Published online Jul 26, 2013. doi: 10.4252/wjsc.v5.i3.86
Revised: June 3, 2013
Accepted: June 8, 2013
Published online: July 26, 2013
Processing time: 120 Days and 18 Hours
AIM: To develop an improved p38 MAPK inhibitor-based serum-free medium for embryoid body cardiomyocyte differentiation of human pluripotent stem cells.
METHODS: Human embryonic stem cells (hESC) differentiated to cardiomyocytes (CM) using a p38 MAPK inhibitor (SB203580) based serum-free medium (SB media). Nutrient supplements known to increase cell viability were added to SB medium. The ability of these supplements to improve cardiomyogenesis was evaluated by measurements of cell viability, total cell count, and the expression of cardiac markers via flow cytometry. An improved medium containing Soy hydrolysate (HySoy) and bovine serum albumin (BSA) (SupSB media) was developed and tested on 2 additional cell lines (H1 and Siu-hiPSC). Characterization of the cardiomyocytes was done by immunohistochemistry, electrophysiology and quantitative real-time reverse transcription-polymerase chain reaction.
RESULTS: hESC cell line, HES-3, differentiating in SB medium for 16 d resulted in a cardiomyocyte yield of 0.07 ± 0.03 CM/hESC. A new medium (SupSB media) was developed with the addition of HySoy and BSA to SB medium. This medium resulted in 2.6 fold increase in cardiomyocyte yield (0.21 ± 0.08 CM/hESC). The robustness of SupSB medium was further demonstrated using two additional pluripotent cell lines (H1, hESC and Siu1, hiPSC), showing a 15 and 9 fold increase in cardiomyocyte yield respectively. The age (passage number) of the pluripotent cells did not affect the cardiomyocyte yields. Embryoid body (EB) cardiomyocytes formed in SupSB medium expressed canonical cardiac markers (sarcomeric α-actinin, myosin heavy chain and troponin-T) and demonstrated all three major phenotypes: nodal-, atrial- and ventricular-like. Electrophysiological characteristics (maximum diastolic potentials and action potential durations) of cardiomyocytes derived from SB and SupSB media were similar.
CONCLUSION: The nutrient supplementation (HySoy and BSA) leads to increase in cell viability, cell yield and cardiac marker expression during cardiomyocyte differentiation, translating to an overall increase in cardiomyocyte yield.
Core tip: Nutrient supplements were screened for improving cell survival during the cardiomyocyte differentiation process of human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC) in a serum-free medium based on the inhibition of p38 MAPK (SB media). Soy hydrolysate and bovine serum albumin supplementation was found to improve cell viability and therefore increased the yield of HES-3 cardiomyocytes by 2.6-fold over non-supplemented SB medium. The enhancing effect of this medium was demonstrated in an additional hESC line (H1) and Siu1-hiPSC cell line (15 and 9 fold respectively). The cardiomyocytes formed expressed canonical cardiac markers (sarcomeric α-actinin, myosin heavy chain and troponin-T) and demonstrated all three major phenotypes: nodal-, atrial- and ventricular-like.
- Citation: Ting S, Lecina M, Chan YC, Tse HF, Reuveny S, Oh SK. Nutrient supplemented serum-free medium increases cardiomyogenesis efficiency of human pluripotent stem cells. World J Stem Cells 2013; 5(3): 86-97
- URL: https://www.wjgnet.com/1948-0210/full/v5/i3/86.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v5.i3.86
Heart disease is one of the most common causes of mortality in the world and accounts for more than 800000 deaths per year on average in the United States alone[1]. After an episode of major cardiac insult (such as a myocardial infarction), the heart can lose up to 2 billion cardiomyocytes. Being an organ that cannot auto-regenerate, progressive heart failure develops. Cardiomyocyte cell therapy can thus be a potential cure for heart failure after myocardial infarction, and it is suggested that at least one billion cardiomyocytes will be required per patient for such treatment[2].
Human embryonic stem cells (hESCs) present an attractive cell source for generating large amounts of cardiomyocytes, due to their pluripotency and ability to proliferate for multiple passages[3]. Several studies have reported techniques to efficiently differentiate hESCs to cardiomyocytes via growth factors and small molecule inhibitors[4-8]. Current cardiomyocyte differentiation protocols can be divided into two groups: differentiating hESC in 2D monolayers culture or in 3D suspended embryoid bodies (EBs) cultures[9]. Although monolayer differentiation protocols have achieved high yields of cardiomyocytes[6,10], the scalability of these methods is problematic and they have limited capability in generating the amounts of cardiomyocytes needed for cell therapy. On the other hand, methods that involve EBs formation, which have better potential for scale up, have lower yields of cardiomyocyte, and requires extensive use of expensive growth factors like BMP4 and activin A at multiple specific time points during differentiation[7,11-13]. In addition, the growth factors have to be optimized for different cell lines, growth platforms or passage numbers[14,15]. As such, there is a lack of protocols for cardiomyocyte differentiation in EB suspended cultures that are cost-effective, scalable and most importantly robust. Previously, we have developed a simple scalable methodology to differentiate hESCs to cardiomyocytes using a serum-free differentiation medium[16,17] containing a small molecule p38 MAP kinase inhibitor SB203580 (SB media)[18,19]. The enhancing effect of SB203580 on cardiomyogenesis of hESC has been correlated to the expected inhibition of the p38 pathway as well as the activation of JNK[20]. This suggests a regulatory interlink between the JNK and p38 pathways during cardiomyogenesis. Compared to protocols based on growth factors, small molecules are less costly and more amenable for good manufacturing practice (GMP) manufacturing of cells[21]. However, the SB medium is essentially protein-free and lacks nutrients (e.g., lipids) and growth factors. From the low cell viability and yield observed, we hypothesized that SB medium has nutritional deficiencies that limit cardiomyogensis, especially in the initial stages of the differentiation process.
In this study, we sought to improve the survival of cells in SB medium and thereby enhance cardiomyogenesis using the embryoid body method of differentiation. For successful growth and maintenance of metabolic functions of differentiated human cells in vitro, appropriate culture conditions are required to mimic the physiological conditions in vivo[22,23]. The culture medium is one of the most important factors in maintaining cell and tissue culture as it provides nutrients and salts, hormones and growth factors, buffering elements and oxygen supply[22-24]. While media supplements have been developed for a variety of cell types, none have been performed for stem cell differentiation.
In this study, nutritional components were screened for an improvement in overall yields of cardiomyocytes of HES-3 cells using serum-free and insulin-free SB medium. Two supplements, Soy-hydrolysate (HySoy) and bovine serum albumin (BSA), resulted in improved cardiomyocyte differentiation efficiency. The concentrations of both supplements were optimized, resulting in an increase in cell growth and differentiation. The robustness of this new medium (SupSB media) was evaluated using the H1 and HES-3 hESC, as well as the Siu1-hiPSC cell lines (2.6 to 15.0 fold increase in cardiomyocyte yield). Similar cell yields and differentiation efficiency were obtained using 9 different batches of BSA and HySoy indicating that batch variability is not a major concern. Cardiomyocytes formed in SupSB media expressed canonical cardiac markers and electrophysiological studies demonstrated successful cardiac differentiation to give all the three major phenotypes of cardiomyocytes: nodal-, atrial- and ventricular-like. In summary, we have developed a cost-effective, scalable and robust protocol for cardiomyocyte differentiation by improving the p38 MAP kinase protocol with the addition of BSA and HySoy. SupSB media increases cell yield and cardiac expression markers during cardiomyocyte differentiation, translating to an overall increase in cardiomyocyte yield.
MATERIALS AND METHODS
HES-3 [(46, XX); ES Cell International], H1 [(46, XY); WiCell], and Siu1-hiPSC (Professor Tse HF, The University of Hong Kong) with normal karyotypes were cultured in KNOCKOUT medium on inactivated immortalized mouse feeders. The medium was refreshed daily and the cells were passaged weekly. Cultures were kept at 37 ˚C with 5% CO2.
hESC cultures were washed using phosphate buffered saline (PBS) (Invitrogen), cut into small clumps (EZ-passage tool; Invitrogen), and seeded at 1.33 × 106 cells/mL in ultra-low attachment 12-well plates (Nunc). The plates were agitated for 1 h and then cultured in static conditions at 37˚C in a humidified atmosphere with 5% CO2. The differentiation medium (SB media) comprised Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 2 mmol/L L-glutamine (Invitrogen), 0.182 mmol/L sodium pyruvate (Invitrogen), 1% nonessential amino acids (Invitrogen), 0.1 mmol/L 2-mercaptoethanol, 5.6 mg/L transferrin (Invitrogen), and 20 mg/L sodium selenite (Sigma). A solution of 5 mmol/L of p38-MAPK inhibitor (SB203580; Sigma), dissolved in dimethylsulfoxide (Sigma), was added to the medium at a final concentration of 5 µmol/L. The medium was refreshed every 2 d following the previously described protocol[16].
Various supplements (Table 1) were added to the SB medium and tested for cell yields and cardiomyogenesis efficiency using the above differentiation protocol.
| Supplements | Concentration/addition | Source |
| MEM vitamin solution (× 100) | 30 μL/mL | Gibco 11120 |
| Yeastolate ultrafiltrate (× 50) | 20 μL/mL | Gibco 18200 |
| Vitamin E | 70 μg/mL | Sigma T3376 |
| Synthechol (cholesterol) (× 500) | 12 μL/mL | Sigma S5442 |
| Yeast extract | 50 μg/mL | Sigma Y1625 |
| Bovine serum albumin | 50 μg/mL | Gibco A10008-01 |
| Soy hydrolysate | 50 μg/mL | Kerry Bio-science 5X59022 |
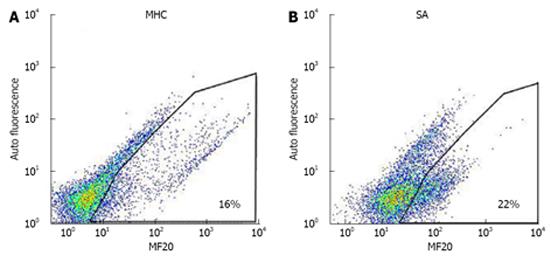
Following the protocol established by Lecina et al[15] and Ting et al[16], beating aggregates collected from 1 well were washed with 5 mL of PBS without Ca2/ Mg2 (PBS-) (Invitrogen), and incubated for 30 mins in 500 µL PBS- solution containing 1.6 mg/mL collagenase (Sigma) and 20% fetal bovine serum (Hyclone). Thereafter, 500 µL of 0.25% trypsin EDTA (Invitrogen) was added to generate single-cell suspensions. Cell suspensions were finally filtered through a 40-µm cell strainer (Becton Dickinson), fixed, and permeabilized (Caltag Laboratories). For FACS analysis, cells were incubated with anti-myosin heavy chain (anti-MHC; MF20, dilution 1:200; Developmental Studies Hybridoma Bank), anti-sarcomeric α-actinin (anti-SA; 1:100; Sigma) and anti-Troponin T (anti-cTnT; 1:200; Thermo Scientific). Fluorescein isothiocyanate-conjugated antimouse antibody (1:500; DAKO) was used as a secondary antibody. Dot plots for both anti-MHC and anti-SA are shown in Figure 1. All incubations were carried out at room temperature for 30 min. FACS measurements were done using Guava (Millipore).
Cell concentration was determined by the nuclei count method using NucleoCounter (Chemometec). Apoptosis and cell proliferation were evaluated using Annexin V (Invitrogen) and Ki-67 (BD Biosciences) respectively via FACS. Cardiomyocyte yields were calculated following published protocols[16,17], namely: CM hESC iield = (cell countDay 16/cell countDay 0: Initial hESC seeded) × (% positive cellsMHC, SA/100); normalised yield = (CM/hESC yieldCondition)/(CM/hESC yieldControl).
Total RNA was isolated from the cells (< 5 × 106) using RNeasy Mini Kit (Qiagen) following the supplier’s protocol. Reverse transcription was carried out with 1 µg total RNA using SuperScript III (Invitrogen). Real-time PCR was performed by applying a standard two-step amplification protocol on an ABI 7500 system (Applied Biosystem) to detect mRNA expression. Normalization of the results was done using a house-keeping gene, GAPDH. Primer sequences for Nanog, OCT 4, T-bra, Mesp 1, Nkx 2.5, MHC and GAPDH are provided in Table 2.
| Primers for qPCR | Forward (5’→3’) | Reverse (5’→3’) |
| Nanog | GAAAAACAACTGGCCGAAGAAT | GGTGCTGAGGCCTTCTGC |
| 4-Oct | AACGACCATCTGCCGCTTT | GGCCGCAGCTTACACATGTT |
| T-bra | AATTTGGTCCAGCCTTGGAAT | CGTTGCTCACAGACCACAG |
| Mesp 1 | GACGTGCTGGCTCTGTTG | TGTCACTTGGGCTCCTCAG |
| Nkx 2.5 | CAAGTGTGCGTCTGCCTTT | TTGTCCGCCTCTGTCTTCTC |
| MHC | ATTGCTGAAACCGAGAATGG | CGCTCCTTGAGGTTGAAAAG |
| GAPDH | GTCGGAGTCAACGGATTTGG | AAAAGCAGCCCTGGTGACC |
Cell aggregates were harvested, washed with PBS-, mechanically dissociated by pipetting, and plated in 24-well plates for 2 d in the SupSB medium at 37˚C in a humidified atmosphere with 5% CO2. The cells were then washed twice with PBS- and fixed with 4% paraformaldehyde (2 mL for 20 minutes at room temperature). After washing twice with PBS-, permeabilization and blocking was done using 0.1% Triton X-100 and 10% goat serum respectively. The following antibodies were used: anti-MHC (MF20; Developmental Studies Hybridoma Bank), anti-SA (Sigma), and anti-troponin-T (Thermo Scientific). Nuclear staining was done using SlowFade Glow with DAPI (4’6-diamidino-2-phenylindole) (Invitrogen). The fluorescence was observed using an Olympus IX71 fluorescence microscope (Olympus) coupled with Olympus imaging software Cell P.
Standard whole-cell patch-clamp recordings were performed at 37 ± 0.5 °C to record the action potential phenotypes (HEKA Instruments Inc. Southboro, MA, United States) of beating cardiomyocyte aggregates as previously described[25,26] . Patch pipettes were prepared from 1.5-mm thin-walled borosilicate glass tubes using a Sutter micropipette puller P-97 and had typical resistances of 3-4 MΩ when filled with an internal solution containing (mmol/L): 110 K+ aspartate, 20 KCl, 1 MgCl2, 0.1 Na-GTP, 5 Mg-ATP, 5 Na2-phosphocreatine, 5 EGTA, 10 HEPES, and pH adjusted to 7.3 with KOH. The external Tyrode’s bath solution consisted of (mmol/L): 140 NaCl, 5 KCl, 1 MgCl2, 0.4 KH2PO4, 1.8 CaCl2, 10 Glucose, 5 HEPES, with pH adjusted to 7.4 with NaOH. Twenty consecutive action potentials from spontaneously firing HES3-derived cardiomyocytes were recorded per cell to ensure stable waveforms for analysis. For the electrically quiescent cardiomyocytes, a stimulation of 0.1-1 nA for 5 ms was given to elicit an action potential. The sampling frequency was 2.00 kHz and data were corrected for the liquid junction potentials of +15.9 mV. Maximal diastolic potential as well as action potential duration at 90% (APD90) and 50% repolarization level (APD50) were measured.
Experiments were done using three independent replicates. The significance of the results was calculated by Student’s t-test or by one-way ANOVA (P < 0.05, P < 0.01).
RESULTS
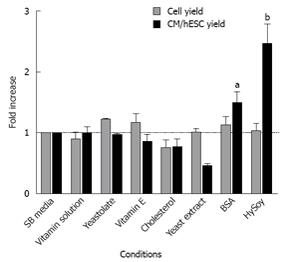
In order to identify elements that can increase cardiomyocyte differentiation, we selected a panel of defined and non-defined nutritional supplements (Table 1) which are known to support cell growth of a variety of cell lines[27]. The supplements were used at concentrations typically reported for animal cell culture.
HES-3 cells were seeded at a concentration of 1.33 × 106 cells/mL and differentiated in SB medium supplemented with the various supplements described in Table 1. Control cultures were differentiated in non-supplemented SB medium. After 16 d, cells were harvested and measured for cell yield and expression of the cardiac markers sarcomeric α-actinin (SA) and myosin heavy chain (MHC). A third antibody, cTnT which detects cardiac troponin was also used to verify the results (data not shown). The differentiation efficiency of the supplement was evaluated by dividing the yields of cardiomyocytes produced per seeded hESC in the supplemented culture with the one in the control culture (normalized cardiomyocyte yield). This parameter considers both the differentiation efficiency (percentage of cardiomyocytes) and final number of total cells, making it meaningful for process analysis and evaluation[16,17].
The nutritional supplements did not significantly affect cell counts. However, supplementing the media with BSA and HySoy improved the metabolic state of the cells, leading to an increase in normalized cardiomyocyte yield with improvements of 1.47 ± 0.20 and 2.45 ± 0.33, fold on average respectively, compared to the control (Figure 2). Addition of vitamins had no effect on the differentiation process. Yeastolate, Yeast extract, and Vitamin E reduced cardiomyocyte yields showing a negative effect on cardiac marker expression (data not shown), while cholesterol had a negative effect on cell yields.
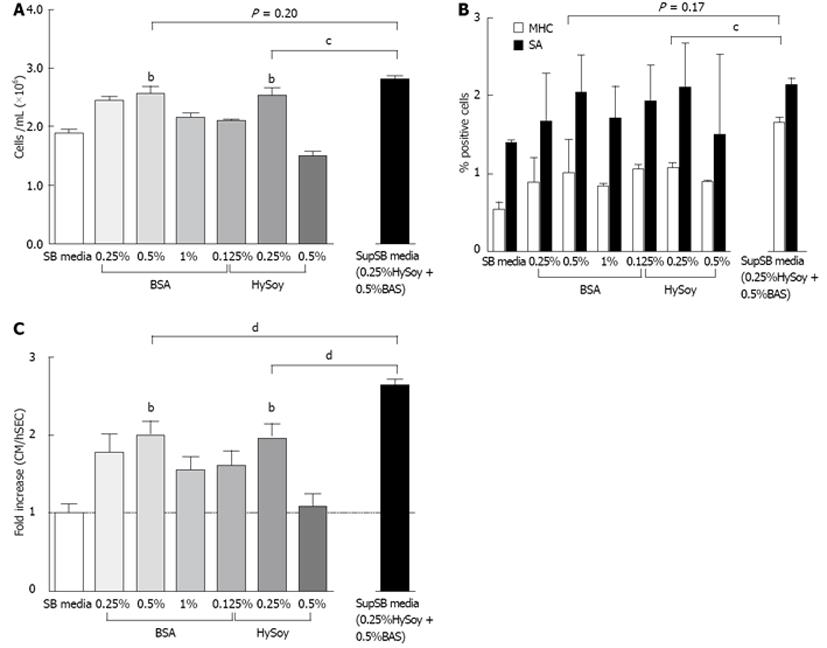
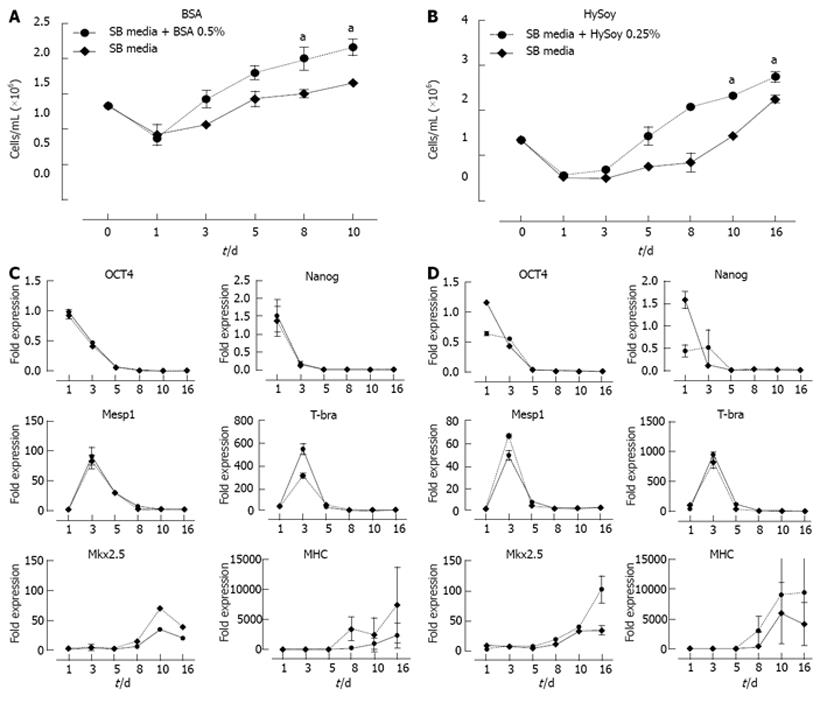
In order to further increase cardiomyocyte yields, the effect of HySoy and BSA concentration on cardiomyocyte yield was further investigated (Figure 3). At a BSA concentration of 0.5% v/v, an increase in cell growth compared to the control was observed, achieving an average of 2.54 × 106 cells/mL (Figure 3A). Similarly, cultures supplemented with 0.25% w/v HySoy achieved a maximum cell density of 2.51 × 106 cells/mL. The supplements also improved cell viability, showing > 85% viability as compared to 65% in the controls (SB media). This increase in cell growth and viability resulted in a 2-fold increase in normalized cardiomyocyte yield for both BSA and HySoy (Figure 3C). Although there was no statistical difference in the level of expression of cardiac markers (SA and MHC) between the cultures supplemented with different concentrations of BSA and HySoy as compared to the control, a general trend of increased level of expression was observed in cultures supplemented with 0.25% w/v HySoy and 0.5% v/v BSA (Figure 3B). Embryoid bodies of all cultures started beating at day 9, indicating that the supplements did not affect the process of cardiac differentiation temporally.
Next, we explored the effects of combining both supplements on cell yield and cardiac marker expression (SA and MHC). HES-3 cells were seeded in medium containing both 0.25% w/v HySoy and 0.5% v/v BSA (SupSB media) and compared to control cultures supplemented by HySoy and BSA alone or not supplemented at all.
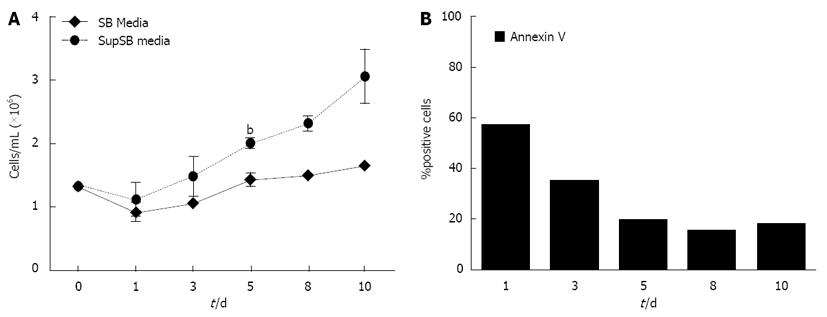
Results show a further increase in normalized cardiomyocyte yield (2.6-fold) when HySoy and BSA were combined, compared to only a 2-fold increase with the individual supplements alone (P < 0.01) (Figure 3C). This increase in cardiomyocyte yield can be attributed to a higher percentage of differentiated cardiomyocytes as indicated by a higher percentage of MHC-expressing cells (16.5% of cells) cultured in the SupSB medium vs. the individual supplements (10%-12%) (Figure 3B). In addition, there was also an increase in cell yield of cultures in SupSB media resulting in 2.79 × 106 cells/mL (Figures 3A and Figure 4), that was statistically significant (P < 0.01). The variability between different batches of BSA and HySoy were also tested. Differentiation experiments using 9 different batches of BSA and HySoy were conducted and little variability was observed. A high level of apoptotic cells (60% of total cells), measured by annexin V, was observed during the first day of differentiation, which correlates to cell death probably as a result of mechanical manipulation of the culture during seeding. Thereafter, gradual increase in cell yields and down regulation of apoptotic markers were observed (Figure 4). In summary, the additive effect of HySoy and BSA increased cardiomyocyte yield further over individual supplements alone, through increasing cell yield and expression of cardiac markers.
Next, we investigated the underlying factors that resulted in the overall increase in cardiomyocyte yields using the optimal concentrations of both supplements. Differentiating HES-3 cultures supplemented with optimal concentrations of HySoy and BSA were monitored for 10-16 d. Every 2-3 d cells were harvested and analyzed for cell yield, expression of pluripotent markers (Nanog and OCT4), mesoderm markers (Mesp 1 and T-bra), the early cardiac marker Nkx 2.5 and the late cardiac marker MHC via qRT-PCR (Figure 5).
More than 30% of cell death was observed during the first day in both the supplemented as well as the control cultures. This phenomenon can be attributed to the mechanical cutting of the HES-3 cultures and the adaptation of the cells to the new differentiation media. Thereafter, BSA and HySoy supplemented cultures showed 34% and 39% increase in cell yields respectively by day 3 and achieved cell densities of 2.16 × 106 and 2.32 × 106 cells/mL on day 10, as compared to 1.65 × 106 and 1.44 × 106 cells/mL in the control cultures respectively (Figure 5A and B). At the end of the culture, addition of BSA and HySoy resulted in a rise in cell yield by 23% and 19% respectively as compared to the controls.
qRT-PCR analysis show that both supplemented cultures and SB control culture displayed similar trends of down regulation of pluripotent markers (Nanog and OCT4) as well as up-regulation of mesoderm markers (Mesp 1 and T-bra) (Figure 5C and D). However, cultures with supplements showed a significantly higher expression of cardiac progenitor gene Nkx 2.5 as well as a higher general trend of late cardiomyocyte gene MHC expression during the later stages as compared to SB control cultures (Figure 5C and D). In summary, the addition of supplements, BSA and HySoy, not only enhanced cardiomyogenesis via improvement in cell yield, but also increased the expressions of both Nkx 2.5 and MHC.
In order to demonstrate the universality and robustness of SupSB media for increased cardiomyogenesis induction efficiency, we cultured two additional cell lines, H1 hESC and Siu1-hiPSC. These cells were differentiated in SupSB medium for 16 d and tested for cell yields and cardiac marker expression, with cells differentiated in SB medium used as controls. Results show that culturing cells in SupSB medium led to increased cell density from 1.10 × 106 to 3.12 × 106 cells/mL for H1 and from 0.67 × 106 to 1.22 × 106 cells/mL for Siu1-hiPSC as compared to SB media (Table 3). Moreover, significantly higher expression of the cardiac specific marker MHC was observed in cultures with SupSB medium (Table 3). H1 cultures differentiated in SupSB medium showed a significant increase in normalized cardiomyocyte yield of 15-fold over the control, while Siu1-hiPSC showed a 9-fold increase (Table 3). Cardiomyocyte yield per hESC seeded was 0.59 cardiomyocyte/hESC for H1 cultures and 0.04 cardiomyocyte/hESC for Siu1-hiPSC cultures.
| Conditions | Cell expansion | Differentiation efficiency | CM yield | |||
| Cell type | Medium | Cell yield (106 cells/mL) | Cell expansion fold | Sarcomeric actinin | MHC | Cardiomyocyte/hESC |
| HES-3 | SupSB | 2.27 ± 0.58 | 1.65 ± 0.47 | 14.70% ± 6.30% | 13.59% ± 3.40% | 0.21 ± 0.08 |
| SB | 1.66 ± 0.48 | 1.25 ± 0.36 | 9.25% ± 6.19% | 6.17% ± 2.95% | 0.07 ± 0.03 | |
| H1 | SupSB | 3.14 ± 0.24 | 2.35 ± 0.18 | 26.70% ± 2.09% | 25.20% ± 5.41% | 0.59 ± 0.01 |
| SB | 1.10 ± 0.19 | 0.82 ± 0.14 | 20.23% ± 1.87% | 4.76% ± 1.62% | 0.04 ± 0.01 | |
| Siu1-hiPSC | SupSB | 1.22 ± 0.15 | 0.92 ± 0.11 | 20.76% ± 4.74% | 2.11% ± 0.73% | 0.04 ± 0.04 |
| SB | 0.67 ± 0.26 | 0.51 ± 0.20 | 16.90% ± 1.95% | 1.01% ± 0.20% | 0.004 ± 0.001 | |
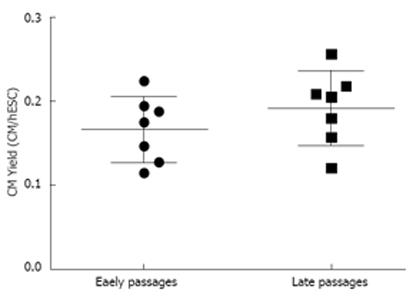
Another factor that can influence the yield of cardiomyocyte differentiation is the age or passage number of the cells. As such, HES-3 cells at early (P12 to P13) and late (P21 to P39) passages were differentiated using SupSB medium and cardiomyocyte yields were measured at day 16. Results in Figure 6 show that cells from early or late passage showed similar cardiomyocyte yields.
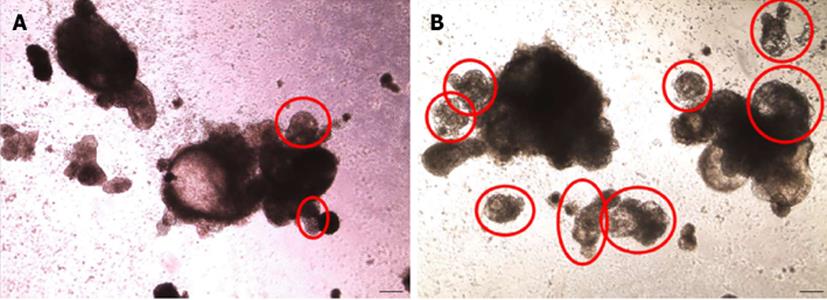
HES-3, H1 and Siu1-hiPSC cells differentiated in SupSB medium all showed increases in cell yield, expression of cardiac markers and cardiomyocyte yields (Table 3). The increase in cell yield can be attributed to better metabolic conditions manifested by an increase of 20%-30% in cell viability over the control and an increased expression of proliferation marker Ki-67[28]. Specifically, at day 2 of differentiation, 12% increase in Ki-67 expression was observed in cultures differentiated in SupSB medium compared to SB medium. By day 4 onward, Ki-67 expression was similar in both cultures (data not shown).The percentage of beating aggregates in cultures with SupSB medium was also higher than that in SB medium (Figure 7).
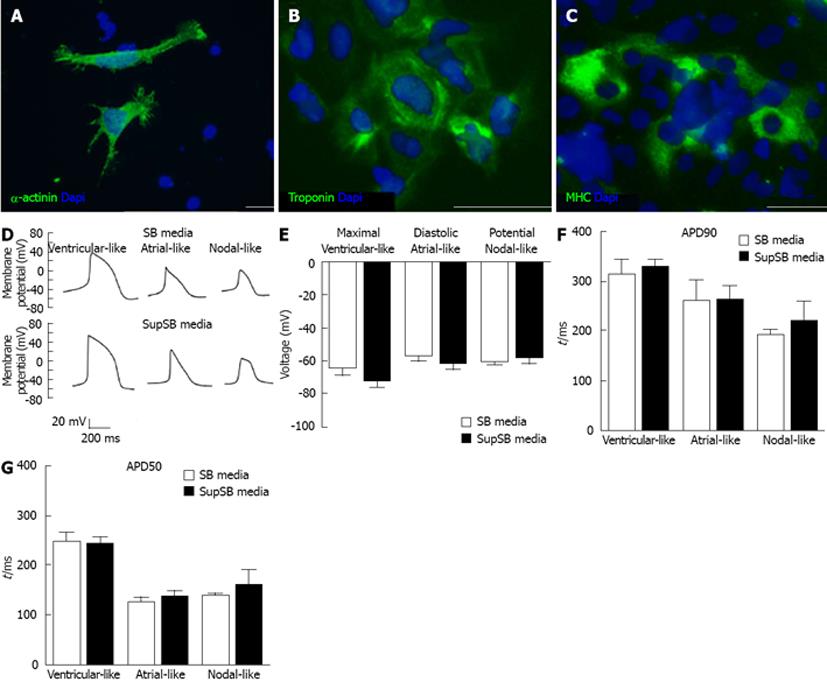
Cardiomyocytes differentiated in SupSB medium were also characterized via immunostaining of cardiac proteins. 16 d old EBs were harvested, mechanically dissociated, and plated on 0.1% gelatin-coated 24-well plates before staining with a set of antibodies against cardiac-specific markers (Figure 8A-C). Cells stained positive for cytoskeleton structural proteins (SA) and contractile functional proteins responsible for motility (MHC, and troponin-T)[29].Electrophysiological study via whole-cell patch-clamping was performed. Action potential durations at 90% and 50% repolarization levels as well as maximal diastolic potential were recorded using HES-3 cells differentiated for 23 d. Cells differentiated in both SB and SupSB medium gave similar results. All the three major phenotypes of cardiomyocytes: nodal-, atrial- and ventricular-like were obtained (Figure 8D). The APD and maximal diastolic potential of the cardiomyocytes were similar for cells cultured in both SB and SupSB media (Figure 8E-G) and are comparable to those of the control hESC cell line H7 (data not shown).
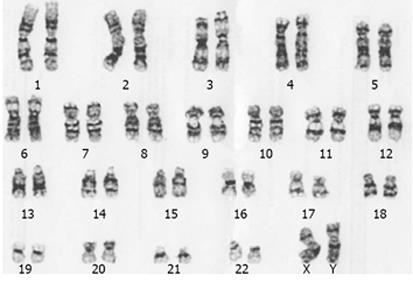
Furthermore, a normal karyotype was observed in
20 d old cardiomyocytes differentiated in SupSB medium (Figure 9).
DISCUSSION
In this paper, we have demonstrated that the media supplements, HySoy and BSA, can improve the differentiation efficiency of hESCs into cardiomyocytes when added to SB media containing the p38 MAP kinase inhibitor, SB203580. In particular, when combined at optimal concentrations (0.25%HySoy and 0.50%BSA), the cardiomyocyte yield is enhanced by 2.6-fold compared to the SB medium without supplements for the HES-3 cell line. This increase is due to the improvement in cell growth, as well as differentiation efficiency. Cell lines such as, H1 and Siu1-hiPSC, which initially did not differentiate efficiently to cardiomyocytes in the SB media, showed significant improvements in yields of cardiomyocyte/hESC or cardiomyocyte/hiPSC (15- and 9-fold respectively) when these supplements were added. The variability in cardiomyocyte differentiation yields between cell lines is not surprising and it likely reflects genetic and epigenetic differences between pluripotent stem cell lines[30-32] that influences their cardiac differentiation efficiencies[14,15,33]. Moreover, the level of differentiation obtained in SupSB medium was consistent and not affected by cell age (passages 12 to 39). We have observed varying ratios of expression levels for MHC and SA, ranging from 1:1 to 1:16 (MHC: SA) (Table 3). These differences can be attributed to the stages in the differentiation process which the cells are at. SA is expressed earlier than MHC during cardiac differentiation[34]. Cultures expressing a higher ratio of SA to MHC (16:1) indicate an earlier stage of differentiation in comparison to cultures with lower ratios.
The results from qRT-PCR analysis showed no acceleration in the differentiation process when cells cultured with supplements (BSA and HySoy) were compared to SB media, with expression of pluripotent and mesoderm markers showing similar trends. However, there was a significantly higher expression of Nkx 2.5, indicating a higher population of cardiac progenitor cells in the cultures with supplements. MHC expression showed a higher general trend, but not a significant increase, when comparing cells cultured with individual supplements to those cultured in SB media only. However, when the supplements were combined, their additive effect produced a significantly higher expression of MHC, indicating that SupSB media improves the differentiation efficiency of cardiomyocytes.
Albumin, as a major serum protein with a typical concentration of 50 mg/mL in blood, accounts for approximately 60% of the total protein in mammals[35]. Although there is such an abundance of albumin, its physiological actions and molecular mechanisms are not fully understood[36,37]. The main functions of albumin have been summarized to include (1) maintenance of blood oncotic pressure and pH; (2) binding and transport of physiologically important nutrients, including lipids, metal ions, amino acids and other factors; and (3) antioxidant functions but mainly from the perspective of its role in blood circulation[35].
Protein hydrolysates are known as a potential source of metabolizable materials including amino acids, oligopeptides, iron salts, lipids and trace elements. Their beneficial effects to the growth of animal cell culture have been known for more than two decades[38] and are generally thought to act as a concentrated balanced nutrient mixture that may partly or fully replace serum[39,40]. In recent years suppliers have improved their production processes to generate more consistent products with less batch-to-batch variability for the biopharma industry[41]. Multiple reports have shown the effect of protein hydrolysates on growth in a variety of cell lines including both animal and rodent cells[40,42]. This study further validates HySoy as a suitable supplement for cardiomyocyte differentiation.
The increase in cardiomyocyte efficiency was attributed to two reasons: (1) increased cell growth; and (2) increased differentiation efficiency, probably due to the improved metabolic state of the cells. Both BSA and HySoy have been repeatedly reported to increase cell densities in various different cell lines, and thus, the increase in cell growth observed during cardiomyocyte differentiation is not surprising. BSA and HySoy are also more defined in comparison to fetal bovine Serum, which is widely used in current EB and monolayer differentiation protocols[4,10]. We assume that the addition of albumin and HySoy to the differentiation medium improves cardiomyocyte cell yields and viability by their antioxidant activity, conferring needed metabolized nutrients and the ability to transport nutrients (e.g., lipids) to the cells. Previously it has been shown that insulin inhibits the process of cardiomyogenesis, therefore it was removed from the SB media[43]. BSA and HySoy supplements were able to restore the proliferative capacity of cardiomyocytes in this insulin-free SupSB media. Moreover, it was reported that BSA can also help in the transport of small molecules such as the p38 MAPK inhibitor to the cells[35]. Maturation of cardiomyocytes into ventricular, atrial and nodal phenotypes occurred within 16 d of differentiation as shown by the electrophysiology characterization. Ventricular phenotype maturation was much faster in comparison to other works which indicated a time frame of 60 d[8].
Currently, there are multiple companies offering complete medium for hESC cardiac differentiation, these media use expensive ingredients and thus they are considerably more expensive then SupSB media. This work is the first step in developing an inexpensive and efficient cardiomyocyte differentiation protocol that can be used by commercial companies to produce cardiomyocytes. To the best of our knowledge, this is the first report of the benefits of BSA and HySoy on improving cardiomyogenesis of pluripotent human stem cells. Future works into cardiomyocyte purification and increase cardiomyocyte yield is needed.
In conclusion, we have created a simple, robust and cost effective media that significantly improves cardiomyocyte differentiation over many passages for multiple pluripotent cell lines that will be useful for research and cell therapy applications.
ACKNOWLEDGEMENTS
The authors want to acknowledge Dr. Allen Chen and Dr. Hia Hui Ching for their advice as well as Dr. Filip Laco for reviewing this article.
COMMENTS
Heart disease is one of the most common causes of mortality in the world and accounts for more than 800000 deaths per year on average in the United States alone. After an episode of major cardiac insult (such as a myocardial infarction), the heart can lose up to 2 billion cardiomyocytes. Being an organ that cannot auto-regenerate, progressive heart failure develops. Cardiomyocyte cell therapy can thus be a potential cure for heart failure after myocardial infarction, and it is suggested that at least one billion cardiomyocytes will be required per patient for such treatment
Human embryonic stem cells (hESCs) present an attractive cell source for generating large amounts of cardiomyocytes, due to their pluripotency and ability to proliferate for multiple passages. As such, several studies have reported techniques to efficiently differentiate hESCs to cardiomyocytes via growth factors and small molecule inhibitors. Specifically, a protocol based on the inhibition of p38 MAPK with small molecules displayed the ability to be scaled up in a cost efficient manner. However, low cell yield and viability were inherent to this protocol, reducing the output of cardiomyocytes.
Soy hydrolysate (HySoy) and bovine serum albumin (BSA) were found to improve cell viability during cardiomyogenesis of human embryonic and induced pluripotent stem cells in serum free medium. The addition of both supplements leads to an increase in cell viability, cell yield and cardiac marker expression during cardiomyocyte differentiation, translating to an overall increase in cardiomyocyte yield (2.6 fold increase over non supplemented medium).
Authors have created a simple, robust and cost effective serum free medium (SupSB media) that significantly improves cardiomyocyte differentiation of human pluripotent stem cell in suspended embryoid bodies culture. The new medium can be used for research and cell therapy applications using human embryonic and induced pluripotent cultures.
p38 MAPK are a class of protein kinases involved in pathways that deal with differentiation, apoptosis and autophagy. p38 MAPK was inhibited with small molecule, SB203580.
The authors have carried out an interesting study in which they developed an inexpensive and efficient cardiomyocyte differentiation protocol that can be used to produce cardiomyocytes. They demonstrated the benefits of BSA and HySoy on improving cardiomyogenesis of pluripotent human stem cells.
P- Reviewers Houria B, Placantonakis DG, Resende R S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 912] [Cited by in RCA: 950] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 2. | Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Zweigerdt R. The art of cobbling a running pump--will human embryonic stem cells mend broken hearts? Semin Cell Dev Biol. 2007;18:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Burridge PW, Anderson D, Priddle H, Barbadillo Muñoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 220] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 6. | Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1672] [Cited by in RCA: 1598] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 7. | Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1172] [Cited by in RCA: 1070] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 8. | Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA. 2012;109:E1848-E1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1272] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 9. | Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 10. | Tohyama S, Hattori F, Sano M, Hishiki T, Nagahata Y, Matsuura T, Hashimoto H, Suzuki T, Yamashita H, Satoh Y. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell. 2013;12:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 752] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 11. | Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 866] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 12. | Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, Cashman J, Mercola M. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res. 2011;109:360-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 559] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 14. | Moore JC, Fu J, Chan YC, Lin D, Tran H, Tse HF, Li RA. Distinct cardiogenic preferences of two human embryonic stem cell (hESC) lines are imprinted in their proteomes in the pluripotent state. Biochem Biophys Res Commun. 2008;372:553-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Pekkanen-Mattila M, Kerkelä E, Tanskanen JM, Pietilä M, Pelto-Huikko M, Hyttinen J, Skottman H, Suuronen R, Aalto-Setälä K. Substantial variation in the cardiac differentiation of human embryonic stem cell lines derived and propagated under the same conditions--a comparison of multiple cell lines. Ann Med. 2009;41:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Lecina M, Ting S, Choo A, Reuveny S, Oh S. Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng Part C Methods. 2010;16:1609-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Ting S, Lecina M, Reuveny S, Oh S. Differentiation of human embryonic stem cells to cardiomyocytes on microcarrier cultures. Curr Protoc Stem Cell Biol. 2012;Chapter 1:Unit1D.7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Graichen R, Xu X, Braam SR, Balakrishnan T, Norfiza S, Sieh S, Soo SY, Tham SC, Mummery C, Colman A. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Xu XQ, Graichen R, Soo SY, Balakrishnan T, Rahmat SN, Sieh S, Tham SC, Freund C, Moore J, Mummery C. Chemically defined medium supporting cardiomyocyte differentiation of human embryonic stem cells. Differentiation. 2008;76:958-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Kempf H, Lecina M, Ting S, Zweigerdt R, Oh S. Distinct regulation of mitogen-activated protein kinase activities is coupled with enhanced cardiac differentiation of human embryonic stem cells. Stem Cell Res. 2011;7:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Davis JM. Basic cell culture: a practical approach. 2nd. Oxford: Oxford University Press 2002; . |
| 23. | Masters JRW. Animal cell culture: a practical approach. 3rd. Oxford: Oxford University Press 2000; . |
| 24. | Freshney RI. Culture of animal cells: a manual of basic technique. 4th. New York, Chichester: Wiley-Liss 2005; . |
| 25. | Ng KM, Chan YC, Lee YK, Lai WH, Au KW, Fung ML, Siu CW, Li RA, Tse HF. Cobalt chloride pretreatment promotes cardiac differentiation of human embryonic stem cells under atmospheric oxygen level. Cell Reprogram. 2011;13:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Li W, Wang X, Fan W, Zhao P, Chan YC, Chen S, Zhang S, Guo X, Zhang Y, Li Y. Modeling abnormal early development with induced pluripotent stem cells from aneuploid syndromes. Hum Mol Genet. 2012;21:32-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010;27:53-62. [PubMed] |
| 28. | Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710-1715. [PubMed] |
| 29. | Ebashi S. Ca2+ and the contractile proteins. J Mol Cell Cardiol. 1984;16:129-136. [PubMed] |
| 30. | Allegrucci C, Young LE. Differences between human embryonic stem cell lines. Hum Reprod Update. 2007;13:103-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 31. | Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 793] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 32. | Skottman H, Mikkola M, Lundin K, Olsson C, Strömberg AM, Tuuri T, Otonkoski T, Hovatta O, Lahesmaa R. Gene expression signatures of seven individual human embryonic stem cell lines. Stem Cells. 2005;23:1343-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 646] [Cited by in RCA: 631] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 34. | Gregorio CC, Antin PB. To the heart of myofibril assembly. Trends Cell Biol. 2000;10:355-362. [PubMed] |
| 35. | Francis GL. Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology. 2010;62:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 36. | Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 640] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 37. | Ahn SM, Byun K, Cho K, Kim JY, Yoo JS, Kim D, Paek SH, Kim SU, Simpson RJ, Lee B. Human microglial cells synthesize albumin in brain. PLoS One. 2008;3:e2829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Mizrahi A. Primatone RL in mammalian cell culture media. Biotechnol Bioeng. 1977;19:1557-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Jan DC, Jones SJ, Emery AN, al-Rubeai M. Peptone, a low-cost growth-promoting nutrient for intensive animal cell culture. Cytotechnology. 1994;16:17-26. [PubMed] |
| 40. | Schlaeger EJ. The protein hydrolysate, Primatone RL, is a cost-effective multiple growth promoter of mammalian cell culture in serum-containing and serum-free media and displays anti-apoptosis properties. J Immunol Methods. 1996;194:191-199. [PubMed] |
| 41. | Pasupuleti VK, Braun S. State of the Art Manufacturing of Protein Hydrolysates. New York: Springer 2010; 11-33. |
| 42. | Franek F, Hohenwarter O, Katinger H. Plant protein hydrolysates: preparation of defined peptide fractions promoting growth and production in animal cells cultures. Biotechnol Prog. 2000;16:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Freund C, Ward-van Oostwaard D, Monshouwer-Kloots J, van den Brink S, van Rooijen M, Xu X, Zweigerdt R, Mummery C, Passier R. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells. 2008;26:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |