Published online Oct 26, 2013. doi: 10.4252/wjsc.v5.i4.229
Revised: October 4, 2013
Accepted: October 17, 2013
Published online: October 26, 2013
Processing time: 128 Days and 18.1 Hours
AIM: To develop an in vitro model based on neural stem cells derived from transgenic animals, to be used in the study of pathological mechanisms of Alzheimer’s disease and for testing new molecules.
METHODS: Neural stem cells (NSCs) were isolated from the subventricular zone of Wild type (Wt) and Tg2576 mice. Primary and secondary neurosphere generation was studied, analysing population doubling and the cell yield per animal. Secondary neurospheres were dissociated and plated on MCM Gel Cultrex 2D and after 6 d in vitro (DIVs) in mitogen withdrawal conditions, spontaneous differentiation was studied using specific neural markers (MAP2 and TuJ-1 for neurons, GFAP for astroglial cells and CNPase for oligodendrocytes). Gene expression pathways were analysed in secondary neurospheres, using the QIAGEN PCR array for neurogenesis, comparing the Tg2576 derived cell expression with the Wt cells. Proteins encoded by the altered genes were clustered using STRING web software.
RESULTS: As revealed by 6E10 positive staining, all Tg2576 derived cells retain the expression of the human transgenic Amyloid Precursor Protein. Tg2576 derived primary neurospheres show a decrease in population doubling. Morphological analysis of differentiated NSCs reveals a decrease in MAP2- and an increase in GFAP-positive cells in Tg2576 derived cells. Analysing the branching of TuJ-1 positive cells, a clear decrease in neurite number and length is observed in Tg2576 cells. The gene expression neurogenesis pathway revealed 11 altered genes in Tg2576 NSCs compared to Wt.
CONCLUSION: Tg2576 NSCs represent an appropriate AD in vitro model resembling some cellular alterations observed in vivo, both as stem and differentiated cells.
Core tip: In this study neural stem cells isolated from Tg2576 mice are characterized as an in vitro model for Alzheimer’s disease. These cells represent a robust system for studying pathological mechanisms related to Aβ intracellular accumulation, such as stem cell status, or during differentiation processes. This model could provide a new cell platform for developing and screening new molecules.
- Citation: Baldassarro VA, Lizzo G, Paradisi M, Fernández M, Giardino L, Calzà L. Neural stem cells isolated from amyloid precursor protein-mutated mice for drug discovery. World J Stem Cells 2013; 5(4): 229-237
- URL: https://www.wjgnet.com/1948-0210/full/v5/i4/229.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i4.229
Alzheimer’s disease (AD) is the most common cause of dementia, affecting 24.3 million people worldwide[1] with a 10%-11% and 14%-17% estimated lifetime risk respectively for males and females at age 85[2]. The cost of caring for afflicted patients is enormous and beyond the capability of most developing countries[3] and poses a serious problem also for the gross national product (GNP) of western countries[4]. The prevalence and incidence rates increase exponentially with age[5] and ageing is actually the only risk factor identified with certainty[6].
Today the pathophysiology of this disease is still to be elucidated, resulting in a total absence of disease-modifying therapies. However, the finding of amyloid β (Aβ) peptide deposits in the brains of affected individuals[7] and the study of the causes of the Familial AD (FAD) indicated Aβ peptide production as a possible therapeutic target[8].
The lack of effective therapies leads to the need for new models for drug discovery and screening. In this way stem cells represent a promising tool to create in vitro models suitable for studying disease mechanisms, for pharmacological target identification and for drug screening[9].
In particular, the possibility of deriving primary cultures of neural stem cells (NSCs) from animal models of disease at different ages provides an opportunity to derive cell types carrying human mutations. In this context, the Tg2576 mice, expressing the 695 isoform of the human Amyloid precursor protein (APP) gene carrying the Swedish mutation[10], represent a well-characterized model for AD. These mice accumulate Aβ in an age-related manner, particularly in the hippocampus[11], the cerebral cortex[12] and the olfactory bulb[13]. NSCs derived from these animals could be a robust tool for studying the disease mechanisms related to Aβ intra- and extra-cellular accumulation[14]. Cell platforms derived from stem cells can be used at different stages of the differentiation process, thus acting as a useful tool for pharmacological agents active on proliferation, differentiation, functions and pathways of the mature Wild type (Wt) and pathological phenotype.
In this study we have characterized neural stem cells derived from adult Tg2576 AD mice in terms of self-renewal, gene expression profile, multipotency and differentiation capability, compared to neural stem cells derived from Wt age-matched animals.
We derived NSCs from the subventricular zone (SVZ). Neuroblasts generated in this area migrate to the olfactory bulb, renewing inhibitory interneurons in the primary olfactory nucleus and olfactory glomerula, thus contributing to the preservation of the olfactory function. The interest in studying these cells derives from the early olfactory impairment in AD patients[15], and from the possibility to use olfaction as a response marker for novel treatments[16]. Moreover, the neurosphere assay is almost exclusively used in the SVZ, while neuroblast and neural stem cells derived from the subgranular zone of the dentate gyrus of the hippocampus (e.g., the second brain area for constitutive neurogenesis) are predominantly cultured as adherent cells[17].
The term neural stem cell (NSC) has been used throughout the text to refer to a heterogeneous population of neural stem cells (NSCs), neural precursors and progenitor cells (NPCs)[18].
Tg2576 mice and their non-transgenic littermates (001349-W) were purchased from Taconic Europe (Lille Skensved, Denmark). Animal care and treatment were in accordance with the EU Directive 2010/63/EU for animal experiments and in conformity with protocols approved by the Ethical Committee of Animal Experimentation, University of Bologna.
Adult NSCs were isolated following the Ahlenius and Kokaia protocol[19] with some modifications[20]. Brains from six month old mice were collected in a 50 mL tube containing ice-cold HBSS (Life Technologies, Milan, Italy).
Using a lancet, the olfactory bulbs were removed. Two 1 mm thick coronal slices were prepared from the rest of the brain and the SVZ was isolated and triturated in cold PBS using scissors. SVZ tissues were transferred to a 15 ml tube and allowed to settle. The PBS was then removed and the tissues incubated with the dissociation buffer consisting of: 1x HBSS; 5.4 mg/mL D-Glucose (SIGMA, St. Louise, MO, United States); 15 mmol/L HEPES (Life Technologies); 1.33 mg/mL Trypsin (SIGMA); 0.7 mg/mL hyaluronidase (SIGMA); 80U/mL DNase (SIGMA). After 15 min incubation at 37 °C tissues were pipetted several times to favour dissociation and incubated again at 37 °C for 10 min. In order to remove the undissociated tissue fragments, the solution was filtered through a 70 µm filter paper and then centrifuged at 400 ×g for 5 min. The resulting pellet was washed twice, first with a sucrose-HBSS solution (HBSS 0.5 ×; 0.3 g/mL sucrose), 500 ×g 10 min, then with a solution consisting of BSA (40 mg/mL), HEPES (0.02 mol/L) in EBSS. After 7 min centrifugation at 400 ×g, the cellular pellet was resuspended in serum-free medium (DMEM/F12 GlutaMAX 1 ×; 8 mmol/L HEPES; 100 U/100 μg Penicillin/Streptomycin; 0.1 × B27; 1 × N-2; 10 ng/mL bFGF; 20 ng/mL EGF) and, after cell count, cells were plated at a density of 50 cells/µl in a final volume of 3 mL in low-attachment 6-well plates (NUNC). Medium was changed every three days, centrifuging the cell suspension at 300 ×g for 5 min and gently resuspending the cellular pellet in fresh medium.
To obtain secondary neurospheres, cells were centrifuged at 300 ×g for 5 min and incubated in a 0.5 mg/mL trypsin - 0.2 mg/mL EDTA solution in HBSS at 37 °C for 15 min. After inhibiting trypsinization and subsequent centrifugation, the cellular pellet was resuspended in half fresh/half old medium. Cells were counted and re-plated at the same density.
Three different cultures were prepared; all experiments were performed in duplicate.
For population doubling, cell yields and mRNA analysis, undifferentiated neurospheres were used, while for morphology studies, secondary neurosphere derived cells were analysed during spontaneous differentiation.
Cells were counted from primary and secondary neurospheres 3, 4 and 5 dafter plating. Counting procedure was performed taking images of all neurospheres and statistically calculating the cell number based on single cell and sphere area using Image ProPlus software (Media Cybernetics Inc, Bethesda, MD, United States).
Population doubling was calculated using the following formula[21]: PD = log10(N/N0)*3.33
Where PD is the Population Doubling, N and N0 are the final and initial number of cells, respectively.
The RNeasy Micro Kit (QIAGEN) was used for total RNA extraction and 300 ng were retrotranscribed using the RT2 First Strand Kit (QIAGEN) following the manufacturer’s instructions.
For the study of NSC gene expression, the 96-well QIAGEN PCR array for neurogenesis was used in combination with the RT2 SYBR Green qPCR Mastermix (QIAGEN), using 10 ng of cDNA per well.
In brief, 5 d in vitro (DIV) secondary neurospheres were dissociated as described above and plated on 0.25 mg/mL MCM Gel 2D Cultrex (TREVIGEN, Helgerman Court, Gaithersburg, MD USA) in 24-well plates at a density of 1 × 104 cells/cm2. Cells were grown in the same culture medium without mitogens. After this, 6 DIV cells were fixed (cold 4% paraformaldehyde, 20 min), washed (two PBS washes, 10 min each) and incubated overnight at 4 °C with primary antibodies diluted in PBS/0.3% Triton x-100. After two washes, incubation with the secondary antibodies was performed at 37°C for 30 min. Cells were then washed and incubated with Hoechst 33258 (1 µg/mL in PBS/0.2% Triton x-100) for 30 min at RT. Finally, cells were washed again and mounted with phenylendiamine solution (0.1% 1,4-phenylendiamine -Sigma-, 50% glycerine -Sigma-, carbonate/bicarbonate buffer pH 8.6). Controls were always performed on secondary antibodies. The primary and secondary antibodies used are described in Table 1, as well as the species in which they were produced, the manufacturers and the working dilutions.
| Antibody | Specie | Supplier | Dilution | |
| Primary antibody | ||||
| β-III-Tubulin (TuJ-1) | Mouse | R and D | 1:1000 | |
| GFAP | Rabbit | Eurodiagnostic | 1:100 | |
| MAP2 | Rabbit | S.Cruz | 1:250 | |
| 6E10 | Mouse | Covance | 1:1000 | |
| CNPase | Mouse | Chemicon | 1:250 | |
| Secondary antibody | ||||
| Anti-Mo RRX | Donkey | Alexa | 1:600 | |
| Anti-Mo Cy2 | Donkey | Jackson | 1:100 | |
| Anti-Rb Cy2 | Donkey | Jackson | 1:100 | |
| Anti-Rb RRX | Donkey | Jackson | 1:100 | |
In order to study the development of the filaments net of differentiated neurons, cells positive for the β-tubulin marker (TuJ-1) were analysed using the NIS-Elements Microscope Imaging Software (NIKON). Three random fields from each well were analysed, counting total neurite length and the number of branches per neuron. Hoechst 33258 nuclear staining was used to identify the total number of cells. At least 20 TuJ-1 positive cells per group were analysed.
To quantify the percentage of GFAP, CNPase and MAP2 positive cells, three random fields per well were considered.
In order to identify the expression of the Aβ/APP transgenic protein, the 6E10 antibody was used. This antibody recognizes the APP whole protein, its cleavage products Aβ (40 and 42) and the CTFβ (C-terminal fragment β), although it is specific for the human protein, identifying in this case only the transgenic protein[22].
Student’s t-test was used to statistically analyse data obtained from Wt and Tg2576 stem cell cultures. Data were considered significant when P < 0.05.
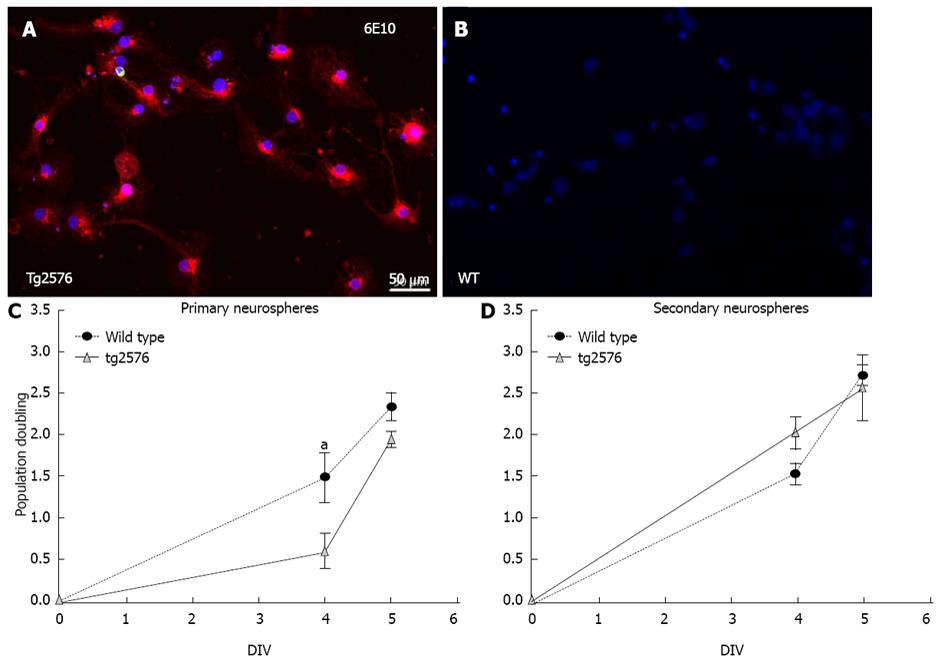
Cells from Tg2576 mice neurospheres express the human transgenic protein APP/Aβ visualized using the 6E10 antibody (Figure 1A) whereas cells differentiated from Wt neurospheres do not (Figure 1B).
In order to characterize the neural stem cell model, the proliferation and differentiation capability of cultured NSCs derived from Tg2576 compared to Wt cultures were studied. Up to two generations of neurospheres, primary and secondary, were derived from Wt and Tg2576 animals in the presence of EGF and bFGF, and the population doubling calculated. The secondary neurosphere formation is considered the directly related parameter to the self-renewal activity[23]. When comparing Wt versus Tg2576 derived neurospheres, a statistically significant impairment was observed in population doubling of Tg2576 primary neurospheres (Figure 1C). A decrease in the yield of cells per animal in Tg2576 secondary neurospheres (Table 2) was also observed, even if the population doubling rate was restored during the generation of secondary neurospheres (Figure 1D).
| Wild type | Tg2576 | |
| Primary neurospheres | 1.7 × 105± 5 × 104 | 1.7 × 105± 1×104 |
| Secondary neurospheres | 1.7 × 105± 1.4× 104 | 1.3×105± 9.7×103a |
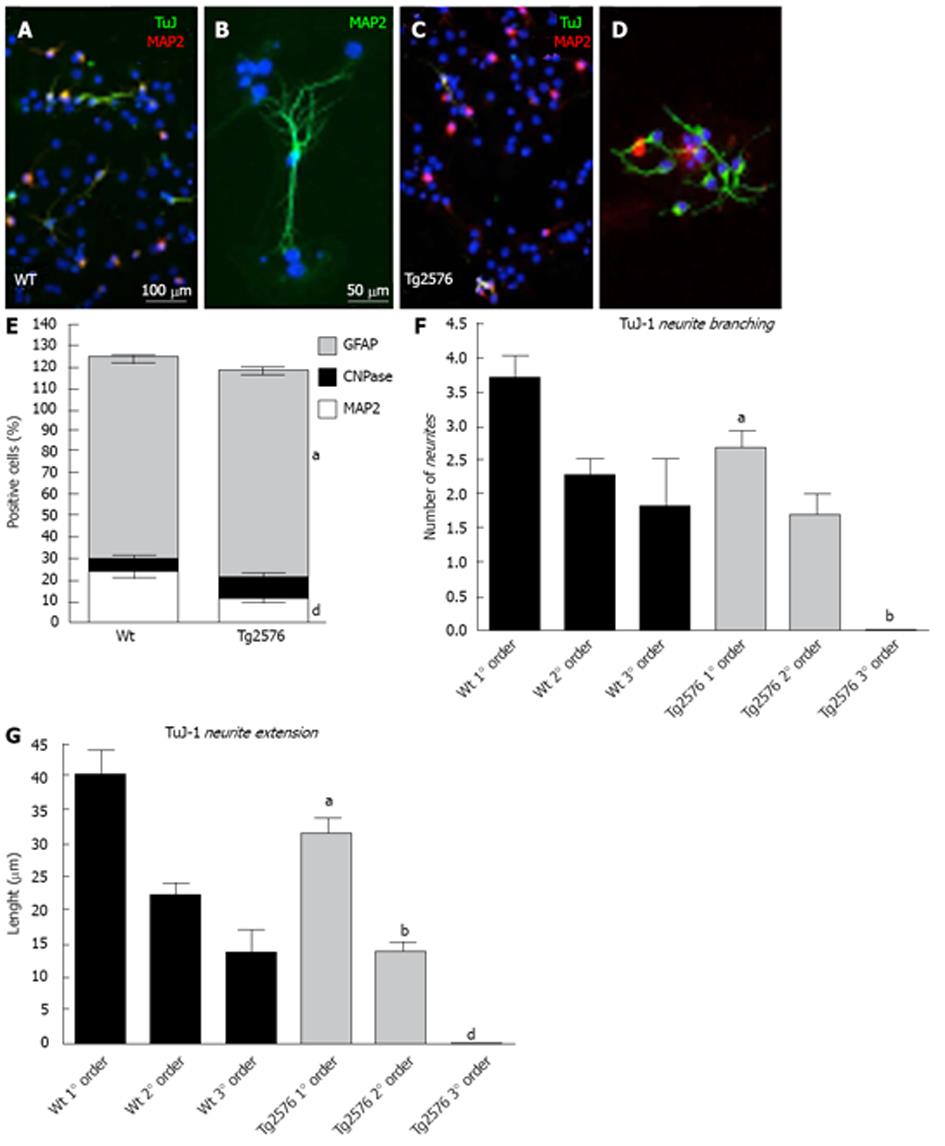
Specific markers for neurons (Tuj-1 and MAP2), oligodendrocytes (CNPase) and astroglial cells (GFAP) were used to study the differentiation capability of NSCs from primary and secondary neurospheres after mitogen withdrawal (Figure 2). Significant differences were found in the expression of GFAP (glial fibrillary acidic protein) and MAP2 (microtubule associated protein 2) between Wt and Tg2576 differentiated cells, with a lower number of MAP2-positive cells and a higher number of GFAP-positive cells derived from Tg2576 mice (Figure 2E) being observed. Differentiated neurons from these animals also show a lower number (Figure 2F) and length (Figure 2G) of neurites marked using an antibody against the β-III-tubulin (Tuj-1).
In order to detect gene expression alterations between Tg2576 and Wt derived NSCs, a QIAGEN PCR array for neurogenesis was used.
Different genes are affected by the transgenic human mutated APP gene expressed in Tg2576 mice. According to the manufacturer’s instructions, a difference two times greater is considered biologically significant (Table 3). All the genes differently expressed in the two genotypes are overexpressed in Tg2576 cells. These include genes involved in neurotransmission (Acetylcholinesterase and Dopamine receptor D2), growth factors (Glial cell line derived neurotrophic factor, V-erb-b2 and Vascular endothelial growth factor A), cell growth and neuronal differentiation (CDK5, interleukin 3 and Notch gene homolog 2) and an APP-related protein (Amyloid beta precursor protein-binding-1).
| Gene | Regulation |
| Acetylcholinesterase | 3.41 |
| Amyloid beta (A4) precursor protein-binding, family B, member 1 | 2.33 |
| CDK5 regulatory subunit associated protein 1 | 2.07 |
| Dopamine receptor D2 | 2.62 |
| V-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian) | 2.17 |
| Glial cell line derived neurotrophic factor | 3.13 |
| Interleukin 3 | 2.69 |
| Notch gene homolog 2 (Drosophila) | 2.29 |
| Odd Oz/ten-m homolog 1 (Drosophila) | 3.81 |
| Par-3 (partitioning defective 3) homolog (C. elegans) | 2.39 |
| Vascular endothelial growth factor A | 3.01 |
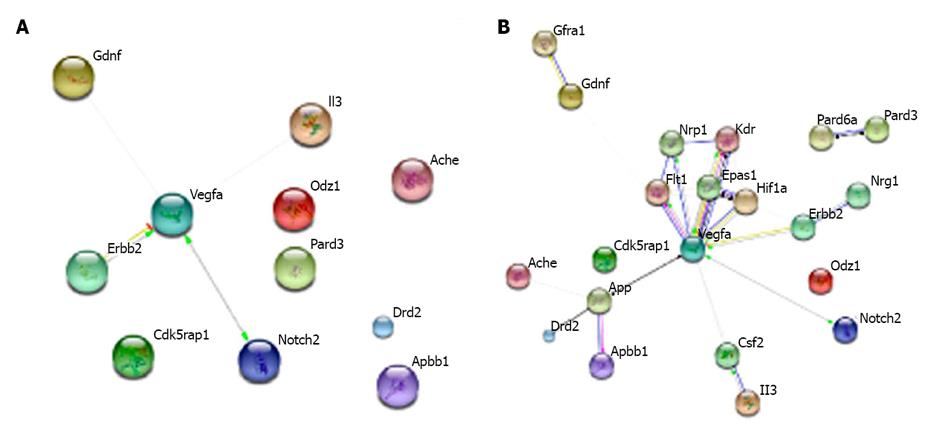
Using the web-software STRING [http://string-db.org/], proteins derived from the genes of interest were connected in clusters according to their interactions and involvement in biological processes (Figure 3A). The software allows the net of interactions including other proteins closely linked to the one analysed to be extended, in order to obtain a better understanding of the possible pathways affected by the transgene effect in Tg2576 mice (Figure 3B). The analysis shows that almost all genes upregulated in Tg2576 NSCs interact with each other along different pathways, situating VEGF (vascular endothelial growth factor) as a node of the net.
At the present time, medical research relies on appropriate model systems to study disease mechanisms and to develop novel therapies. In particular, complex pathologies, such as AD, with no certainly ascertained causes and with no available cure, need new models for in vitro studies and for fast screening new molecules. Even if a number of animal models allow us to recreate diseases in vivo, in vitro systems are still needed to study pathologies at cellular and molecular level and to generate screening platforms for drug discovery.
The Tg2576 mice, which express the human APP695 transgene carrying the Swedish mutation, display several neuropathological features of AD, including plaque deposition[8] and early synaptic abnormalities[24-28]. This transgenic mouse is widely used as an animal model of AD, resembling the inherited form of the pathology related to the APP gene.
In the present study NSCs isolated from Tg2565 mice were characterized as an in vitro model for AD due to their ability to preserve transgenic Aβ protein expression. Notably, an altered neurogenesis was showed in AD patients and in different animal models.
The neurospheres generated from the primary culture of adult SVZ mice when cells are grown in the presence of mitogens (EGF and bFGF) are composed of a heterogeneous population of neural stem cells (NSCs), neural precursors and progenitor cells (NPCs). However, this mixed cell population is usually denoted as “neurostem cells”. Neurosphere assay is an accepted model for the study of NSC self-renewal through the formation of different neurosphere generations (primary, secondary neurospheres) and also for the study of their capability to differentiate into neural cell phenotypes (neurons, astrocytes and oligodendrocytes)[18].
The complex pathology of AD is thought to include an impairment of neurotrophic signaling leading to alterations in the neurogenesis process[26]. Tg2576 derived cells show a significant decrease in cell doubling during the first stages of primary neurosphere development and a decreased yield of isolated neurons, thus resembling impairment of neurotrophic alterations in the neurogenesis process in Tg2576 mice[29] and in AD brains[30].
The ability of these cells to spontaneously differentiate when plated on a matrix mimicking the extracellular space allows us to study possible lineage and maturation differences between Tg2576- and wild-type-derived cells. A significant decrease was found in MAP2 positive cells in Tg2576 cultures, indicating a defect in neural lineage. Also neuron maturation is altered in Tg2576 compared to wild-type-derived animals. These cultures actually display a decrease in neurite extension of β-III-tubulin positive cells.
The role of APP in neurogenesis and neuron maturation processes has still to be fully elucidated, but a number of data suggest that the soluble APP (sAPP) alpha and the APP intracellular domain (AICD) affect proliferation, survival and migration of the NSC population[31], as well as neuronal maturation. The sAPP seems to promotes gliogenesis, whereas AICD negatively modulates proliferation and maturation of the neural precursor[32]. This observation suggests that an APP overexpression could influence the cell fate of the NSCs, decreasing their number and impairing maturation.
The effect of the human APP transgene expression was thus analysed using neurogenesis-related PCR arrays, resulting in 11 genes overexpressed in the Tg2576 neural stem related to Wt cultures. Among those, APP- binding-protein-1 (APP-BP1) is more than two-fold up-regulated in Tg2576-derived neurospheres. Overexpression of the APP-BP1 gene has already been described in cortices and hippocampus of this animal model[33] and, in addition, overexpression of this gene in primary neurons is related to apoptosis induction and increase in DNA synthesis[34]. The expression of the APP-BP1 gene in Tg2576 NSCs seems also to resemble the up-regulated expression found in the lipid rafts in the hippocampi of AD brains[35].
Genes involved in the neurotransmission and differentiation processes are also affected by the presence of the transgene. Factors involved in driving neurogenesis in AD include the cholinergic system since acetylcholine acts as a growth-regulatory signal in the brain[36]. The overexpression of the acetylcholinesterase gene found in this in vitro model can partially represent an AD environment. Notably, treatments involving acetylcholinesterase inhibitors in clinical use for the symptomatic treatment of memory defects have shown a potential for stimulating neurogenesis[37]. Overexpression of neurotrophins and growth factors like GDNF[38] and VEGF[39] also mimic mechanisms acting to compensate the neurotrophic and differentiation deficits in these cells.
Other factors, such as interleukin-3[40], dopamine receptor D2[41], and cyclin-dependent kinase-5 (CDK5)[42], have already been shown to be involved in neurogenesis and AD models. With regard to CDK5, the Aβ-induced neurogenesis is coupled with an increase of inhibition of CDK[43]. Thus, the overexpression of CDK5 in these cells could explain the delay in the population doubling of Tg2576 primary neurospheres, which is restored in secondary, where Aβ accumulation probably could balance the CDK5 overexpression.
In conclusion, we propose that NSCs derived from animal models carrying human mutations possibly represent a novel and useful tool for drug discovery and drug screening in AD. In particular these cells might be source of mature neurons as a robust model of intraneuronal Aβ accumulation. Due to the key pathogenetic role of intraneuronal Aβ in neurodegeneration and AD pathology[25,44,45], the possibility to derive mature neurons from animal of different age could allow to generate cell systems with different Aβ overleading, thus providing ad ideal system to investigate Aβ intraneuronal clearance.
Alzheimer’s disease is the most common form of dementia. Genetic studies indicate a possible role of the amyloid precursor protein in the disease mechanisms, thus indicating this protein and derived amyloid-β fragments as potential pharmacological targets. However, the lack of effective therapies leads to the need of new models for drug discovery and for the study of pathological processes.
Stem and re-programmed cells are looked as robust platforms and promising tools to create in vitro models suitable to study disease mechanisms, for pharmacological target identification and drug screening.
Neural stem cells isolated from transgenic animal models of diseases carrying human gene mutations could be used also to mimic in vitro neural ageing. Neural stem cells isolated from Tg2576 mice recapitulate aspects of in vivo patology, e.g., APP processing alterations, population doubling and neuronal differentiation process.
The study results suggest that neural stem cells derived from a mouse model of Alzheimer’s disease could be used as a platform for drug screening and to study disease mechanisms.
Neural stem cells are multipotent stem cells capable to generate the main central nervous system phenotypes (neurons, astrocytes and oligodendrocytes). Adult neurogenesis: new neurons are formed and integrated in specific brain areas during adulthood. Subventricular zone: this is one of the neurogenic areas of the adult central nervous system.
This is a scientific paper that illustrates an in vitro model based on neural stem cells derived from transgenic animals of interest in the study of pathological mechanisms of Alzheimer’s disease and for testing new molecules for therapeutic purposes.
P- Reviewers Kan L, Serra PA S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Stozická Z, Zilka N, Novák M. Risk and protective factors for sporadic Alzheimer’s disease. Acta Virol. 2007;51:205-222. [PubMed] |
| 2. | Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O, Bullido MJ, Engelborghs S, De Deyn P, Berr C. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16:903–907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 527] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 3. | Zhu CW, Sano M. Economic considerations in the management of Alzheimer’s disease. Clin Interv Aging. 2006;1:143-154. [PubMed] |
| 4. | Banerjee S. The macroeconomics of dementia--will the world economy get Alzheimer’s disease? Arch Med Res. 2012;43:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Alves L, Correia AS, Miguel R, Alegria P, Bugalho P. Alzheimer’s disease: a clinical practice-oriented review. Front Neurol. 2012;3:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20 Suppl 2:S499-S512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Marchesi VT. Alzheimer’s disease 2012: the great amyloid gamble. Am J Pathol. 2012;180:1762-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Reitz C. Alzheimer’s disease and the amyloid cascade hypothesis: a critical review. Int J Alzheimers Dis. 2012;2012:369808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Fernández M, Paradisi M, Del Vecchio G, Giardino L, Calzà L. Thyroid hormone induces glial lineage of primary neurospheres derived from non-pathological and pathological rat brain: implications for remyelination-enhancing therapies. Int J Dev Neurosci. 2009;27:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3070] [Cited by in RCA: 3252] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 11. | Ihunwo AO, Schliebs R. Cell proliferation and total granule cell number in dentate gyrus of transgenic Tg2576 mouse. Acta Neurobiol Exp (Wars). 2010;70:362-369. [PubMed] |
| 12. | Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18:423-431. [PubMed] |
| 13. | Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer‘s disease mouse model. J Neurosci. 2010;30:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 14. | Dottori M, Familari M, Hansson S, Hasegawa K. Stem cells as in vitro models of disease. Stem Cells Int. 2012;2012:565083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Peters JM, Hummel T, Kratzsch T, Lötsch J, Skarke C, Frölich L. Olfactory function in mild cognitive impairment and Alzheimer’s disease: an investigation using psychophysical and electrophysiological techniques. Am J Psychiatry. 2003;160:1995-2002. [PubMed] |
| 16. | Velayudhan L, Lovestone S. Smell identification test as a treatment response marker in patients with Alzheimer disease receiving donepezil. J Clin Psychopharmacol. 2009;29:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Pastrana E, Silva-Vargas V, Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 665] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 18. | Deleyrolle LP, Reynolds BA. Isolation, expansion, and differentiation of adult Mammalian neural stem and progenitor cells using the neurosphere assay. Methods Mol Biol. 2009;549:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Ahlenius H, Kokaia Z. Isolation and generation of neurosphere cultures from embryonic and adult mouse brain. Methods Mol Biol. 2010;633:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Fernández M, Paradisi M, D'Intino G, Del Vecchio G, Sivilia S, Giardino L, Calzà L. A single prenatal exposure to the endocrine disruptor 2,3,7,8-tetrachlorodibenzo-p-dioxin alters developmental myelination and remyelination potential in the rat brain. J Neurochem. 2010;115:897-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 21. | Fierabracci A, Puglisi MA, Giuliani L, Mattarocci S, Gallinella-Muzi M. Identification of an adult stem/progenitor cell-like population in the human thyroid. J Endocrinol. 2008;198:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Jung JH, An K, Kwon OB, Kim HS, Kim JH. Pathway-specific alteration of synaptic plasticity in Tg2576 mice. Mol Cells. 2011;32:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Xu G, Shen J, Ishii Y, Fukuchi M, Dang TC, Zheng Y, Hamashima T, Fujimori T, Tsuda M, Funa K and Sasahara M. Dunctional analysis of platelet-derived growth factor receptor-β in neural stem/progenitor cells. Neuroscience. 2013;238:195-208. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer’s disease through 19 months. Physiol Behav. 2002;75:627-642. [PubMed] |
| 25. | Imbimbo BP, Giardino L, Sivilia S, Giuliani A, Gusciglio M, Pietrini V, Del Giudice E, D'Arrigo A, Leon A, Villetti G. CHF5074, a novel gamma-secretase modulator, restores hippocampal neurogenesis potential and reverses contextual memory deficit in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2010;20:159-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Sivilia S, Lorenzini L, Giuliani A, Gusciglio M, Fernandez M, Baldassarro VA, Mangano C, Ferraro L, Pietrini V, Baroc MF. Multi-target action of the novel anti-Alzheimer compound CHF5074: in vivo study of long term treatment in Tg2576 mice. BMC Neurosci. 2013;14:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Giuliani A, Beggiato S, Baldassarro VA, Mangano C, Giardino L, Imbimbo BP, Antonelli T, Calzà L, Ferraro L. CHF5074 restores visual memory ability and pre-synaptic cortical acetylcholine release in pre-plaque Tg2576 mice. J Neurochem. 2013;124:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Lazarov O, Marr RA. Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol. 2010;223:267-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 29. | Lilja AM, Röjdner J, Mustafiz T, Thomé CM, Storelli E, Gonzalez D, Unger-Lithner C, Greig NH, Nordberg A, Marutle A. Age-dependent neuroplasticity mechanisms in Alzheimer Tg2576 mice following modulation of brain amyloid-β levels. PLoS One. 2013;8:e58752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Rodríguez JJ, Verkhratsky A. Neurogenesis in Alzheimer’s disease. J Anat. 2011;219:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Lazarov O, Demars MP. All in the Family: How the APPs Regulate Neurogenesis. Front Neurosci. 2012;6:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Trazzi S, Fuchs C, Valli E, Perini G, Bartesaghi R, Ciani E. The amyloid precursor protein (APP) triplicated gene impairs neuronal precursor differentiation and neurite development through two different domains in the Ts65Dn mouse model for down syndrome. J Biol Chem. 2013;288:20817-20829. [PubMed] |
| 33. | Yang HJ, Joo Y, Hong BH, Ha SJ, Woo RS, Lee SH, Suh YH and Kim HS. Amyloid precursor protein binding protein-1 is up-regulated in brains of Tg2576 mice. Korean J Physiol Pharmacol. 2010;14:229-233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Chen Y, Liu W, Naumovski L, Neve RL. ASPP2 inhibits APP-BP1-mediated NEDD8 conjugation to cullin-1 and decreases APP-BP1-induced cell proliferation and neuronal apoptosis. J Neurochem. 2003;85:801-809. [PubMed] |
| 35. | Chen Y, Liu W, McPhie DL, Hassinger L, Neve RL. APP-BP1 mediates APP-induced apoptosis and DNA synthesis and is increased in Alzheimer’s disease brain. J Cell Biol. 2003;163:27-33. [PubMed] |
| 36. | Ma W, Maric D, Li BS, Hu Q, Andreadis JD, Grant GM, Liu QY, Shaffer KM, Chang YH, Zhang L. Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation. Eur J Neurosci. 2000;12:1227-1240. [PubMed] |
| 37. | Lilja AM, Luo Y, Yu QS, Röjdner J, Li Y, Marini AM, Marutle A, Nordberg A, Greig NH. Neurotrophic and neuroprotective actions of (-)- and (+)-phenserine, candidate drugs for Alzheimer’s disease. PLoS One. 2013;8:e54887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Kim S, Chang KA, Kim Ja, Park HG, Ra JC, Kim HS, Suh YH. The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS One. 2012;7:e45757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 39. | Bürger S, Noack M, Kirazov LP, Kirazov EP, Naydenov CL, Kouznetsova E, Yafai Y, Schliebs R. Vascular endothelial growth factor (VEGF) affects processing of amyloid precursor protein and beta-amyloidogenesis in brain slice cultures derived from transgenic Tg2576 mouse brain. Int J Dev Neurosci. 2009;27:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Zambrano A, Otth C, Maccioni RB, Concha II. IL-3 controls tau modifications and protects cortical neurons from neurodegeneration. Curr Alzheimer Res. 2010;7:615-624. [PubMed] |
| 41. | Tam P, Kaghazwala R, Easwaramoorthy B, Nistor M, Head E and Mukheriee J. Reduction of dopamine D2/D3 receptors in transgenic mouse models of Alzheimer’s disease. J Nucl Med. 2007;48:11. |
| 42. | Crews L, Patrick C, Adame A, Rockenstein E, Masliah E. Modulation of aberrant CDK5 signaling rescues impaired neurogenesis in models of Alzheimer’s disease. Cell Death Dis. 2011;2:e120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Calafiore M, Copani A, Deng W. DNA polymerase-β mediates the neurogenic effect of β-amyloid protein in cultured subventricular zone neurospheres. J Neurosci Res. 2010;90:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Echeverria V, Cuello AC. Intracellular A-beta amyloid, a sign for worse things to come? Mol Neurobiol. 2002;26:299-316. [PubMed] |
| 45. | Hashimoto M, Bogdanovic N, Volkmann I, Aoki M, Winblad B, Tjernberg LO. Analysis of microdissected human neurons by a sensitive ELISA reveals a correlation between elevated intracellular concentrations of Abeta42 and Alzheimer’s disease neuropathology. Acta Neuropathol. 2010;119:543-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |