Published online Sep 26, 2014. doi: 10.4252/wjsc.v6.i4.421
Revised: August 29, 2014
Accepted: September 4, 2014
Published online: September 26, 2014
Processing time: 61 Days and 18.6 Hours
B lymphocytes differentiate from hematopoietic stem cells through a series of distinct stages. Early B cell development proceeds in bone marrow until immature B cells migrate out to secondary lymphoid tissues, such as a spleen and lymph nodes, after completion of immunoglobulin heavy and light chain rearrangement. Although the information about the regulation by numerous factors, including signaling molecules, transcription factors, epigenetic changes and the microenvironment, could provide the clinical application, our knowledge on human B lymphopoiesis is limited. However, with great methodological advances, significant progress for understanding B lymphopoiesis both in human and mouse has been made. In this review, we summarize the experimental models for studies about human adult B lymphopoiesis, and the role of microenvironment and signaling molecules, such as cytokines, transforming growth factor-β superfamily, Wnt family and Notch family, with point-by-point comparison between human and mouse.
Core tip: There are several species differences between human and mouse, while the mouse studies precede those of human. Recent progresses of experimental techniques have made it possible to understand the biology in human B lymphopoiesis deeply. Various phenotype markers, which can define the distinct developmental stages, and requirement of cytokines are distinguishable. More common issues are observed in the role of signaling molecules, including transforming growth factor-β superfamily, Wnt family, and Notch family, which have been known the high conservation among mammals. The knowledge on niches for human hematopoietic stem cell and B cell development is still limited.
- Citation: Ichii M, Oritani K, Kanakura Y. Early B lymphocyte development: Similarities and differences in human and mouse. World J Stem Cells 2014; 6(4): 421-431
- URL: https://www.wjgnet.com/1948-0210/full/v6/i4/421.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v6.i4.421
B lineage cells develop from hematopoietic stem cells (HSCs) in adult bone marrow (BM) through several well-characterized stages before migrating to secondary lymphoid tissues such as a spleen and lymph nodes. Once HSC divides asymmetrically into one stem cell and one differentiating cell, it gives rise to progenitor cells that undergo lineage commitment and the production of specific lineage blood cells starts. Multipotent progenitors (MPP), which lose the reconstituting capacity, differentiate sequentially into lymphoid-committed progenitors, and B lineage-restricted progenitors originate from the lymphoid-primed multipotent/ early lymphoid progenitors (LMPP/ELP), followed by common lymphoid progenitors (CLP), pro-B cells, pre-B cells and immature B cells (Figure 1). Immunoglobulin gene rearrangements are required for the process of B lymphopoiesis[1-3]. The activation of the recombination enzymes, such as recombination-activating gene (RAG)-1, RAG-2 and terminal deoxynucleotidyl transferase, promotes the D-to-J and V-to-DJ rearrangements in the immunoglobulin heavy (IgH) chain locus during the differentiation from CLP to pro-B stage. Signaling through the pre-B-cell antigen receptor (pre-BCR), composed of IgH chains and surrogate light (L) chains, induces VJL rearrangements and allelic excision at IgH chain locus leading the functional BCR expression on immature B cells. This rearrangement machinery is precisely regulated by several transcription factors including PU.1, E2A, early B cell factor (EBF) and Pax5[2,3]. For example, Pax5 activates the expression of Cd19, Cd79a, Blnk, Igll5 (lamda5) and VpreB1 involving in the pre-BCR signaling. Although it was believed that the fate decision of B cell commitment would occur after becoming CLP, recent studies have shown the lineage skewing begins earlier than previously expected[3-7]. The expression of lymphoid-lineage priming genes like Satb1 and Ikaros in HSC is recognized[8,9]. During the differentiation from HSC to CLP, lymphopoiesis proceeds in asynchronous ways. These developmental procedures are regulated by signaling molecules, transcription factors, epigenetic changes and the microenvironment[6,7,10,11].
It has been known that HSC are extremely heterogeneous. Those can be subdivided to long-term and short-term HSC based on reconstitution time periods in transplantation assays[12-16]. Recent studies suggest that HSC compartment also contains distinct subtypes with different developmental preferences[15-18]. Myeloid-biased HSC produce greater numbers of myeloid than lymphoid lineage cells and tend to be quiescent. On the other hand, lymphoid-biased HSC generate more lymphoid cells and have shorter duration of reconstitution than myeloid-biased HSC. In aged mice, which reduce production of B and T cells and diminish function of mature lymphocytes, the number of myeloid-biased HSC increases[17,19,20]. The distribution of HSC subsets is at least partly responsible for homeostasis of B lymphopoiesis.
The evidences about hematopoietic biology have been accumulated from murine experiments and primary deficiencies in humans. However, recent advances in biological analysis techniques including xenotransplantation model, in vitro clonal assays and flow cytometric analysis and sorting made great progress for understanding normal hematopoiesis in human. Mouse and human are obviously different in size, ecology, and lifespan. It has been known that human B lymphopoiesis differs from that in mice with requirement of cytokines and the role of microenvironment. To apply the findings about the regulation of B lymphopoiesis for clinical settings, studies in human are necessary.
In this article, we focus on common and distinct features in human and mouse early B lymphopoiesis. First we discuss the differences of adult B cell development from HSC between these two species. In the late sections, we describe the role of microenvironment in BM including the cellular components and signaling molecules, especially about members of TGF-β superfamily, Wnt family, and Notch family, which have been known the importance in regulating proliferation, differentiation, and survival.
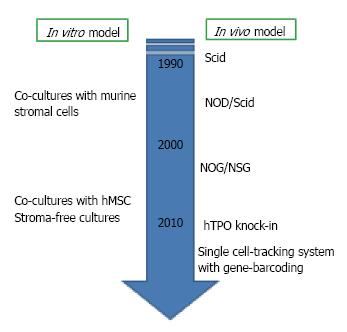
As we mentioned above, there are several species differences in B cell lymphopoiesis between human and mouse. The development of human study has been relatively slow with several reasons. The most critical one is the lack of adequate experimental models for evaluating molecular mechanisms in vivo and in vitro. For murine studies, various in vitro assays, such as Whitlock-Witte long-term cultures, cultures of BM cells with or without stromal cell lines, and colony assays for IL-7-responding progenitors are available[21,22]. However, cultures to generate human B lymphocyte have not been well established. Although murine stromal cell lines can support human B cell development from hematopoietic stem/progenitor cells (HSPC), the species differences make the precise evaluation about some necessary cytokines and interaction with the microenvironment difficult[23-26]. The establishment of new culture systems reported from our group and others hampered this problem[27-29]. We established co-culture with human mesenchymal stem cells (MSC) and stromal cell-free culture systems. Our co-culture or stromal cell-free culture systems in the presence of stem cell factor (SCF) and Flt3 ligand (Flt3L) are successfully produced CD10+ CD19+ B cells within 4 wk from human umbilical cord blood (CB) CD34+ CD38- HSC. Surface IgM+ immature B cells begin to appear after 4 wk of co-cultures. Although lymphocyte production from adult BM-derived HSC in the stromal cell-free culture is much more difficult than CB cells, both are responsive to granulocyte colony stimulating factor (G-CSF). Our data showed that human MSC can efficiently support commitment and differentiation of human HSC into B lymphocytes, and human does not require the direct interactions with stromal cells for B cell generation.
Concerning about in vivo studies, humanized mouse models were established around 1990s with the discovery of the severe combined immune-deficient (Scid) mouse lacking B and T cells[30,31]. Since then, a variety of xenograft models including nonobese diabetic (NOD)-Scid mice and NOD-Scid with either truncation (NOG) or deletion (NSG) in the IL-2 receptor common gamma chain have been generated to improve the efficiency of human HSC engraftment and long-term reconstitution[32,33]. With humanized model, we can observe multi-lineage reconstitution from human HSC in vivo. Newer generation of transplantation methods are now being developed. To elucidate the role of cytokines which are not cross-reactive, transgenic mice producing human cytokines such as thrombopoietin, IL-3 and GM-CSF, have been generated[34]. The viral integration site tracking system and the use in combination with massively parallel sequencing make it possible to track human HSC clones in transplanted Scid mice[35].
Another obstacle to studying human lymphopoiesis is genetic and biological diversity. Human BM samples are all different in age, sex, body size, genetic and epigenetic background and health condition when samples are collected. The development of highly purifying techniques with flow cytometry, single-cell assay methods and gene sequencing would help this problem solved[36].
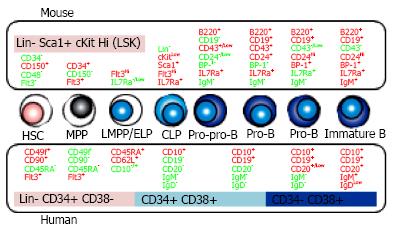
HSC is an extremely rare subset. The frequency of HSC in human BM is only 1 in 106 cells[37]. In mice, lineage (Lin)-/Low Sca-1+ c-KitHi (LSK) fraction contains multipotent cells such as HSC and MPP[38]. Using CD34, Flt3, SLAM family markers (CD150, CD48, CD229 and CD244) and Hoechst 33342 efflux, HSPC in LSK cells can be resolved into several subsets with distinct level of reconstituting potential and lineage preference[12,39-41]. According to c-Kit intensity decline, lymphoid committed cells are differentiated. Kondo et al[42] defined CLP in mice as Lin- IL-7 receptor alpha (IL-7Ra)+ Sca-1+ c-KitLow cells that appear to produce mainly B, T and natural killer (NK) cells. B lineage-restricted progenitors are fractionated based on the developmental stage and surface expression of CD45R/B220, CD19, CD24 (heat-stable antigen), CD43 and BP-1[43,44]. Mouse lymphopoietic hierarchy with cell surface markers is shown in Figure 1.
In human, HSPC markers are quite different from murine ones (Figure 1). Unlike mice, human HSPC can be enriched with CD34 expression although a very rare subset of HSC are devoid of that[45-47]. Other phenotypes of HSC are Flt3+, CD38- and CD150-, in great contrast with the expression on murine one[48,49]. CD133 helps the isolation of human HSPC and the rare CD34- HSC subset[46,50]. Recently, Dick and colleagues subdivided human Lin- CD34+ HSPC into long-term HSC, short-term HSC/MPP, and 6 lineage progenitor subsets on the basis of expression of the markers CD34, CD38, CD90 (Thy-1), CD49f, CD135 (Flt3), CD45RA, CD10, and CD7[51,52].
CD10 and CD45RA are often used as human-specific markers of lymphoid progenitors at early stages. Doulatov et al[51] described CD34+ CD38- CD45RA+ CD10+ fraction as multilymphoid progenitor and CD34+ CD38+ CD45RA+ CD10- fraction as granulocyte and monocyte progenitor. It is known that CD34+ CD10+ cells have a strong bias toward B cell development with relatively little T or NK cell potential[53,54]. We previously reported CD34+ early lymphocyte progenitors differ in CD10 expression[53]. CD34+ CD10Hi and CD34+ CD10Low populations have unique patterns depending on their sources; CB, BM and G-CSF mobilized peripheral blood, and increasing level of CD10 corresponds to expression of B lymphoid related transcription factors and markers, as well as loss of proliferative potential. Recently, Kohn et al[55] reported that L-selectin (CD62L) is expressed at the earliest stage of lymphoid priming before starting CD10 positive. CD34+ CD45RA+ CD62LHi CD10- cells showed lymphoid skewing although they produced both of myeloid and lymphoid cells in transplanted NSG mice. The differentiation potential and gene profiling indicated that CD34+ CD45RA+ CD62LHi CD10- cells are placed between HSC and CD34+ CD10+ lymphoid progenitors.
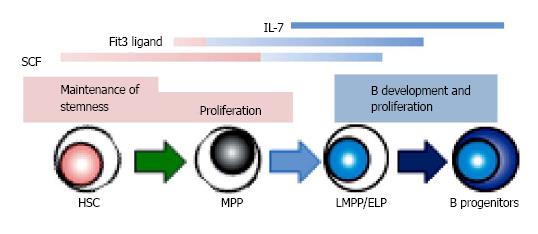
In mice, two cytokines, IL-7 and Flt3L, are known to be essential for adult B lymphopoiesis[56-58]. The loss of these receptors completely blocks B cell development. The up-regulation of IL-7Ra with Flt3 signaling induces EBF expression in B lineage progenitors, and that allows differentiation with the consequent expression of B cell-specific genes[10,11]. Moreover, recent studies have shown that the expression of IL-7Ra denotes the transition from LMPP to CLP, and Flt3L regulates the survival and proliferation of MPP/LMPP with commitment to B lineage fate[42,59,60]. The combination of crucial cytokines changes during ontogeny. Studies using IL-7 knockout mice showed that only adult but not fetal or neonatal B development is inhibited[61]. Thymic stromal lymphopoietin (TSLP) regulates IL-7-independent fetal B lymphopoiesis. SCF and chemokines recognized by the CXCR4 receptor also affect the differentiation[62].
In contrast, IL-7 is not required for human B cell development[63-66]. Crucial transcription factors including E2A, EBF and Pax5 are expressed during the differentiation from HSC to B lineage progenitors before acquisition of CD19, in the same manner as mouse B lymphopoiesis[67]. The importance of SCF and CXCL12 for B cell development has been recognized[68-70]. In human, Flt3L is critical to cell survival and proliferation of HSPC as well as to B lymphopoiesis[68,69]. Several groups reported that human HSPC could develop B lineage cells independently of IL-7 stimulation, and IL-7 induces little increase of B production in co-cultures[64]. Moreover, patients with disruption of the human IL-7 receptor spared B lymphopoiesis while development of T and NK cells was severely impaired[65,66]. Some groups questioned about the interpretation because in these studies fetal materials or murine stromal cells might influence the consequences[71,72]. However, we found that addition of neutralizing antibody to IL-7 or TSLP has no effect in stromal cell-free cultures we established[28,29]. In our study, hMSC conditioned medium could support human B lineage generation, indicating the existence of unknown stromal cell-derived factors facilitating B lymphopoiesis. Interestingly, we and others reported that G-CSF promotes human B production from HSC in vitro[23,28]. G-CSF is originally cloned as a glycoprotein which stimulates the production of granulocytes, and now is known the important role in HSC proliferation and mobilization, and bone resorption. For now, nothing has been reported about the influences on early B lymphopoiesis in vivo while clinical studies showed a higher proportion of Th2 cells present in peripheral blood cell grafts from G-CSF-stimulated donors and T cell hyporesponsiveness in association with increase of Th2-inducing dendritic cell[73,74]. There are several possibilities about the mechanism how G-CSF affects B lymphocyte generation in vitro. It might have direct effects on cultured cells. Another speculation is that HSC or progenitors with the specific stage or lineage stimulated by G-CSF might regulate B generation indirectly. In B cell cultures, short-term expansion of myeloid cells is observed before emerging B lineage cells[27,28].
Collectively, the essential key of human B lymphopoiesis is still remained unknown. The recent study with the depth of single-cell mass cytometry and an algorithm analysis of human BM showed the exclusive activation of STAT5, which phosphorylation is known to be induced by IL-7, in early B progenitors[75]. Using novel technologies, the precise biology could be unveiled in the near future.
In 1978, Schofield proposed the hypothesis that a specialized niche in BM preserves the reconstituting and differentiating ability of HSC, but could not prove that[76]. It is believed that bone marrow contains specialized niches for differentiation of specific lineage progenitors[77-79]. With the great advances of gene-modified mice generation and imaging techniques, the anatomical location and cellular components of HSC niches have been elucidated since 2000s, although our understanding is still incomplete and novel analysis tools are needed[80,81]. In parallel, the roles of molecular and environmental factors in the niches have been extensively studied. Niches make specialized environments, consisting of soluble or surface-bound signaling factors, cell-cell contacts, extracellular matrix (ECM) proteins, and local mechanical environments such as the concentration of oxygen and calcium.
In marrow, there are many types of non-hematopoietic cells including mesenchymal stem/progenitor cells, osteoblastic lineage cells, adipocytes, endothelial cells, reticular cells, pericytes, fibroblasts and nerve cells[80,81]. The effects of several molecular regulators produced by niche cells, such as chemokines like CXCL12, cytokines (SCF, thrombopoietin, angiopoietin-2, and angiopoietin-like 3), Wnt, Notch, TGF-β and hedgehog signaling, and ECM proteins (osteopontin, decorin, and tenascin C) have been reported. Based on the concept of HSC niche, the cellular components are supposed to neighbor with HSC, and more importantly, the influence on HSC maintenance should be direct.
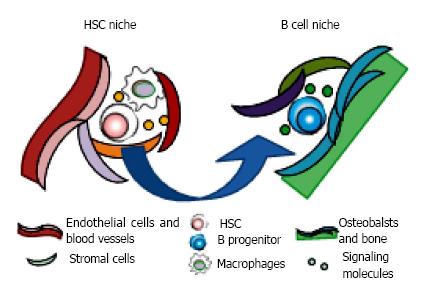
Several immunofluorescence imaging studies showed that HSC is consistently located adjacent to the sinusoidal vasculature[39,82]. In perivascular niches, mesenchymal stem/progenitors which express Nestin, leptin receptor, or fibroblast activation protein (FAP), CXCL12-abundant reticular cells, and endothelial cells are co-localized with HSC and secrete HSC supporting factors like SCF or CXCL12[39,83-86]. The sympathetic neurons, arteries, macrophages such as osteoclasts and regulatory T cells in the niches affect the frequency, function and localization of HSC[87-91]. Surrounded by these cells and molecular components, HSC can maintain the capacity of self-renew and multipotent differentiation.
On the other hand, whether the osteoblastic lineage cells at the endosteal surface of the bone, described first as the place where HSC reside, could be the niche is under debate[82,92]. Although osteoblasts may not to be adjacent to HSC, they do have the distinct influences on HSPC through the production of CXCL12 and SCF, and expression of adhesion molecules. It is known that HSPC frequently move out from their own niche[93,94]. Thirty percent of IL-7Ra+ B progenitors are co-localized with bone-lining cells, and acute depletion of them are observed when osteoblastic cells are conditionally deleted[95]. Interestingly, the deletion of CXCL12 from osteoblasts depletes early lymphoid progenitors, but not HSC or myeloerythroid progenitors[95,96]. These findings suggest that osteoblasts could be the niches for B lymphopoiesis in endosteal area (Figure 4).
As well as the identification of cellular components of niches, the anatomical localization of HSC in BM has been the subject of intense researches. The initial studies indicated that HSC might reside in the endosteum, adjacent cortical bones with osteoblasts[92]. With the great advance in immunostaining methods and understanding HSC characteristics, however, others showed that most of accurate HSC localize adjacent to sinusoid vessels while less than 20% to bone-lining cells[82,84]. It is consistent that HSC are found in the trabecular regions at metaphysis.
Inside marrow, HSC is mobile when HSC divides or starts to differentiate. Interestingly, it is known that HSC periodically leave and reenter the niches for circulation with circadian oscillation and in response to infection or G-CSF stimulation[87,91]. In vivo time-lapse imaging makes it possible to observe HSC motility and localization of activating HSPC. Another unanswered question is skeletal localization. In human adult, the sternum is active hematopoietic site while long bones are occupied by adipocytes with aging. The three-dimensional, whole-mount confocal immunofluorescence imaging techniques showed the same is true in mice[89].
In clinical settings, hematopoietic stem cell transplantation offers patients with refractory hematological diseases a curative treatment option. Several types of stem cell sources, CB, BM and G-CSF mobilized peripheral blood are used for the therapy, although differences among sources are still remained unclear[97]. After transplantation, HSC migrate, localize in niches and start to proliferate and reconstitute all lineage bloods in the recipient BM damaged by the conditioning. A full understanding of the whole process is critical for choosing the adequate strategy of donor sources, conditioning and immunosuppressive therapy before or after transplantation.
Several types of mesenchymal stem/progenitors, osteoblast, and endothelial cells in human have been reported the supportive effects on HSC maintenance or specific lineage differentiation[27,98,99]. Imaging analysis using bone biopsy specimens to evaluate the actual distance between HSPC and niche component showed that human CD45+ CD34+ CD38- HSC localize in the trabecular area similar to mice HSC, while CD34+ CD38+ HPC are dispersed evenly in BM[99]. HSC in the trabecular area own better HSC functions compared to those in long bone area. There is no information about the niches for human B lymphopoiesis.
Signaling molecule families like TGF-β, Wnt, Notch and hedgehog are highly conserved in mammals, and control proliferation or cell fate determination during embryonic development and adult homeostasis. In hematopoiesis, the specific ligand-receptor interactions regulate the maintenance of HSC stemness and differentiation through direct and indirect effects via the affected microenvironments.
TGF-βsuperfamily
The TGF-β superfamily is composed of more than 20 members, including three TGF-βs, bone morphogenetic proteins (BMP), growth and differentiation factors (GDF), Activins, and Nodal. TGF-β signaling regulates HSC quiescence, and is reduced in aged HSC[88,100]. The activation is restricted although many cells can produce TGF-β ligands and express the receptors. For HSC maintenance, the latent type of ligand is produced from the HSC microenvironment and activated by the nonmyelinating Schwann cells ensheathing sympathetic nerves in contact with HSC[88]. We and others reported the effects of TGF-β signaling for mouse and human B lymphopoiesis[27,101-103]. We showed that both Activin A and TGF-β1 inhibit generation of B cells from CB CD34+ cells in cultures. The receptors are expressed by not only CD34+ HSPC but also CD34- CD10+ cells, and we observed the same effects of the signaling when the inhibitor was added at the later periods of the co-cultures. These findings indicate TGF-β superfamily might affect early B lymphocyte progenitors. Transition into IgM+ immature B-cells was not influenced by the TGF-β superfamily in our culture systems.
Wnt is a large family of glycoproteins. Canonical pathway used by Wnt3a has been most studied in hematopoiesis[104]. After Wnt3a binds Frizzled receptor, this canonical signaling stabilizes intracellular β-catenin by inhibition of GSK-3β, and then β-catenin translocates to the nucleus and interacts with transcription factors. The role of Wnt for HSC maintenance has been a debatable issue. Constitutively active β-catenin blocks their differentiation, but induces exhaustion by shifting HSC cell-cycling status, although some of conditional deletion of β-catenin mouse have no abnormality in hematopoiesis[105,106]. However, now it is known that these discrepant results from studies using gain or loss of functions reflect the sensitivity to the dosage[107]. Wnt5a associated with noncanonical pathway also regulates HSC maintenance and differentiation[108-110]. Recent studies showed that noncanonical signaling is balanced with canonical signaling under inflammatory and aging condition[109].
Our and other groups reported the inhibitory effects on B lymphopoiesis[108,110,111]. Wnt3a, using canonical pathway, inhibits B and pDC but not cDC development, and Wnt5a promotes B lymphopoiesis in vitro. The observations about canonical Wnt signaling can translate from mouse to human[111]. It is known that Wnt ligands and receptors are expressed in both of hematopoietic tissues and the niche cells, and Wnt3a regulates mesenchymal lineage differentiation[111,112]. While hematopoietic cells themselves are Wnt responsive, we showed that the regulation of niches by Wnt3a mediates the effects. Specifically, Wnt3a strongly induces the production of ECM protein, decorin, which inhibits B lymphopoiesis and retains the HSC phenotype, from stromal cells. Decorin is a small leucine-rich proteoglycan secreted by MSC, and regulates TGF-β signaling, although the detailed mechanisms have not been elucidated[111,113]. Collectively, the findings suggest that Wnt signaling is important for maintaining not only hematopoiesis but also the niches.
The ligands of Notch signaling are membrane bound proteins, and the function depends on the type of ligand, such as Delta-like and Jagged, and responsive receptors, Notch 1-3. Notch is essential for early T lymphopoiesis, and B lymphopoiesis is suppressed by the interactions between Delta-like and Notch1 to avoid B cell generation in thymus[114]. The precise role in adult HSC at physiological levels is still controversial. Loss of the function in HSC did not show any influences for reconstitution and differentiation in mice, while in vitro expansion of HSC is promoted by the signaling[115,116]. The same is true in human[117]. The two recent studies published in 2013 emphasized the importance of Notch signaling in the interaction between human HSC and the microenvironment. Human CD146+ perivascular cells maintain stemness of HSC via Notch activation[98]. Bhatia and colleagues showed that in the trabecular bone area where HSC can hold the regenerative and self-renewing capacity, 3-fold greater of proportion of mesenchymal cells express Jagged-1 compared to those in long bone area[99]. More recently, it is reported that Notch signaling in HSC stimulated after the activating mutation of β-catenin in mouse osteoblasts induces the leukemogenesis[118]. The mutation induces Jagged-1 expression in osteoblast leading the Notch activation in HSC, and the inhibition of Notch signaling prevents the onset of leukemia. According to this study, 38% of patients with acute myeloid leukemia or myelodysplastic syndromes showed increased β-catenin in osteoblasts and increased Notch signaling in hematopoietic cells. The cooperation between Wnt and Notch is also reported in HSC maintenance[119]. Further study about the role of Notch signaling is warranted.
Although hedgehog signaling is also important for the development, stem cell maintenance, and tumorigenesis in various organs, the detailed effects on hematopoiesis have remained unclear. In mice, hedgehog signaling in HSC is not required for hematopoiesis although several studies showed the effects on cell-cycle and differentiation of HSC[120,121]. The activation of hedgehog signaling in stromal cells promotes B lymphopoiesis and HSC expansion[122]. Both of cell-extrinsic and cell-autonomous effects might be critical.
B lineage commitment starts at the early stage of HSC in asynchronous ways. The fate decision and development are affected by the microenvironmental factors including cellular niche components and signaling molecules. In this review, we described the common and different features in early B lymphopoiesis between human and mouse. The surface phenotypes on human HSC and B progenitors and requirement of cytokines are distinct while many effects of signaling molecules are consistent with mice.
It is known that immune system can be harmed by malignant disease, chronic inflammation and normal aging. Many studies concerning impairments in cellular and humoral immunity have focused on regulation of mature lymphocyte function. Recent studies, however, revealed that the earliest stage of B lymphopoiesis plays an important role in the immune decline. Understanding the precise mechanism in human and mouse BM, and the assessment of species variations with novel technologies would make the potential applications to cancer immunotherapy and the discovery of novel treatment for autoimmune diseases possible.
P- Reviewer: Borrione P, Regueiro JR, Song J S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Clark MR, Mandal M, Ochiai K, Singh H. Orchestrating B cell lymphopoiesis through interplay of IL-7 receptor and pre-B cell receptor signalling. Nat Rev Immunol. 2014;14:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Rolink AG, Schaniel C, Busslinger M, Nutt SL, Melchers F. Fidelity and infidelity in commitment to B-lymphocyte lineage development. Immunol Rev. 2000;175:104-111. [PubMed] |
| 3. | Tudor KS, Payne KJ, Yamashita Y, Kincade PW. Functional assessment of precursors from murine bone marrow suggests a sequence of early B lineage differentiation events. Immunity. 2000;12:335-345. [PubMed] |
| 4. | Kawamoto H, Katsura Y. A new paradigm for hematopoietic cell lineages: revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. 2009;30:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Mansson R, Zandi S, Welinder E, Tsapogas P, Sakaguchi N, Bryder D, Sigvardsson M. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115:2601-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Ichii M, Shimazu T, Welner RS, Garrett KP, Zhang Q, Esplin BL, Kincade PW. Functional diversity of stem and progenitor cells with B-lymphopoietic potential. Immunol Rev. 2010;237:10-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Yokota T, Sudo T, Ishibashi T, Doi Y, Ichii M, Orirani K, Kanakura Y. Complementary regulation of early B-lymphoid differentiation by genetic and epigenetic mechanisms. Int J Hematol. 2013;98:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Ferreirós-Vidal I, Carroll T, Taylor B, Terry A, Liang Z, Bruno L, Dharmalingam G, Khadayate S, Cobb BS, Smale ST. Genome-wide identification of Ikaros targets elucidates its contribution to mouse B-cell lineage specification and pre-B-cell differentiation. Blood. 2013;121:1769-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Satoh Y, Yokota T, Sudo T, Kondo M, Lai A, Kincade PW, Kouro T, Iida R, Kokame K, Miyata T. The Satb1 protein directs hematopoietic stem cell differentiation toward lymphoid lineages. Immunity. 2013;38:1105-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Medina KL, Pongubala JM, Reddy KL, Lancki DW, Dekoter R, Kieslinger M, Grosschedl R, Singh H. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607-617. [PubMed] |
| 11. | Busslinger M. Transcriptional control of early B cell development. Annu Rev Immunol. 2004;22:55-79. [PubMed] |
| 12. | Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242-245. [PubMed] |
| 13. | Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929-1939. [PubMed] |
| 14. | Yang L, Bryder D, Adolfsson J, Nygren J, Månsson R, Sigvardsson M, Jacobsen SE. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717-2723. [PubMed] |
| 15. | Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 475] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 16. | Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173-1182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 17. | Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553-5561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 266] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 18. | Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 458] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 19. | Geiger H, Rudolph KL. Aging in the lympho-hematopoietic stem cell compartment. Trends Immunol. 2009;30:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273-1280. [PubMed] |
| 21. | Whitlock CA, Witte ON. Long-term culture of murine bone marrow precursors of B lymphocytes. Methods Enzymol. 1987;150:275-286. [PubMed] |
| 22. | Kouro T, Yokota T, Welner R, Kincade PW. In vitro differentiation and measurement of B cell progenitor activity in culture. Curr Protoc Immunol. 2005;Chapter 22:Unit 22F.2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Nishihara M, Wada Y, Ogami K, Ebihara Y, Ishii T, Tsuji K, Ueno H, Asano S, Nakahata T, Maekawa T. A combination of stem cell factor and granulocyte colony-stimulating factor enhances the growth of human progenitor B cells supported by murine stromal cell line MS-5. Eur J Immunol. 1998;28:855-864. [PubMed] |
| 24. | Kurosaka D, LeBien TW, Pribyl JA. Comparative studies of different stromal cell microenvironments in support of human B-cell development. Exp Hematol. 1999;27:1271-1281. [PubMed] |
| 25. | Ohkawara JI, Ikebuchi K, Fujihara M, Sato N, Hirayama F, Yamaguchi M, Mori KJ, Sekiguchi S. Culture system for extensive production of CD19+IgM+ cells by human cord blood CD34+ progenitors. Leukemia. 1998;12:764-771. [PubMed] |
| 26. | Rawlings DJ, Quan SG, Kato RM, Witte ON. Long-term culture system for selective growth of human B-cell progenitors. Proc Natl Acad Sci USA. 1995;92:1570-1574. [PubMed] |
| 27. | Ichii M, Oritani K, Yokota T, Nishida M, Takahashi I, Shirogane T, Ezoe S, Saitoh N, Tanigawa R, Kincade PW. Regulation of human B lymphopoiesis by the transforming growth factor-beta superfamily in a newly established coculture system using human mesenchymal stem cells as a supportive microenvironment. Exp Hematol. 2008;36:587-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Ichii M, Oritani K, Yokota T, Schultz DC, Holter JL, Kanakura Y, Kincade PW. Stromal cell-free conditions favorable for human B lymphopoiesis in culture. J Immunol Methods. 2010;359:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Kraus H, Kaiser S, Aumann K, Bönelt P, Salzer U, Vestweber D, Erlacher M, Kunze M, Burger M, Pieper K. A feeder-free differentiation system identifies autonomously proliferating B cell precursors in human bone marrow. J Immunol. 2014;192:1044-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Fulop GM, Phillips RA. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479-482. [PubMed] |
| 31. | Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527-530. [PubMed] |
| 32. | Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175-3182. [PubMed] |
| 33. | Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477-6489. [PubMed] |
| 34. | Rongvaux A, Willinger T, Takizawa H, Rathinam C, Auerbach W, Murphy AJ, Valenzuela DM, Yancopoulos GD, Eynon EE, Stevens S. Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci USA. 2011;108:2378-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 35. | Cheung AM, Nguyen LV, Carles A, Beer P, Miller PH, Knapp DJ, Dhillon K, Hirst M, Eaves CJ. Analysis of the clonal growth and differentiation dynamics of primitive barcoded human cord blood cells in NSG mice. Blood. 2013;122:3129-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell. 2012;10:120-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 591] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 37. | Wang JC, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919-3924. [PubMed] |
| 38. | Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502-1506. [PubMed] |
| 39. | Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109-1121. [PubMed] |
| 40. | Adolfsson J, Borge OJ, Bryder D, Theilgaard-Mönch K, Astrand-Grundström I, Sitnicka E, Sasaki Y, Jacobsen SE. Upregulation of Flt3 expression within the bone marrow Lin(-)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659-669. [PubMed] |
| 41. | Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797-1806. [PubMed] |
| 42. | Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661-672. [PubMed] |
| 43. | Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213-1225. [PubMed] |
| 44. | Li YS, Wasserman R, Hayakawa K, Hardy RR. Identification of the earliest B lineage stage in mouse bone marrow. Immunity. 1996;5:527-535. [PubMed] |
| 45. | Anjos-Afonso F, Currie E, Palmer HG, Foster KE, Taussig DC, Bonnet D. CD34(-) cells at the apex of the human hematopoietic stem cell hierarchy have distinctive cellular and molecular signatures. Cell Stem Cell. 2013;13:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Takahashi M, Matsuoka Y, Sumide K, Nakatsuka R, Fujioka T, Kohno H, Sasaki Y, Matsui K, Asano H, Kaneko K. CD133 is a positive marker for a distinct class of primitive human cord blood-derived CD34-negative hematopoietic stem cells. Leukemia. 2014;28:1308-1315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y, Fujimura Y, Tsuji T, Ikehara S, Sonoda Y. SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood. 2003;101:2924-2931. [PubMed] |
| 48. | Larochelle A, Savona M, Wiggins M, Anderson S, Ichwan B, Keyvanfar K, Morrison SJ, Dunbar CE. Human and rhesus macaque hematopoietic stem cells cannot be purified based only on SLAM family markers. Blood. 2011;117:1550-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Sitnicka E, Buza-Vidas N, Larsson S, Nygren JM, Liuba K, Jacobsen SE. Human CD34+ hematopoietic stem cells capable of multilineage engrafting NOD/SCID mice express flt3: distinct flt3 and c-kit expression and response patterns on mouse and candidate human hematopoietic stem cells. Blood. 2003;102:881-886. [PubMed] |
| 50. | Görgens A, Radtke S, Möllmann M, Cross M, Dürig J, Horn PA, Giebel B. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Rep. 2013;3:1539-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 51. | Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 373] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 52. | Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 622] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 53. | Ichii M, Oritani K, Yokota T, Zhang Q, Garrett KP, Kanakura Y, Kincade PW. The density of CD10 corresponds to commitment and progression in the human B lymphoid lineage. PLoS One. 2010;5:e12954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 54. | Six EM, Bonhomme D, Monteiro M, Beldjord K, Jurkowska M, Cordier-Garcia C, Garrigue A, Dal Cortivo L, Rocha B, Fischer A. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204:3085-3093. [PubMed] |
| 55. | Kohn LA, Hao QL, Sasidharan R, Parekh C, Ge S, Zhu Y, Mikkola HK, Crooks GM. Lymphoid priming in human bone marrow begins before expression of CD10 with upregulation of L-selectin. Nat Immunol. 2012;13:963-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955-1960. [PubMed] |
| 57. | von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519-1526. [PubMed] |
| 58. | Sitnicka E, Bryder D, Theilgaard-Mönch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463-472. [PubMed] |
| 59. | Gwin KA, Shapiro MB, Dolence JJ, Huang ZL, Medina KL. Hoxa9 and Flt3 signaling synergistically regulate an early checkpoint in lymphopoiesis. J Immunol. 2013;191:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Dolence JJ, Gwin KA, Shapiro MB, Medina KL. Flt3 signaling regulates the proliferation, survival, and maintenance of multipotent hematopoietic progenitors that generate B cell precursors. Exp Hematol. 2014;42:380-393.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Kikuchi K, Kondo M. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc Natl Acad Sci USA. 2006;103:17852-17857. [PubMed] |
| 62. | Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463-471. [PubMed] |
| 63. | Lundström W, Fewkes NM, Mackall CL. IL-7 in human health and disease. Semin Immunol. 2012;24:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 64. | Prieyl JA, LeBien TW. Interleukin 7 independent development of human B cells. Proc Natl Acad Sci USA. 1996;93:10348-10353. [PubMed] |
| 65. | Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394-397. [PubMed] |
| 66. | Giliani S, Mori L, de Saint Basile G, Le Deist F, Rodriguez-Perez C, Forino C, Mazzolari E, Dupuis S, Elhasid R, Kessel A. Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev. 2005;203:110-126. [PubMed] |
| 67. | Hystad ME, Myklebust JH, Bø TH, Sivertsen EA, Rian E, Forfang L, Munthe E, Rosenwald A, Chiorazzi M, Jonassen I. Characterization of early stages of human B cell development by gene expression profiling. J Immunol. 2007;179:3662-3671. [PubMed] |
| 68. | Kikushige Y, Yoshimoto G, Miyamoto T, Iino T, Mori Y, Iwasaki H, Niiro H, Takenaka K, Nagafuji K, Harada M. Human Flt3 is expressed at the hematopoietic stem cell and the granulocyte/macrophage progenitor stages to maintain cell survival. J Immunol. 2008;180:7358-7367. [PubMed] |
| 69. | Nakamori Y, Liu B, Ohishi K, Suzuki K, Ino K, Matsumoto T, Masuya M, Nishikawa H, Shiku H, Hamada H. Human bone marrow stromal cells simultaneously support B and T/NK lineage development from human haematopoietic progenitors: a principal role for flt3 ligand in lymphopoiesis. Br J Haematol. 2012;157:674-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784-3793. [PubMed] |
| 71. | Taguchi T, Takenouchi H, Shiozawa Y, Matsui J, Kitamura N, Miyagawa Y, Katagiri YU, Takahashi T, Okita H, Fujimoto J. Interleukin-7 contributes to human pro-B-cell development in a mouse stromal cell-dependent culture system. Exp Hematol. 2007;35:1398-1407. [PubMed] |
| 72. | Parrish YK, Baez I, Milford TA, Benitez A, Galloway N, Rogerio JW, Sahakian E, Kagoda M, Huang G, Hao QL. IL-7 Dependence in human B lymphopoiesis increases during progression of ontogeny from cord blood to bone marrow. J Immunol. 2009;182:4255-4266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 73. | Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484-2490. [PubMed] |
| 74. | Klangsinsirikul P, Russell NH. Peripheral blood stem cell harvests from G-CSF-stimulated donors contain a skewed Th2 CD4 phenotype and a predominance of type 2 dendritic cells. Exp Hematol. 2002;30:495-501. [PubMed] |
| 75. | Bendall SC, Davis KL, Amir el-AD, Tadmor MD, Simonds EF, Chen TJ, Shenfeld DK, Nolan GP, Pe’er D. Single-cell trajectory detection uncovers progression and regulatory coordination in human B cell development. Cell. 2014;157:714-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 669] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 76. | Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7-25. [PubMed] |
| 77. | Tang J, Scott G, Ryan DH. Subpopulations of bone marrow fibroblasts support VLA-4-mediated migration of B-cell precursors. Blood. 1993;82:3415-3423. [PubMed] |
| 78. | Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707-718. [PubMed] |
| 79. | Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706-3712. [PubMed] |
| 80. | Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1759] [Article Influence: 159.9] [Reference Citation Analysis (0)] |
| 81. | Lo Celso C, Scadden DT. The haematopoietic stem cell niche at a glance. J Cell Sci. 2011;124:3529-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 82. | Kiel MJ, Radice GL, Morrison SJ. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1:204-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 83. | Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2810] [Cited by in RCA: 2580] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 84. | Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1291] [Cited by in RCA: 1532] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 85. | Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJ. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med. 2013;210:1137-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 338] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 86. | Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 623] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 87. | Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407-421. [PubMed] |
| 88. | Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 575] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 89. | Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637-643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 940] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 90. | Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 638] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 91. | Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, Gao W, Saito TI, Lo Celso C, Tsuyuzaki H. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 452] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 92. | Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836-841. [PubMed] |
| 93. | Lai AY, Watanabe A, O’Brien T, Kondo M. Pertussis toxin-sensitive G proteins regulate lymphoid lineage specification in multipotent hematopoietic progenitors. Blood. 2009;113:5757-5764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 94. | Rashidi NM, Scott MK, Scherf N, Krinner A, Kalchschmidt JS, Gounaris K, Selkirk ME, Roeder I, Lo Celso C. In vivo time-lapse imaging shows diverse niche engagement by quiescent and naturally activated hematopoietic stem cells. Blood. 2014;124:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 956] [Cited by in RCA: 977] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 96. | Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 915] [Cited by in RCA: 1061] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 97. | Tomblyn MB, Arora M, Baker KS, Blazar BR, Brunstein CG, Burns LJ, DeFor TE, Dusenbery KE, Kaufman DS, Kersey JH. Myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia: analysis of graft sources and long-term outcome. J Clin Oncol. 2009;27:3634-3641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 98. | Corselli M, Chin CJ, Parekh C, Sahaghian A, Wang W, Ge S, Evseenko D, Wang X, Montelatici E, Lazzari L. Perivascular support of human hematopoietic stem/progenitor cells. Blood. 2013;121:2891-2901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 99. | Guezguez B, Campbell CJ, Boyd AL, Karanu F, Casado FL, Di Cresce C, Collins TJ, Shapovalova Z, Xenocostas A, Bhatia M. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell. 2013;13:175-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 100. | Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, Wang H, Le T, Faull KF, Chen R. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 503] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 101. | Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood. 2000;96:2022-2036. [PubMed] |
| 102. | Zipori D, Barda-Saad M. Role of activin A in negative regulation of normal and tumor B lymphocytes. J Leukoc Biol. 2001;69:867-873. [PubMed] |
| 103. | Bouchard C, Fridman WH, Sautès C. Effect of TGF-beta1 on cell cycle regulatory proteins in LPS-stimulated normal mouse B lymphocytes. J Immunol. 1997;159:4155-4164. [PubMed] |
| 104. | Malhotra S, Kincade PW. Wnt-related molecules and signaling pathway equilibrium in hematopoiesis. Cell Stem Cell. 2009;4:27-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 105. | Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160-164. [PubMed] |
| 106. | Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat Immunol. 2006;7:1048-1056. [PubMed] |
| 107. | Luis TC, Naber BA, Roozen PP, Brugman MH, de Haas EF, Ghazvini M, Fibbe WE, van Dongen JJ, Fodde R, Staal FJ. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 108. | Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci USA. 2007;104:15436-15441. [PubMed] |
| 109. | Florian MC, Nattamai KJ, Dörr K, Marka G, Uberle B, Vas V, Eckl C, Andrä I, Schiemann M, Oostendorp RA. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;503:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 110. | Malhotra S, Baba Y, Garrett KP, Staal FJ, Gerstein R, Kincade PW. Contrasting responses of lymphoid progenitors to canonical and noncanonical Wnt signals. J Immunol. 2008;181:3955-3964. [PubMed] |
| 111. | Ichii M, Frank MB, Iozzo RV, Kincade PW. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood. 2012;119:1683-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 112. | Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp Hematol. 2009;37:19-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Ständer M, Naumann U, Wick W, Weller M. Transforming growth factor-beta and p-21: multiple molecular targets of decorin-mediated suppression of neoplastic growth. Cell Tissue Res. 1999;296:221-227. [PubMed] |
| 114. | Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547-558. [PubMed] |
| 115. | Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 234] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 116. | Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 510] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 117. | Benveniste P, Serra P, Dervovic D, Herer E, Knowles G, Mohtashami M, Zúñiga-Pflücker JC. Notch signals are required for in vitro but not in vivo maintenance of human hematopoietic stem cells and delay the appearance of multipotent progenitors. Blood. 2014;123:1167-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 118. | Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506:240-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 420] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 119. | Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 389] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 120. | Trowbridge JJ, Scott MP, Bhatia M. Hedgehog modulates cell cycle regulators in stem cells to control hematopoietic regeneration. Proc Natl Acad Sci USA. 2006;103:14134-14139. [PubMed] |
| 121. | Hofmann I, Stover EH, Cullen DE, Mao J, Morgan KJ, Lee BH, Kharas MG, Miller PG, Cornejo MG, Okabe R. Hedgehog signaling is dispensable for adult murine hematopoietic stem cell function and hematopoiesis. Cell Stem Cell. 2009;4:559-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 122. | Cooper CL, Hardy RR, Reth M, Desiderio S. Non-cell-autonomous hedgehog signaling promotes murine B lymphopoiesis from hematopoietic progenitors. Blood. 2012;119:5438-5448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |