Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.107202
Revised: April 3, 2025
Accepted: June 4, 2025
Published online: July 26, 2025
Processing time: 128 Days and 19.9 Hours
Autoimmune diseases are complex clinical conditions that present significant therapeutic challenges due to their intricate immunological mechanisms. Conventional treatment strategies, such as immunosuppressive drugs and anti-inflammatory therapies, often demonstrate limited efficacy and are associated with considerable side effects. Recently, mesenchymal stem cells (MSCs) have attracted growing interest as a promising therapeutic approach, owing to their immuno
Core Tip: Autoimmune diseases result from dysregulated immune responses directed against self-antigens, leading to chronic inflammation and tissue damage. Mesenchymal stem cells (MSCs) and MSC-derived exosomes have demonstrated significant potential as therapeutic agents due to their strong immunomodulatory and regenerative properties. MSC-derived exosomes modulate immune responses by regulating cytokine secretion, suppressing inflammatory cell activity, and promo
- Citation: Sengul Bag F, Bag OF. Molecular and therapeutic effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. World J Stem Cells 2025; 17(7): 107202
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/107202.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.107202
Autoimmune diseases are a group of disorders resulting from an abnormal immune response against the body’s own tissues. These conditions are particularly common in females, affect all age groups, and are typically characterized by the production of autoantibodies and the dysregulation of B cell and T cell activity[1,2]. Their pathophysiology is complex, involving interactions between genetic, environmental, and immunological factors[3]. Clinically, autoimmune diseases may be organ-specific or systemic and can present as inflammatory, neurological, gastrointestinal, or systemic conditions such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), type 1 diabetes, multiple sclerosis (MS), Behçet’s disease (BD), psoriasis, and irritable bowel syndrome[4,5]. This overview specifically focused on RA, SLE, MS, and BD.
The molecular mechanisms underlying autoimmune diseases include the loss of autoantigen tolerance, cytokine dysregulation, molecular mimicry, and epitope spreading. The breakdown in tolerance of B and T cells to autoantigens leads to the production of autoantibodies, while specific variants of the human leukocyte antigen (HLA) genes are key in presenting these autoantigens. Elevated levels of proinflammatory cytokines and deficient anti-inflammatory cytokines contribute to chronic inflammation. Additionally, structural similarities between autoantigens and infectious agents can mislead the immune system into attacking healthy tissue, a phenomenon known as molecular mimicry. Epitope spreading refers to the expansion of the immune response from a single antigen to multiple autoantigens over time, further exacerbating disease progression. These processes are fundamental to the development and progression of autoimmune diseases[3].
Various therapeutic strategies aim to suppress abnormal immune responses and alleviate symptoms. Traditional treatments include corticosteroids and immunosuppressive drugs, but in recent years biological agents and targeted therapies have also been developed. In particular, tumor necrosis factor (TNF) inhibitors, therapies that target B cells, and agents that modulate cytokine signaling pathways have made significant advances in the treatment of autoimmune disease treatment. Regardless of all the advances, the treatment of autoimmune diseases still faces challenges, and a better understanding of the pathogenesis of these diseases requires the development of new and more effective therapeutic approaches. In this context mesenchymal stem cells (MSCs) are emerging as one of the most promising cellular therapies in the treatment of autoimmune diseases due to their immunomodulatory and regenerative properties[6].
MSCs were first isolated from bone marrow (BM) in the 1960s and 1970s and are defined as non-hematopoietic, multipotent cells capable of self-renewal and differentiation into various lineages[7]. They are present in multiple tissues, including BM, placenta, adipose tissue (AD), Wharton’s jelly, umbilical cord (UC), and teeth[8]. According to the International Society for Cellular Therapy, MSCs must adhere to plastic surfaces, express specific surface markers (positive for CD73, CD90, and CD105; negative for CD45, CD34, CD14, CD11b, CD79a, CD19, and HLA-DR), and differentiate into mesodermal cell types under in vitro conditions[9]. Beyond their differentiation potential, MSCs have immunomodulatory properties, enabling them to modulate both innate and adaptive immune responses[10]. Research has shown that MSCs can suppress the activation, proliferation, and differentiation of natural killer (NK) cells, dendritic cells (DCs), macrophages, and B and T lymphocytes[11]. Their therapeutic effects are largely mediated by paracrine factors and exosomes, small extracellular vesicles that regulate inflammation, reprogram immune cells, and support tissue repair via their rich cargo of immunoregulatory molecules[12]. MSC-derived exosomes (MSC-Exos) offer advantages over whole-cell MSC therapies, including lower immunogenicity, the ability to cross the blood-brain barrier, no risk of unwanted differentiation, and the capacity to regulate inflammation through multiple mechanisms[13,14].
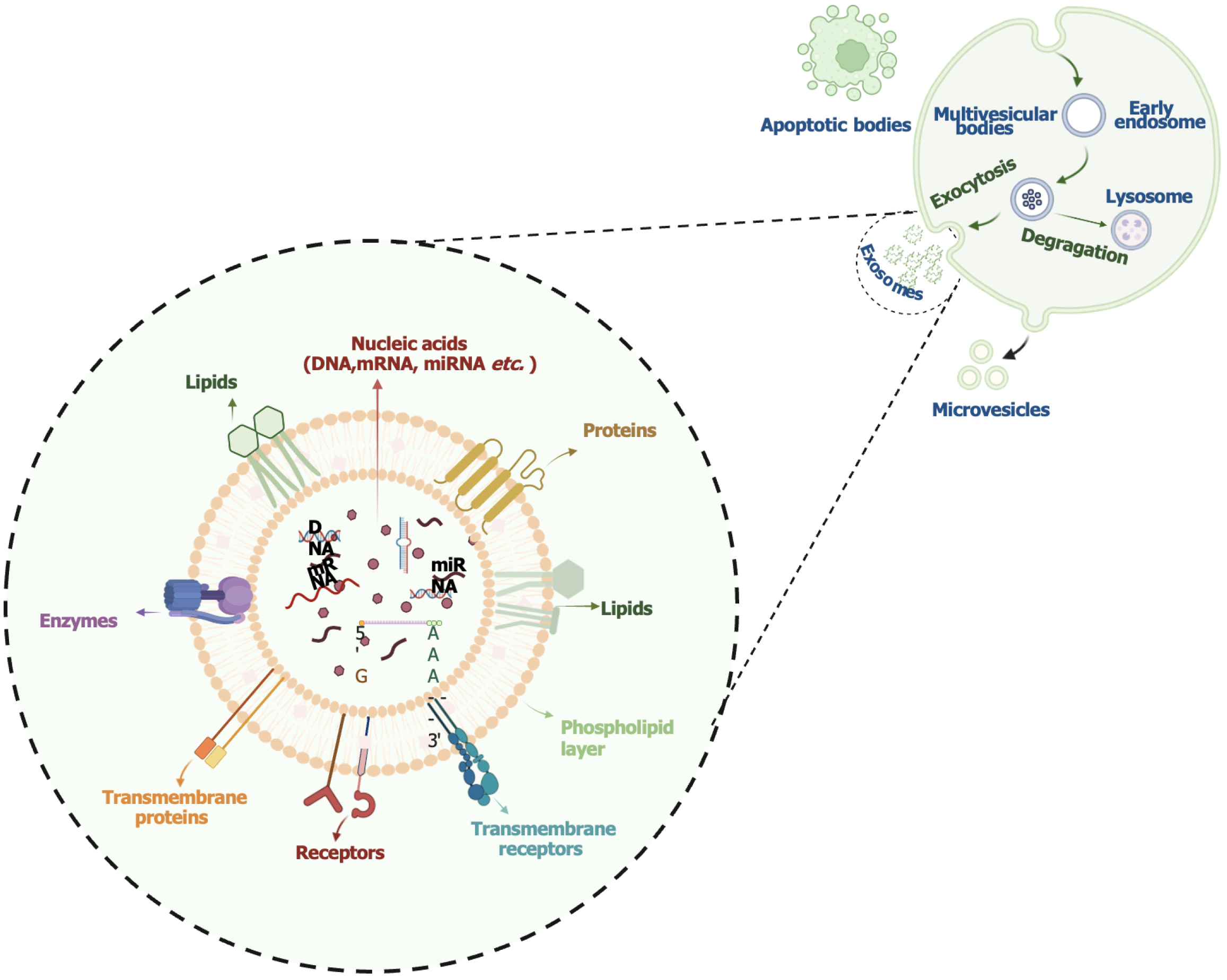
Extracellular vesicles are classified based on their biogenesis and size into exosomes, microvesicles, and apoptotic bodies (Figure 1)[15]. Exosomes (about 30-150 nm) originate from endosomes and are released when multivesicular bodies fuse with the plasma membrane[16]. They are formed via both the endosomal sorting complex required for transport-dependent pathway and the endosomal sorting complex required for transport-independent pathway. Initially thought to be cellular waste products, exosomes are now known to play essential roles in intercellular communication and immune regulation[17,18].
Exosomes are secreted by both hematopoietic (e.g., reticulocytes, B and T lymphocytes, platelets, mast cells, DCs, macrophages) and non-hematopoietic cells (e.g., epithelial cells, Schwann cells, astrocytes, neurons, melanocytes, mesothelial cells, adipocytes, fibroblasts, and tumor cells)[19]. They are present in biological fluids such as plasma, urine, cerebrospinal fluid, saliva, and breast milk. Their cargo [proteins, lipids, mRNAs, microRNAs (miRNAs), and DNA] reflects the physiological state of the parent cell and contributes to immune regulation, metabolism, and pathological processes (Figure 1)[20]. Exosome isolation methods include centrifugation, chromatography, polymer-based precipitation, and immunological techniques. Characterization is typically performed using nanoparticle tracking analysis, flow cytometry, electron microscopy, and western blotting[21,22]. Recent advances in high-throughput analysis of exosome content have led to the creation of dedicated databases such as ExoCarta, Vesiclepedia, and EVpedia[23-25].
The immunosuppressive effect of MSCs is one of the key mechanisms underlying their immunotherapeutic potential. MSCs secrete various immunomodulatory factors to suppress the immune response and prevent autoimmune attacks in cases of excessive inflammation. These cells regulate the inflammatory response by secreting immunosuppressive mediators such as prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), nitric oxide, transforming growth factor-beta (TGF-β), hepatocyte growth factor, interleukin-10 (IL-10), CD39, CD73, and programmed cell death ligands (PD-L1, PD-L2). The immunomodulatory effects of MSCs on immune cells are mediated through direct cell-to-cell interactions and the suppression of proinflammatory cytokine production via extracellular vesicles.
Notably, MSCs possess the ability to recognize danger signals and modulate immune responses through toll-like receptors (TLRs), enhancing their therapeutic potential[26]. MSC-derived extracellular vesicles contain various miRNAs, proteins, and lipids that regulate the immune response and exert anti-inflammatory effects. While miRNAs such as let-7b, miR-146a, miR-181c and miR-122 reduce inflammation by inhibiting the polarization of proinflammatory macrophage (M1), miR-21, miR-146a, miR-182 and miR-223 modulate the immune response by regulating TLR signaling pathways[27-29]. While miR-125a and miR-99b regulate TLR-4 signaling, miR-182 can affect MSC differentiation by decreasing TLR-4 expression[30,31]. In addition, miR-17 inhibits the release of proinflammatory mediators by suppressing the activation of the NOD-like receptor protein-3 inflammasome[32].
MSC-derived extracellular vesicles reduce IL-1β production by suppressing the activation of nuclear factor kappa B (NF-κB) with their protein contents such as TGF-β and PGE2, and they calm inflammation[33,34]. These vesicles suppress the activation of T and B cells by acting directly on immune cell activity, support tissue regeneration by increasing the proportion of anti-inflammatory macrophage (M2), and reduce mast cell activity[35,36]. While M1 macrophages secrete proinflammatory cytokines such as TNF-α and IL-1β, M2 macrophages produce immunosuppressive factors such as IL-10. MSC-Exos increase the expression of IL-10 and simultaneously decrease the levels of vascular endothelial growth factor (VEGF)-A, interferon-gamma (IFN-γ), IL-12, and TNF-α by promoting the conversion of M1 to M2[6].
Vasandan et al[37] showed that human MSCs modulate the immune response by modulating the metabolic phenotype of macrophages through PGE2. PGE2 secreted by MSCs promotes the conversion of macrophages from a proinflammatory (M1) to an anti-inflammatory (M2) phenotype by promoting the transition from glycolysis to oxidative phos
Moreover, MSC-derived extracellular vesicles carry anti-inflammatory proteins such as TNF-stimulated gene 6 and cyclooxygenase-2, inhibit the migration of polymorphonuclear granulocytes into tissues, and regulate the inflammatory immune response[38,39]. The C-C motif chemokine receptor 2 in their membrane structure inhibits inflammatory macrophage functions by interacting with monocyte chemoattractant protein 1[27,40].
MSC-Exos suppress the proliferation and differentiation of B cells and the secretion of immunoglobulins in a dose-dependent manner. CD19+/CD86+ inhibit activated B cells and promote IL-10-producing regulatory B cells. They also decrease B cell activity by inhibiting the phosphatidylinositol 3-kinase/protein kinase B signaling pathway via miR-155-5p and increasing the expression of genes involved in immune regulation[6].
One of the mechanisms of MSC-Exos is to prevent the progression of autoimmune diseases by suppressing the proliferation and activation of T cells and restoring immune homeostasis. MSC-Exos, which carry immunosuppressive molecules such as TGF-β, IDO protein, and miR-125a-3p, regulate the T helper 1/2 (Th1/Th2) balance, promote regu
In experimental autoimmune encephalomyelitis (EAE) and colitis models, MSC-Exos have been shown to suppress CD4+ T cell proliferation, decrease IL-17 and IFN-γ production, and stabilize inflammation by increasing TGF-β and IL-10 levels. In addition, it indirectly supports Treg cell formation by directing monocytes into the M2 macrophage phenotype and suppresses autoimmune responses by increasing immune tolerance[6,41].
The ability of MSC-Exos to regulate the immune response is also mediated by NF-κB-mediated, MyD88-dependent mechanisms. These exosomes suppress proinflammatory cytokine levels while increasing the expression of IL-10 and TGF-β1. Monocytes interacting with exosomes contribute to immune tolerance by directing CD4+ T cells to the Treg phenotype. In animal models, MSC-Exos have been shown to prolong the survival of allogeneic skin grafts and regulate the immune response[42]. These mechanisms explain the immunosuppressive effect of MSC-Exos and reveal their therapeutic potential in autoimmune diseases and inflammatory processes.
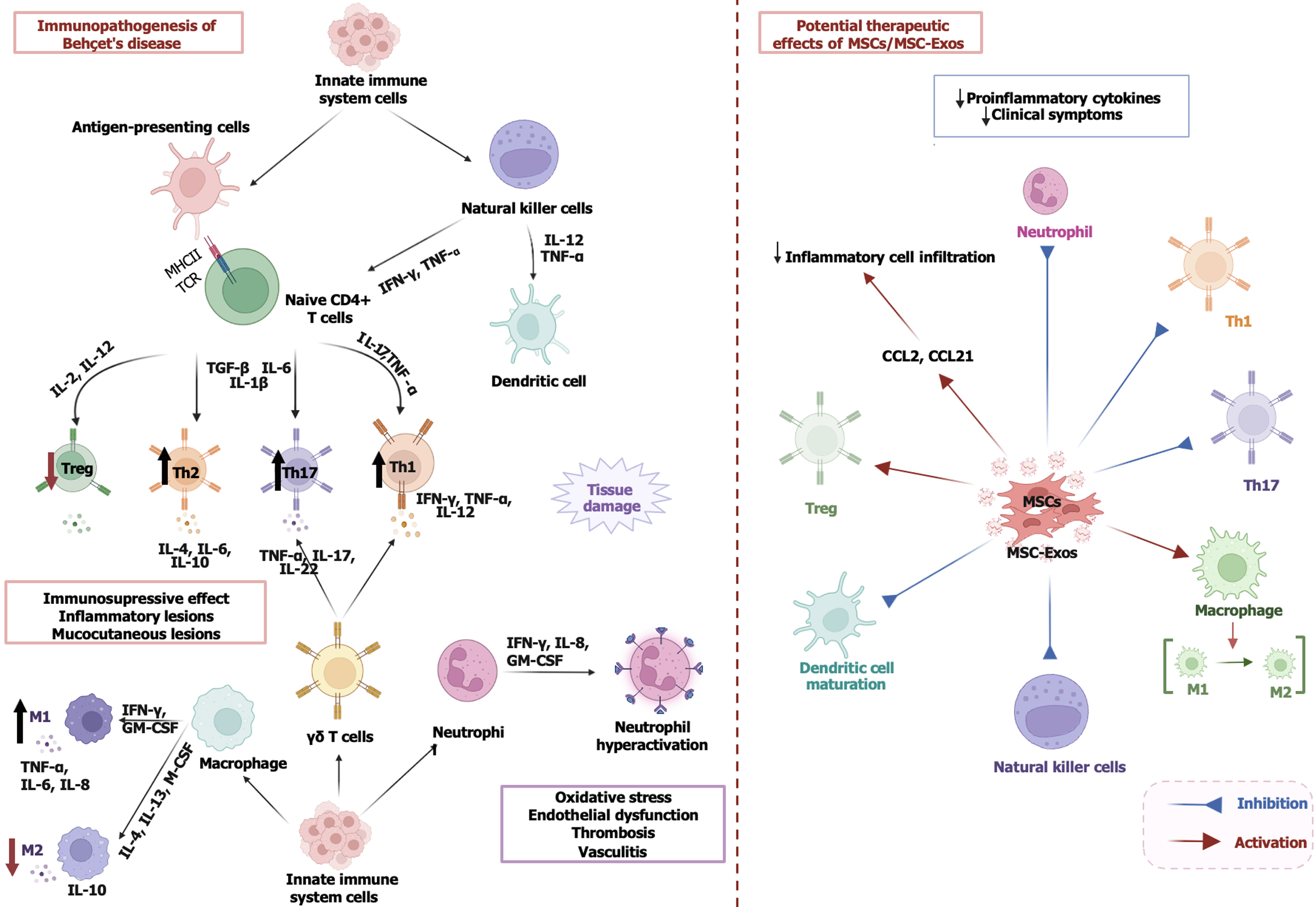
BD is a relapsing autoinflammatory disorder with a broad clinical spectrum that includes oral and genital ulceration, skin lesions, uveitis, and vascular involvement[5]. Although the etiopathogenesis of the disease is not yet fully understood, it is believed that HLA-B51-related genetic predisposition, epigenetic alterations, environmental factors, and infectious agents trigger the immune system and lead to an increase in proinflammatory cytokines[43,44]. The disruption of the Th1/Th2 balance, the proliferation of Th17 cells, and the dysregulation of Treg cell functions play an important role in immunopathogenesis[45,46]. This process is characterized by an overactivation of the innate and adaptive immune system.
The interaction between major histocompatibility complex-II and the T cell receptor promotes Th1 cell differentiation (IFN-γ, TNF-α, IL-12) via antigen-presenting cells, IL-12, and IL-2 and Th17 cell differentiation (IL-17, TNF-α, IL-22) via TGF-β, IL-6, and IL-1β. Th2 (IL-4, IL-6, IL-10) cells play a limited role, while loss of function of IL-2 and IL-12-dependent Treg cells causes uncontrolled progression of inflammation[47-49]. Macrophages exacerbate inflammation by switching to the M1 phenotype (TNF-α, IL-6, IL-8) under the influence of IFN-γ and granulocyte-macrophage colony-stimulating factor, while IL-4, IL-13, and macrophage colony-stimulating factor-mediated M2 (IL-10) are suppressed. Gamma delta T and NK cells (IFN-γ, TNF-α) increase Th1/Th17 activity. Neutrophils (IFN-γ, IL-8, granulocyte-macrophage colony-stimulating factor) are overactivated and lead to oxidative stress, endothelial dysfunction, thrombosis, and vasculitis, which exacerbates the mucocutaneous lesions and inflammatory damage[48,49] (Figure 2).
Currently, various drugs such as colchicine, corticosteroids, immunosuppressants (azathioprine, cyclophosphamide), IFN, and anti-TNF biologics (infliximab, adalimumab, etanercept) are used to treat BD[50]. However, current treatments generally only alleviate symptoms, many patients develop resistance, and the need for more effective, less toxic new treatment options is increasing, especially in cases of severe organ involvement[47]. MSCs and their exosomes can suppress the autoimmune response by restoring the functions of Treg cells[51]. Considering the dysregulation of the innate immune response and the abnormalities in the adaptive immune system in the pathogenesis of BD, MSC-Exos could suppress the autoinflammatory process and alleviate the clinical symptoms by regulating the phenotype of immune cells[49]. In addition, it can suppress the activation of Th1 and Th17 cells and reduce inflammation by promoting the immunosuppressive functions of Treg cells[52].
In the study conducted by Mazaheri et al[47] in C57BL/6 mice in which herpes simplex virus type 1 was used as a model for BD, intraperitoneal MSCs were administered at different time points (before, simultaneously, and after herpes simplex virus type 1 infection). MSCs decreased the production of proinflammatory cytokines by suppressing TNF-α, IFN-γ, and IL-17 expression and regulated the immune response by inhibiting DC maturation and T cell, B cell, and NK cell activity. The results showed that MSCs slow disease progression by suppressing inflammation and have therapeutic potential[47].
In this context MSC-Exos have the potential as a novel therapeutic approach for patients resistant to conventional therapies in the treatment of BD. In a clinical case report, leg ulcers associated with BD that were resistant to conventional therapies were successfully healed by treatment with MSCs. A 55-year-old patient with recurrent oral and genital ulcers, papulopustular lesions, and walking disability was diagnosed with BD due to recurrent leg ulcers. Previously, conventional immunosuppressive therapy and anti-TNF agents (adalimumab, etanercept) were administered, but no clinical improvement was achieved. The patient received MSC injections in combination with low-dose prednisone and thalidomide. After treatment complete healing of ulcers was observed on one leg and significant improvement on the other leg. During the follow-up period, remission of the lesions and preservation of leg function was reported for 34 months. The results suggest that MSC infusion could be a safe and effective therapeutic option for the treatment of refractory leg ulcers due to BD[53].
BD-associated uveitis (BU) is characterized by severe inflammation that can lead to permanent vision loss and is refractory in some patients despite current immunosuppressive therapies[54]. Therefore, the need for new immunomodulatory treatment approaches is increasing. To investigate the therapeutic effect of MSC-Exos on experimental autoimmune uveitis (EAU), Bai et al[55] established an EAU model in rats immunized with the peptide interphotoreceptor retinol-binding protein 1177-1191 and administered periocular MSC-Exos injections for 7 days after disease onset. After treatment, a significant decrease in the severity of EAU was observed, and T cell subsets and inflammatory cell infiltration in ocular tissues were suppressed. MSC-Exos inhibited the chemotactic effects of C-C motif chemokine ligand 2 and C-C motif chemokine ligand 21 on inflammatory cells and thus reduced the migration of immune cells into the eye. However, no direct inhibitory effect on interphotoreceptor retinol-binding protein-specific T cell proliferation was observed[55]. These results suggest that MSC-Exos alleviates the severity of EAU by regulating inflammation and protects ocular tissue by reducing immune cell infiltration. Since BU results from similar immune mechanisms, this study suggests that MSC-Exos could be considered as a potential immunomodulatory agent for the treatment of BU.
In a study published in the preprint, the immunomodulatory effect of exosomes derived from AD-derived MSCs, specifically IFN-γ-pre-stimulated exosomes (IFN + Exo), on BU was investigated. Peripheral blood mononuclear cells from patients with BU and healthy individuals were cultured with exosomes, and their effects on lymphocyte proliferation, cell viability, and apoptosis were evaluated. IFN + Exo increased apoptosis of T lymphocytes in patients with BU, suppressed proinflammatory cytokines such as IL-17 and TGF-β, and stabilized the immune response by increasing IL-10 expression. These effects have been shown to be mediated by the induction of apoptosis via the Fas/FasL signaling pathway and the suppression of proinflammatory cytokines. The results suggest that IFN + Exo can be used as a potential therapeutic agent by controlling inflammation in patients with BU[56].
Intravitreal BM-MSCs (1.8 × 106 cells) were injected in 3 patients with advanced retinal vasculitis due to BU and severe vision loss unresponsive to conventional immunosuppressive therapies. However, no significant improvement in visual acuity was achieved during the 12-month follow-up period. However, it was observed that MSC treatment partially modulated the inflammatory response[57]. The results suggest that MSCs are not sufficient to reverse vision loss in advanced retinal vasculitis but that they have the potential to suppress inflammation.
Although studies related to BD are limited, the fact that MSC-Exos suppress IL-17 and TGF-β levels, regulate Th1/Th17 cell balance, and reduce inflammatory cell infiltration[6] can be considered a promising approach for the treatment of BD. Further clinical studies on the efficacy and long-term effects of MSC-Exos in different phenotypes of BD are needed. Figure 2 summarizes the immunopathogenesis of BD and potential therapeutic effects of MSCs/MSC-Exos.
SLE is a chronic autoimmune disease characterized by a dysregulation of the immune system leading to the production of autoantibodies (such as anti-double-stranded DNA and antinuclear antibodies) and subsequent inflammation affecting multiple organ systems[58]. This immunological imbalance is due to the presence of autoreactive T and B lymphocytes, leading to polyclonal activation of B cells and excessive production of autoantibodies and proinflammatory cytokines by plasma cells[59].
The pathogenesis of SLE is complex and multifactorial. It includes a genetic predisposition, environmental factors, and hormonal influences, which together contribute to impaired immune tolerance and activation of autoreactive T and B cells[60]. One of the most important clinical manifestations of SLE is lupus nephritis, which occurs in about 40%-60% of patients and can lead to end-stage renal disease if not treated effectively[61]. Treatment is usually with corticosteroids, nonsteroidal anti-inflammatory drugs, and immunosuppressants (cyclophosphamide, mycophenolate mofetil, azathioprine, and leflunomide); intravenous (IV) immunoglobulin is rarely preferred. However, more than one-third of patients show resistance to these therapies or relapse[62,63], contributing to the need for new treatment strategies.
Recent studies have shown that MSCs can improve clinical outcomes in patients with SLE, especially in cases of refractory lupus nephritis[64]. This therapeutic efficacy of MSCs is largely regulated by paracrine factors, while their immunoregulatory activity has been shown to be mainly mediated by MSC-Exos[65]. For example, Liu et al[66] emphasized the potential of MSC-Exos to deliver miRNAs and other bioactive molecules that can regulate immune responses and promote tissue repair. Dou et al[67] demonstrated that tsRNA-21109 contained in MSC-Exos inhibited the conversion of macrophages to the proinflammatory M1 phenotype, thereby reducing inflammation and thus alleviating SLE symptoms.
The therapeutic effects of MSCs in SLE are mediated by different mechanisms. Geng et al[68] demonstrated that reduced Let-7f levels in BM-derived MSCs contribute to the Th17/Treg imbalance observed in patients with SLE, suggesting that MSCs may play a role in restoring immune homeostasis. AD-derived MSCs treatment suppressed autoimmunity by increasing IL-10-producing regulatory B cells, decreasing proinflammatory cytokine levels, increasing the proportion of Treg cells, and decreasing autoantibody production and tissue damage[69].
In a study conducted by Wang et al[70] in patients with active and refractory SLE, transplantation of UC-derived MSC was shown to significantly improve immune tolerance and clinical parameters by regulating T and B cells, suppressing the production of proinflammatory cytokines and increasing Treg cells, with a low side effect profile. Furthermore, in patients with severe and drug-resistant SLE, allogeneic transplantation of BM and/or UC-derived MSCs restored the balance of the immune system by increasing Treg cells and modulating the autoimmune response, while decreasing disease activity and improving renal function[71]. The 5-year overall survival rate was reported to be 84%[70]. Some representative clinical studies are listed in Table 1.
| Study type | MSC source | Disease | Patients (treatment/control) | Administration | Follow-up (months) | Mechanism of action | Outcome measures | Conclusion | Trial registration number | Ref. |
| - | BM-MSCs (autologous) | SLE | 2 (2/0) | 1 × 106 cells/kg, IV infusion | 4 | Immunomodulation | Safety and efficacy, Selena SLEDAI and BILAG scores | CD4+CD25+FoxP3+ cells increased, but the disease did not show remission | [121] | |
| Phase I/II | UC-MSCs (allogeneic) | SLE | 244 (211/78) | 1 × 106 cells/kg, IV infusion | 6 | FLT3 L production, CD1c+ DC proliferation, IFN-γ effect | Clinical improvement, CD1c+ DC and FLT3 L levels | Increased FLT3 L levels and increased number of tolerant CD1c+ DCs, suppression of inflammation | NCT01741857 | [122] |
| Phase II | BM-MSCs | SLE | 3 (3/0) | 9 × 107 cells/kg, IV infusion | 9 | Immunomodulation | SLEDAI, proteinuria values, renal function | Significant reduction in disease activity and improvement in kidney function | [123] | |
| - | UC-MSCs, BM-MSCs | Persistently active SLE | 87 (87/0) | 1 × 106 cells/kg, IV infusion | 48 | Suppression of the proliferation of T and B lymphocytes, modulation of the inflammatory reaction and proliferation of Treg cells | Selena SLEDAI, ANA, dsDNA, clinical remission, relapse, and survival rate | Prolonged clinical remission and improvement in organ function | [124] | |
| Phase I | AD-MSCs (allogeneic) | Refractory lupus nephritis | 9 (9/0) | 2 × 106 cells/kg, IV infusion | 12 | Immunomodulation | SLEDAI, 24-h urinary protein excretion, serum creatinine, anti-dsDNA antibodies | Effective in reducing urinary protein excretion and disease activity in the short term, single dose limited for long-term remission | [125] | |
| - | UC-MSCs | Lupus nephritis | 37 (17/20) | 3 × 107 cells/kg, IV infusion | 12 | Immunomodulation | SLEDAI, ANA, dsDNA urinary protein excretion, safety, and tolerability | Reduction of disease activity, regulation of the balance of inflammatory cytokines, improvement of serological markers and renal function | [126] | |
| Phase I/II | BM-MSCs (autologous) | RA with knee involvement | 30 (15/15) | Intra-articular implantation of 40 million autologous BM-MSCs per knee joint | 12 | Immunomodulation, suppression of inflammatory cytokines | WOMAC, VAS, time to gelling, pain-free walking distance, standing time | Safe and well tolerated, with a trend towards clinical efficacy with improvements in WOMAC, VAS, gelling time, and pain-free walking distance | NCT01873625 | [127] |
| Phase I/II | UC-MSCs | RA | 63 (32 MSC monotherapy group, 31 MSC + IFN-γ group) | UC-MSC transplantation, with some patients receiving recombinant human IFN-γ 1 × 106 cells/kg 1 dose | 3 | IFN-γ enhanced MSC therapeutic efficacy | EULAR response rates, ACR20 response rates | The combination therapy of MSC and IFN-γ improved RA outcomes with an ACR20 response of 93.3% at 3 months vs 53.3% with MSC alone, with no major safety concerns at 1 year | ChiCTR-INR-17012462 | [128] |
| Phase I/IIa | AD-MSCs (autologous) | DMARD-resistant RA | 54 (392/15) | 2.0 or 2.86 × 106 cells/kg, IV infusion | 12 | Immunomodulation | RAPID3, DAS28, and ACR20 | Study ongoing | NCT04170426 | |
| Phase I/II | UC-MSCs | RA | 172 (136/36) | IV infusion of 4 × 107 UC-MSCs in combination with DMARDs | Follow-up examinations after 3, 6, and 8 months | Immunomodulation, suppression of inflammatory cytokines | ACR improvement criteria, DAS28, HAQ, TNF-α, IL-6 levels, CD4+CD25+FoxP3+ Treg percentage | Safe, TNF-α and IL-6 levels decreased, the proportion of Tregs increased, and a clinical improvement was observed. The therapeutic effect lasted 3-6 months and repeated infusions increased the efficacy | NCT01547091 | [89] |
| Phase I/II | MSCs (allogeneic) | RA | 30 (15/15) | Dose not specified IV infusion | 1 | Immunomodulation, suppression of inflammatory response | Safety, tolerability, preliminary efficacy, DAS28 | Study ongoing; results not yet published | NCT05925647 | |
| Phase I | UC-MSCs (allogeneic) (BX-U001) | RA | 16 (8/8) | (0.75-1.5) × 106 cells/kg, BX-U001 IV infusion | 24 | Immunomodulation and anti-inflammatory effect | Safety and tolerability, ACR20, HAQ, DAS28, CRP, ESR, SDAI, anti-CCP | Study ongoing | NCT03828344 | |
| Phase I/II | UC-MSCs (allogeneic) | RA | 105 (52/53) | 1 × 106 cells/kg, IV infusion | 12 | Immunomodulation, regulation of Treg/Th17 balance, suppression of inflammatory cytokines | DAS28, HAQ, serum cytokine levels (IFN-γ, IL-10, IL-6), Treg/Th17 ratio | Safe and effective, clinical improvement persisted for 48 weeks, and high IFN-γ levels correlated with better treatment response | ChiCTR-ONC-16008770 | [129] |
| Phase Ib/IIa | AD-MSCs (allogeneic) (Cx611) | Refractory RA | 53 (46/7) | 1, 2 or 4 × 106/kg, IV infusion | 6 | Immunomodulation, suppression of inflammatory response | Safety, tolerability, pre-activity | Well tolerated, with no dose-dependent toxicity observed. A trend towards clinical efficacy was observed | NCT01663116 | [130] |
| Phase Ia | UC-MSCs | RA | 9 (9/0) | 2.5, 5.0 or 10.0 × 107 cells/patient, IV infusion | 1 | Immunomodulation | Safety, DAS28, ESR, and CRP levels | Significant decrease in DAS28 score at week 4, decrease in ESR and CRP values | NCT02221258 | [131] |
| Phase I | BM-MSCs (autologous) | RA | 9 (9/0) | 1 × 106 cells/kg, IV injection | 12 | Immunomodulation | DAS28-ESR, VAS, and ESR | Significant reduction in DAS28-ESR, VAS, and ESR | NCT03333681 | [132] |
| Phase I | Placenta derived MSCs (allogeneic) | SPMS | 5 (5/0) | 3 × 106 cells/kg, IV injection | 6 | Immunomodulation | EDSS, cytokines, DTI, fMRI, cognitive and psychological evaluations | Results not yet published | NCT06360861 | |
| Phase I/II | MSCs (autologous) | MS | 24 (24/0) | 1 × 106 cells/kg, IV or intrathecal injection | 48 | Immunomodulation | EDSS, adverse events | Results not yet published | NCT04823000 | |
| Phase I | BM-MSCs (autologous) | MS | 7 (7/0) | (1-2) × 106 cells/kg, IV infusion | 12 | Immunomodulation | EDSS, MRI, adverse events | Results not yet published | NCT03778333 | |
| Phase II | BM-MSCs (autologous) | MS | 48 (32/16) | 1 × 106 cells/kg, IV or intrathecal injection | 12 | Immunomodulation | EDSS, MRI, ambulation score, relapse rate | Results not yet published | NCT02166021 | |
| Phase I/II | UC-MSCs (allogeneic) | MS | 20 (20/0) | Dose not specified IV injection | 12 | Immunomodulation | EDSS, NRS, PASAT, the nine-hole peg test, and 25-foot walking time. Short-form 36 | Results not yet published | NCT02034188 |
MSC-Exos have been shown to have anti-inflammatory properties and can inhibit the proliferation of autoreactive B cells, which play a central role in the pathogenesis of SLE. Ng et al[72] reported that MSCs can inhibit B cell proliferation and antibody production through T cell-mediated mechanisms (suppression of Th1/Th2/Th17 populations and increase in Treg cells), highlighting the importance of MSCs in regulating humoral immunity in SLE. This is particularly important given the role of autoantibodies in disease progression and associated organ damage.
Despite the promising results there are still some challenges in the clinical application of MSC therapies in SLE. The variability in the origin and preparation of MSCs may affect their therapeutic efficacy, and further research is needed to standardize protocols for the isolation and characterization of MSCs[73,74]. It is important that SLE is not recognized today as a uniform disease but as a heterogeneous spectrum of clinical phenotypes with different immunopathological courses. This phenotypic diversity poses a major challenge to standard treatment protocols and underscores the need for personalized medical approaches in the treatment of the disease[75].
MSC-Exos may offer phenotype-specific therapeutic benefits due to various immunoregulatory molecules such as miRNAs and tRNA fragments. Therefore, it is recommended that future clinical trials stratify patients according to their disease phenotype to further evaluate the efficacy of MSC-Exos treatment and tailor treatment strategies to individual immune profiles. This approach could significantly help to improve clinical outcomes and reduce treatment resistance in SLE.
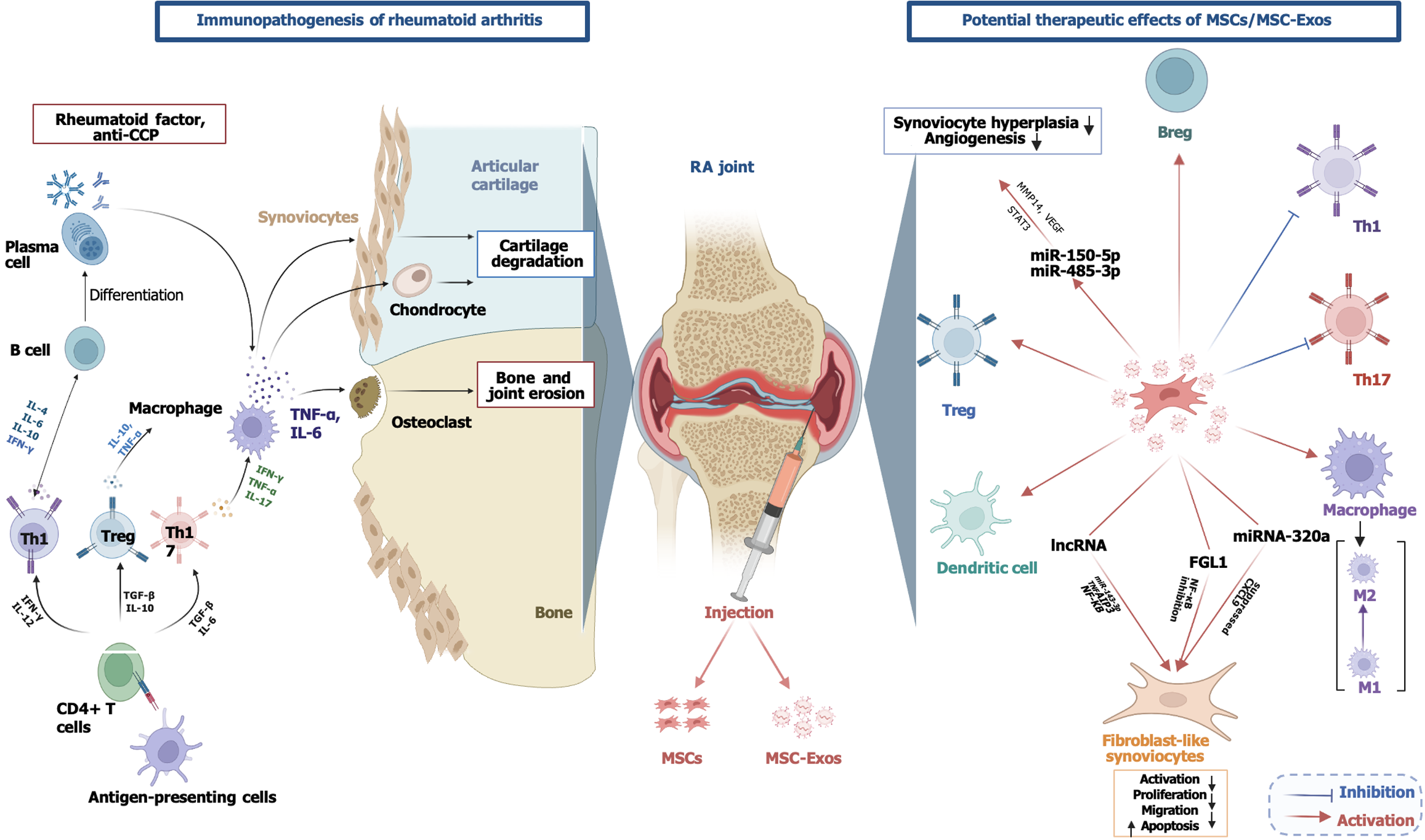
RA is a chronic, systemic, autoimmune, inflammatory disease that mainly affects the synovial joints and is associated with genetic and environmental factors although its etiology is not yet fully understood[76]. Epidemiologic data show that RA occurs in 0.5%-1.0% of the adult population and can affect any age group although it is more common in females and the elderly[77]. Synovial hyperplasia, cell activation, joint inflammation, and the invasion of synovial tissue into adjacent bone and cartilage are characteristic of RA[77]. Many different cell types such as macrophages, DCs, B lymphocytes, T lymphocytes, chondrocytes, fibroblasts, and osteoclasts are involved in the pathophysiology. Macro
In RA fibroblast-like synoviocytes (FLS) initiate inflammatory and destructive processes in the joint and play an important role in cartilage damage[83]. Treatment includes synthetic disease-modifying antirheumatic drugs (DMARDs/methotrexate, leflunomide, sulfasalazine, hydroxychloroquine), biological DMARDs (anti-TNF drugs, tocilizumab, anakinra, abatacept, rituximab), and corticosteroids, which aim to improve disease progression by suppressing inflammation[84]. However, despite current advances many patients with RA do not respond to treatment and suffer from unwanted side effects[85].
MSCs have shown promise as a target for the treatment of RA as they can effectively regulate cartilage bone formation and immune response[86]. The first pilot study, published in 2011[87] investigated the safety and efficacy of autologous AD-derived MSCs in patients with RA. Four patients with RA received single, double, or quadruple dose IV infusions of 2 × 108 to 3.5 × 108 cells. Two patients also received an intra-articular injection. During a 13-month follow-up period, an improvement in the visual analog scale and Korean Western Ontario McMaster Score was observed. Multiple infusions increased efficacy, and up to 8 × 108 cells were shown to be safe. The study was considered the first demonstration of the safety of autologous MSC therapy in RA[87].
In patients with refractory RA, infusion of allogeneic MSCs from the BM and endometrium was reported to reduce erythrocyte sedimentation rate, improved the 28-joint Disease Activity Score, and reduced anti-cyclic citrullinated peptide levels although this clinical improvement was transient due to the short follow-up period[88]. A study in a larger group of patients showed that coadministration of allogeneic UC-derived MSCs with DMARDs increased CD4+ Treg cells and suppressed disease activity for up to 6 months, and repeated MSC infusions increased therapeutic efficacy[89]. In vitro UC-derived MSCs suppressed FLS proliferation and IL-6 secretion from patients with RA via IL-10, IDO, and TGF-β1, induced T cell hyporesponsivity via PGE2, TGF-β1, and nitric oxide, and increased CD4+FoxP3+Treg cells.
In a mouse model systemic infusion of UC-derived MSCs reduced the severity of arthritis, decreased proinflammatory cytokines, and regulated the Th1/Th2 balance by increasing IL-10 levels[90]. In another study IL-1β-stimulated human UC-derived MSCs alleviated RA symptoms by inducing apoptosis of FLS[91]. BM-derived MSCs suppressed the production of TNF-α, IL-17, IL-6, IL-2, IFN-γ, and IL-9 in T cells from patients with RA, and this effect was observed in all T cell subsets. It also supported immune tolerance by increasing the mRNA expression of IL-10 and TGF-β[92].
UC-derived MSCs with increased TNF receptor 2 expression reduced joint inflammation and cartilage destruction by blocking TNF-α[93]. Orthotopic transplantation of BM-derived MSCs with microfracturing and thermal gel reduced joint inflammation by lowering the concentration of inflammatory cytokines[94]. Encapsulation of BM-derived MSCs in alginate hydrogel suppressed inflammation by inhibiting DCs[95]. MSCs with increased expression of CXC chemokine receptor 7 alleviated arthritis symptoms by suppressing inflammation, while BM-derived MSCs expressing IL-10 promoted articular cartilage repair by inhibiting inflammation[96,97]. Consistent with these findings, the immunomodulatory and anti-inflammatory effects of MSC-Exos in RA have been extensively studied. Other representative clinical studies in this field are summarized in Table 1 (http://www.clinicaltrials.gov/).
Studies have reported that these exosomes can suppress the activation and proliferation of synovial FLS involved in RA pathology. For example, Su et al[98] showed that MSC-derived exosomal long non-coding RNA HAND2-AS1 suppressed FLS activation and proliferation via miR-143-3p/TNFAIP3/NF-κB pathways, while reducing inflammation and inducing apoptosis[98]. Exosomal miRNA-320a derived from BM-derived MSC suppressed the expression of CXC motif chemokine ligand 9, a chemokine involved in inflammatory responses, and halted the progression of RA by reducing FLS activation and inflammatory response[86].
These exosomes also suppressed apoptosis and promoted FLS proliferation by inhibiting the NF-κB signaling pathway via the expression of fibrinogen-like protein 1, thereby reducing RA-induced joint damage[99]. In addition, MSC exosomes containing miR-150-5p inhibited synoviocyte hyperplasia and angiogenesis by suppressing the expression of matrix metalloproteinase 14 and VEGF, thereby slowing RA progression[100]. MiR-21-containing exosomes have been shown to alleviate RA symptoms by regulating immune imbalance via the ten-eleven translocation protein 1/Kruppel-like factor 4 axis[101]. Exosomes from UC-derived MSCs inhibited the development of RA by exerting a regulatory effect on the balance of Th1/Th17 and Treg cells. MiRNA-140-3p from UC-derived MSC exosomes reduced joint damage by suppressing serum/glucocorticoid regulated kinase 1 expression[102]. Synovium-derived MSC-Exos suppressed signal transducer and activator of transcription 3 activity by targeting miR-485-3p via the circEDIL3 molecule and limited RA-induced angiogenesis by reducing VEGF levels[103]. MiR-146a-modified MSC-Exos have been shown to maintain immune balance by restoring the immunologic potential of MSCs[104].
MSC-derived exosomal circFBXW7 suppressed FLS proliferation, movement, and inflammation in RA. CircFBXW7 levels were low, miR-216a-3p levels were high, and histone deacetylation 4 levels were low in patients with RA. CircFBXW7, which is carried by MSC-Exos, bound miR-216a-3p, reduced its effect, and increased histone deacetylation 4 levels. This suppressed the excessive proliferation and inflammatory activity of FLS[105]. Meng et al[106] produced exosomes from MSCs overexpressing miRNA-124a and investigated the effects of these exosomes on RA-associated FLS cells. These exosomes inhibited the proliferation and migration of FLS cells and promoted their apoptosis. These results suggested that MSC-Exos are a suitable vector for the delivery of therapeutic miRNA-124a and may represent a novel strategy for the treatment of RA[106].
In a study on inhibition of bone and cartilage destruction, You et al[107] showed that dextran sulphate-modified MSC-Exos (DS-EXOs) have an anti-inflammatory effect in the treatment of RA by regulating macrophage heterogeneity. DS-EXOs accumulated in inflamed joints after systemic administration, converted M1 macrophages to an M2 phenotype, decreased TNF-α and IL-6 levels, and increased IL-10 production. It also provided an immunomodulatory effect in the synovial microenvironment by promoting the activation of Treg cells and suppressing the autoimmune response by inhibiting Th17. In a collagen-induced arthritis model, DS-EXOs, which showed similar therapeutic efficacy at a 10-fold lower dose compared with naked exosomes, reduced joint damage[107]. Figure 3 summarizes the immunopathogenesis of RA and potential therapeutic effects of MSCs/MSC-Exos.
MS is a chronic, progressive disease of the central nervous system characterized by degeneration of the myelin sheath, axonal damage, and neuroinflammation through autoimmune mechanisms. It occurs in three clinical forms. Relapsing-remitting MS, the most common form, progresses in relapses and complete/partial remissions and affects 85%-90% of patients. Over time, it progresses to secondary progressive MS, which occurs without remission in 50%-60% of cases. Primary progressive MS, on the other hand, is a form that occurs in 15% of patients and in which neurological function gradually deteriorates[108].
Th1 and Th17 cells cause demyelination by secreting IFN-γ, IL-17, TNF-α, and IL-1, while CD8+ T cells contribute to axonal damage. Autoimmune B cells drive the disease through antigen presentation, autoantibody production, and cytokine secretion[109]. To investigate the immune-mediated mechanisms of MS, the EAE model is used as the gold standard in preclinical studies[110]. MSC-derived microvesicles carry immunosuppressive molecules such as PD-L1, galectin-1, and TGF-β and through these mechanisms reduce disease activity in the EAE model[111]. In addition, MSC-Exos containing immunosuppressive cytokines (TGF-β, IL-10) and anti-inflammatory biomolecules (PGE2, miR-155, miR-146a, miR-181c, miR-17, miR-21) suppressed T cell proliferation and effector T cell activity through these factors. These mechanisms contribute to the attenuation of the inflammatory process in the central nervous system by reducing the activity of Th1 and Th17 cells[41].
Intranasal administration of MSC-Exos by Fathollahi et al[112] reduced disease severity, increased the ratio of CD25+FoxP3+ Treg cells, and showed a significant immunomodulatory effect by increasing TGF-β levels. After administration of placental MSC-Exos, improvement of motor function, activation of oligodendrocyte progenitor cells, and increased myelin repair were observed[113]. In mice with EAE, human AD-derived MSCs and MSC-Exos have been shown to reduce disease severity, attenuate myelin damage, and suppress inflammatory responses[114]. BM-derived MSCs promoted remyelination by promoting oligodendrocyte progenitor cell proliferation and reduced neuroinflammation by shifting the microglia/macrophage phenotype from M1 to anti-inflammatory M2. Exosomes promoted the maturation of oligodendrocytes by increasing myelin-associated miRNAs such as miR-219 and miR-338 and suppressed inflammation by inhibiting the NF-κB/TLR4 signaling pathway[115].
The periodontal ligament stem cell-derived secretome inhibited NOD-like receptor protein-3 inflammasome activation, decreased the production of proinflammatory cytokines (IL-1β, IL-18), increased the proportion of Treg cells, and suppressed Th1 and Th17 responses[116]. Riazifar et al[14] observed that IV injection of IFNγ-Exo decreased the clinical score, reduced demyelination, and suppressed neuroinflammation. It also increased the number of CD4+CD25+FoxP3+ Tregs in spinal cord tissue. In vitro studies have shown that IFNγ-Exo decreased the levels of proinflammatory Th1 and Th17 cytokines in peripheral blood mononuclear cells and increased IDO levels. In addition, exosomes have been shown to suppress inflammatory processes by regulating cellular immune responses and could potentially be used in the treatment of autoimmune diseases and central nervous system disorders[14]. Therefore, MSC-Exos are thought to have important therapeutic potential in the regulation of neuroinflammation and the treatment of autoimmune diseases.
In the study conducted by Li et al[117], an improvement in the Expanded Disability Status Scale scores, a reduction in relapse frequency and a shift in immune response from Th1 to Th2 were observed in patients receiving UC-derived MSC infusions at a dose of 4 × 106 cells/kg every 2 weeks for a total of 6 weeks. However, in a triple-blind, placebo-controlled, randomized phase I/II clinical trial, AD-derived MSC (1 × 106 or 4 × 106 cells/kg) were administered to patients with secondary progressive MS, and it was reported that MSC treatment was safe but did not provide significant clinical improvement during the 12-month follow-up period[118]. In another phase I/II branch group study (NCT03326505), intrathecally administered doses of UC-derived MSCs (group A: Two doses; group B: One dose), and secretomes of UC-derived MSCs were administered 3 months later. Significant clinical improvements were observed in group A, particularly positive changes in CD3+CD4+ T cells and neurocognitive function. Magnetic resonance imaging data demon
Current approaches to the treatment of autoimmune diseases are still inadequate, and long-term effective solutions are limited. In this review the immunomodulatory effects of MSC-Exos on immune cells and their potential in the treatment of autoimmune diseases were discussed. The results suggested that MSC-Exos hold promise as cell-free biological treatment options and may offer a novel therapeutic approach for autoimmune diseases. However, despite the promising potential of MSC-Exos therapies, the lack of standardized protocols, isolation methods, and dosages pose a major challenge. In addition, the source of MSCs used for exosome production (autologous or allogeneic) is an important factor that may influence therapeutic consistency, production scalability, and clinical applicability. While autologous sources increase costs and complicate the process, allogeneic sources allow for standardization and off-the-shelf product development. Exosome engineering makes it possible to direct and optimize immunomodulatory components to the target tissue, while combination therapies with biological agents can improve treatment efficacy. Long-term clinical trials could provide more data on the safety and efficacy of MSC exosomes and clarify their role in autoimmune diseases.
| 1. | Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 761] [Cited by in RCA: 768] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 2. | Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278:369-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 707] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 3. | Pisetsky DS. Pathogenesis of autoimmune disease. Nat Rev Nephrol. 2023;19:509-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 236] [Reference Citation Analysis (0)] |
| 4. | Mastrandrea LD. An Overview of Organ-Specific Autoimmune Diseases Including Immunotherapy. Immunol Invest. 2015;44:803-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Pineton de Chambrun M, Wechsler B, Geri G, Cacoub P, Saadoun D. New insights into the pathogenesis of Behçet's disease. Autoimmun Rev. 2012;11:687-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 6. | Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of Mesenchymal Stem Cell-Derived Exosomes on Autoimmune Diseases. Front Immunol. 2021;12:749192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 181] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 7. | Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1248] [Cited by in RCA: 1126] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 8. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1256] [Article Influence: 209.3] [Reference Citation Analysis (0)] |
| 9. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 10. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1335] [Article Influence: 74.2] [Reference Citation Analysis (1)] |
| 11. | Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016;8:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 12. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1230] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 13. | El Andaloussi S, Lakhal S, Mäger I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 410] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 14. | Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI, McIntyre LL, Shelke GV, Hutchins E, Hamamoto A, Calle EN, Crescitelli R, Liao W, Pham V, Yin Y, Jayaraman J, Lakey JRT, Walsh CM, Van Keuren-Jensen K, Lotvall J, Zhao W. Stem Cell-Derived Exosomes as Nanotherapeutics for Autoimmune and Neurodegenerative Disorders. ACS Nano. 2019;13:6670-6688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 412] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 15. | Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3378] [Cited by in RCA: 3537] [Article Influence: 321.5] [Reference Citation Analysis (0)] |
| 16. | Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4254] [Cited by in RCA: 4121] [Article Influence: 179.2] [Reference Citation Analysis (0)] |
| 17. | Burtenshaw D, Regan B, Owen K, Collins D, McEneaney D, Megson IL, Redmond EM, Cahill PA. Exosomal Composition, Biogenesis and Profiling Using Point-of-Care Diagnostics-Implications for Cardiovascular Disease. Front Cell Dev Biol. 2022;10:853451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 18. | Dilsiz N. Hallmarks of exosomes. Future Sci OA. 2022;8:FSO764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Frydrychowicz M, Kolecka-Bednarczyk A, Madejczyk M, Yasar S, Dworacki G. Exosomes - structure, biogenesis and biological role in non-small-cell lung cancer. Scand J Immunol. 2015;81:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 20. | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 2141] [Article Influence: 356.8] [Reference Citation Analysis (35)] |
| 21. | Momen-Heravi F, Balaj L, Alian S, Tigges J, Toxavidis V, Ericsson M, Distel RJ, Ivanov AR, Skog J, Kuo WP. Alternative methods for characterization of extracellular vesicles. Front Physiol. 2012;3:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 870] [Cited by in RCA: 1388] [Article Influence: 173.5] [Reference Citation Analysis (1)] |
| 23. | Chitti SV, Gummadi S, Kang T, Shahi S, Marzan AL, Nedeva C, Sanwlani R, Bramich K, Stewart S, Petrovska M, Sen B, Ozkan A, Akinfenwa M, Fonseka P, Mathivanan S. Vesiclepedia 2024: an extracellular vesicles and extracellular particles repository. Nucleic Acids Res. 2024;52:D1694-D1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 91] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 24. | Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 1065] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 25. | Kim DK, Kang B, Kim OY, Choi DS, Lee J, Kim SR, Go G, Yoon YJ, Kim JH, Jang SC, Park KS, Choi EJ, Kim KP, Desiderio DM, Kim YK, Lötvall J, Hwang D, Gho YS. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles. 2013;2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 371] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 26. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 391] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 27. | Brennan MÁ, Layrolle P, Mooney DJ. Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv Funct Mater. 2020;30:1909125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 28. | Curtale G, Rubino M, Locati M. MicroRNAs as Molecular Switches in Macrophage Activation. Front Immunol. 2019;10:799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 29. | O'Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 707] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 30. | Curtale G, Renzi TA, Mirolo M, Drufuca L, Albanese M, De Luca M, Rossato M, Bazzoni F, Locati M. Multi-Step Regulation of the TLR4 Pathway by the miR-125a~99b~let-7e Cluster. Front Immunol. 2018;9:2037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115:1205-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Lou G, Li A, Zhang T, Qi J, Ye D, Zheng M, Chen Z. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 33. | Pizzinat N, Ong-Meang V, Bourgailh-Tortosa F, Blanzat M, Perquis L, Cussac D, Parini A, Poinsot V. Extracellular vesicles of MSCs and cardiomyoblasts are vehicles for lipid mediators. Biochimie. 2020;178:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Izquierdo-Altarejos P, Cabrera-Pastor A, Martínez-García M, Sánchez-Huertas C, Hernández A, Moreno-Manzano V, Felipo V. Extracellular vesicles from mesenchymal stem cells reduce neuroinflammation in hippocampus and restore cognitive function in hyperammonemic rats. J Neuroinflammation. 2023;20:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 35. | Lin TY, Chang TM, Huang HC. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Attenuate Mast Cell Activation. Antioxidants (Basel). 2022;11:2279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Crain SK, Robinson SR, Thane KE, Davis AM, Meola DM, Barton BA, Yang VK, Hoffman AM. Extracellular Vesicles from Wharton's Jelly Mesenchymal Stem Cells Suppress CD4 Expressing T Cells Through Transforming Growth Factor Beta and Adenosine Signaling in a Canine Model. Stem Cells Dev. 2019;28:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci Rep. 2016;6:38308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 38. | Chaubey S, Thueson S, Ponnalagu D, Alam MA, Gheorghe CP, Aghai Z, Singh H, Bhandari V. Early gestational mesenchymal stem cell secretome attenuates experimental bronchopulmonary dysplasia in part via exosome-associated factor TSG-6. Stem Cell Res Ther. 2018;9:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 39. | Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS Jr, Olson SD. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells. 2018;36:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 40. | Shen B, Liu J, Zhang F, Wang Y, Qin Y, Zhou Z, Qiu J, Fan Y. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int. 2016;2016:1240301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 41. | Baharlooi H, Azimi M, Salehi Z, Izad M. Mesenchymal Stem Cell-Derived Exosomes: A Promising Therapeutic Ace Card to Address Autoimmune Diseases. Int J Stem Cells. 2020;13:13-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 42. | Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 520] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 43. | Mattioli I, Bettiol A, Saruhan-Direskeneli G, Direskeneli H, Emmi G. Pathogenesis of Behçet's Syndrome: Genetic, Environmental and Immunological Factors. Front Med (Lausanne). 2021;8:713052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | van der Houwen TB, van Hagen PM, van Laar JAM. Immunopathogenesis of Behçet's disease and treatment modalities. Semin Arthritis Rheum. 2022;52:151956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (1)] |
| 45. | Hamzaoui K, Hamzaoui A, Houman H. CD4+CD25+ regulatory T cells in patients with Behçet's disease. Clin Exp Rheumatol. 2006;24:S71-S78. [PubMed] |
| 46. | Gündüz E, Teke HU, Bilge NS, Cansu DU, Bal C, Korkmaz C, Gülbaş Z. Regulatory T cells in Behçet's disease: is there a correlation with disease activity? Rheumatol Int. 2013;33:3049-3054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Mazaheri T, Esmaeilzadeh A, Mirzaei MH. Introducing the immunomodulatory effects of mesenchymal stem cells in an experimental model of Behçet’s disease. J Med Hypotheses Ideas. 2012;6:23-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Hu D, Guan JL. The roles of immune cells in Behçet's disease. Adv Rheumatol. 2023;63:49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 49. | Tong B, Liu X, Xiao J, Su G. Immunopathogenesis of Behcet's Disease. Front Immunol. 2019;10:665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 50. | Alibaz-Oner F, Direskeneli H. Advances in the Treatment of Behcet's Disease. Curr Rheumatol Rep. 2021;23:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 51. | Zhang R, Miao J, Zhu P. Regulatory T cell heterogeneity and therapy in autoimmune diseases. Autoimmun Rev. 2021;20:102715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 53. | Li Y, Wang Z, Zhao Y, Luo Y, Xu W, Marion TN, Liu Y. Successful mesenchymal stem cell treatment of leg ulcers complicated by Behcet disease: A case report and literature review. Medicine (Baltimore). 2018;97:e0515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Çakar Özdal P. Behçet's Uveitis: Current Diagnostic and Therapeutic Approach. Turk J Ophthalmol. 2020;50:169-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 55. | Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X. Effects of Mesenchymal Stem Cell-Derived Exosomes on Experimental Autoimmune Uveitis. Sci Rep. 2017;7:4323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 56. | Gozel M, Aydemir D, Kaleli HN, Cosar F, Guleser UY, Unlu EK, Kesim C, Sezgin B, Sahin A, Ucar D, Hatemi G, Hasanreisoglu M. Effects of Primed Adipose Mesenchymal Stem Cell-Derived Exosomes on Immunomodulation in Behcet Uveitis. 2024 Preprint. Available from: bioRxiv:2024.11.24.625042. [DOI] [Full Text] |
| 57. | Davatchi F, Nikbin B, Shams H, Sadeghi Abdollahi B, Mohyeddin M, Shahram F. Mesenchymal stem cell therapy unable to rescue the vision from advanced Behcet's disease retinal vasculitis: report of three patients. Int J Rheum Dis. 2013;16:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Crow MK. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis. 2023;82:999-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 178] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 59. | Hoffman RW. T cells in the pathogenesis of systemic lupus erythematosus. Clin Immunol. 2004;113:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Ameer MA, Chaudhry H, Mushtaq J, Khan OS, Babar M, Hashim T, Zeb S, Tariq MA, Patlolla SR, Ali J, Hashim SN, Hashim S. An Overview of Systemic Lupus Erythematosus (SLE) Pathogenesis, Classification, and Management. Cureus. 2022;14:e30330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 97] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 61. | Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. 2020;6:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 577] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 62. | Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | Relle M, Weinmann-Menke J, Scorletti E, Cavagna L, Schwarting A. Genetics and novel aspects of therapies in systemic lupus erythematosus. Autoimmun Rev. 2015;14:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Wang D, Wang S, Huang S, Zhang Z, Yuan X, Feng X, Lu L, Sun L. Serum IFN-γ Predicts the Therapeutic Effect of Mesenchymal Stem Cells Transplantation in Systemic Lupus Erythematosus Patients. Stem Cells Transl Med. 2017;6:1777-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, Li H, Liu Z, Shi C, Duan F, Xiao Y. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 66. | Liu YJ, Miao HB, Lin S, Chen Z. Current Progress in Treating Systemic Lupus Erythematosus Using Exosomes/MicroRNAs. Cell Transplant. 2023;32:9636897221148775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 67. | Dou R, Zhang X, Xu X, Wang P, Yan B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol Immunol. 2021;139:106-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 68. | Geng L, Tang X, Wang S, Sun Y, Wang D, Tsao BP, Feng X, Sun L. Reduced Let-7f in Bone Marrow-Derived Mesenchymal Stem Cells Triggers Treg/Th17 Imbalance in Patients With Systemic Lupus Erythematosus. Front Immunol. 2020;11:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Park MJ, Kwok SK, Lee SH, Kim EK, Park SH, Cho ML. Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant. 2015;24:2367-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 70. | Wang D, Zhang H, Liang J, Wang H, Hua B, Feng X, Gilkeson GS, Farge D, Shi S, Sun L. A Long-Term Follow-Up Study of Allogeneic Mesenchymal Stem/Stromal Cell Transplantation in Patients with Drug-Resistant Systemic Lupus Erythematosus. Stem Cell Reports. 2018;10:933-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 71. | Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 463] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 72. | Ng J, Hynes K, White G, Sivanathan KN, Vandyke K, Bartold PM, Gronthos S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J Cell Biochem. 2016;117:2844-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 73. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 427] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 74. | Li A, Guo F, Pan Q, Chen S, Chen J, Liu HF, Pan Q. Mesenchymal Stem Cell Therapy: Hope for Patients With Systemic Lupus Erythematosus. Front Immunol. 2021;12:728190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 75. | Fava A, Petri M. Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun. 2019;96:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 417] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 76. | Gao Y, Zhang Y, Liu X. Rheumatoid arthritis: pathogenesis and therapeutic advances. MedComm (2020). 2024;5:e509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 35] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 77. | Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1567] [Article Influence: 223.9] [Reference Citation Analysis (0)] |
| 78. | Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:472-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 487] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 79. | Bugatti S, Vitolo B, Caporali R, Montecucco C, Manzo A. B cells in rheumatoid arthritis: from pathogenic players to disease biomarkers. Biomed Res Int. 2014;2014:681678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 80. | Podojil JR, Miller SD. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol Rev. 2009;229:337-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 723] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 82. | Schulze-Koops H, Kalden JR. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2001;15:677-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 200] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 83. | Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 707] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 84. | Koenders MI, van den Berg WB. Novel therapeutic targets in rheumatoid arthritis. Trends Pharmacol Sci. 2015;36:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 85. | Shams S, Martinez JM, Dawson JRD, Flores J, Gabriel M, Garcia G, Guevara A, Murray K, Pacifici N, Vargas MV, Voelker T, Hell JW, Ashouri JF. The Therapeutic Landscape of Rheumatoid Arthritis: Current State and Future Directions. Front Pharmacol. 2021;12:680043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 86. | Meng Q, Qiu B. Exosomal MicroRNA-320a Derived From Mesenchymal Stem Cells Regulates Rheumatoid Arthritis Fibroblast-Like Synoviocyte Activation by Suppressing CXCL9 Expression. Front Physiol. 2020;11:441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 87. | Ra JC, Kang SK, Shin IS, Park HG, Joo SA, Kim JG, Kang BC, Lee YS, Nakama K, Piao M, Sohl B, Kurtz A. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J Transl Med. 2011;9:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 88. | Liang J, Li X, Zhang H, Wang D, Feng X, Wang H, Hua B, Liu B, Sun L. Allogeneic mesenchymal stem cells transplantation in patients with refractory RA. Clin Rheumatol. 2012;31:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 89. | Wang L, Wang L, Cong X, Liu G, Zhou J, Bai B, Li Y, Bai W, Li M, Ji H, Zhu D, Wu M, Liu Y. Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells Dev. 2013;22:3192-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 90. | Liu Y, Mu R, Wang S, Long L, Liu X, Li R, Sun J, Guo J, Zhang X, Guo J, Yu P, Li C, Liu X, Huang Z, Wang D, Li H, Gu Z, Liu B, Li Z. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 91. | Chiu YH, Liang YH, Hwang JJ, Wang HS. IL-1β stimulated human umbilical cord mesenchymal stem cells ameliorate rheumatoid arthritis via inducing apoptosis of fibroblast-like synoviocytes. Sci Rep. 2023;13:15344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 92. | Pedrosa M, Gomes J, Laranjeira P, Duarte C, Pedreiro S, Antunes B, Ribeiro T, Santos F, Martinho A, Fardilha M, Domingues MR, Abecasis M, P da Silva JA, Paiva A. Immunomodulatory effect of human bone marrow-derived mesenchymal stromal/stem cells on peripheral blood T cells from rheumatoid arthritis patients. J Tissue Eng Regen Med. 2020;14:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 93. | Zhao Y, Yang X, Li S, Zhang B, Li S, Wang X, Wang Y, Jia C, Chang Y, Wei W. sTNFRII-Fc modification protects human UC-MSCs against apoptosis/autophagy induced by TNF-α and enhances their efficacy in alleviating inflammatory arthritis. Stem Cell Res Ther. 2021;12:535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Liu H, Ding J, Wang J, Wang Y, Yang M, Zhang Y, Chang F, Chen X. Remission of collagen-induced arthritis through combination therapy of microfracture and transplantation of thermogel-encapsulated bone marrow mesenchymal stem cells. PLoS One. 2015;10:e0120596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Shi G, Zhou Y, Liu W, Chen C, Wei Y, Yan X, Wu L, Wang W, Sun L, Zhang T. Bone-derived MSCs encapsulated in alginate hydrogel prevent collagen-induced arthritis in mice through the activation of adenosine A(2A/2B) receptors in tolerogenic dendritic cells. Acta Pharm Sin B. 2023;13:2778-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 96. | Wei ST, Huang YC, Chiang JY, Lin CC, Lin YJ, Shyu WC, Chen HC, Hsieh CH. Gain of CXCR7 function with mesenchymal stem cell therapy ameliorates experimental arthritis via enhancing tissue regeneration and immunomodulation. Stem Cell Res Ther. 2021;12:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Tian S, Yan Y, Qi X, Li X, Li Z. Treatment of Type II Collagen-Induced Rat Rheumatoid Arthritis Model by Interleukin 10 (IL10)-Mesenchymal Stem Cells (BMSCs). Med Sci Monit. 2019;25:2923-2934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Su Y, Liu Y, Ma C, Guan C, Ma X, Meng S. Mesenchymal stem cell-originated exosomal lncRNA HAND2-AS1 impairs rheumatoid arthritis fibroblast-like synoviocyte activation through miR-143-3p/TNFAIP3/NF-κB pathway. J Orthop Surg Res. 2021;16:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 99. | Xu W, Liu X, Qu W, Wang X, Su H, Li W, Cheng Y. Exosomes derived from fibrinogen-like protein 1-overexpressing bone marrow-derived mesenchymal stem cells ameliorates rheumatoid arthritis. Bioengineered. 2022;13:14545-14561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 100. | Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J Immunol. 2018;201:2472-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 101. | Li GQ, Fang YX, Liu Y, Meng FR, Wu X, Zhang CW, Zhang Y, Liu YQ, Liu D. MicroRNA-21 from bone marrow mesenchymal stem cell-derived extracellular vesicles targets TET1 to suppress KLF4 and alleviate rheumatoid arthritis. Ther Adv Chronic Dis. 2021;12:20406223211007369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 102. | Huang Y, Chen L, Chen D, Fan P, Yu H. Exosomal microRNA-140-3p from human umbilical cord mesenchymal stem cells attenuates joint injury of rats with rheumatoid arthritis by silencing SGK1. Mol Med. 2022;28:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 103. | Zhang J, Zhang Y, Ma Y, Luo L, Chu M, Zhang Z. Therapeutic Potential of Exosomal circRNA Derived from Synovial Mesenchymal Cells via Targeting circEDIL3/miR-485-3p/PIAS3/STAT3/VEGF Functional Module in Rheumatoid Arthritis. Int J Nanomedicine. 2021;16:7977-7994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 104. | Tavasolian F, Hosseini AZ, Soudi S, Naderi M. miRNA-146a Improves Immunomodulatory Effects of MSC-derived Exosomes in Rheumatoid Arthritis. Curr Gene Ther. 2020;20:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 105. | Chang L, Kan L. Mesenchymal Stem Cell-Originated Exosomal Circular RNA circFBXW7 Attenuates Cell Proliferation, Migration and Inflammation of Fibroblast-Like Synoviocytes by Targeting miR-216a-3p/HDAC4 in Rheumatoid Arthritis. J Inflamm Res. 2021;14:6157-6171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 106. | Meng HY, Chen LQ, Chen LH. The inhibition by human MSCs-derived miRNA-124a overexpression exosomes in the proliferation and migration of rheumatoid arthritis-related fibroblast-like synoviocyte cell. BMC Musculoskelet Disord. 2020;21:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 107. | You DG, Lim GT, Kwon S, Um W, Oh BH, Song SH, Lee J, Jo DG, Cho YW, Park JH. Metabolically engineered stem cell-derived exosomes to regulate macrophage heterogeneity in rheumatoid arthritis. Sci Adv. 2021;7:eabe0083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 108. | Sheikhi K, Ghaderi S, Firouzi H, Rahimibarghani S, Shabani E, Afkhami H, Yarahmadi A. Recent advances in mesenchymal stem cell therapy for multiple sclerosis: clinical applications and challenges. Front Cell Dev Biol. 2025;13:1517369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 109. | Liu R, Du S, Zhao L, Jain S, Sahay K, Rizvanov A, Lezhnyova V, Khaibullin T, Martynova E, Khaiboullina S, Baranwal M. Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target. Front Immunol. 2022;13:996469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 110. | Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164:1079-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 1103] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 111. | Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 112. | Fathollahi A, Hashemi SM, Haji Molla Hoseini M, Tavakoli S, Farahani E, Yeganeh F. Intranasal administration of small extracellular vesicles derived from mesenchymal stem cells ameliorated the experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2021;90:107207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 113. | Clark K, Zhang S, Barthe S, Kumar P, Pivetti C, Kreutzberg N, Reed C, Wang Y, Paxton Z, Farmer D, Guo F, Wang A. Placental Mesenchymal Stem Cell-Derived Extracellular Vesicles Promote Myelin Regeneration in an Animal Model of Multiple Sclerosis. Cells. 2019;8:1497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 114. | Jafarinia M, Alsahebfosoul F, Salehi H, Eskandari N, Azimzadeh M, Mahmoodi M, Asgary S, Ganjalikhani Hakemi M. Therapeutic effects of extracellular vesicles from human adipose-derived mesenchymal stem cells on chronic experimental autoimmune encephalomyelitis. J Cell Physiol. 2020;235:8779-8790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 115. | Zhang J, Buller BA, Zhang ZG, Zhang Y, Lu M, Rosene DL, Medalla M, Moore TL, Chopp M. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp Neurol. 2022;347:113895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 116. | Soundara Rajan T, Giacoppo S, Diomede F, Bramanti P, Trubiani O, Mazzon E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int J Immunopathol Pharmacol. 2017;30:238-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 117. | Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, Zhang YZ, Lou JY, Cao B, Wang YL. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014;23 Suppl 1:S113-S122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 118. | Fernández O, Izquierdo G, Fernández V, Leyva L, Reyes V, Guerrero M, León A, Arnaiz C, Navarro G, Páramo MD, Cuesta A, Soria B, Hmadcha A, Pozo D, Fernandez-Montesinos R, Leal M, Ochotorena I, Gálvez P, Geniz MA, Barón FJ, Mata R, Medina C, Caparrós-Escudero C, Cardesa A, Cuende N; Research Group Study EudraCT 2008-004015-35. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One. 2018;13:e0195891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 119. | Jamali F, Aldughmi M, Atiani S, Al-Radaideh A, Dahbour S, Alhattab D, Khwaireh H, Arafat S, Jaghbeer JA, Rahmeh R, Abu Moshref K, Bawaneh H, Hassuneh MR, Hourani B, Ababneh O, Alghwiri A, Awidi A. Human Umbilical Cord-Derived Mesenchymal Stem Cells in the Treatment of Multiple Sclerosis Patients: Phase I/II Dose-Finding Clinical Study. Cell Transplant. 2024;33:9636897241233045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 120. | Islam MA, Alam SS, Kundu S, Ahmed S, Sultana S, Patar A, Hossan T. Mesenchymal Stem Cell Therapy in Multiple Sclerosis: A Systematic Review and Meta-Analysis. J Clin Med. 2023;12:6311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 121. | Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D, Mönckeberg G, Figueroa FE. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 122. | Yuan X, Qin X, Wang D, Zhang Z, Tang X, Gao X, Chen W, Sun L. Mesenchymal stem cell therapy induces FLT3L and CD1c(+) dendritic cells in systemic lupus erythematosus patients. Nat Commun. 2019;10:2498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 123. | Barbado J, Tabera S, Sánchez A, García-Sancho J. Therapeutic potential of allogeneic mesenchymal stromal cells transplantation for lupus nephritis. Lupus. 2018;27:2161-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 124. | Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22:2267-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 125. | Ranjbar A, Hassanzadeh H, Jahandoust F, Miri R, Bidkhori HR, Monzavi SM, Sanjar-Moussavi N, Matin MM, Shariati-Sarabi Z. Allogeneic adipose-derived mesenchymal stromal cell transplantation for refractory lupus nephritis: Results of a phase I clinical trial. Curr Res Transl Med. 2022;70:103324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 126. | Yang GX, Pan LP, Zhou QY, Song W, Chen ZQ, Wang CX, Wu YB, Wang X, Chen Q. [Therapeutic effects of umbilical cord mesenchymal stem cells transplantation on systemic lupus erythematosus]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:338-341, 350. [PubMed] |
| 127. | Shadmanfar S, Labibzadeh N, Emadedin M, Jaroughi N, Azimian V, Mardpour S, Kakroodi FA, Bolurieh T, Hosseini SE, Chehrazi M, Niknejadi M, Baharvand H, Gharibdoost F, Aghdami N. Intra-articular knee implantation of autologous bone marrow-derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: Results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. 2018;20:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 128. | He X, Yang Y, Yao M, Yang L, Ao L, Hu X, Li Z, Wu X, Tan Y, Xing W, Guo W, Bellanti JA, Zheng SG, Xu X. Combination of human umbilical cord mesenchymal stem (stromal) cell transplantation with IFN-γ treatment synergistically improves the clinical outcomes of patients with rheumatoid arthritis. Ann Rheum Dis. 2020;79:1298-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 129. | Yang Y, He X, Zhao R, Guo W, Zhu M, Xing W, Jiang D, Liu C, Xu X. Serum IFN-γ levels predict the therapeutic effect of mesenchymal stem cell transplantation in active rheumatoid arthritis. J Transl Med. 2018;16:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 130. | Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, Blanco F, Martínez-Taboada VM, Taylor P, Martín-Martín C, DelaRosa O, Tagarro I, Díaz-González F. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 131. | Park EH, Lim HS, Lee S, Roh K, Seo KW, Kang KS, Shin K. Intravenous Infusion of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Rheumatoid Arthritis: A Phase Ia Clinical Trial. Stem Cells Transl Med. 2018;7:636-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 132. | Ghoryani M, Shariati-Sarabi Z, Tavakkol-Afshari J, Ghasemi A, Poursamimi J, Mohammadi M. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow-derived mesenchymal stem cells: A successful clinical trial in Iran. Biomed Pharmacother. 2019;109:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |