin of CSCs and their distinct role in thyroid cancer still uphold a great interest.
Stem cells are the population of cells that have a tremendous potential for self-renewal and can differentiate into various specialized cells in the body. These are distinguished from other cell types by two important properties. Firstly, they have the ability for self-renewal through continuous cell division and secondly, under specialized circumstances, they can be induced to become tissue/organ specific cells carrying their designated functions. Among these cells, of particular importance are (1) Embryonic stem cells (ESCs) - which are pluripotent cells that divide infinitely and give rise to ectodermal, endodermal and mesodermal cells; and (2) Somatic stem cells (SSC) - also known as adult stem cells, are tissue specific cells with limited life-span that give rise to all cells in a particular lineage, for instance thyroid follicular cells or hematopoietic cells. However, the putative role of ESC and SSC in adult thyroid pathophysiology still remains to be proven.
A sub-type of cancer cells that has recently gained much recognition are CSCs, also referred to as Tumor-initiating cells (TICs)[6-8]. These cells possess characteristics associated with normal stem cells with a remarkable potential to reconstitute and sustain tumor growth. However, it does not infer their origin from a normal stem cell. It has been reported that basal-like epithelial cells can de-differentiate into stem-like cell[9]. Moreover, existing literature illustrates that CSCs may depend on a specific microenvironment or the niche for sustained stem-cell like properties[6,10]. One such example of CSCs niche is hypoxia of cancer where these cells undergo continued proliferation on exposure to increased free radical generation within the tumor. Therefore, several studies have attempted to identify the niche that necessitates these cells to sustain and promote tumor growth.
In 1997, Bonnet et al[11] were the first to provide conclusive evidence of CSCs in leukemia. The isolated leukemic cells expressed cell surface markers CD34 but lacked CD38. On injection into an immunodeficient mice, these cells initiated tumor with similar histological features of the parental tumor[11]. In 2002, Ignatova et al[12] were the first to isolate CSCs from human brain gliomas which were described to be clonogenic with special sphere-forming property. Since then there have been many published clinical researches that have successfully identified CSCs in solid cancers of breast, colon, pancreas, prostate and ovary[13-15]. Most recently, existence of stem cells in thyroid cancer with an understanding of its developmental biology, especially from the perspective of innovation of newer cell-replacement therapies for aggressive thyroid cancer, has been laid much importance in the field of regenerative medicine and tissue engineering[3-8].
Organogenesis of thyroid gland is dependent on specific transcription factors which are responsible for differentiation of progenitor cells. Certain cell-specific transcription factors namely thyroid transcription factor (TTF) 1, TTF 2, Hhex factor, pax 8, fgfr-2, and Eya1 possess distinctive roles in thyroid development[10,16]. However, their combined expression through a controlled regulation is essential to carry out cellular differentiation and expression of thyroid specific genes. Mature thyroid cells display a number of markers of differentiation such as thyroglobulin (Tg), thyroid peroxidase (TPO), and thyroid stimulating hormone receptor.
The origin of cancer cells in thyroid has been described in various literatures; however, it still maintains its avenue for debate. The basic concept of multistep carcinogenesis considers transformation of well differentiated thyroid cancer cells of follicular origin into undifferentiated cells through sequential events which occur during maturation of thyroid epithelial cells[6]. On the contrary, others propose that these well-differentiated follicular cells rarely proliferate and thus carry limited accumulated mutations in the cells. Also, the genetic mutations that are seen in well-differentiated cancers are not evident in anaplastic cancers[17]. Some authors favor the notion of fetal carcinogenesis which postulates that thyroid CSCs originate from abnormal transformation of fetal cells: (1) fetal thyroid stem cells, the primitive cells that express onco-fetal protein responsible for the origin of ATC; (2) Thyroblasts, which express fetal protein and Tg give rise to PTC; and (3) Prothyrocytes, which are differentiated cells responsible for FTC/follicular adenoma[8,18]. Once they follow aberrant pathways of malignant transformation, these cells lose their ability to differentiate further and become a potential source of CSCs.
Another concept of CSC theory, which has been proposed previously, suggests that these cells originate either from stem cells, progenitor cells or from de-differentiated mature thyroid cells[19]. Because of a shorter lifespan of somatic cells, researchers claim that stem cells or progenitor cells represent their most likely the source. Much evidence exists on the fact that cancer comprises of heterogeneous cells out of which only a sub-population with stem-cell like characteristics are tumorigenic[6,10,19]. However, the concept of CSC in the cellular origin for thyroid tumors, in particular, cannot be clearly demonstrated using this model. Because CSC are isolated at an advanced-stage of the tumor, these cells, though, capable of initiating new tumor formation, are not described for cellular-origin by some authors[20]. This contemplates the use of term TICs or tumor-propagating cells by some authors.
According to Zhang et al[21] because of loss of specific markers that govern degree of differentiation, thyroid CSCs undertake aberrant differentiation pathways and suffer maturation arrest. If this arrest is seen late in the differentiation process, they give rise to well differentiated carcinoma[21], when encountered early in the process, poorly differentiated carcinoma results. Therefore, different oncological pathways are responsible for providing diverse histological and morphological patterns to thyroid cancer.
Other studies demonstrate that stem cells can be recruited to the site of tumor and probably can acquire tumor-like properties and acting as parental tumor cells. Moreover, these cells have internal driven-force for supporting tumor progression and metastasis and they have the power to communicate with other cells through exosomes[22,23].
ISOLATION AND IDENTIFICATION OF CANCER STEM CELLS
Various pre-clinical in vivo and in vitro models have been designed by the researchers to determine thyroid cancer progression and their response to treatment. According to the American Association for Cancer Research, ‘cancer stem cell can only be defined experimentally by their ability to recapitulate the generation of a continuously growing tumor’, proving the term TIC’s[6,24]. The commonest and most definite way to confirm their presence is by isolating cells and then serially injecting them into immuno-deficient, for example non-obese diabetic mice or severe combined immunodeficiency (SCID) mice, to identify tumor initiation. CSCs isolated by flowcytometry are sorted according to CSC-specific surface markers, thyrosphere formation assay, aldehyde dehydrogenase activity (ALDH) and ATP-binding cassette sub-family G member 2 (ABCG2) efflux-pump mediated Hoechst 33342 dye exclusion[6,9,10]. The sphere-forming assays are the best in vitro strategy to study clonal behavior and multi-potential of thyroid stem cells. There are different CSC-specific markers proposed by different authors such as side population (SP), CD-133+, CD-44, POU5F1, ALDH, insulin and insulin-like factor (IGF). The existence of embryonic remnants with stem-cell properties in mature thyroid gland has already been hypothesized using Oct-4, ABCG-2, GATA-4, HNF-4α, α-fetoprotein and p63 markers[10,25,26]. Malguanera et al[25] demonstrated expression of various stemness markers (Oct-4, NANOG, Sox-2, CD44, and CD133) in follicular thyrospheres. However, the sphere cultures displayed very low levels of thyroid differentiation markers (Tg and TPO). Additionally, their findings also displayed higher expression of IGF components in the stem cells suggesting their important role in the regulation of precursor cells in follicular cancer[25]. Specific genetic alterations such as RET/PTC and PAX8/PPARγ rearrangements play a crucial role in thyroid carcinogenesis. These alterations prevent differentiation of thyroid fetal cells, leading to their uncontrolled proliferation and malignant transformation within the gland. Moreover, dysregulation in signaling pathways of stem cell renewal (Wnt/β-catenin, Hedgehog and Notch pathways) may contribute to malignant transformation of normal thyroid resident cells.
The existence of CSCs has been considered in several thyroid cell lines. Mistutake et al[26] reported ability of SP cells to efflux Hoechst 33342, a DNA-binding dye. They demonstrated that SP cells were enriched with stem-cell like characteristics. These cells were clonogenic that could give rise to both SP and non-SP cells. Additionally, SP cells showed up-regulation of “stemness” genes including those found in Notch and Wnt signaling pathways. However, both sub-population of cells (SP and non-SP cells) were tumorigenic on injection in a nude mice[26]. A research demonstrated a function role of CSCs in human ATC cell line (THJ-11T, THJ-16T, THJ-21T, THJ-29T). In their study, 3%-9% of cells formed thyrospheres expressing NANOG and Oct4 markers, which possessed the ability to self-renew. On orthotopic thyroid transplantation of thyrospheres in NOD/SCID Il2rg-/- mice, aggressive and metastatic tumors were generated depicting that thyroid provides the niche for these thyrospheres derived cells[3]. Another such results were recently displayed by Todaro et al[8] using 3 histological variants (PTC, FTC, ATC). They demonstrated that only a small population of cells (1.2-3.5%) retains tumorigenic potential in thyroid cancer. Cells with ALDH(high) expression were associated with unlimited replication potential and self-renewing property in serum-free media with highest percentage in ATC tissues. On orthotopic thyroid injection of thyrospheres in immunodeficient mice, these cells were able to reproduce similar phenotypic characteristics of parental tumor cells with ALDH(high) UTC spheres exhibiting cervical nodal and distant metastasis[8]. Accordingly, these results were also reported by Shimamura where their results displayed higher sphere forming ability with ALDHpos in FRO, KTC3 and ACT1 and CD326high in FRO cell lines[27].
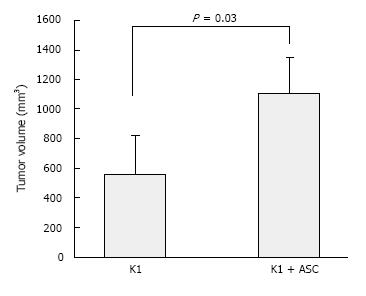
Although PTC accounts for majority of thyroid cancers, the data on CSCs existence in PTC cell lines is currently limited. A recent in vivo model has been designed by our group, where we described a subcutaneous mouse model of metastatic human thyroid cancer by combining human adipose-derived stromal/stem cells (ASCs) with the human mutant BRAF V600E PTC cell line K1 (Figure 1A). Over a period of six weeks, we observed development of large tumors with distant metastasis in mice that were concomitantly injected with ASCs (5 × 105 cells) and K1 cells (5 × 105 cells). About 100% of lung metastasis was identified in ASCs + K1 group (Figure 1B) compared to 40% in mice receiving only K1 cells. Tumors in ASCs + K1 were significantly larger (P < 0.05) (Figure 2A) and developed earlier than the group of K1 alone (Figure 2B) demonstrating the role of ASCs in promoting dramatic tumor growth and seeding within the metastatic organs (Figure 3). To date, our model is the first model to display the use of ASCs to produce metastatic thyroid cancer[28]. Zhu et al[29] demonstrated the existence of CSCs in MTC cell lines. These cells showed positivity for CD133 and displayed that RET proto-oncogene with basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) favor self-renewal in MTC cells. Additionally, these cells also expressed neuron specific markers namely β-tubulin isotype III and glial fibrillar acidic protein[29]. The purpose of demonstration of stemness markers using different cell lines in the models suggests that these markers should be targeted with anintent to develop new efficacious treatment for refractory tumors.
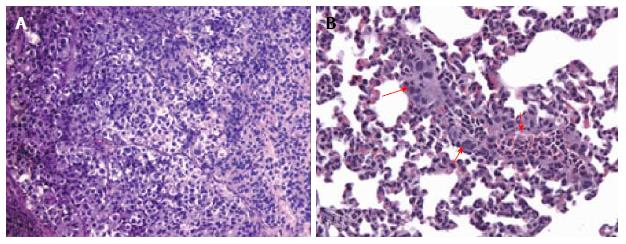
Figure 1 Histological images showing subcutaneous thyroid cancer mouse model.
A: Hematoxylin and eosin stained microphotograph of tumor xenografts engrafted human thyroid cancer cell line (K1) with adipose-derived stem cells (ASCs) (× 100); B: HE microphotograph of lung metastasis (red arrows) in the group transplanted with K1 cells and ASCs (× 200). Methods of image acquisition: Tumor and organs removed from mouse, photographed, and stored in 10% neutral buffered formalin for paraffin sectioning and HE staining. Tumor tissue were sectioned and stained with HE[28].
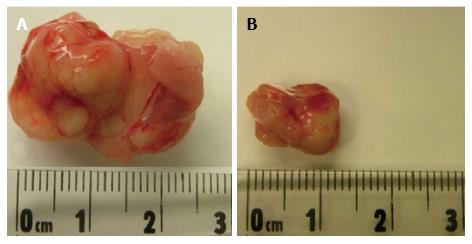
Figure 2 Representative tumor from severe combined immunodeficiency mice injected with: (A) K1 + ASCs and (B) K1 alone.
ASCs: Adipose-derived stem cells.
Figure 3 Adipose-derived stem cells promote tumor growth of papillary thyroid cancer cells.
K1 cells alone and K1 cells with ASCs (5 x 105 cells each) were injected subcutaneously into nude mice (n = 5, each group)[28]. ASC: Adipose-derived stem cell.
FUTURE PERSPECTIVES
Various genetic alterations defining oncogenic pathways in aggressive thyroid cancer have been recognized, yet, the ability to decode these mutations into novel anti-cancer therapies is limited. This recent discovery of thyroid CSCs marks an imperative stage for innovation of efficient anti-cancer treatment for resistant tumors. It is a well-known fact that conventional anti-cancer therapies target differentiating/differentiated cells, which form the bulk of the tumor but are unable to generate new cells. If CSCs remain in the quiescent stage (dormant cells), they resist the therapy targeted for dividing cells. CSCs self-renewal and ability to constitute a very small proportion of the tumor, they might develop resistance to chemo- and radiotherapy, ultimately causing the disease to relapse. It is possible that these cells may repair DNA damage more rapidly than normal cells[30]. A newer concept has been postulated by various authors that points out the metastatic potential of CSCs secondary to epithelial-mesenchymal transition (EMT) and the inverse [mesenchymal-epithelial transition (MET)] at an advanced stage of the disease[6,10,31,32]. A strong association between EMT/MET and CSCs has been highlighted recently, suggesting that EMT increases epithelial plasticity, confers tumor progression and therapeutic resistance to cancer cells. These transformed cells, then, behave like stem-cells similar to those seen in normal thyroid tissue. In a study done by Vasko et al[31] PTC was associated with EMT due to overexpression of vimentin which led to regional lymph node invasion by the tumor[31]. EMT is also associated with loss of E-cadherin, SNAIL, Twist and activation of β-catenin gene expression which make cells lose their adhesion and facilitate metastasis[32]. However, some studies claim that the process of EMT is only observed in ATC. Authors have scripted the role of microRNAs in this transition process which makes CSCs undergo unlimited proliferation and capable of initiating tumor growth at metastatic sites[10,25,31]. However, this area needs to be further explored before designing therapies aimed at the eradication of transformed cells.
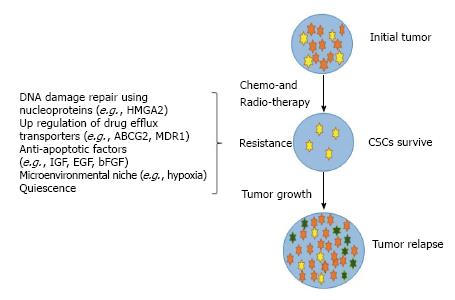
In an attempt to develop targeted therapeutic strategies to eradicate this subset of CSCs, it becomes essential to determine their origin and whether they differ in various sub-types of thyroid cancer. Studies have shown overexpressed multi-drug resistance protein 1 (MDR 1) and ABCG2 transporters to cause resistance to cytotoxic drugs. With this concept, Zheng et al[32] displayed how doxorubicin becomes ineffective and fail to eradicate CSC population. Because these drugs can specifically kill cancer cells, it provides a major space for CSCs to proliferate making the tumor resistant to chemotherapy, thus causing the disease to relapse[32] (Figure 4).
Figure 4 A schematic representation showing cancer stem cells resistance to chemo-and radio-therapy causing tumor to relapse.
Cancer stem cells (CSCs) (yellow) with differentiated cells committed to a particular lineage (red). Ability of CSCs to resist anti-cancer therapy due to various mechanisms and ability to proliferate into heterogeneous group of cells cause tumor to relapse. HMGA2: High mobility group A2; ABCG2: ATP-binding cassette sub-family G member 2; MDR1: Multi-drug resistance protein-1; IGF: Insulin-derived growth factor; EGF: Epidermal growth factor; bFGF: Basic fibroblast growth factor.
In conclusion, therapeutic rationale should be laid, specifically, on destruction of CSCs by abruption of self-renewal signaling pathways, induction of differentiation of cancer cells and inhibition of survival-related mechanisms. Another venue to develop specific targeted therapies is by identification and destruction of the niche that nourishes CSCs for tumor growth. Because CSCs are heterogeneous and the cell-specific markers vary enormously amongst different tumor-types, there is an urgent need to identify further specific markers to support their existence. Further advancements in stem cell technology should focus on conglomerated existence of factors responsible for failure of current therapies in eradicating thyroid CSCs with the aim to target specific subpopulation of cells in the patients with refractory thyroid cancers.