Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.108519
Revised: May 27, 2025
Accepted: June 16, 2025
Published online: July 26, 2025
Processing time: 99 Days and 15 Hours
Exosome-based therapies represent a promising approach for hair regeneration. Unlike conventional treatments such as minoxidil and finasteride, exosomes deliver bioactive cargo that can stimulate dermal papilla cells, enhance angio
To synthesize findings from in vitro, preclinical and clinical studies, and to evaluate the efficacy, mechanisms, and challenges associated with exosome-based hair restoration therapies.
A literature search was conducted using multiple databases (PubMed/Medline, Embase, Scopus, and Web of Science) employing terms for exosomes and hair regeneration for articles published in English to February 2025, following Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
A total of 27 studies (three in vitro, three pre-clinical, 18 with both in vitro and pre-clinical component and three clinical) met the pre-defined search and inclusion criteria and were included in this review.
Exosome-based therapies hold immense promise for hair regeneration by leveraging their ability to modulate key signaling pathways and enhance hair follicle regeneration. While in vitro and preclinical studies demonstrate consistent efficacy across diverse exosome sources, methodological heterogeneity and a limited number of clinical studies warrant further clinical research to realize their full clinical potential for hair regeneration.
Core Tip: This review synthesizes findings from in vitro, preclinical, and clinical studies to evaluate the efficacy, mechanisms, and challenges associated with exosome-based hair restoration therapies. A total of 27 studies met the pre-defined search and inclusion criteria and were included in this review. Exosome-based therapies hold immense promise for hair regeneration by leveraging their ability to modulate key signaling pathways and enhance hair follicle regeneration. While in vitro and preclinical studies demonstrate consistent efficacy across diverse exosome sources, methodological heterogeneity and limited number of clinical studies warrant further clinical research to realize their full clinical potential for hair regeneration.
- Citation: Poddar N, Aratikatla A, Gupta A. Therapeutic potential of stem cell-derived exosomes in hair regeneration: A systematic review. World J Stem Cells 2025; 17(7): 108519
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/108519.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.108519
Hair loss, or alopecia, is a widespread condition affecting millions of individuals worldwide, with significant physical, psychological, and economic implications[1]. It is estimated that androgenetic alopecia alone impacts up to 50% of men and 25% of women by the age of 50, contributing to an expanding market for hair loss treatments[2]. Beyond its cosmetic concerns, hair loss can lead to scarring, inflammation, and profound emotional distress, reducing overall quality of life[3,4]. The global burden of alopecia extends to healthcare costs and lost productivity, further highlighting the need for effective and long-lasting treatments[5-7].
Hair loss manifests in various forms, including progressive hair thinning, increased shedding, or complete bald patches, depending on the underlying cause[8]. Androgenetic alopecia, commonly known as male or female pattern baldness, results from genetic predisposition and hormonal influences[9]. In contrast, autoimmune conditions such as alopecia areata occur when the immune system mistakenly attacks hair follicles, leading to patchy hair loss[1,9,10]. Other causes include telogen effluvium, which results from metabolic stress, hormonal imbalances, or medication use, and Tinea capitis, a fungal infection of the scalp. Trichorrhexis nodosa, characterized by dry, brittle hair, is often associated with underlying scalp infections, while alopecia totalis manifests as complete body hair loss[11-13]. In addition to the physical symptoms, individuals with hair loss frequently experience psychological consequences, including anxiety, depression, and social withdrawal, with studies reporting a high prevalence of depression among affected individuals, particularly women[3,14].
Several treatment modalities have been developed to combat hair loss, ranging from pharmaceutical interventions to surgical procedures. Food and Drug Administration-approved medications such as minoxidil and finasteride have been widely used, though their effectiveness is limited[15,16]. Minoxidil works by stimulating hair follicles through potassium channel activation, but long-term use has been associated with paradoxical hair shedding and scalp irritation[17,18]. Finasteride, a 5α-reductase inhibitor, is effective in some cases but carries risks of sexual dysfunction and mood disorders, particularly in men[19,20]. Alternative therapies such as herbal compounds - quercitrin, proanthocyanidins, and astragaloside IV - have been explored for their ability to modulate key hair growth signaling pathways, including protein kinase B (AKT), insulin-like growth factor 1, and vascular endothelial growth factor (VEGF)[21-24]. However, these treatments often provide only temporary relief and do not consistently stimulate new hair follicle formation.
For patients seeking more definitive solutions, hair transplant surgery remains an option. While effective, it is invasive, expensive, and limited by donor hair availability[25,26]. Less invasive approaches such as microneedling and low-level laser therapy have shown some promise in stimulating hair growth, particularly when combined with minoxidil, though clinical evidence remains inconclusive[27,28]. Given these limitations, regenerative medicine has emerged as a promising field for hair restoration, leveraging biologics such as platelet-rich plasma, stem cells, and growth factor-based therapies to enhance hair follicle regeneration[29-31]. These treatments capitalize on bioactive molecules - including cytokines, extracellular vesicles, and exosomes - that modulate cellular signaling, reduce inflammation, and promote tissue repair[32,33].
Among these biologics, exosomes have garnered increasing attention as potential therapeutic agents for hair loss. Exosomes are lipid bilayer vesicles that play a critical role in intercellular communication by transporting proteins, RNAs, and microRNAs (miRNAs)[34]. Recent studies suggest that exosome-based therapies may effectively stimulate hair follicle stem cells, induce the Wnt/β-catenin signaling pathway, and promote hair regrowth[33,35,36]. Dermal papilla cell-derived exosomes in particular can accelerate the transition from the resting phase (telogen) to the active growth phase (anagen) of the hair cycle[33,37,38]. Despite these promising findings, challenges such as the standardization of exosome isolation methods and the need for robust clinical trials remain hurdles to widespread clinical application.
The primary objective of this systematic review is to evaluate the current body of evidence on exosome-based therapies for hair regeneration. Specifically, we aim to document findings from basic science/in vitro investigations, pre-clinical studies, and clinical trials assessing the efficacy and safety of exosomes for hair restoration. Additionally, we summarize on-going registered clinical trials investigating exosome-based interventions in hair loss treatment.
A systematic search was conducted across PubMed/Medline, Embase, Scopus and Web of Science databases for articles published in English prior to February 2025. The Preferred Reporting Items for Systematic Reviews and Meta-analyses statement and guidelines were followed, using the following search terms: (‘exosome’ OR ‘small extracellular vesicle’ OR ‘small EV’ OR ‘microsome’ OR ‘endosome’ OR ‘secretome’ OR ‘extracellular vesicle’ OR ‘vesicle’ OR ‘microvesicle’ OR ‘ectosome’) AND (‘pilus’ OR ‘pili’ OR ‘hair’ OR ‘triches’ OR ‘trich’ OR ‘capillus’ OR ‘capilli’ OR ‘pilosity’ OR ‘follicle’ OR ‘shaft’ OR ‘cortex’ OR ‘cuticle’ OR ‘medulla’ OR ‘anagen’ OR ‘catagen’ OR ‘telogen’) AND (‘regeneration’ OR ‘rejuvenation’ OR ‘restoration’ OR ‘repair’ OR ‘regrowth’ OR ‘revitalization’ OR ‘revitalization’). All basic science, preclinical, and clinical studies using the aforementioned terms for exosomes for the purpose of hair regeneration were included. There were no control groups or alternative treatments used for comparison purposes, nor were there any specific outcomes or metrics that were assessed or measured.
To minimize the risk of bias, the first two authors discussed and reviewed all selected articles, references and excluded articles from the study. Any disagreements among authors were resolved by the last author, who made the final decision on inclusion or exclusion. In addition, on-going clinical studies involving the use of exosomes for the purpose of hair regeneration, registered on publicly available clinical trial repositories (ClinicalTrials.gov, Clinical Trials Registry - India, and Chinese Clinical Trial Register using the aforementioned search terms were documented.
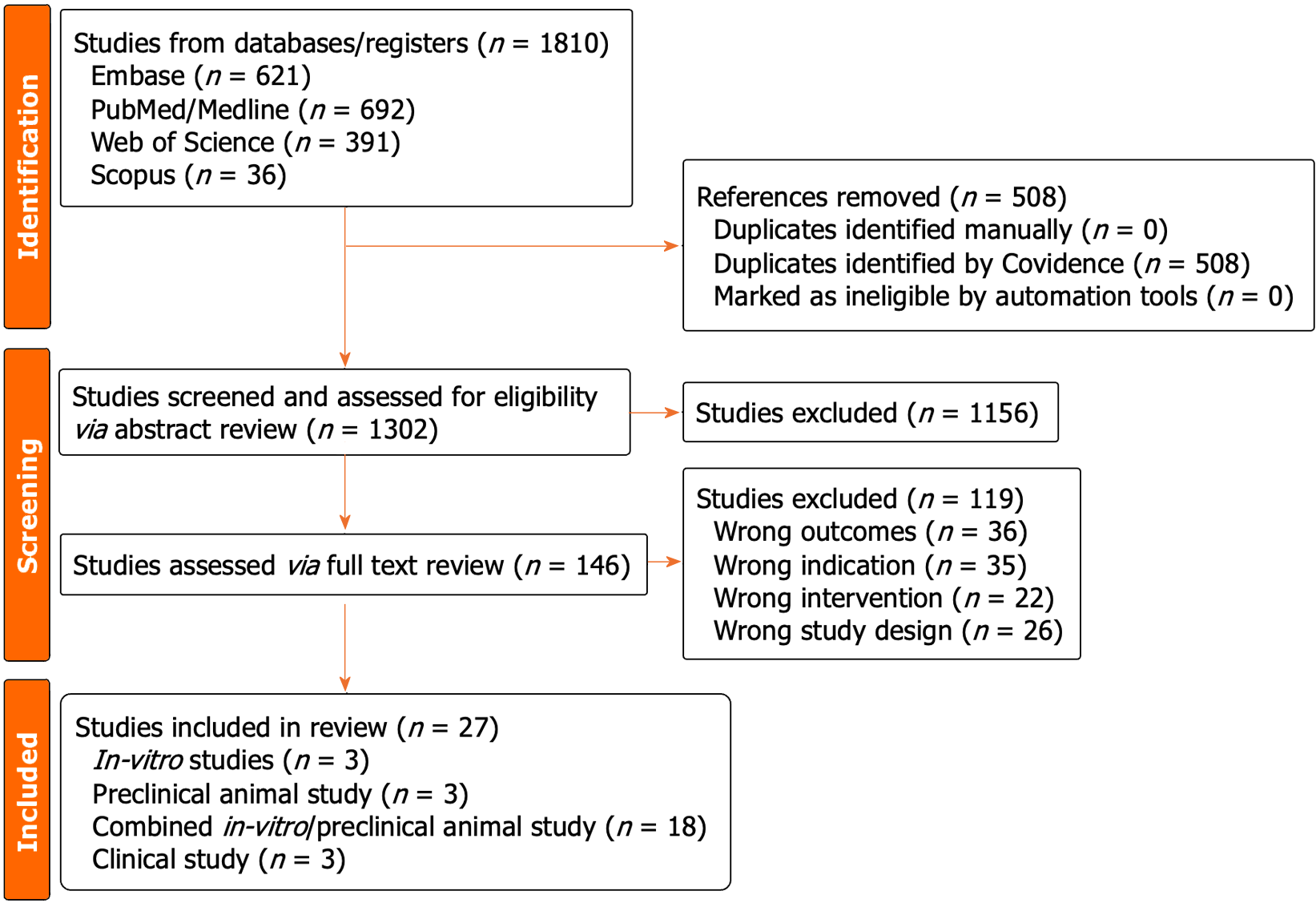
Our initial literature search uncovered 1810 articles potentially relevant to the search question. A total of 508 duplicates were eliminated, and the remaining 1302 articles were screened according to their abstracts, of which 1156 were excluded. The full text was reviewed for the remaining 146 articles, and 119 were excluded due to wrong outcomes (36 articles), wrong indications (35 articles), wrong intervention (22 articles) and wrong study design (26 articles). A total of 27 studies met our pre-defined search and inclusion criteria and were included in this review (Figure 1).
Three in vitro studies met our search criteria and are included in this review. They are summarized in Table 1.
| Ref. | Title | Country | Source of exosomes | Groups included in study | Results/key outcomes |
| Kazi et al[72], 2022 | Dermal papilla cell-derived extracellular vesicles increase hair inductive gene expression in adipose stem cells via β-catenin activation | Japan | Dermal papilla cell-derived extracellular vesicles | Control group: ASCs without treatment; CAO1/2FP group: ASCs treated only with CAO1/2FP; DPC-EVs group: ASCs treated with 5 μg/mL of DPC-EVs and CAO1/2FP; P4 DPC-EVs group: ASCs treated with 5 μg/mL of early-passage (P4) DPC-EVs and CAO1/2FP; P5 DPC-EVs group: ASCs treated with 5 μg/mL of late-passage (P5) DPC-EVs and CAO1/2FP | Cell proliferation: P4 DPC-EVs significantly increased ASC proliferation compared to P5 DPC-EVs (P < 0.01); optimal concentration of 5 μg/mL DPC-EVs enhanced proliferation without toxicity |
| Gene & protein expression: P4 DPC-EVs upregulated VCAN, α-SMA, OPN, and NCAM genes in ASCs (P < 0.05); western blot confirmed increased β-catenin and VCAN expression in treated ASCs | |||||
| MiRNA regulation: P4 DPC-EVs contained higher levels of mir-195-5P and mir-218-5P, both of which regulate hair follicle induction; mir-214-5P (a type of inhibitor of keratinocyte proliferation) was lower in P4 DPC-EVs compared to P5 | |||||
| Exosome uptake & characterization: TEM analysis showed that DPC-EVs were 80-170 nm in diameter; CD63 and TSG101 confirmed as exosome markers via western blot; fluorescence microscopy demonstrated efficient DPC-EV uptake by ASCs | |||||
| Nilforoushzadeh et al[73], 2020 | Human hair outer root sheath cells and platelet-lysis exosomes promote hair inductivity of dermal papilla cell | Iran | HHORSCs and PL exosomes | Control group: hDPCs cultured without exosomes; HHORSC-Exo group: hDPCs treated with 25, 50, 100 μg/mL HHORSC-derived exosomes; PL-Exo group: hDPCs treated with 25, 50, 100 μg/mL PL-derived exosomes | Cell proliferation: HHORSC-Exo significantly increased hDPC proliferation compared to control and PL-Exo groups; MTS assay confirmed higher metabolic activity in HHORSC-Exo-treated |
| Migration & inductive capacity: Transwell migration assay showed increased hDPC migration in HHORSC-Exo-treated cells compared to controls; PL-Exo had a less significant effect in both migration and proliferation analyses compared to HHORSC-Exo | |||||
| Gene & protein expression: HHORSC-Exo treatment upregulated ALP (by 2.1-fold), VCAN (by 1.7-fold), and α-SMA (by 1.3-fold) compared to control; flow cytometry confirmed high expression of VCAN (77%) and α-SMA (55.2%) in hDPCs | |||||
| Exosome characterization & internalization: Nanoparticle tracking analysis showed HHORSC- and PL-derived exosomes were 30-150 nm in size; western blot confirmed the presence of CD9, CD63, and CD81 in both HHORSC- and PL-Exos; fluorescence microscopy demonstrated efficient uptake of exosomes by hDPCs, with PL-Exo showing higher internalization efficiency (59.93%) than HHORSC-Exo (47.73%), but had weaker biological effects | |||||
| Shieh et al[74], 2022 | Bio-pulsed stimulation effectively improves the production of avian mesenchymal stem cell-derived extracellular vesicles that enhance the bioactivity of skin fibroblasts and hair follicle cells | Taiwan | Bio-pulsed AMSC-sEVs and control AMSC-sEVs were isolated from the bio-pulsed AMSC CM and control AMSC CM | Control group: HSFs without treatment; HFDPCs treated with control AMSC-sEV; HFDPCs treated with AMSC-sEVs, treatment with the bio-pulsed AMSC-sEVs | Furthermore, the bio-pulsed AMSC-sEVs, especially at the concentration of 70 μg/mL, enhanced wound healing in the HSFs within 24 hours |
Three pre-clinical studies met our search criteria and are included in this review. They are summarized in Table 2.
| Ref. | Title | Country | Sources of exosomes | Treatment groups | Main study outcomes |
| Rosalina et al[75], 2023 | Placenta extract-loaded novasome significantly improved hair growth in a rat in vivo model | Indonesia | Bovine PE-loaded novasomes | Control group: No treatment | Hair growth acceleration: The PE-novasome group showed earlier and more even hair growth compared to other groups. By day 14, the control group still had incomplete hair coverage, while all the treatment groups had full coverage. Hair length & diameter: At week 4, the PE-novasome group had the longest hair (14.19 mm), followed by PE-liposome (12.54 mm), minoxidil (12.05 mm), and control (8.50 mm). Hair diameter was significantly thicker in the PE-novasome group (120.68 μm) compared to PE-liposome (90.06 μm), minoxidil (49.98 μm), and control (38.59 μm). Anagen-telogen ratio: PE-novasome group had the highest anagen/telogen ratio (4.25), followed by PE-liposome (3.41), minoxidil (2.66), and control (1.88). Hair weight evaluation: Hair weight on day 28 was highest in the PE-novasome group (128.6 mg), significantly greater than PE-liposome (110.7 mg), minoxidil (104 mg), and control (83.4 mg). Novasome characterization & stability: PE-loaded novasomes had 155.0 nm particle size, polydispersity index of 0.139, and entrapment efficiency of 79.60%. Transmission electron microscopy confirmed non-aggregating, oligolamellar nanovesicles. Stable at 4 °C for 90 days with minimal changes in size and entrapment efficiency |
| Minoxidil group: Treated with 2% minoxidil solution | |||||
| PE-liposome group: Treated with PE-loaded liposomes | |||||
| PE-novasome group: Treated with PE-loaded novasomes | |||||
| Zöller et al[39], 2018 | Immunoregulatory effects of myeloid-derived suppressor cell exosomes in mouse model of autoimmune alopecia areata | Germany | MDSCs isolated from bone marrow cells of healthy donor mice | Control group: Mice with alopecia areata receiving no treatment | Hair growth acceleration: MDSC-Exo-treated mice showed partial hair regrowth, preventing the progression of alopecia areata. MDSC-Exo homing was the strongest in activated immune cells. Live imaging confirmed exosome uptake in skin-draining lymph nodes and near hair follicles. Histological analysis: H&E staining showed increased Treg infiltration in MDSC-Exo-treated mice. T helper cell proliferation was significantly reduced, and cytotoxic T-cell activity was suppressed. Immunomodulation & gene expression: FoxP3 (Treg marker) and arginase 1 mRNA levels increased in MDSC-Exo-treated mice. Treg expansion was a dominant feature, supporting immune tolerance in AA. Cytotoxic activity of T cells was suppressed, reducing inflammation. Exosome characterization & targeting: MDSC-Exo were 30-100 nm in diameter, confirmed via transmission electron microscopy. Flow cytometry showed that MDSC-Exo preferentially targeted Tregs, macrophages, and NK cells. Exosome uptake exceeded the binding of MDSCs themselves, suggesting stronger immunoregulatory effects. In vivo distribution & effects: MDSC-Exo were detected in lymphoid tissues and near hair follicles 8-48 hours after injection. Repeated MDSC-Exo injections reduced inflammatory cytokines (IL-1β, IL-6) and increased IL-10 expression |
| MDSC-Exo group: AA mice treated with MDSC-derived exosomes | |||||
| MDSC group: AA mice treated with MDSCs | |||||
| SADBE group: AA mice treated with squaric acid dibutylester, a known therapeutic agent for AA | |||||
| MDSC group: AA mice treated with MDSCs | |||||
| Mao et al[46], 2024 | Exosomes derived from umbilical cord mesenchymal stem cell promote hair regrowth in C57BL6 mice through upregulation of the RAS/ERK signaling pathway | China | Umbilical cord mesenchymal stem cells | Blank group: No treatment | Hair growth acceleration: Model group had significantly shorter and thinner hairs compared to the blank group. Exosome hydrogel-treated mice showed longer hair length, greater hair diameter, and more hair follicles than the model group. Histological analysis: H&E staining revealed increased hair follicle number and size in the exosome group compared to the model group. AR expression: Model group exhibited significantly higher AR mRNA and protein levels, which were reduced in the exosome hydrogel group. HFSC markers: K15 and CD200 expression increased in the exosome group, indicating improved stemness of HFSCs. PCNA expression (a marker of proliferation) was significantly upregulated in exosome-treated mice. Molecular mechanism (transcriptomics & pathway analysis): KEGG analysis identified the RAS/ERK pathway as significantly activated in the exosome group. Exosome treatment upregulated p-Raf, p-MEK1/2, p-ERK1/2, and RAS expression, confirming ERK/MAPK pathway activation. Western blot showed reduced expression of AR and increased expression of MAPK-related proteins in exosome-treated mice |
| Model group: Injected with dihydrotestosterone solution (AGA induction) | |||||
| Positive control group: Treated with 5% minoxidil solution | |||||
| Exosome hydrogel group: Treated with hUCMSC-derived exosome hydrogel |
Eighteen studies with both in vitro and pre-clinical component met our search criteria and are included in this review. They are summarized in Table 3.
| Ref. | Title | Country | Sources of exosomes | Groups in study | Results/key outcomes |
| Xiong et al[61], 2024 | Bioinspired engineering ADSC nanovesicles thermosensitive hydrogel enhance autophagy of dermal papilla cells for androgenetic alopecia treatment | China, United Kingdom | Adipose-derived stem cell NVs | In vitro: (1) Control group: Untreated hDPCs; (2) AGA model: HDPCs cells damaged by DHT; (3) ADSC-NV treatment group; (4) JAM-AOE group; (5) JAM-A interference group; and (6) JAM-A control group. In vivo: (1) Model group: Mice injected with DHT to induce AGA; (2) Minoxidil group: Treated with topical minoxidil (positive control); (3) HSF-NVs group: Treated with HSF-NVs; (4) ADSC-NVs group: Treated with ADSC-NVs; (5) JAM-AOE@NV group: Treated with JAM-AOE ADSC NVs; and (6) JAM-AOE@NV Gel group: Treated with JAM-AOE@NV encapsulated in thermosensitive hydrogel | Hair growth acceleration: JAM-AOE@NV Gel-treated mice exhibited the highest hair regrowth rate, with significantly greater hair follicle density than other groups. By day 15, ADSC-NVs showed superior hair growth compared to HSF-NVs |
| Histological analysis: H&E staining demonstrated more intact and larger hair follicles in the JAM-AOE@NV Gel group. Ki67 immunofluorescence staining confirmed higher proliferation in JAM-AOE@NV-treated hair follicles | |||||
| Autophagy activation: JAM-AOE@NV treatment increased autophagosome formation in DHT-injured DPCs. LC3-II expression was significantly upregulated, while p62 levels decreased, which confirmed induction of autophagic processes | |||||
| Wnt/β-catenin pathway modulation: β-catenin and cyclin D1 were significantly upregulated, promoting HFSC activation | |||||
| AR & inflammation reduction: JAM-AOE@NV reduced inflammation by suppressing TGF-β1 and IL-6, decreasing the amount of androgen-induced follicular damage. Exosome characterization: NV size: About 110 nm, confirmed via TEM. Western blotting identified exosome markers CD63, TSG101, and calnexin. Hydrogel properties & drug release: JAM-AOE@NV Gel exhibited sustained release, achieving 60% release at day 1 and full release at day 4. Hydrogel was biocompatible, with no significant toxicity in dermal papilla cells | |||||
| Tang et al[40], 2025 | Baricitinib-loaded EVs promote alopecia areata mouse hair regrowth by reducing JAK-STAT-mediated inflammation and promoting hair follicle regeneration | China | Mesenchymal stem cells derived from human placenta | In vitro: (1) Control group: Untreated DPCs; (2) DPCs treated baricitinib solution; (3) DPCs treated with EVs; and (4) DPCs treated with EV-B. In vivo: (1) Control group: Injected with saline; (2) Baricitinib group: Injected with baricitinib solution (86.37 μg/mL); (3) EV group: Injected with EVs (2.48 × 1011 particles/mL); and (4) EV-B group: Injected with EV-B (86.37 μg baricitinib + 2.48 × 1011 EV particles) | Enhanced hair regrowth: EV-B group showed the most significant hair regrowth compared to all other groups. By day 20, hair fully covered the previously bald area |
| Improved drug delivery: EV-B demonstrated higher delivery efficiency than baricitinib alone, leading to greater therapeutic effects | |||||
| JAK-STAT pathway inhibition: EV-B significantly downregulated IFN-γ, Jak-2, Stat-1, and IL-15, reducing inflammation in the alopecia areata mouse model | |||||
| Wnt/β-catenin pathway activation: EV-B upregulated β-catenin, promoting hair follicle regeneration | |||||
| Synergistic effect: The combination of EVs and baricitinib led to better inflammation control and hair regrowth compared to either treatment alone | |||||
| Kwak et al[76], 2024 | Development of pluripotent stem cell-derived epidermal organoids that generate effective extracellular vesicles in skin regeneration | South Korea | iEpiOs | In vitro: (1) Control group: PBS only; (2) Cells were treated with 2D-cultured iEpiO-derived EVs; (3) Cells treated with 3D-cultured iEpiO-derived EVs; (4) Cells cultured in full growth medium; and (5) Cells treated with miRNA inhibitors. In vivo: (1) Control PBS group: Received 100 μL PBS; (2) 2D-EV group: Received 100 μg of 2D-cultured iEpiO-derived EVs in 100 μL PBS; and (3) 3D-EV group: Received 100 μg of 3D-cultured iEpiO-derived EVs in 100 μL PBS | Wound healing acceleration: 3D-EVs increased wound closure 1.6-fold faster than PBS and 2D-EVs by days 3, 5, and 7 |
| EV yield: 3D-cultured iEpiOs produced 2 × more EVs than 2D cultures | |||||
| Angiogenesis: 3D-EVs contained higher VEGF levels and enhanced endothelial tube formation in HUVECs | |||||
| Cellular proliferation: 3D-EVs significantly upregulated Ki67 and COL1A1 expression in fibroblasts | |||||
| Migration: 3D-EVs promoted fibroblast migration in scratch-wound and transwell assays | |||||
| MiRNA content: 3D-EVs were enriched in miR-31-5p, miR-146a-5p, which regulate proliferation, migration, and differentiation | |||||
| Epidermal regeneration: iEpiOs formed multi-layered epidermal structures, closely mimicking native skin morphology | |||||
| Shang et al[52], 2024 | Exosomes derived from mouse vibrissa dermal papilla cells promote hair follicle regeneration during wound healing by activating Wnt/β-catenin signaling pathway | China | Dermal papilla cells from mouse vibrissa follicles | In vitro: (1) Fibroblasts without exosomes; (2) Fibroblasts treated with various concentrations of DPC-exos; (3) Fibroblasts treated with the Wnt/β-catenin pathway inhibitor; and (4) Fibroblasts treated with both DPC-Exos and XAV939. In vivo: (1) PBS group: Received PBS (control); (2) DPC-Exos group: Received 100 μg of DPC-Exos in 100 μL PBS; (3) XAV939 group: Received Wnt/β-catenin pathway inhibitor (XAV939); and (4) DPC-Exos + XAV939 group: Received DPC-Exos + XAV939 | Wound healing acceleration: DPC-Exos enhanced wound closure, with significantly smaller wound areas on days 7 and 10 compared to PBS (P < 0.05) |
| Hair follicle regeneration: DPC-Exos increased new hair follicle formation at wound sites compared to control, confirmed by H&E staining | |||||
| Fibroblast proliferation and migration: DPC-Exos promoted fibroblast proliferation and migration in a concentration-dependent manner (10-40 μg/mL) | |||||
| Hair-inducing capacity: DPC-Exos-treated fibroblasts significantly upregulated β-catenin, ALP, Lef1, and Noggin, key markers for hair follicle neogenesis | |||||
| In vivo hair reconstitution: Exos-treated fibroblasts combined with neonatal mouse ECs induced hair follicle formation in nude mice, comparable to dermal papilla cells | |||||
| Collagen deposition: Masson staining showed reduced collagen deposition in the DPC-Exos group, indicating lower fibrosis and improved skin regeneration | |||||
| Wnt/β-catenin activation: DPC-Exos upregulated β-catenin and Lef1, while XAV939 inhibited these effects, confirming the pathway’s role in hair follicle regeneration | |||||
| Effect of Wnt inhibition: XAV939 significantly slowed wound healing, reduced fibroblast activity, and decreased new hair follicle formation, confirming that DPC-Exos act via Wnt/β-catenin signaling | |||||
| Zhang et al[62], 2024 | Engineered exosomes biopotentiated hydrogel promote hair follicle growth via reprogramming the perifollicular microenvironment | China | mDPCs | In vitro: (1) NC: PBS group; (2) Group treated with 5 μg/mL minoxidil; (3) Group treated with 20 μg/mL nanoparticles consisting of liposomes and exosomes without minoxidil; and (4) Group treated with 25 μg/mL of engineered exosomes consisting of liposomes and exosomes loaded with minoxidil. In vivo: (1) Model group: Received testosterone solution (AGA induction); (2) Minoxidil group: Treated with 5% minoxidil; (3) Gel group: Treated with blank hydrogel (no active components); (4) Gel@MNs group: Treated with hydrogel containing exosomes but no minoxidil; and (5) Gel@MNs group: Treated with hydrogel containing both exosomes and minoxidil | Hair regrowth acceleration: Gel@MNs group showed greater hair coverage and density on day 28 compared to other groups. Hair follicles in Gel@MNs group were larger and more numerous than in the minoxidil group |
| Histological analysis: H&E staining confirmed more hair follicle units in Gel@MNs-treated mice compared to other groups. Epidermal thickness was significantly increased in Gel@MNs and Gel@MNs groups | |||||
| Angiogenesis and microenvironment changes: CD31-positive blood vessels were more abundant around hair follicles in the Gel@MNs group. VEGF expression was significantly higher in Gel@MNs-treated skin tissues than in untreated AGA skin | |||||
| Transcriptomic analysis: RNA-seq revealed that Gel@MNs modulated gene expression, particularly downregulating IL-17 signaling (inflammation suppression). Genes associated with lipid biosynthesis, glucose metabolism, and protein synthesis were regulated after treatment. Circadian rhythm pathway alterations were noted, indicating possible involvement in hair follicle cycling | |||||
| Safety and biocompatibility: No significant toxicity was observed in major organs (heart, liver, spleen, lung, kidney). Blood counts (RBC, WBC, PLT, HGB) remained unchanged post-treatment. No allergic reactions (IgE levels unchanged) were detected in Gel@MNs-treated mice | |||||
| Jiao et al[57], 2024 | Stimulation of mouse hair regrowth by exosomes derived from human umbilical cord mesenchymal stem cells | China | hUCMSCs | In vitro: (1) Control group: Only PBS; and (2) Fibroblasts cultured with hUCMSC-Exos (50 μg/mL, 100 μg/mL, 200 μg/mL). In vivo: (1) PBS group: Received PBS (control); and (2) hUCMSC-Exos group: Received 200 μg/mL hUCMSC-derived exosomes | Fibroblast proliferation: Fibroblast proliferation was significantly increased in the hUCMSC-Exos (200 μg/mL) group compared to controls (P = 0.017 at 72 hours) |
| Hair growth acceleration: Mice treated with hUCMSC-Exos showed faster transition into the anagen phase, with thicker skin, larger hair bulbs, and more follicles in the subcutis | |||||
| Hair follicle stem/progenitor cells: Increased expression of CD34, K15, Lgr5, and Lrig1 in hUCMSC-Exos-treated skin (qPCR analysis). Flow cytometry confirmed higher numbers of CD49f+CD34+ and CD34-CD49f+Pcad^hi stem cell populations in treated areas. Immunofluorescence showed elevated Lgr5 expression in epidermal and bulge regions | |||||
| Wnt/β-catenin pathway activation: β-catenin, Lrp5, Wnt5, and Lef1 expression significantly increased in hUCMSC-Exos-treated regions (qPCR analysis). Immunohistochemistry revealed higher β-catenin staining in epidermal and follicular regions of treated mice | |||||
| Li et al[50], 2025 | Decorin-mediated dermal papilla cell-derived exosomes regulate hair follicle growth and development through miR-129-2-3p/SMAD3/TGF-β axis | China, Japan | Dermal papilla cells derived from angora rabbit vibrissa follicles | In vitro: (1) Control-Exos group: DPC-derived exosomes without treatment; (2) DCN-Exos group: DPC-derived exosomes treated with 200 nM rhDCN; (3) SRI-011381 group: Treated with a TGF-β pathway activator (SRI-011381 hydrochloride, 10 μM); (4) MiR-129-2-3p mimic/inhibitor groups: Cells transfected with miR-129-2-3p mimic/inhibitor; and (5) SMAD3 OE/KD groups: Cells with SMAD3 gene modulation. In vivo: Mice injected with fluorescently labelled DPC-Exos | Hair follicle growth: DCN-Exos significantly promoted hair shaft elongation compared to untreated exosomes (P < 0.05) |
| Cell proliferation & apoptosis: DPC proliferation was enhanced following DCN treatment (P < 0.05). MiR-129-2-3p mimic increased HFSC proliferation and reduced apoptosis, whereas its inhibitor had the opposite effect | |||||
| Gene & protein expression: MiR-129-2-3p was significantly upregulated in DCN-Exos compared to control-Exos. SMAD3, E2F4, RBL1, TFDP2, and TGF-β1 were downregulated in the miR-129-2-3p mimic group and upregulated in the inhibitor group. Western blot confirmed lower SMAD3 and TGF-β1 expression in DCN-Exos-treated HFSCs | |||||
| Wnt/TGF-β pathway modulation: β-catenin and LEF1 were upregulated, while SMAD3 and TGF-β1 were downregulated in DCN-Exos-treated HFSCs. SRI-011381 restored SMAD3/TGF-β1 expression, counteracting miR-129-2-3p effects | |||||
| Exosome uptake & function: DiI-labeled DPC-Exos were successfully internalized by HFSCs within 24 hours. Live imaging confirmed in vivo uptake of exosomes after dorsal skin injection in mice | |||||
| Wu et al[45], 2024 | Hybrid hair follicle stem cell extracellular vesicles co-delivering finasteride and gold nanoparticles for androgenetic alopecia treatment | China | HFSC-derived EVs | In vitro: (1) Control group: Untreated cells; (2) HDPCs treated with AuNPs; (3) HDPCs treated with HFSC-derived EV; (4) HDPCs treated with finasteride; (5) HVs group: Fusion vesicles of EVs and liposomes; and (6) HVs co-loaded with AuNPs and finasteride. In vivo: (1) Blank group: No treatment after depilation; (2) Model group: Topically applied 0.1 mL/cm2 of 0.5% testosterone solution (AGA induction); (3) Minoxidil (Mino) group: Treated with 5% minoxidil solution; (4) AuNPs group: Treated with AuNPs gel; (5) EVs group: Treated with HFSC-derived EVs gel; (6) Fi group: Treated with finasteride-loaded gel; (7) HVs group: Treated with HVs containing EVs and liposomes; and (8) Hybrid/Au@Fi group: Treated with HVs containing AuNPs and finasteride | Hair regrowth acceleration: Hybrid/Au@Fi-treated mice had significantly greater hair regrowth coverage on day 11 (23.04% ± 11.17%) and day 14 (53.88% ± 15.25%) than the minoxidil group (31.92% ± 18.09% on day 14). Regrown hair was thicker and more uniform in the hybrid/Au@Fi group compared to other treatments |
| Histological analysis: H&E staining showed more intact and well-structured hair follicles in the hybrid/Au@Fi group. Bulb diameter was larger in hybrid/Au@Fi-treated mice compared to other treatment groups | |||||
| Microenvironment modulation: CD31-positive blood vessels were significantly increased in hybrid/Au@Fi-treated skin, suggesting enhanced angiogenesis. SOX9 expression was more evenly distributed in hybrid/Au@Fi-treated follicles, indicating a well-maintained follicular niche | |||||
| Cell proliferation & apoptosis: Ki67 (proliferation marker) expression in hair bulbs was significantly higher in hybrid/Au@Fi-treated mice than in the minoxidil group. TUNEL staining showed more controlled apoptosis in the ORS, suggesting healthy hair follicle cycling | |||||
| Exosome stability & drug encapsulation: HVs provided higher follicle retention, storage stability, and finasteride encapsulation efficiency (45.33%) compared to EVs alone. AuNPs mimicked LLLT, stimulating vascularization and follicular activation | |||||
| Oh et al[77], 2024 | Improvement of androgenic alopecia by extracellular vesicles secreted from hyaluronic acid-stimulated induced mesenchymal stem cells | South Korea | iMSCs stimulated with HA | In vitro: (1) Control group: No testosterone or treatment; (2) HFDPCs treated with 50 μM testosterone; (3) Positive control: HFDPCs treated with 50 μM testosterone and 100 nM finasteride; (4) HFDPCs treated with 50 μM testosterone and 25 μg/mL iMSC-derived EVs; (5) HFDPCs treated with 50 μM testosterone and 25 μg/mL HA-stimulated iMSC-derived EVs; and (6) HFDPCs treated with testosterone and HA-iMSC-EVs and rhDKK-1. In vivo: (1) Vehicle control group: Received 50% ethanol + DPBS injections; (2) Testosterone-treated group: Received 0.5% testosterone propionate topically; (3) Finasteride group: Received 0.5% testosterone + 1 mg/kg finasteride injections; (4) iMSC-EVs group: Received 0.5% testosterone + 0.2 mg/kg iMSC-EVs injections; and (5) HA-iMSC-EVs group: Received 0.5% testosterone + 0.2 mg/kg HA-iMSC-EVs injections | Hair growth acceleration: HA-iMSC-EVs significantly restored hair growth in testosterone-induced AGA mice. Hair growth was comparable to the finasteride group on day 27 |
| Anagen phase restoration: Testosterone reduced the anagen ratio (0.53 vs 1.55 in normal mice, P < 0.0001). HA-iMSC-EVs increased the anagen ratio to 1.19, comparable to finasteride (1.29) | |||||
| AR & Wnt/β-catenin pathway activation: AR expression was significantly reduced in HA-iMSC-EVs-treated mice. β-catenin and phosphorylated GSK3β levels were increased, confirming Wnt/β-catenin pathway reactivation. Immunofluorescence showed increased β-catenin-positive hair follicles in HA-iMSC-EVs-treated skin | |||||
| HFDPC response: Testosterone-induced increases in AR, TGF-β1, and IL-6 were blocked by HA-iMSC-EVs. IGF1, FGF7, and VEGF expression was upregulated, promoting hair growth | |||||
| Exosome characterization: HA-iMSC-EVs were about 135.3 nm in diameter and contained CD63, CD81, and TSG101 markers. Proteomic analysis identified 44 differentially expressed proteins, linked to ECM interactions, PI3K/AKT signaling, and proteasome function | |||||
| Chu et al[44], 2025 | Exosome-derived long non-coding RNA AC010789.1 modified by FTO and hnRNPA2B1 accelerates growth of hair follicle stem cells against androgen alopecia by activating S100A8/Wnt/β-catenin signaling | China | Exosomes derived from human HFSCs | In vitro: (1) Control group: HFSCs transfected with NC plasmids or siRNAs; (2) AGA group: HFSCs from androgenetic alopecia patients; (3) AC010789.1 OE group: HFSCs transfected with AC010789.1 OE plasmids; (4) AC010789.1 KD group: HFSCs transfected with si-AC010789.1; and (5) Exosome-treated groups: HFSCs treated with exosomes containing AC010789.1 (Exo-AC010789.1). In vivo: (1) Hairless mice injected with Exo-AC010789.1 or Exo-NC; (2) Positive control: Hairless mice treated with 5% minoxidil; (3) Exo-AC010789.1 group: Hairless mice injected with exosomes containing AC010789.1; and (4) Exo-NC group: Hairless mice injected with control exosomes | LncRNA AC010789.1 expression: Downregulated in AGA hair follicle tissues but upregulated in HFSCs from normal scalp tissue |
| HFSC proliferation & migration: AC010789.1 OE increased proliferation and migration, while KD inhibited growth. Exo-AC010789.1 significantly enhanced HFSC proliferation compared to control exosomes (P < 0.05) | |||||
| m6A modification & molecular interactions: FTO demethylase suppressed AC010789.1 expression, reducing HFSC growth. hnRNPA2B1 bound to AC010789.1, enhancing its stability and upregulating Wnt/β-catenin signaling | |||||
| Gene & protein expression: AC010789.1 OE upregulated K6HF, Lgr5, and Wnt/β-catenin pathway components (β-catenin, Wnt10b, c-myc). KD of S100A8 reversed AC010789.1-induced HFSC proliferation, confirming its role in hair growth signaling | |||||
| Exosome uptake & function in mice: Exo-AC010789.1 was internalized by HFSCs, leading to increased proliferation and migration. Exo-AC010789.1-treated mice showed significantly more hair regrowth than controls. Immunohistochemistry confirmed increased K6HF, Lgr5, Wnt10b, and β-catenin expression in hair follicles | |||||
| Hu et al[78], 2024 | Exosomes derived from uMSCs promote hair regrowth in alopecia areata through accelerating human hair follicular keratinocyte proliferation and migration | China | HUCMSCs | In vitro: (1) Human hair follicle KCs treated with PBS; and (2) KCs treated with various concentrations of exosomes. In vivo: (1) PBS group: Injected with 100 μL PBS (control); (2) UMSC-Exos group: Injected with 100 μL of 30 μg UMSC-derived exosomes | Hair regrowth acceleration: On day 14, the UMSC-Exos group achieved about 95% hair coverage, compared to about 70% in the PBS group. Hair follicle density and skin thickness were significantly higher in the UMSC-Exos group (P < 0.05) |
| Exosome uptake & function: DiI-labeled UMSC-Exos were internalized by hair follicle KCs in vitro within 24 hours. Injected UMSC-Exos integrated into mouse hair follicles after 24 hours | |||||
| Hair follicle regeneration: H&E staining showed a higher number of anagen-stage hair follicles in the UMSC-Exos group. Western blot confirmed increased expression of FGF-7, BMP-4, and VCAN, key factors for hair follicle development | |||||
| KC proliferation & apoptosis: Ki67-positive KCs increased to about 70% in the UMSC-Exos group, compared to about 45% in controls. TUNEL staining showed decreased KC apoptosis in UMSC-Exos-treated cells (P < 0.05) | |||||
| Wound healing & migration: KCs treated with UMSC-Exos exhibited faster migration in a wound scratch assay | |||||
| Liu et al[48], 2024 | Combatting ageing in dermal papilla cells and promoting hair follicle regeneration using exosomes from human hair follicle dermal sheath cup cells | China | Human hair follicle DSCCs | In vitro: (1) Control group: Normal DPCs; (2) H2O2 group: DPCs treated with 600 μmol/L H2O2 to induce senescence; (3) H2O2 + ExoDSCCs (P3) group: Senescent DPCs treated with exosomes from P3 DSCCs; (4) Long-generation DPCs (P10) group: Aged DPCs from hair follicles in bald areas; and (5) P10 + ExoDSCCs (P3) group: Aged DPCs treated with exosomes from P3 DSCCs. In vivo: (1) Control group: Normal DPCs combined with neonatal mouse ECs; (2) Senescent DPC group: Senescent DPCs + neonatal mouse ECs; (3) ExoDSCCs group: Senescent pre-treated with ExoDSCCs and neonatal mouse ECs; and (4) ECs group: Neonatal mouse ECs alone | DPC proliferation & migration: ExoDSCCs (P3) significantly increased cell viability and proliferation in both H2O2-treated and P10 DPCs (P < 0.01). Scratch wound healing assay confirmed enhanced migration of senescent DPCs after ExoDSCCs (P3) treatment |
| Senescence marker expression: SA-β-Gal staining showed a significant reduction in senescent cells after ExoDSCCs (P3) treatment (P < 0.05). p16, p21, and p53 expression levels were reduced, indicating delayed cellular ageing | |||||
| Hair follicle induction markers: ALP and VCAN expression were upregulated in ExoDSCCs (P3)-treated DPCs (P < 0.001). Hanging drop assay showed that ExoDSCCs (P3)-treated senescent DPCs formed spheroids, mimicking functional dermal papilla aggregates | |||||
| In vivo hair follicle regeneration: ExoDSCCs (P3)-treated DPCs successfully induced new hair follicles when co-transplanted with neonatal ECs in nude mice. Untreated senescent DPCs failed to induce hair follicle neogenesis | |||||
| Wnt/β-catenin pathway activation: β-catenin, Wnt7a, Wnt10b, and LEF1 were significantly upregulated in ExoDSCCs (P3)-treated DPCs (P < 0.01). GSK-3β phosphorylation (Ser-9) increased, indicating Wnt pathway activation | |||||
| Chen et al[43], 2020 | Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth | China | Human DP-EVs | In vitro: (1) NC: PBS group; (2) DP-EVs group: Cells treated with DP-EVs; (3) OSA group: Treated with OSA hydrogel; (4) OSA-EVs group: Treated with DP-EVs encapsulated in OSA hydrogel; (5) DF-EVs group: Treated with dermal fibroblast-derived EVs; and (6) KC-EVs group: Treated with KC-derived EVs. In vivo: (1) PBS group: Control group with PBS injection; (2) DP-EVs group: Injected with naked DP-EVs; (3) OSA-EVs group: Injected with OSA-encapsulated DP-EVs; and (4) Minoxidil group: Treated with 3% minoxidil | Hair growth acceleration: OSA-EVs significantly accelerated hair regrowth in depilated mice compared to DP-EVs alone; increased hair follicle density and larger hair bulb diameters observed in OSA-EVs-treated mice |
| Histological analysis: H&E staining showed more anagen-phase follicles in OSA-EVs-treated mice compared to controls | |||||
| Hair matrix cell proliferation: Ki67-positive cells were significantly increased in OSA-EVs-treated follicles (P < 0.01); wound healing assays showed enhanced migration of hair matrix cells with OSA-EVs | |||||
| Molecular mechanisms: OSA-EVs upregulated Wnt3a and β-catenin expression, activating the Wnt/β-catenin signaling pathway; BMP2 (inhibitory molecule) was significantly downregulated in OSA-EVs-treated follicles; MMP3 (a hair growth-promoting enzyme) expression was elevated, enhancing ECM remodeling | |||||
| Exosome retention & stability: OSA hydrogels provided sustained release of DP-EVs, ensuring longer bioavailability compared to free DP-EVs; encapsulated DP-EVs were retained in hair follicles for up to 8 days, compared to 3 days for naked DP-EVs; encapsulation protected EV proteins from degradation, maintaining their biological activity | |||||
| Kim et al[79], 2022 | Potential of colostrum-derived exosomes for promoting hair regeneration through the transition from telogen to anagen phase | South Korea | Milk-exo | In vitro: (1) DPCs without treatment; (2) DPCs treated with various concentrations of colostrum-derived milk exosomes; (3) DPCs treated with exosome-free milk; (4) DPCs pre-treated with DHT (30 μM) and then with Milk-exo; and (5) DPCs pre-treated with DHT and then treated with Exo-free milk. In vivo: (1) PBS group: Injected with PBS (control); (2) Exo-free milk group: Injected with milk fraction without exosomes; (3) Milk-exo group: Injected with 200 μg of colostrum-derived exosomes; and (4) Minoxidil group: Topically applied 2.5% minoxidil solution | Hair growth acceleration: Milk-exo significantly promoted hair regrowth in mice, with results comparable to minoxidil treatment; by day 15, the Milk-exo group had the highest hair coverage, reaching about 50%, while the control group had about 25% (P < 0.0001) |
| Histological analysis: H&E staining showed more hair follicles in the anagen phase in the Milk-exo group compared to controls; Ki67 staining indicated increased dermal papilla cell proliferation in the Milk-exo-treated mice | |||||
| Telogen-to-anagen transition: Milk-exo accelerated the transition from telogen to anagen phase, evidenced by increased hair bulb size and deeper follicles; CD31 staining showed increased angiogenesis in Milk-exo-treated skin | |||||
| Wnt/β-catenin pathway activation: Western blot analysis showed increased β-catenin and Wnt3a expression in the Milk-exo group; Milk-exo inhibited DHT-induced suppression of β-catenin, preventing hair follicle miniaturization | |||||
| Exosome characterization & stability: Milk-exo were 50-100 nm in size, confirmed by TEM and dynamic light scattering analysis; western blot identified exosomal markers TSG101 and Alix, confirming successful isolation; lyophilized Milk-exo maintained stability and hair growth efficacy after reconstitution | |||||
| Liang et al[49], 2023 | Adipose mesenchymal stromal cell-derived exosomes carrying miR-122-5p antagonize the inhibitory effect of dihydrotestosterone on hair follicles by targeting the TGF-β1/SMAD3 signaling pathway | China | ADSCs | In vitro: (1) Control group: Normal untreated DPCs; (2) DHT group: DPCs treated with 10-5 M DHT; (3) ADSC-Exos group: DPCs treated with 10 μg/mL ADSC-Exos; (4) ADSC-Exos + DHT group: Co-treatment with ADSC-Exos and DHT; (5) Exo-miR-122-5p group: DPCs treated with exosomes enriched in miR-122-5p; and (6) Exo-in-miR-122-5p group: DPCs treated with exosomes with inhibited miR-122-5p. In vivo: (1) Control group: PBS only; (2) DHT group to induce hair loss; (3) Mice given DHT + Exo-miR-122-5p; (4) Mice treated with DHT + minoxidil; and (5) Mice treated with DHT and exosomes lacking miR-122-5p | Hair growth acceleration: Exo-miR-122-5p significantly reversed DHT-induced hair follicle miniaturization. Hair bulb size and dermal thickness were restored in Exo-miR-122-5p-treated mice |
| DPC proliferation & migration: Ki67-positive cells increased in ADSC-Exos-treated DPCs compared to the DHT group (P < 0.05). Wound healing assay confirmed enhanced DPC migration in the ADSC-Exos group | |||||
| Gene & protein expression: β-catenin and VCAN were upregulated in ADSC-Exos and Exo-miR-122-5p groups (P < 0.01). SMAD3 and p-SMAD3 levels were significantly reduced in Exo-miR-122-5p-treated cells, confirming suppression of the TGF-β1/SMAD3 axis. BCL2 expression increased, while Bax (an apoptosis marker) was downregulated, indicating reduced DHT-induced apoptosis | |||||
| MiRNA regulation: MiR-122-5p was highly enriched in ADSC-Exos, and luciferase assays confirmed SMAD3 as its direct target. Exo-miR-122-5p reduced SMAD3 levels, rescuing β-catenin expression and DPC proliferation | |||||
| In vivo hair growth model: Exo-miR-122-5p-treated mice exhibited significantly greater hair regrowth than DHT-treated controls. Immunofluorescence analysis showed increased β-catenin and reduced SMAD3 in hair follicles of Exo-miR-122-5p-treated mice | |||||
| Wang et al[60], 2023 | Treatment of androgenetic alopecia by exosomes secreted from hair papilla cells and the intervention effect of LTF | China | DPC-Exo treated with LTF, a traditional Chinese medicine formulation | In vitro: (1) Control group: DPCs without treatment; and (2) DPCs cultured with various LTF concentrations. In vivo: (1) Control group: Normal untreated mice; (2) Model group: Mice injected with testosterone propionate to induce AGA; (3) Minoxidil group: Treated with 5% minoxidil solution; (4) LTF group: Treated with LTF herbal extract; and (5) LTF-DPC-Exo group: Treated with exosomes derived from LTF-treated DPCs | Hair growth acceleration: LTF, minoxidil, and LTF-DPC-Exo groups showed significant hair regrowth, while the model group had only localized hair growth. Live imaging confirmed sustained fluorescence signals of exosomes in treated mice, with disappearance in areas of full hair regrowth |
| Histological analysis: H&E staining showed denser, larger anagen-phase hair follicles in the LTF-DPC-Exo group. Model group had reduced and miniaturized follicles, indicative of AGA | |||||
| Serum hormone levels: Testosterone levels were significantly reduced, and estradiol levels were increased in all treatment groups compared to the model group (P < 0.01). LTF-DPC-Exo group had the greatest reduction in androgen levels, suggesting an anti-AGA effect | |||||
| Gene & protein expression: LTF-DPC-Exo significantly upregulated VEGF and AKT1, key regulators of hair follicle growth (P < 0.01). Caspase-3 (apoptosis marker) expression was significantly downregulated in the LTF-DPC-Exo group, indicating reduced follicular apoptosis. Western blot confirmed increased VEGF and AKT1 levels, with reduced caspase-3 expression | |||||
| Molecular mechanism: LTF-DPC-Exo activated the PI3K/AKT pathway, promoting hair follicle regeneration. Inhibition of caspase-3 prevented hair follicle apoptosis, maintaining follicular viability | |||||
| Wu et al[80], 2021 | Adipose-derived stem cell exosomes promoted hair regeneration | China | ADSC-Exos isolated from 6-week-old C57BL/6 mice | Control group: Grafted with DCs and ECs only. ADSC-Exos group: Grafted with DCs, ECs, and 50 μg/mL ADSC-Exos | Hair growth acceleration: By week 2, the ADSC-Exos group had significantly more regenerated hairs than the control group (P < 0.001). By week 3, the ADSC-Exos group showed uniform, denser hair regrowth compared to the control |
| Histological analysis: H&E staining confirmed that ADSC-Exos promoted terminal hair formation, while the control group had immature follicles. Follicle count per vertical section was significantly higher in the ADSC-Exos group vs control group (17.70 ± 2.67 vs 11.22 ± 2.37, P < 0.001) | |||||
| Cytokine expression & molecular markers: PDGF and VEGF expression was significantly increased in ADSC-Exos-treated skin (P < 0.05), indicating enhanced angiogenesis. TGF-β1 levels were significantly lower in the ADSC-Exos group compared to controls (P < 0.001), suggesting reduced fibrosis and improved follicular health | |||||
| Exosome characterization: ADSC-Exos were 20-130 nm in diameter, confirmed by TEM. Western blot detected exosome markers CD63, ALX1, and CD9, verifying successful isolation | |||||
| Zhang et al[47], 2016 | iTRAQ-based quantitative proteomic comparison of early- and late-passage human dermal papilla cell secretome in relation to inducing hair follicle regeneration | China | Secretome (conditioned media) from early-passage (P3) and late-passage (P9) human DPCs | P3 DPC-CM group: Conditioned medium from early-passage (P3) DPCs. P9 DPC-CM group: Conditioned medium from late-passage (P9) DPCs | Hair growth acceleration: P3 DPC-CM induced hair follicle regeneration in NU/NU mice, with white hair appearing by day 3 post-injection. P9 DPC-CM failed to induce hair regrowth, confirming loss of inductive potential in late-passage DPCs |
| Histological analysis: H&E staining showed newly formed hair follicle structures in P3 DPC-CM-treated mice, absent in P9 DPC-CM-treated mice | |||||
| Proteomic analysis (iTRAQ-based quantification): 1360 proteins identified, with 213 proteins differentially expressed between P3 and P9 DPC-CM. SDF1, MMP3, Biglycan, and LTBP1 were significantly upregulated in P3 DPC-CM, suggesting key roles in hair follicle regeneration | |||||
| Molecular mechanisms: SDF1 (CXCL12) upregulation in P3 DPC-CM promoted Wnt/β-catenin activation, facilitating dermal-epidermal crosstalk for hair follicle induction. MMP3 enhanced Wnt signaling by antagonizing Wnt5b, a non-canonical Wnt inhibitor. Biglycan promoted β-catenin/TCF-mediated transcription via LRP6 receptor activation, further stimulating follicle development. LTBP1 was highly expressed in P3 DPC-CM and localized in hair follicles, supporting its role in TGF-β/BMP pathway regulation |
Three clinical studies met our search criteria and are included in this review. They are summarized in Table 4.
| Ref. | Title | Country | Sources of exosomes | Groups in study | Results/key outcomes |
| Gentile et al[29], 2015 | The effect of platelet-rich plasma in hair regrowth: A randomized placebo-controlled trial | Italy, India, Turkey (multicentric study) | Autologous micrografts containing EVs derived from human follicle mesenchymal stem cells | MPHL group: 40 males (Norwood-Hamilton stages I-III vertex); FPHL group: 20 females (Ludwig stages I-II) | HD increase: FPHL group: HD increased by 28 ± 4 hairs/cm2 after 12 months (P = 0.0429). MPHL group: HD increased by 30 ± 5 hairs/cm2 after 12 months (P = 0.0012) |
| Trichoscopic analysis: Baseline HD (T0): 114 ± 5 hairs/cm2 (FPHL), 108 ± 3 hairs/cm2 (MPHL). Post-treatment HD (T4, 12 months): 142 ± 4 hairs/cm2 (FPHL), 138 ± 4 hairs/cm2 (MPHL). No significant changes in vellus HD, hair thickness, or anagen/telogen ratio | |||||
| Physician’s global assessment: 70% of patients (42/60) reported improved global scalp coverage (P = 0.045) | |||||
| Patient satisfaction: 80% of patients (48/60) reported a good level of satisfaction (P = 0.021) | |||||
| In vitro exosome analysis: EVs were 95.9-123.2 nm in size, with concentrations ranging from 108 to 1010 particles/mL | |||||
| Transmission electron microscopy confirmed lipid bilayer vesicle morphology. Fluorescence microscopy showed EV uptake by fibroblasts, confirming cellular interaction | |||||
| Lee et al[51], 2024 | The efficacy of adipose stem cell-derived exosomes in hair regeneration based on a preclinical and clinical study | South Korea | Adipose stem cell derived exosomes | In-vitro groups: HDPCs treated with ASC-exosomes (8 μg or 40 μg); ex-vivo groups: Human hair follicles treated with ASC-exosomes (8 μg or 40 μg) or 20 μmol/L minoxidil; clinical groups: 30 patients with androgenetic alopecia treated with ASC-exosomes (ASCE + HRLV®) over 24 weeks | In vitro & ex vivo findings: (1) hDPC proliferation: ASC-exosomes increased hDPC proliferation in a concentration-dependent manner (P < 0.05) compared to controls; (2) Hair follicle growth: Hair shaft elongation was significantly greater in the ASC-exosome 40 μg group than in controls (P = 0.03); (3) Gene & protein expression: ALP, VCAN, β-catenin, and LEF-1 mRNA levels were upregulated in ASC-exosome-treated hDPCs (P < 0.05). Western blot confirmed increased β-catenin and phosphorylated GSK3β, indicating Wnt/β-catenin pathway activation; and (4) Immunostaining: Ki67 and β-catenin expression was highest in ASC-exosome-treated follicles, suggesting enhanced cell proliferation |
| Clinical findings: (1) HD increase: Baseline: 158.03 ± 16.48 hairs/cm2. Week 12: 161.90 ± 17.78 hairs/cm2 (P = 0.033). Week 24: 166.14 ± 19.57 hairs/cm2 (P < 0.001); (2) Global photographic assessment: Week 12: 31.03% of patients showed slight improvement, 3.45% showed marked improvement (P = 0.023). Week 24: 41.38% had slight improvement, 10.34% had marked improvement (P = 0.004); (3) Subjective satisfaction: 52.72% of patients reported improved HD (P < 0.001). 55.17% noted reduced daily hair loss (P = 0.003). 58.62% reported thicker hair strands (P < 0.001); and (4) Adverse reactions: Mild scalp tingling and erythema were observed but resolved quickly. No severe adverse effects reported | |||||
| Lueangarun et al[81], 2024 | Rose stem cell-derived exosomes for hair regeneration enhancement via noninvasive electroporation in androgenetic alopecia | Thailand, South Korea | RSCEs | Single case study: 54-year-old male with Norwood-Hamilton scale V androgenetic alopecia | Hair growth improvement: After three treatment sessions, the patient showed increased HD and thickness. Significant regrowth observed by the 6th and 12th sessions, with continued improvement three months post-treatment |
| Dermoscopy & clinical evaluation: Dermoscopic images showed increased HD and shaft thickness after six and twelve treatment sessions | |||||
| Exosome characterization: RSCEs were 100-200 nm in diameter, confirmed by nanoparticle tracking analysis and cryo-electron microscopy. Proteomic analysis identified 206 proteins, including cell membrane components, enzymes, and RNA-binding proteins. RSCEs contained Let-7 family miRNAs, miR-8485, miR-574-5p, and miR-1246, which are linked to cell proliferation and inflammation regulation | |||||
| Mechanism of action: RSCEs activated the Wnt/β-catenin signaling pathway, promoting dermal papilla proliferation and follicular development. Reduced inflammation and oxidative stress, supporting hair regeneration | |||||
| Treatment protocol: 5 mL of lyophilized RSCEs (20 mg) applied via electroporation every 3 weeks for 12 sessions. Electroporation enhanced exosome penetration, avoiding the need for invasive injections | |||||
| Adverse events & safety: No serious adverse effects reported |
Five clinical trials were registered on ClinicalTrials.gov. They are summarized in Table 5. No clinical trials were found on Clinical Trials Registry - India and Chinese Clinical Trial Register.
| Study identifier | Title of the study | Source of exosomes | Study phase; estimated enrollment (n) | Primary outcome measure(s) | Recruitment status | Study location(s) |
| NCT06571799 | Study evaluating the efficacy and safety of BENEV exosome regenerative complex+ for self-perceived thinning hair | Adipose tissue stem cell-derived exosomes | Not applicable; n = 30 | Change in terminal hair counts, change in vellus hair counts, change in total hair counts (time frame: Baseline to day 120 or end of study visit) | Completed | United States |
| NCT05658094 | Exosome effect on prevention of hair loss | Human amniotic mesenchymal stem cells-derived exosomes | Not applicable; n = 20 | Change in mean total hair density (hair/cm2) (time frame: 0, 3 and 6 months), visual assessment before and after (time frame: 6 months) | Unknown | Iran |
| NCT06482541 | Efficacy and safety of AGE ZERO™ exosomes to treat men and women with androgenetic alopecia | Wharton’s jelly mesenchymal stem cell-derived exosome | Phase I; n = 100 | Hair count, hair thickness, hair color (time frame: Evaluated on monthly visits for a year) | Not yet recruiting | United States |
| NCT06539273 | Exosome treatment in androgenetic alopecia | Foreskin-derived mesenchymal stromal cells derived exosome | Phase III; n = 30 | Hair density (time frame: 4 and 12 weeks) | Completed | Turkey |
| NCT06697080 | Umbilical cord-derived mesenchymal stem cell exosomes on hair growth in patients with androgenetic alopecia | Umbilical cord-derived mesenchymal stem cell exosomes | Not applicable; n = 50 | Hair diameters changed over time (time frame: 0 days, 1 month, 3 months, 6 months) | Active, not recruiting | China |
The current study investigated the therapeutic potential of exosomes for hair regeneration. All in vitro, pre-clinical and clinical studies using exosomes for hair regeneration were included. A total of 27 studies (three in vitro, three pre-clinical, eighteen with both in vitro and pre-clinical component and three clinical) fulfilled the scope of our manuscript. Five on-going clinical trials, registered on ClinicalTrials.gov, were also identified.
Exosomes demonstrate potent immunomodulatory capabilities essential for treating inflammatory hair loss conditions such as alopecia areata. Zöller et al[39] found that myeloid-derived suppressor cell-derived exosomes significantly promoted T regulatory cell expansion, decreased pro-inflammatory cytokines [interleukin (IL)-1β, IL-6)], and enhanced anti-inflammatory cytokines (IL-10), effectively restoring immune tolerance. Similarly, Tang et al[40] showed that baricitinib-loaded extracellular vesicles effectively suppressed Janus kinase-signal transducer and activator of the transcription-mediated inflammation, reducing cytokines, including interferon-γ and IL-15, which are critical mediators of inflammation in alopecia. Collectively, these findings underscore the therapeutic potential of exosomes in resolving follicular inflammation by recalibrating immune responses and restoring a conducive microenvironment for follicular regeneration[33,35,41,42].
Optimizing delivery strategies is crucial for translating exosome therapies into clinical applications. Chen et al[43] demonstrated that encapsulating dermal papilla cell-derived exosomes within injectable hydrogels significantly enhanced follicular retention, provided sustained release, and extended the anagen phase. Similarly, Chu et al[44] reported improved therapeutic outcomes by integrating engineered adipose-derived stem cell nanovesicles into thermosensitive hydrogels, enhancing follicular targeting and stability. Wu et al[45] further confirmed that hybrid vesicles encapsulating exosomes, finasteride, and gold nanoparticles considerably improved follicular delivery, bioavailability, and efficacy compared to conventional solutions. These advanced delivery approaches illustrate the importance of developing targeted, stable, and biocompatible exosome formulations to maximize clinical effectiveness.
Cellular origin critically impacts exosome function and therapeutic potential in hair regeneration. Mao et al[46] utilized exosomes from umbilical cord mesenchymal stem cells, showing robust promotion of keratinocyte proliferation and reduced apoptosis, translating into significantly improved hair regeneration. Similarly, Zhang et al[47] demonstrated that dermal papilla cell-derived exosomes effectively restored hair growth by modulating the perifollicular environment. Rajendran et al[37] showed that fibroblast-derived engineered nanovesicles significantly induced hair growth ex vivo, while Liu et al[48] reported human dermal sheath cup cell-derived exosomes notably reversed dermal papilla cell senescence, underscoring varied cellular sources’ regenerative potential in hair regrowth therapy.
Exosomal cargo, particularly miRNAs, critically regulates hair regeneration via precise genetic modulation. Liang et al[49] reported that adipose mesenchymal stromal cell-derived exosomes enriched with miR-122-5p antagonized dihydrotestosterone-induced hair follicle suppression by targeting transforming growth factor (TGF)-β1/SMAD3 signaling. Li et al[50] found that decorin-modified dermal papilla cell-derived exosomes enhanced hair follicle proliferation through miR-129-2-3p mediated downregulation of SMAD3 expression. Chu et al[44] illustrated that the exosome-derived long non-coding RNA AC010789.1 promoted hair growth by activating S100A8/Wnt/β-catenin signaling. These findings underscore the potent regenerative effects of exosomes, heavily influenced by their unique cellular sources and miRNA content, emphasizing the necessity of source-specific characterization to achieve consistent clinical outcomes.
A significant limitation in the current body of literature - and consequently in this review - is the substantial methodological heterogeneity across relevant studies. Variability exists in several key domains, including exosome isolation techniques, dosing regimens, and outcome measures. This lack of standardization limits the comparability of findings across studies and introduces uncertainty regarding the reproducibility of therapeutic effects. Moreover, inconsistent reporting of exosome characterization data further complicates efforts to draw definitive conclusions. As a result, the generalizability of the preclinical and early clinical findings remains limited, and the translation of these therapies into standardized clinical protocols is hindered.
A crucial challenge in exosome-based hair regeneration therapy is the lack of standardized characterization methods, hindering reproducibility and comparability across studies. Consistency in exosome size, protein markers, cargo profiling, and bioactivity assessment is necessary for clinical translation. Studies by Lee et al[51], Hu et al[38], and Liang et al[49] consistently utilized nanoparticle tracking analysis, electron microscopy, and western blotting for exosomal markers (CD63, tumor susceptibility gene 101, Alix), illustrating the importance of systematic characterization. Nevertheless, variations in isolation techniques and analytical methods across studies underscore the urgent need for universally accepted standardization protocols to ensure reproducible, high-quality exosome preparations capable of consistent therapeutic outcomes.
One of the most important limitations hindering the clinical translation of exosome-based hair regeneration therapies is the lack of standardized methodologies for exosome isolation, characterization, and bioactivity assessment. As noted in our review, while many studies have demonstrated promising regenerative effects of exosomes derived from various cellular sources, the absence of universally accepted criteria for defining and validating exosomal preparations makes it difficult to compare results across studies or establish reproducible protocols for clinical application, especially considering country-specific regulatory protocols.
Exosome isolation methods vary widely across the literature, ranging from ultracentrifugation and size-exclusion chromatography to polymer-based precipitation and microfluidic-based separation[36,52]. Each technique offers distinct advantages and limitations in terms of yield, purity, and scalability. For example, ultracentrifugation remains the most commonly used approach, but it often results in co-isolation of protein aggregates and other vesicular contaminants, leading to decreased homogeneity of exosomal contents[53]. Furthermore, size-exclusion chromatography and immunoaffinity capture offer greater specificity but are less scalable for therapeutic production[54].
Characterization of exosomes similarly lacks standardization. While many studies report using nanoparticle tracking analysis, transmission electron microscopy, and western blotting to identify canonical exosome markers, there is no consensus on the minimal set of criteria required to confirm the identity or integrity of therapeutic exosomes. Moreover, few studies report quantitative measures of cargo content (i.e., specific miRNAs) or functional assays to assess biological activity. As a result, it is very difficult to determine whether the therapeutic effects observed in the studies are due to the exosomal cargo itself or artifacts from the isolation process.
Fortunately, efforts have recently been made to address these gaps. The International Society for Extracellular Vesicles has written and proposed the Minimal Information for Studies of Extracellular Vesicles guidelines, which suggest standardized criteria for the isolation, characterization, and reporting of extracellular vesicle studies. These recommendations encourage and allow researchers to validate particle identity, assess purity, and provide detailed methodological transparency, as they realized the necessity for a standardized exosome processing method[55].
Exosomes modulate several intracellular signaling cascades, of which we found Wnt/β-catenin and TGF-β to be among the most critical and consistent in regulating hair follicle regeneration[35]. The Wnt/β-catenin pathway is a well-established regulator of the anagen (growth) phase in the hair cycle. Activation of this pathway promotes proliferation and differentiation of dermal papilla cells and hair follicle stem cells, both of which are essential for initiating new follicular growth[56]. Studies included in this review have demonstrated that exosomes derived from adipose-derived stem cells, dermal papilla cells, and mesenchymal stromal cells can upregulate β-catenin and its downstream effectors, thereby increasing activation of the follicle and proliferative capability of the relevant matrix cells[44,52,57].
The TGF-β/SMAD pathway, on the other hand, has dual and context-dependent roles. While TGF-β1 is generally associated with catagen induction and follicular regression, exosomes enriched with specific miRNAs inhibit TGF-β1 or its downstream mediator SMAD3, thereby preventing premature follicle apoptosis. By rebalancing this pathway, exosomes may help sustain the anagen phase and promote healthier follicular environments[58,59].
Beyond the Wnt/β-catenin pathway, exosomes modulate multiple signaling cascades crucial for hair follicle regene
Recent research has investigated the use of biomaterials and scaffold systems to enhance the therapeutic potential and application of exosomes in hair regeneration. Chen et al[43] highlighted oxidized sodium alginate-based injectable microgels that significantly prolonged the release and retention of dermal papilla-derived extracellular vesicles, maintaining their biological activity and optimizing their efficacy. Similarly, Xiong et al[61] introduced thermosensitive hydrogels encapsulating JAM-A-overexpressing adipose mesenchymal stromal cell nanovesicles, which increased stability, ensured controlled release, and significantly enhanced dermal papilla cell proliferation and autophagy activation. Wu et al[45] developed hybrid exosomes combined with finasteride-loaded gold nanoparticles within vesicles, substantially improving follicular delivery and bioavailability. These advanced biomaterial-integrated exosome formulations clearly indicate their potential to augment exosome therapeutic efficiency, suggesting crucial implications for enhancing clinical applicability.
Despite promising results, the long-term safety and durability of exosome-based hair regeneration therapies remain insufficiently studied. Lee et al[51] reported that patients treated with adipose-derived stem cell-derived exosomes did not experience any severe adverse effects, though mild scalp tingling was noted. Zhang et al[62] confirmed that there was no systemic toxicity in major organs following exosome administration in animal models, yet long-term biodistribution and potential off-target effects remain unclear. Wu et al[45] demonstrated that encapsulating exosomes in hybrid vesicles improved stability and biocompatibility, reducing degradation risks. While no studies reported immune rejection, rigorous clinical trials are essential to assess prolonged safety, immune responses, and sustained efficacy over time.
While short-term safety data from preclinical and early-phase clinical studies suggest that exosome-based therapies are generally well-tolerated, the long-term risks associated with their use remain insufficiently studied. In our review, we noted that clinical trials have reported minimal adverse effects, such as mild scalp tingling or localized irritation following administration of adipose- or dermal papilla cell-derived exosomes[51]. Additionally, even though animal models have not demonstrated evidence of systemic toxicity or immune rejection, the absence of long-term follow-up and rigorous post-treatment monitoring limits our understanding of potential delayed adverse outcomes[45,62].
One of the primary safety concerns involves the biological complexity and heterogeneity of exosome cargo[63]. Exosomes carry a range of molecules - including miRNAs, proteins, and lipids - which can affect various signaling pathways and can potentially influence cellular behavior[64]. The potential for adverse side effects including oncogenic activation or immune dysregulation cannot be ruled out, particularly with increased frequency and dosage. Furthermore, batch-to-batch variability and inconsistencies in isolation methods may lead to unpredictable therapeutic profiles, as we have previously described[65].
Determining the optimal dosing and treatment regimen for exosome-based hair regeneration remains an unresolved challenge. Studies vary widely in exosome concentrations, frequencies, and delivery methods, making direct comparisons difficult. Zhang et al[62] showed that higher concentrations of dermal papilla-derived exosomes (200 μg/mL) enhanced follicle proliferation, whereas lower doses had limited effects. Lee et al[51] reported that multiple sessions over 24 weeks improved hair density, suggesting that repeated administrations may be necessary for sustained results. However, excessive dosing risks immune activation or unintended signaling disruptions. Establishing standardized dosing guidelines through controlled clinical trials is essential for optimizing efficacy while ensuring safety.
Integrating exosome therapy with complementary treatments may enhance hair regeneration outcomes by targeting multiple biological pathways. Several studies suggest that microneedling, pharmacological agents, and growth factor delivery can amplify exosome efficacy. Wu et al[45] demonstrated that hybrid vesicles co-delivering exosomes and finasteride enhanced follicular penetration and prolonged therapeutic effects compared to exosomes alone. Similarly, Wang et al[60] found that combining exosomes with traditional Chinese medicine (Liao Tuo Fang) improved VEGF expression and hair follicle proliferation. Microneedling, which is known to stimulate dermal papilla activation, could further enhance exosome uptake[35,66]. Future studies should explore optimized combination protocols for maximum therapeutic synergy[67,68].
Despite promising preclinical data, translating exosome therapies into clinical practice remains complex. Key barriers include lack of large-scale human trials, variability in exosome isolation, and uncertain long-term effects. Lee et al[51] highlighted the absence of standardized dosing regimens, while Wu et al[45] emphasized the need for optimized delivery systems to enhance follicular uptake. Additionally, regulatory uncertainties slow clinical adoption. Potential solutions include developing GMP-compliant production methods, conducting multicenter trials, and integrating exosome therapies with existing dermatological treatments. Addressing these challenges is crucial for ensuring safe, scalable, and effective clinical implementation of exosome-based hair regeneration therapies[69-71].
Animal models play a crucial role in exosome research for hair regeneration, yet they often fail to fully replicate human hair follicle biology. Most studies, including those by Wu et al[45] and Zhang et al[47], rely on murine models, which lack human-like follicular cycling and immune responses. While rodent models provide insights into exosome-induced follicular proliferation, they do not account for androgen-driven miniaturization seen in androgenetic alopecia. Large-animal models, such as pigs, may offer better translational relevance. Future research must focus on developing humanized models or organoid-based systems to improve predictive validity for clinical applications.
One of the primary limitations in the current body of literature is the extremely limited number of published clinical studies evaluating exosome-based therapies for hair regeneration. Of the studies included in this review, only three met eligibility criteria and each had small cohorts, varying methodologies, and short-term follow-up. The current evidence reported does not allow for forming strong conclusions regarding safety, efficacy, or long-term outcomes of exosome-based therapies for hair regeneration. This is particularly important as these therapies begin to attract increasing clinical interest and patient demand increases. Without larger, well-designed randomized trials, there remains a risk of premature clinical application based on early-stage data that may not hold up under more rigorous testing. Furthermore, the lack of standardized dosing protocols, manufacturing consistency, and long-term safety data compounds the uncertainty.
Exosome therapy shows some promise for hair regeneration, but there are many limitations that hinder its widespread clinical use. First, the lack of standardized protocols for exosome isolation, purification, and dosage makes treatment outcomes inconsistent. Additionally, the potential for immune reactions or unwanted side effects remains unclear due to limited long-term studies. Scalability and cost are also major concerns, as high-quality exosome production is expensive and complex. Furthermore, regulatory hurdles pose challenges, as exosome-based therapies require rigorous safety and efficacy testing before approval.
A key limitation of this review is the absence of formal statistical analysis or meta-analytic synthesis of the included studies. Due to substantial heterogeneity in study designs, exosome sources, outcome measures, and reporting formats, quantitative comparison or pooling of effect sizes across studies was not feasible. Most studies reported qualitative or semi-quantitative outcomes without standardized metrics, further limiting our ability to perform a rigorous comparative analysis. While we aimed to provide a comprehensive overview of current findings, future research in this field would benefit from greater methodological consistency and standardized reporting, which would enable meaningful statistical evaluation and evidence-based comparison of therapeutic efficacy across different exosome-based interventions. Lastly, while early research is promising, robust clinical trials with large patient populations are needed to confirm its long-term benefits and mechanisms in hair regeneration[71].
Exosome-based therapies hold immense promise for the treatment of hair loss by leveraging their ability to modulate key signaling pathways and enhance hair follicle regeneration. While pre-clinical studies demonstrate consistent efficacy across diverse exosome sources, methodological heterogeneity and the limited amount of clinical data highlight the need for further research. Addressing these challenges through standardized protocols, early-phase, adequately powered, randomized clinical trials with longer follow-up, and combinatorial approaches will be critical in realizing the full potential of exosome therapies in clinical practice.
| 1. | National Institute of Arthritis and Musculoskeletal and Skin Diseases. Alopecia Areata. [cited 28 December 2024]. Available from: https://www.niams.nih.gov/health-topics/alopecia-areata. |
| 2. | Asfour L, Cranwell W, Sinclair R. Male Androgenetic Alopecia. 2023 Jan 25. In: Feingold KR, Ahmed SF, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Muzumdar R, Purnell J, Rey R, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. [PubMed] |
| 3. | Dhami L. Psychology of Hair Loss Patients and Importance of Counseling. Indian J Plast Surg. 2021;54:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004;123:455-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Mostaghimi A, Xenakis J, Meche A, Smith TW, Gruben D, Sikirica V. Economic Burden and Healthcare Resource Use of Alopecia Areata in an Insured Population in the USA. Dermatol Ther (Heidelb). 2022;12:1027-1040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 6. | Muntyanu A, Gabrielli S, Donovan J, Gooderham M, Guenther L, Hanna S, Lynde C, Prajapati VH, Wiseman M, Netchiporouk E. The burden of alopecia areata: A scoping review focusing on quality of life, mental health and work productivity. J Eur Acad Dermatol Venereol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 7. | Thompson AR, Tziotzios C, Nesnas J, Randall R, Czachorowski M, Messenger AG. Lifetime incidence and healthcare disparities in alopecia areata: a UK population-based cohort study. Br J Dermatol. 2024;191:924-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Phillips TG, Slomiany WP, Allison R. Hair Loss: Common Causes and Treatment. Am Fam Physician. 2017;96:371-378. [PubMed] |
| 9. | Ho CH, Sood T, Zito PM. Androgenetic Alopecia. 2024 Jan 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 10. | Pratt CH, King LE Jr, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:17011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 467] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 11. | Hughes EC, Syed HA, Saleh D. Telogen Effluvium. 2024 May 1. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 12. | Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: An update. Pediatr Dermatol. 2022;39:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 13. | Haskin A, Kwatra SG, Aguh C. Breaking the cycle of hair breakage: pearls for the management of acquired trichorrhexis nodosa. J Dermatolog Treat. 2017;28:322-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Aukerman EL, Jafferany M. The psychological consequences of androgenetic alopecia: A systematic review. J Cosmet Dermatol. 2023;22:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 15. | Randolph M, Tosti A. Oral minoxidil treatment for hair loss: A review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 16. | Gupta AK, Venkataraman M, Talukder M, Bamimore MA. Finasteride for hair loss: a review. J Dermatolog Treat. 2022;33:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Patel P, Nessel TA, Kumar D D. Minoxidil. 2023 Feb 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 18. | Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 367] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 19. | Zito PM, Bistas KG, Patel P, Syed K. Finasteride. 2024 Feb 28. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 20. | McClellan KJ, Markham A. Finasteride: a review of its use in male pattern hair loss. Drugs. 1999;57:111-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Kim J, Kim SR, Choi YH, Shin JY, Kim CD, Kang NG, Park BC, Lee S. Quercitrin Stimulates Hair Growth with Enhanced Expression of Growth Factors via Activation of MAPK/CREB Signaling Pathway. Molecules. 2020;25:4004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Takahashi T, Kamiya T, Yokoo Y. Proanthocyanidins from grape seeds promote proliferation of mouse hair follicle cells in vitro and convert hair cycle in vivo. Acta Derm Venereol. 1998;78:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Takahashi T, Kamiya T, Hasegawa A, Yokoo Y. Procyanidin oligomers selectively and intensively promote proliferation of mouse hair epithelial cells in vitro and activate hair follicle growth in vivo. J Invest Dermatol. 1999;112:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kim MH, Kim SH, Yang WM. Beneficial effects of Astragaloside IV for hair loss via inhibition of Fas/Fas L-mediated apoptotic signaling. PLoS One. 2014;9:e92984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Pothula SR, Jayanth BS. Hair Transplantation. In: Oral and Maxillofacial Surgery for the Clinician. Singapore: Springer, 2021. |
| 26. | Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021;20:3759-3781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 27. | Fertig RM, Gamret AC, Cervantes J, Tosti A. Microneedling for the treatment of hair loss? J Eur Acad Dermatol Venereol. 2018;32:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 28. | Lueangarun S, Visutjindaporn P, Parcharoen Y, Jamparuang P, Tempark T. A Systematic Review and Meta-analysis of Randomized Controlled Trials of United States Food and Drug Administration-Approved, Home-use, Low-Level Light/Laser Therapy Devices for Pattern Hair Loss: Device Design and Technology. J Clin Aesthet Dermatol. 2021;14:E64-E75. [PubMed] |
| 29. | Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl Med. 2015;4:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 30. | York K, Meah N, Bhoyrul B, Sinclair R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. 2020;21:603-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 31. | English RS Jr, Ruiz S, DoAmaral P. Microneedling and Its Use in Hair Loss Disorders: A Systematic Review. Dermatol Ther (Heidelb). 2022;12:41-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 32. | Sun T, Li M, Liu Q, Yu A, Cheng K, Ma J, Murphy S, McNutt PM, Zhang Y. Insights into optimizing exosome therapies for acute skin wound healing and other tissue repair. Front Med. 2024;18:258-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Norouzi F, Aghajani S, Vosoughi N, Sharif S, Ghahremanzadeh K, Mokhtari Z, Verdi J. Exosomes derived stem cells as a modern therapeutic approach for skin rejuvenation and hair regrowth. Regen Ther. 2024;26:1124-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Nail HM, Chiu CC, Leung CH, Ahmed MMM, Wang HD. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J Biomed Sci. 2023;30:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 35. | Cheng M, Ma C, Chen HD, Wu Y, Xu XG. The Roles of Exosomes in Regulating Hair Follicle Growth. Clin Cosmet Investig Dermatol. 2024;17:1603-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Zhou Y, Seo J, Tu S, Nanmo A, Kageyama T, Fukuda J. Exosomes for hair growth and regeneration. J Biosci Bioeng. 2024;137:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 37. | Rajendran RL, Gangadaran P, Bak SS, Oh JM, Kalimuthu S, Lee HW, Baek SH, Zhu L, Sung YK, Jeong SY, Lee SW, Lee J, Ahn BC. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017;7:15560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 38. | Hu S, Li Z, Lutz H, Huang K, Su T, Cores J, Dinh PC, Cheng K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:eaba1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 39. | Zöller M, Zhao K, Kutlu N, Bauer N, Provaznik J, Hackert T, Schnölzer M. Immunoregulatory Effects of Myeloid-Derived Suppressor Cell Exosomes in Mouse Model of Autoimmune Alopecia Areata. Front Immunol. 2018;9:1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Tang H, Wang F, Yang R, Zhao Z, Zhang Y, Yang L, Li B. Baricitinib-loaded EVs promote alopecia areata mouse hair regrowth by reducing JAK-STAT-mediated inflammation and promoting hair follicle regeneration. Drug Discov Ther. 2025;18:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Li G, Zhang S, Zou Y, Ai H, Zheng X, Qian K, Lei C, Fu W. The therapeutic potential of exosomes in immunotherapy. Front Immunol. 2024;15:1424081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 42. | Saleem M, Shahzad KA, Marryum M, Singh S, Zhou Q, Du S, Wang S, Shao C, Shaikh II. Exosome-based therapies for inflammatory disorders: a review of recent advances. Stem Cell Res Ther. 2024;15:477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 43. | Chen Y, Huang J, Chen R, Yang L, Wang J, Liu B, Du L, Yi Y, Jia J, Xu Y, Chen Q, Ngondi DG, Miao Y, Hu Z. Sustained release of dermal papilla-derived extracellular vesicles from injectable microgel promotes hair growth. Theranostics. 2020;10:1454-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 44. | Chu S, Jia L, Li Y, Xiong J, Sun Y, Zhou Q, Du D, Li Z, Huang X, Jiang H, Wu B, Li Y. Exosome-derived long non-coding RNA AC010789.1 modified by FTO and hnRNPA2B1 accelerates growth of hair follicle stem cells against androgen alopecia by activating S100A8/Wnt/β-catenin signalling. Clin Transl Med. 2025;15:e70152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Wu X, Huang X, Zhu Q, Zhang J, Hu J, Song Y, You Y, Zhu L, Lu J, Xu X, Chen M, Wang W, Song X, Ji J, Du Y. Hybrid hair follicle stem cell extracellular vesicles co-delivering finasteride and gold nanoparticles for androgenetic alopecia treatment. J Control Release. 2024;373:652-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 46. | Mao Y, Liu P, Wei J, Xie Y, Zheng Q, Hu X, Yao J, Meng W. Exosomes derived from Umbilical cord mesenchymal stem cell promote hair regrowth in C57BL6 mice through upregulation of the RAS/ERK signaling pathway. J Transl Int Med. 2024;12:478-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 47. | Zhang H, Zhu NX, Huang K, Cai BZ, Zeng Y, Xu YM, Liu Y, Yuan YP, Lin CM. iTRAQ-Based Quantitative Proteomic Comparison of Early- and Late-Passage Human Dermal Papilla Cell Secretome in Relation to Inducing Hair Follicle Regeneration. PLoS One. 2016;11:e0167474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Liu F, Liu S, Luo X, Fan Z, Huang S, Deng F, Liu H, Shi G. Combatting ageing in dermal papilla cells and promoting hair follicle regeneration using exosomes from human hair follicle dermal sheath cup cells. Exp Dermatol. 2024;33:e14948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 49. | Liang Y, Tang X, Zhang X, Cao C, Yu M, Wan M. Adipose Mesenchymal Stromal Cell-Derived Exosomes Carrying MiR-122-5p Antagonize the Inhibitory Effect of Dihydrotestosterone on Hair Follicles by Targeting the TGF-β1/SMAD3 Signaling Pathway. Int J Mol Sci. 2023;24:5703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 50. | Li J, Zhao B, Yu Y, Bao Z, Zheng F, Cai J, Chen Y, Wu X. Decorin-mediated dermal papilla cell-derived exosomes regulate hair follicle growth and development through miR-129-2-3p/SMAD3/TGF-β axis. Int J Biol Macromol. 2025;295:139292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Lee E, Choi MS, Cho BS, Won YJ, Lee JH, Kim SR, Kim MH, Jeon JH, Park GH, Kwon HH, Lee J, Park KY, Park BC. The efficacy of adipose stem cell-derived exosomes in hair regeneration based on a preclinical and clinical study. Int J Dermatol. 2024;63:1212-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 52. | Shang Y, Li M, Zhang L, Han C, Shen K, Wang K, Li Y, Zhang Y, Luo L, Jia Y, Guo K, Cai W, Zhang J, Wang X, Wang H, Hu D. Exosomes derived from mouse vibrissa dermal papilla cells promote hair follicle regeneration during wound healing by activating Wnt/β-catenin signaling pathway. J Nanobiotechnology. 2024;22:425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 53. | Chen Y, Qi W, Wang Z, Niu F. Exosome Source Matters: A Comprehensive Review from the Perspective of Diverse Cellular Origins. Pharmaceutics. 2025;17:147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Lei L, Zhou S, Zeng L, Gu Q, Xue H, Wang F, Feng J, Cui S, Shi L. Exosome-Based Therapeutics in Dermatology. Biomater Res. 2025;29:0148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 55. | Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku'ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ; MISEV Consortium, Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1603] [Cited by in RCA: 1392] [Article Influence: 1392.0] [Reference Citation Analysis (0)] |
| 56. | Shin DW. The Molecular Mechanism of Natural Products Activating Wnt/β-Catenin Signaling Pathway for Improving Hair Loss. Life (Basel). 2022;12:1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 57. | Jiao Y, Sun QM, Shen YC, Li QS, Piao YJ, Gong L. Stimulation of mouse hair regrowth by exosomes derived from human umbilical cord mesenchymal stem cells. Acta Histochem. 2024;126:152184. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Soma T, Tsuji Y, Hibino T. Involvement of transforming growth factor-beta2 in catagen induction during the human hair cycle. J Invest Dermatol. 2002;118:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol. 2016;8:a021873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 972] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 60. | Wang G, Wang Z, Zhang J, Shen Y, Hou X, Su L, Chen W, Chen J, Guo X, Song H. Treatment of androgenetic alopecia by exosomes secreted from hair papilla cells and the intervention effect of LTF. J Cosmet Dermatol. 2023;22:2996-3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 61. | Xiong J, Liu Z, Jia L, Sun Y, Guo R, Xi T, Li Z, Wu M, Jiang H, Li Y. Bioinspired engineering ADSC nanovesicles thermosensitive hydrogel enhance autophagy of dermal papilla cells for androgenetic alopecia treatment. Bioact Mater. 2024;36:112-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 62. | Zhang H, Yao J, Jiang Q, Shi Y, Ge W, Xu X. Engineered Exosomes Biopotentiated Hydrogel Promote Hair Follicle Growth via Reprogramming the Perifollicular Microenvironment. Pharmaceutics. 2024;16:935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Lee HK, Lim SH, Chung IS, Park Y, Park MJ, Kim JY, Kim YG, Hong JT, Kim Y, Han SB. Preclinical efficacy and mechanisms of mesenchymal stem cells in animal models of autoimmune diseases. Immune Netw. 2014;14:81-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 64. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6565] [Article Influence: 1313.0] [Reference Citation Analysis (0)] |
| 65. | Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2014;184:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 297] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 66. | Sreeraj H, Anukiruthika R, Tamilselvi KS, Subha D. Exosomes for skin treatment: Therapeutic and cosmetic applications. Nano TransMed. 2024;3:100048. [DOI] [Full Text] |
| 67. | Jaiswal S, Jawade S. Microneedling in Dermatology: A Comprehensive Review of Applications, Techniques, and Outcomes. Cureus. 2024;16:e70033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Guermazi D, Khan S, Shah A, Saliba E. Tiny messengers, big results: A review of exosome-mediated treatments and considerations in dermatology. Gene Protein Dis. 2024;3:3230. [DOI] [Full Text] |
| 69. | Chen YS, Lin EY, Chiou TW, Harn HJ. Exosomes in clinical trial and their production in compliance with good manufacturing practice. Tzu Chi Med J. 2020;32:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 70. | Sharma A, Yadav A, Nandy A, Ghatak S. Insight into the Functional Dynamics and Challenges of Exosomes in Pharmaceutical Innovation and Precision Medicine. Pharmaceutics. 2024;16:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 71. | Buontempo MG, Alhanshali L, Ide M, Shapiro J, Lo Sicco K. Examining the Uncertainties Surrounding Exosome Therapy in Androgenetic Alopecia: A Call for Evidence-Based Practice. J Drugs Dermatol. 2024;23:e86-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 72. | Kazi T, Nagata A, Nakagawa T, Matsuzaki T, Inui S. Dermal Papilla Cell-Derived Extracellular Vesicles Increase Hair Inductive Gene Expression in Adipose Stem Cells via β-Catenin Activation. Cells. 2022;11:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Nilforoushzadeh MA, Aghdami N, Taghiabadi E. Human Hair Outer Root Sheath Cells and Platelet-Lysis Exosomes Promote Hair Inductivity of Dermal Papilla Cell. Tissue Eng Regen Med. 2020;17:525-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Shieh JS, Chin YT, Chiu HC, Hsieh YY, Cheng HR, Gu H, Chang FW. Bio-Pulsed Stimulation Effectively Improves the Production of Avian Mesenchymal Stem Cell-Derived Extracellular Vesicles That Enhance the Bioactivity of Skin Fibroblasts and Hair Follicle Cells. Int J Mol Sci. 2022;23:15010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 75. | Rosalina AI, Iskandarsyah, Sagita E. Placenta extract-loaded novasome significantly improved hair growth in a rat in vivo model. Int J App Pharm. 2023;15:138-145. [DOI] [Full Text] |
| 76. | Kwak S, Song CL, Lee J, Kim S, Nam S, Park YJ, Lee J. Development of pluripotent stem cell-derived epidermal organoids that generate effective extracellular vesicles in skin regeneration. Biomaterials. 2024;307:122522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 77. | Oh HG, Jung M, Jeong SY, Kim J, Han SD, Kim H, Lee S, Lee Y, You H, Park S, Kim EA, Kim TM, Kim S. Improvement of androgenic alopecia by extracellular vesicles secreted from hyaluronic acid-stimulated induced mesenchymal stem cells. Stem Cell Res Ther. 2024;15:287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 78. | Hu S, Zhang J, Ji Q, Xie S, Jiang J, Ni H, He X, Yang Y, Wu M. Exosomes derived from uMSCs promote hair regrowth in alopecia areata through accelerating human hair follicular keratinocyte proliferation and migration. Cell Biol Int. 2024;48:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 79. | Kim H, Jang Y, Kim EH, Jang H, Cho H, Han G, Song HK, Kim SH, Yang Y. Potential of Colostrum-Derived Exosomes for Promoting Hair Regeneration Through the Transition From Telogen to Anagen Phase. Front Cell Dev Biol. 2022;10:815205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 80. | Wu J, Yang Q, Wu S, Yuan R, Zhao X, Li Y, Wu W, Zhu N. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng Regen Med. 2021;18:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 81. | Lueangarun S, Cho BS, Tempark T. Rose stem cell-derived exosomes for hair regeneration enhancement via noninvasive electroporation in androgenetic alopecia. J Cosmet Dermatol. 2024;23:3791-3794. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |