Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.107080
Revised: April 21, 2025
Accepted: June 19, 2025
Published online: July 26, 2025
Processing time: 131 Days and 23.5 Hours
As the role of extracellular vesicles (EVs) in stem cell therapy is increasingly recognized, more researchers regard them as a promising new natural delivery system. When exerting therapeutic effects, EVs exhibit several advantages, including low immunogenicity/cytotoxicity, the ability to traverse biological barriers, and high potential for engineering. However, several challenges remain in the clinical application of EVs. Their plasma half-life is relatively short, and they tend to accumulate in parenchymal organs (e.g., liver and spleen) within a short time. Moreover, their targeting capabilities are neither precise nor highly effective. Additionally, EVs lack controlled and sustained release properties, necessitating the design of effective delivery strategies to ensure that therapeutic concentrations are achieved and maintained at the target site for an adequate duration. This review summarizes the latest drug delivery strategies involving EVs, focusing on systemic and local applications. It introduces various engi
Core Tip: This review summarizes the latest drug delivery strategies involving extracellular vesicles, focusing on systemic and local applications. It introduces various engineering approaches, administration strategies, and auxiliary delivery systems. Finally, the review discusses existing challenges in extracellular vesicle-based drug delivery and provides insights into the trends for future development in this field.
- Citation: Ye YL, Liu L. Engineering the future of nanomedicine: Strategic approaches to extracellular vesicle-based drug administration regimens. World J Stem Cells 2025; 17(7): 107080
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/107080.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.107080
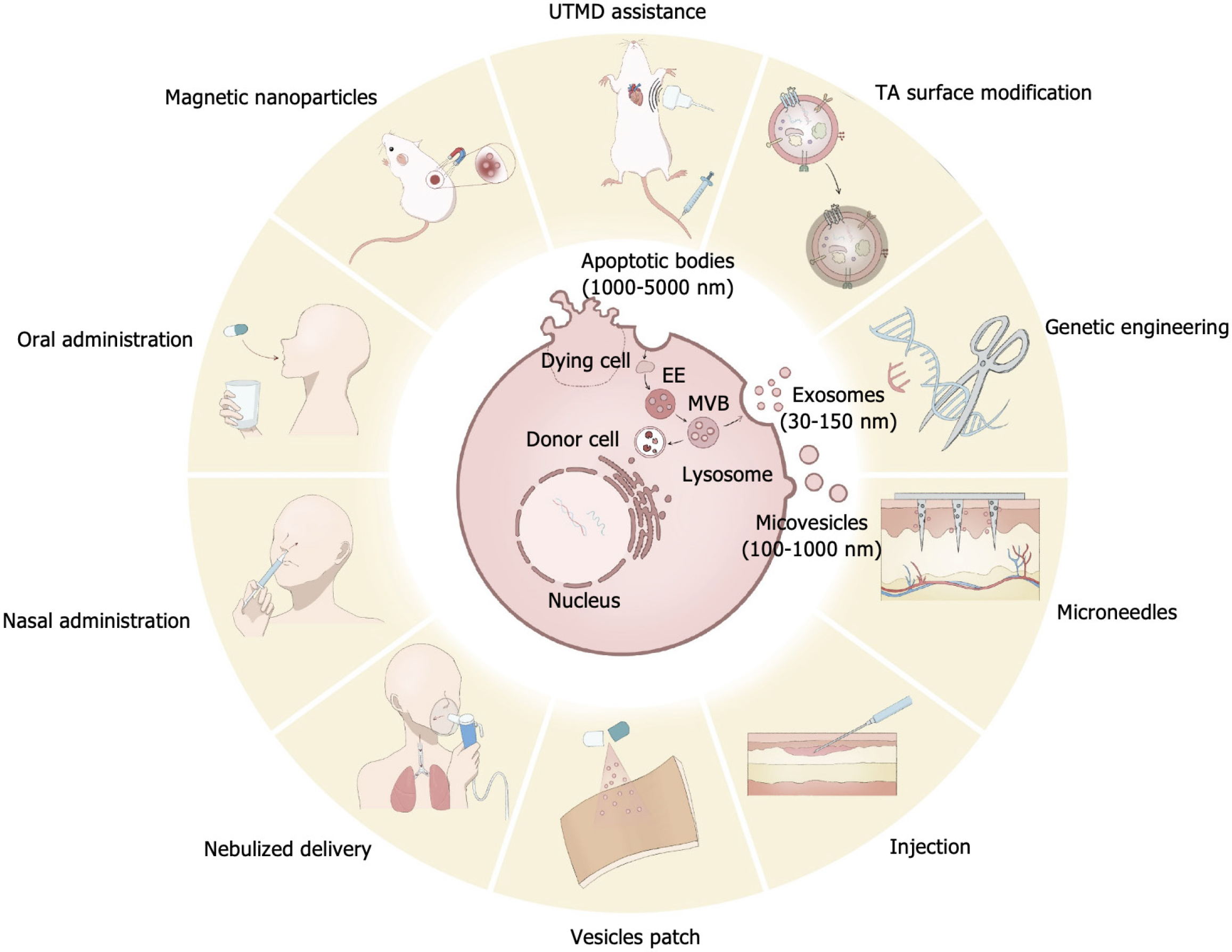
Extracellular vesicles (EVs) are nanoscale lipid bilayer particles that serve as carriers capable of transporting proteins, lipids, RNA, or DNA. Based on their size and origin, EVs are categorized into three main types: Exosomes, which are 30-150 nm in size and are released via multivesicular bodies formed through the endoplasmic reticulum pathway; ectosomes/microvesicles, which range from 100-1000 nm and bud directly from the plasma membrane; and apoptotic bodies, which form during apoptosis and are 50 nm to 5 μm in size[1].
EVs were first described by researchers approximately 40 years ago when Pan and Johnstone[2] and Harding et al[3] independently discovered these structures in sheep reticulocytes and rat reticulocytes, respectively, although they were initially thought to be membrane fragments with no biological significance. It was not until about 20 years ago that the biological roles of EVs began to be understood[4]. With the deepening and expansion of relevant research, EVs have been recognized for their widespread distribution and critical roles in both physiological and pathological processes[5]. Nearly all known cells can secrete various types of EVs to facilitate intercellular communication and accomplish various physiological processes[6].
The therapeutic effects of EVs have been gradually recognized with the advancing research in stem cell therapy. Growing evidence suggests that the therapeutic effects of stem cells may largely result from the paracrine actions of their secreted EVs rather than from the transplantation and proliferation of the stem cells themselves[7]. Courtesy of their innate ability for intercellular delivery, the study of EVs as a novel drug delivery system has become a research hotspot in recent years. EVs have been demonstrated to provide various therapeutic effects in both local and systemic applications, including bone and cartilage regeneration, skin wound healing, treatment of Alzheimer’s disease, and tumor-associated drug delivery. Compared with other nanocarriers for drug delivery, EVs exhibit advantageous properties such as low immunogenicity, high physicochemical stability, the ability to penetrate tissue barriers, high engineering potential, and innate capability for long-distance intercellular communication[8] (Figure 1). However, there are still several challenges associated with the in vivo application of EV-based delivery systems.
First, the plasma half-life of EVs is relatively short, typically 2-30 minutes[9]. Upon entering the bloodstream through injection or other administration routes, EVs are rapidly subjected to early clearance mediated by the macrophage system. Within approximately two hours, EVs are predominantly sequestered in parenchymal organs such as the liver and spleen[10], which significantly limits their efficacy in systemic applications. Second, developing strategies to confer EVs with enhanced targeting capabilities and improve the effective concentration at target sites remains challenging. Additionally, in local applications, optimizing EV administration methods, developing controlled release systems, and maintaining their biological activity are essential aspects that require further refinement.
We conducted a literature search in the PubMed database, focusing on studies published within the last 3 years, supplemented by some earlier foundational research findings. The search strategy incorporated common words in the field and medical subject headings such as “extracellular vesicle”; “exosomes”; “hydrogels”; “drug delivery”; “oral administration”; “engineered”; “injection”; “surface modification”; “magnetic”; “targeting”; “nasal administration”; and “microneedles”. These terms were combined using specific algorithms, such as “extracellular vesicle” and “engineered” (Table 1). From the search results, studies with low relevance were excluded, and the main findings of the selected studies were reviewed. This review summarizes the latest drug delivery strategies involving EVs, focusing on systemic and local applications. It introduces various engineering approaches, administration strategies, and auxiliary delivery systems. Finally, the review discusses existing challenges in EV-based drug delivery and provides insights into the trends for future development in this field.
| EVs | Key components | Target disease | Research focus | Ref. |
| Bone marrow MSC-derived extracellular vesicles | Phase 2 multicenter double-anonymized randomized placebo-controlled dosing trial | Respiratory failure rom COVID-19 | Clinical trial | Lightner et al[18] |
| Human placental mesenchymal stromal cell-derived small extracellular vesicles | Double-blind, randomized, controlled clinical trial | Acute respiratory distress syndrome | Clinical trial | Zamanian et al[19] |
| ExosCD47-HuR | Fusion gene CD47-HuR integrate into LO2 cells via antiviral infection | Hepatic ischemia/reperfusion injury | Engineered vesicles | Liu et al[20] |
| CD9 engineered ExoSmart | Integrate CD47 p110-130 into the exosomal membrane by engineering CD9 E174 | Pancreatic ductal adenocarcinoma | Engineered vesicles | Creeden et al[21] |
| BV2 mouse microglial cell line exosome | Functional oligopeptide-modified exosome loaded with doxorubicin | Glioblastoma | Engineered vesicles | Wang et al[25] |
| Bone marrow MSCs derived exosomes | Genetically engineered exosomes expressing chondrocyte-affinity peptide on the surface | Cartilaginous endplate degeneration | Engineered vesicles | Lin et al[27] |
| MSCs derived exosomes | Anchored cartilage targeting optimally charged arginine-rich cationic motifs into the anionic exosome bilayer | Osteoarthritis | Engineered vesicles | Zhang et al[28] |
| Adipose-derived stem cell-exosome | Conjugate antitumor necrosis factor-α antibodies to the surface of adipose-derived stem cell-exosome | Corneal alkali burns | Engineered vesicles | Yu et al[29] |
| MSCs derived exosomes | Using engineered exosome to modulate ER homeostasis for restoring the function of MSCs | Bone defects in diabetic patients | Engineered vesicles | Liu et al[30] |
| siRNA encapsulated exosomes | UTMD can enhance the local delivery of exosomes via the cavitation effects | DOX-induced cardiomyopathy | UTMD-assisted exosomal delivery | Chen et al[33] |
| The encapsulation of exosomes with ferric ions (Fe3+) and tannic acid | Tannic acid surface modification | Kumar et al[36] | ||
| Tannic acid improves exosome ability to specifically target heart tissue | Heart diseases | Tannic acid surface modification | Shin et al[37] | |
| MSCs derived exosomes | IONP-labeled exosomes intravenous injection followed by the application of a 12 Tesla magnetic field | Clinically relevant model of skin injury | Magnetic nanoparticles targeting | Li et al[39] |
| Bone marrow MSCs derived exosomes | Magnetic guidance: The retention of injected IONP-MSC-derived NVs within the infarcted heart | Myocardial infarction | Magnetic nanoparticles targeting | Lee et al[40] |
| Exosomes from neutrophils | Engineered exosomes selectively accumulate at the tumor sites under an external magnetic field | Tumor | Magnetic nanoparticles targeting | Zhang et al[41] |
| Endogenous extracellular vesicles | Magnetic-guided accumulation of captured CD63-expressing exosomes | Myocardial infarction | Magnetic nanoparticles targeting | Liu et al[42] |
| ANG peptide-modified engineered exosomes | MNP@BQR@ANG-EXOsiGPX4 platform enriched in the brain under magnetic field | Glioblastoma | Magnetic nanoparticles targeting | Li et al[43] |
| Milk-derived exosomes | Milk exosomes are good carriers for chemotherapy drugs | Tumor | Oral administration | Munagala et al[47] |
| Milk-derived exosomes | RSV-loaded milk-derived exosomes to enhance the RSV oral bioavailability | Colon inflammation | Oral administration | Esfahani et al[48] |
| Yam-derived exosome-like nanovesicles | The orally administered YNVs can be transported through the GI tract and absorbed through the small intestine | Osteoporosis | Oral administration | Hwang et al[49] |
| Tea leaves-derived exosome-like nanovesicles | Oral administration is significantly less toxic than intravenous administration | Breast cancer | Oral administration | Chen et al[50] |
| Intranasal delivery provides a practical, noninvasive method for delivering therapeutic agents to the brain | Brain inflammatory-related diseases | Intranasal delivery | Zhuang et al[51] | |
| Adipose MSC-EVs | Intranasally administered via intelligent hydrogel | Alzheimer’s disease | Intranasal delivery | Huang et al[52] |
| Dandelion-derived extracellular vesicle-like nanoparticles | Gelatin methacrylate composite hydrogel | Delayed wound healing caused by bacterial infection | Vesicles patch | Tan et al[58] |
| MSC derived exosomes | Chitosan-based composite hydrogel dressing | Full-thickness skin defects, diabetic wounds, and burn skin injury | Vesicles patch | Shang et al[59] |
| An efficient strategy for delivering exosome-loaded nanoparticles under hypoxic conditions | Poor wound healing following trauma and surgical | Vesicles patch | Han et al[60] | |
| MSCs derived exosomes | HA hydrogel coated with polydopamine and DP7 | Burn injuries | Vesicles patch | Yang et al[61] |
| Curcumin-loaded exosome | An injectable self-healing biphasic hydrogel composed of carboxymethyl chitosan and pullulan | Flap necrosis | Local injection | Liu et al[62] |
| Exosomes originating from decidual stromal cells | An alginate-based hydrogel scaffold system | Intrauterine adhesion | Local injection | Liang et al[63] |
| dECM from human umbilical cord MSCs derived exosomes | Exo-dECM hydrogel is a promising therapeutic strategy for treating SCI | SCI | Local injection | Wang et al[64] |
| Adipose stem cells derived exosomes | Injectable thermosensitive hydrogel system | Androgenic alopecia | Local injection | Xiong et al[65] |
| Bone marrow MSCs derived exosomes | Exosome-loaded hydrogel microparticles using microfluidic technology | Bone fracture | Local injection | Pan et al[68] |
| Hybrid exosome-liposome system | Hyaluronic acid-based hydrogel microspheres via microfluidic techniques | Osteoarthritis | Local injection | Chen et al[69] |
| Adipose stem cells derived exosomes | Clinical efficacy of microneedle-based administration of exosome | Facial skin aging | Microneedles | Park et al[72] |
| Adipose-derived stem cell extracellular vesicles | A chondroitin sulfate C-based dissolving microneedle | Rheumatoid arthritis | Microneedles | Bui et al[75] |
| Bone marrow MSCs derived exosomes | A silk fibroin microneedle patch consisting of LPS-pre-Exos and zeolitic imidazolate framework-8 | Oral ulcers | Microneedles | Ge et al[76] |
| MSCs derived exosomes | Gelatin methacrylate microneedles had excellent 3D-Exo loading capacity and enabled continuous 3D-Exo release to maintain effective therapeutic concentrations | Ischemia reperfusion | Microneedles | Zhang et al[77] |
| Mice breast cancer cell line 4T1 derived exosomes | Nano EXOs loaded within porous microneedles were employed for precise delivery of the STING agonist MSA-2 (MEM) to the tumor site | Tumor | Microneedles | Chen et al[78] |
| Platelet-derived exosomes | A methacrylate-modified decellularized dermal matrix hydrogel-based microneedle patch | Diabetic wounds | Microneedles | Cao et al[79] |
| Microneedle system achieving a balance between mechanical robustness and solubility | Microneedles | Mu et al[80] | ||
| MSCs derived exosomes | A novel core-shell microneedle patch | Scarless skin repair | Microneedles | Lyu et al[81] |
| MSCs derived exosomes | A core-shell microneedle model | Osteoarthritis | Microneedles | Li et al[82] |
| T cells derived exosomes | A nanovesicle-based delivery platform to promote the efficacy of chemotherapeutic drugs with fewer side effects | Non-small cell lung cancer | Nebulizing | Zheng et al[83] |
| MSCs derived exosomes | Explored the safety of nebulized haMSC-EVs in healthy volunteers | Preclinical lung injury model | Nebulizing | Shi et al[84] |
| MSCs derived exosomes | Nebulization of MSC-derived exosomes is a safe, effective, and simple method, and their application at the beginning of treatment may be more beneficial | COVID-19 pneumonia | Nebulizing | Chu et al[85] |
Compared to artificially synthesized drug carriers, EVs derived from cells exhibit several advantages, including higher biocompatibility, lower toxicity, enhanced tissue penetration, and the ability to cross biological barriers such as the blood-brain barrier, enabling long-distance intercellular communication[11,12]. These properties make EVs a promising next-generation drug delivery platform. EVs can be administered through various conventional delivery routes, including intravenous injection, intraperitoneal injection, and even oral administration[13-16]. In the systemic application of EVs, their plasma half-life is typically only 2-30 minutes, and they are rapidly cleared by phagocytosis or collected in parenchymal organs such as the liver and spleen[9]. This sequestration significantly hinders their delivery to target tissues beyond the mononuclear phagocyte system and the liver[17]. How to address these challenges in systemic administration is a key focus of researchers.
Although EVs can be internalized through various strategies, intravenous injection remains one of the most widely used approaches. Lightner et al[18] validated the safety and efficacy of bone marrow mesenchymal stem cell (MSC)-derived EVs in treating acute respiratory distress syndrome in critically ill coronavirus disease 2019 (COVID-19) patients through intravenous injection. Similarly, Zamanian et al[19] demonstrated the efficacy and safety of human placental mesen
Genetic engineering strategies for EVs: One of the primary biological barriers that EVs encounter upon administration is the immune response mediated by the macrophage system, which leads to their rapid early clearance. As a result, EVs injected systemically often struggle to deliver their cargo effectively to distant target sites. Inspired by the immune evasion mechanisms of tumor cells, researchers have engineered EVs to express CD47, acting as a form of ‘camouflage’ that aids in evading macrophage-mediated immune clearance.
Liu et al[20] integrated the CD47 gene into cells via lentiviral transduction, generating CD47-overexpressing cells, and then isolated their secreted exosomes via ultracentrifugation. These engineered exosomes demonstrated immune evasion capability and were successfully used to treat hepatic ischemia-reperfusion injury. Similarly, Creeden et al[21] utilized CD9, a biomarker enriched on the surface of exosomes, as a chassis. By engineering the E174 site of CD9, they integrated CD47 p110-130 (a key segment believed to confer immune evasion) into the exosomal membrane and demonstrated its immune evasion function.
Another critical challenge in the drug delivery process is ensuring that therapeutic agents reach the intended target site with precision, necessitating the design of targeting and controlled release systems. Due to the homing effect, EVs inherently possess a certain degree of targeting capability. The homing effect of exosomes refers to their ability to be specifically transported back to their originating cells or certain target cells and is attributed to the distinct integrin expression patterns on their membranes[22-24]. By selecting EVs from appropriate sources and applying suitable engineering modifications, EVs can achieve enhanced targeting precision in drug delivery.
Wang et al[25] selected BV2 mouse microglial cells as the source of exosomes and designed a functional oligopeptide. This oligopeptide contained cysteine residues that could form disulfide crosslinks, effectively locking the therapeutic drug within the exosome and, preventing its premature release into the bloodstream. Upon crossing the blood-brain barrier and reaching glioblastoma tissues - where the local glutathione concentration is high - the disulfide bonds undergo reductive cleavage, triggering controlled drug release at the target site. This strategy successfully established a targeted and controlled release system for EV-based drug delivery.
Building on this foundation, the targeting capability of EV-based delivery systems can be further enhanced through targeted peptide design. Several membrane proteins, such as CD9, CD63, CD81, and lysosome-associated membrane protein 2b, are ubiquitously expressed on exosomes. Cell-targeting peptides can be displayed on the surface of exosomes through genetic fusion with the extracellular domains of these proteins[26]. Lin et al[27] fused a cartilage-affinity peptide to the N-terminus of lysosome-associated membrane protein 2b, generating cartilage-targeting exosomes by transfecting cells with a plasmid encoding the fusion construct. Similarly, Creeden et al[21] inserted an RGD peptide into the E174 of CD9, increasing CD9’s binding affinity to integrin αvβ3, a well-known RGD peptide receptor, and conferring pancreatic ductal adenocarcinoma-targeting capability.
Engineered vesicles not only address some of the challenges associated with systemic applications but also play a crucial role in the localized application of EVs. Zhang et al[28] anchored an arginine-rich cationic motif onto the negatively charged exosomal bilayer, developing a delivery system capable of reversible binding to cartilage proteoglycan-glycosaminoglycans, which are negatively charged. This approach facilitated deep penetration into early-stage osteoarthritic cartilage, effectively delivering encapsulated mRNA to chondrocytes in deep tissue layers. In addition to functional modifications for targeting, engineered EVs can also exert direct therapeutic effects. Yu et al[29] conjugated an anti-tumor necrosis factor-α antibody to the surface of exosomes using a matrix metalloproteinase-cleavable peptide linker, demonstrating its synergistic therapeutic effects in corneal alkali burns. Likewise, Liu et al[30] loaded Sephin1 onto exosomes via an intermittent ultrasound method, thereby modulating endoplasmic reticulum homeostasis for the treatment of bone defects in diabetes. Through engineering modifications, the issues faced by EV after injection can be largely addressed. However, these methods are often complex, requiring intricate modifications of genes or membrane structure, which pose challenges to the stability and safety of the vesicles.
Ultrasound targeted microbubble destruction assistance: Nearly 30 years ago, researchers observed that microbubbles in ultrasound contrast agents could be disrupted by ultrasound exposure. This phenomenon was subsequently confirmed both in vitro and in vivo. In in vivo experiments, Skyba et al[31] observed microbubble destruction within microvessels ≤ 7 μm in diameter (primarily capillaries), which was accompanied by capillary rupture and red blood cell extravasation. Following this discovery, Price et al[32] applied this ultrasound-mediated microbubble disruption (UTMD) technique to drug delivery research, demonstrating its potential for targeted drug transport. Subsequent studies extended this technique to various targeted therapies, including cancer, rheumatoid arthritis, and cardiac and renal diseases. More recently, Chen et al[33] employed this approach for cardiac-targeted exosome delivery, confirming its efficacy in enhancing targeted exosome transport.
UTMD employs a physical approach to enhance local microvascular permeability, providing a novel strategy for the precise and efficient targeted delivery of EVs. This method is particularly advantageous for targeting organs or deep-seated lesions that are difficult to reach with conventional approaches. Moreover, by adjusting the intensity, frequency, and duration of ultrasound exposure, UTMD enables spatiotemporal control over drug release. However, this technique requires high-precision ultrasound equipment and carries the potential risk of localized tissue damage. For clinical applications, challenges such as dose standardization, long-term safety evaluation, and personalized treatment protocol design still need to be addressed.
Tannic acid surface modification: Tannic acid (TA) is a polyphenolic compound widely found in plants, including in tea leaves, tree bark, fruits, and tree leaves[34]. TA interacts with materials through hydrophobic interactions, electrostatic adsorption, and hydrogen bonding via the abundant phenolic hydroxyl groups it contains. Therefore, it is extensively applied in membrane technology as a multifunctional coating molecule for various substrates in many shapes, including films and particles[35]. However, there is still limited research on surface modification of EVs using TA. Kumar et al[36] developed a droplet-based microvesicle system, where a uniform and robust (TA-Fe3+) protective barrier approximately 10 nm thick was formed on the surface of EVs through coordination complexes between TA and iron ions. This approach demonstrated that the (TA-Fe3+) coating method allows for the customization of exosomes to target specific cells with potential therapeutic anti-tumor effects.
In addition to serving as a protective barrier for EVs, TA holds great potential for cardiac-targeted therapy. Directly targeting the heart via systemic injection presents a significant challenge due to the heart’s continuous dynamic contraction and relaxation cycles, and accompanying large volume changes. Furthermore, rapid and substantial blood flow exchange prevents the therapeutic agents from remaining in the heart for extended periods. Most methods for local delivery of therapeutic agents to the heart require surgical intervention. TA, which contains a portion rich in phenolic hydroxyl groups and five gallic acid groups, can interact with extracellular matrix (ECM) proteins via hydrophobic interactions and hydrogen bonding, exhibiting high affinity for proline-rich proteins in the ECM, such as elastin and collagen. This makes TA a potentially useful agent for targeting heart tissue. Shin et al[37] developed a TA-modification protein technology called TANNylation, which showed stable heart-targeting effects. This method can also be applied to target EVs to heart tissue. Compared to directly modifying the membrane structure of EV, TA surface modification is more conservative. The coating significantly enhances the stability of the EVs; however, further research is needed to confirm how these modifications alter the original delivery capabilities of EVs.
Exosome delivery methods based on MNPs: Through the directional movement of MNPs under an external magnetic field, researchers have developed a novel targeting system. Various methods have been employed to pair MNPs with EVs and guide the EVs to targeted areas using a strong external magnetic field. This approach has gained significant attention, particularly in cancer treatment, not only because of its targeting capability but also due to its potential to kill cancer cells with drugs released from EVs as well as through magnetic hyperthermia induced by MNPs. When enriched in tumor regions, MNPs can be heated under an alternating magnetic field, thereby inducing tumor necrosis[38].
There are many strategies to combine MNPs with EV. Li et al[39] incubated umbilical MSCs together with iron oxide nanoparticles (IONPs) at a concentration of 50 μg/mL for 16 hours. After EV isolation and purification, intravenous injection followed by the application of a 1.2 Tesla magnetic field successfully targeted the EVs to the desired location. Lee et al[40] co-incubated IONPs and bone marrow MSCs at a concentration of 40 μg/mL for 24 hours to produce exosomes containing IONPs, which were subsequently used to reduce ischemic heart injury. In addition to co-incubation, MNPs can also be directly attached to the EV membrane. Zhang et al[41] enhanced the tumor-targeting ability of neutrophil-derived exosomes by modifying them with superparamagnetic IONPs through transferrin-transferrin receptor interactions. Liu et al[42] designed a shuttle system composed of an iron oxide (Fe3O4) core, a silica (SiO2) shell, and a stimulus-cleavable polyethylene glycol crown conjugated with two types of antibodies. This system binds to and enriches EVs through CD63 antibodies, and then guides the EVs to target regions under a magnetic field. Li et al[43] designed a similar platform consisting of an Fe3O4 core and a mesoporous silica shell conjugated with a CD63 antibody, which binds to CD63 antigens on the surface of EVs. Using three-dimensional printing technology, a magnetic helmet was constructed for nude mice, and after applying a local magnetic field, the platform was shown to be accumulated in the brain vasculature.
The cytotoxicity of MNPs to general cells largely depends on factors such as size, shape, surface coating, exposure dose, exposure time, and particle composition. Specific parameters need to be carefully controlled during the design to prevent any adverse effects on cell viability, metabolic activity, oxidative stress, proliferation, and differentiation[44]. Through various strategies, MNP targeting technology imparts the ability for EVs to move directionally in a magnetic field. Compared to direct genetical engineering of EVs, this approach offers a more gentle and conservative engineering process. Additionally, MNP can generate heat under an alternating magnetic field, potentially exerting therapeutic effects in various tumors. However, during the application of this technology, it is crucial to monitor dose toxicity, control particle parameters, and prevent the occurrence of cytotoxicity.
For systemic drug applications, oral administration is generally the preferred route; however, it presents significant challenges. Due to the harsh gastrointestinal environment, it is difficult for drugs to be fully absorbed through the gastrointestinal wall. As a result, the bioavailability of orally administered drugs is very low[45]. Over the years, researchers have been exploring the use of functionalized nanoparticle drug delivery systems to improve drug release in oral administration. With the increasing research into food-derived EVs, EVs have gradually been recognized and studied as a novel drug delivery system for oral administration.
Research on breast milk has revealed that milk-derived EV are signaling bodies from the mother, promoting offspring growth, maturation, immune function, and metabolic programming. A deeper understanding of the molecular biology of milk has led to the conclusion that infants are both “breastfed” and “breast-programmed”[46]. This indirectly proves that certain EVs can resist the complex gastrointestinal environment and maintain efficacy after oral absorption. Similar to breast milk, milk exosomes (MEs) can survive and be absorbed in the highly acidic environment of the stomach and the degradative environment of the intestine. Another prominent feature of MEs is their use of membrane proteins that extend their circulation time in the bloodstream, contributing to more effective drug delivery[45]. In 2016, Munagala et al[47] first demonstrated that MEs are good carriers for chemotherapy drugs. Later, Esfahani et al[48] used MEs to transport resveratrol for the treatment of inflammatory bowel disease, achieving significant therapeutic effects. These studies indicate that the oral administration route of MEs is worthy of attention and further investigation.
In addition to MEs, Hwang et al[49] showed that yam-derived exosome-like nanoparticles can be transported through the gastrointestinal tract, be absorbed by the small intestine, and safely and effectively treat osteoporosis. Chen et al[50] isolated and purified exosome-like nanoparticles from tea leaves, which, through direct apoptosis induction and microbiome modulation, exhibited good therapeutic effects on breast cancer, whether administered intravenously or orally. Furthermore, the significant liver and kidney toxicity seen with intravenous administration was not apparent with oral administration.
Food-derived EVs are widely available, cost-effective, and show significant efficacy in disease treatment. As an oral drug delivery platform, they hold great potential. However, their absorption and mechanism of action remain unclear and require further investigation. Additionally, research on the engineering of orally administrable EVs derived from food sources is still limited, and their therapeutic potential may yet be further enhanced.
Although various brain-targeting drugs have been developed, how to effectively cross the blood-brain barrier and achieve sufficient drug levels in brain tissue remains a challenge. EVs possess the ability to traverse biological barriers and facilitate long-distance transport, making them promising candidates as a delivery system targeting brain tissue. Nasal administration provides a practical, non-invasive method for delivering therapeutic agents to the brain. Zhuang et al[51] alternately administered exosome-based therapeutic agents into the bilateral nostrils of mice and detected labeled exosomes in the brain, demonstrating that this delivery route can quickly and efficiently deliver exosome-based therapeutics to the brain. Furthermore, Huang et al[52] designed and synthesized a hydrogel composed of self-assembling peptides that are degradable by membrane enzymes present in MSC-EVs. After nasal administration, this smart-release hydrogel extended the residence time of MSC-EVs at the site of administration, released MSC-EVs in a controlled manner, and significantly enhanced the therapeutic efficacy of MSC-EVs for Alzheimer’s disease and their clinical translational potential. These results suggest that nasal administration could be an excellent approach for non-invasive brain-targeted drug delivery.
In addition to the direct administration of isolated and purified EVs into the body, researchers have developed assisted delivery strategies to enhance local tissue retention and sustained release of EVs. By combining EVs with these delivery strategies, their therapeutic efficacy can be significantly improved. Furthermore, some delivery strategies possess intrinsic therapeutic properties, further augmenting the overall therapeutic effects of EV-based treatments. Currently, the most used auxiliary delivery systems include hydrogels, microneedle patches and nebulized delivery.
Within localized implantation strategies, patches are the most common and convenient option. Among various types of patches, hydrogel patches are currently the primary focus of research. Hydrogels are three-dimensional networks of hydrophilic polymer chains that are crosslinked through physical or chemical interactions. Due to the presence of numerous hydrophilic functional groups, hydrogels can absorb large amounts of water and swell without dissolving in aqueous environments[53].
Natural hydrogels are typically derived from plant and animal extracts, including chitosan, cellulose, hyaluronic acid (HA), and collagen. In contrast, synthetic hydrogels are composed of materials such as polyacrylamide and its deri
Due to their intrinsic physicochemical properties, hydrogels serve as effective wound dressings by providing a moist and mildly acidic environment, protecting wounds, relieving pain, promoting cell proliferation, preventing tissue necrosis, and mimicking the esophageal ECM structure[57]. Moreover, their porous architecture can effectively encapsulate EVs, offering a stable microenvironment that supports EV retention and sustained release, thereby enhancing their therapeutic efficacy in topical applications. Tan et al[58] synthesized a gelatin methacrylate (GelMA) composite hydrogel and demonstrated its ability to support the loading of plant-derived EVs. The study confirmed that the hydrogel-EV system promoted cell proliferation and migration, suppressed exotoxin-induced inflammation, and ultimately accelerated wound healing.
Beyond directly loading EVs into hydrogels, researchers have developed strategies to incorporate additional components inside of on the surface of hydrogels to enhance exosome adsorption or amplify their therapeutic effects. Shang et al[59] developed a chitosan-based composite hydrogel dressing by integrating chitosan nanoparticles which facilitate exosome adsorption, carboxymethyl chitosan, bioactive glass, and TiO2. This hydrogel was designed for treating deep skin lesions, diabetic wounds, and burns, and it was shown to accelerate wound healing, stimulate angiogenesis, enhance collagen deposition, and increase the expression of anti-inflammatory factors. Han et al[60] encapsulated exosomes within oxygen nanobubbles, which were then incorporated into a PVA/gelatin hydrogel. This approach not only alleviated wound hypoxia but also provided an efficient strategy for delivering exosome-loaded nanoparticles under hypoxic conditions. In another study, Yang et al[61] immobilized exosomes within an HA hydrogel coated with polydopamine and the antimicrobial peptide DP7, achieving the dual function of antimicrobial activity and wound healing. Furthermore, they addressed the challenge of maintaining exosome bioactivity during long-term storage by developing a “rapid freeze-dry-thaw” strategy, which preserved hydrogel integrity and exosome functionality.
Beyond serving as a topical dressing carrier for EV-based wound treatments, hydrogels can also be directly injected in vivo, offering localized therapeutic and sustained-release functions. An ideal injectable hydrogel should possess three characteristics: (1) Bio-adhesiveness and superior tissue integration to prevent hydrogel detachment from defect sites; (2) Appropriate mechanical strength and stability to adapt to complex local tissue environments and self-heal after rupture to maintain structural integrity; and (3) Efficient encapsulation and sustained release of EVs, ensuring long-term therapeutic effects.
Since hydrogels undergo sol-gel transitions via precursor solution crosslinking, they can be designed as injectable biomaterials without compromising functionality. Injectable hydrogels are particularly advantageous for precisely implanting EV-based therapies into deep or enclosed anatomical sites using specialized delivery devices. The fluid properties of hydrogel precursors allow them to conform to irregularly shaped target regions, achieving seamless integration with surrounding tissues upon gelation. Liu et al[30] synthesized a high-performance HA-based hydrogel using ADH-grafted HA and aldehyde-functionalized HA to treat diabetes-related bone defects. Liu et al[62] developed an injectable self-healing biphasic hydrogel composed of carboxymethyl chitosan and pullulan, which was used to encapsulate EVs for promotion of flap survival after skin graft surgery.
In addition to dual-component injectable hydrogels, which undergo mild crosslinking reactions at the injection site, single-component injectable hydrogels have also emerged as a viable alternative. For instance, Liang et al[63] designed an alginate-based hydrogel scaffold system, which, after loading exosomes, was injected with minimal invasiveness into the uterine cavity of mice to form a coating that promoted endometrial repair. Similarly, Wang et al[64] developed an injectable hydrogel derived from decellularized ECM to encapsulate EVs for spinal cord injury treatment. Moreover, Xiong et al[65] incorporated exosomes into a thermosensitive poloxamer 407 hydrogel system, which rapidly gelled post-injection, enabling localized exosome release in situ. This thermosensitive hydrogel platform has also been validated in other studies[66,67]. Huang et al[52] synthesized a self-assembling peptide-based hydrogel, which was temperature-responsive and enzymatically degradable by EV-associated enzymes, thus facilitating the sustained and thermosensitive release of MSC-derived EVs.
Another promising approach in injectable hydrogel design is the development of hydrogel microparticles incor
Microneedles are drug delivery enhancement systems frequently used for transdermal drug administration. They were originally developed to overcome the formidable barrier function of the stratum corneum, the outermost layer of the skin, which is nearly impermeable to large hydrophilic molecules during transdermal drug delivery. A microneedle-based delivery system consists of a series of sub-millimeter-sized needles mounted on a base. These needles penetrate the stratum corneum, enabling painless drug administration without disrupting the dermis[70,71]. Park et al[72] validated the clinical efficacy of microneedle-based administration of the combination of human adipose stem cell-derived exosomes and β-glucan solution in the treatment of facial skin aging.
Hydrogels have increasingly become the ideal material for next-generation microneedles due to their tunable physicochemical properties, design flexibility, and adaptability in biomedical engineering[71,73]. Polysaccharides have been frequently utilized to fabricate dissolvable microneedles, leading to the development of carbohydrate-based microneedle arrays with significant potential in drug administration, diagnostics, and biosensing.
Beyond transdermal drug delivery, microneedles have been explored for ocular, oral mucosal, gastrointestinal, nail, and vaginal drug administration, demonstrating broad applicability in localized treatments. While systemic administration of EVs can be effective, local administration provides higher drug concentrations at the target site with fewer systemic side effects. Among local administration methods, microneedle patches offer distinct advantages, including sustained drug release at the target site, which helps maintain effective therapeutic concentrations over extended periods[74].
Bui et al[75] developed dissolvable microneedles composed of chondroitin sulfate C, which enabled the non-invasive delivery of human adipose stem cell-derived EVs into inflamed joints. Similarly, Ge et al[76] utilized the excellent biocompatibility, biodegradability, and mechanical properties of silk fibroin to fabricate a novel composite microneedle system. In this design, exosomes were loaded into the microneedle tips, while zeolitic imidazolate framework-8 was incorporated into the microneedle base for the treatment of oral ulcers.
Microneedle patches are an optimal platform for enhancing EV uptake and controlled release since EVs can be encapsulated, stored, and protected within the matrix. Yao et al[73] successfully delivered mitochondria-containing EVs using a hydrogel microneedle system, demonstrating its protective and efficient delivery capabilities. Zhang et al[77] fabricated a microneedle system using GelMA loaded with MSC-derived hydrogels, effectively mitigating ischemia-reperfusion injury. Similarly, Yu et al[29] developed PVA-based microneedles for precise and sustained exosome delivery, achieving superior therapeutic outcomes in corneal alkali burn treatment compared with conventional methods. Chen et al[78] utilized an MNP system for delivering MSA-2, replacing the traditional intratumoral injection, to optimize the effects of FLASH radiotherapy. Furthermore, Cao et al[79] designed a methacrylate-modified decellularized dermal matrix hydrogel-based microneedle patch, effectively delivering platelet-derived exosomes and overcoming the limi
Beyond conventional, single-component hydrogel microneedles, researchers have developed dual-component microneedles with a core-shell structure, which offers greater mechanical strength and time-dependent release profiles. Mu et al[80] designed a microneedle system with trehalose and HA as the needle tip material, and polyvinylpyrrolidone as the backing layer, achieving a balance between mechanical robustness and solubility. Lyu et al[81] fabricated a novel shell-core microneedle, where GelMA formed the outer shell loaded with mangiferin for early-stage anti-inflammatory effects, while dimethylacrylamide (sometimes referred to as PGLADMA) constituted the core, encapsulating exosomes for sustained anti-inflammatory and pro-angiogenic effects. Likewise, Li et al[82] developed a core-shell microneedle model, which demonstrated promising therapeutic efficacy in osteoarthritis treatment. Similar to hydrogels, microneedles show significant effects and good feasibility in transdermal drug delivery. However, the underlying mechanism of action is difficult to extend to other drug delivery routes.
Studies have shown that the chemotherapy efficacy for non-small cell lung cancer (NSCLC) is 40%-50%, with paclitaxel (PTX) and platinum-based drugs as first-line strategies. These formulations and routes of administration often induce dose-dependent toxicity, injection extravasation, and phlebitis, while the non-specific delivery of free PTX usually causes inflammation and enhances adverse drug reactions. Zheng et al[83] proposed a new therapeutic strategy by nebulizing PTX-loaded exosomes (PTX@CAR-Exo) for targeted local treatment of lung cancer. Inhalation of chemotherapy drug-loaded CAR-exosomes demonstrates significant clinical potential for treating NSCLC. This strategy not only offers a nanoparticle carrier that enhances the efficacy of anti-cancer drugs but also reduces the clinical adverse reactions associated with PTX chemotherapy in NSCLC. Shi et al[84] demonstrated the biodistribution and therapeutic effects of aerosolized human adipose-derived MSC-EVs in a preclinical lung injury model and explored the safety of aerosolized human adipose-derived MSC-EVs in healthy volunteers. Chu et al[85] conducted a pilot study with seven COVID-19 pneumonia patients, administering aerosolized MSC-derived exosomes, and confirmed that aerosolization of MSC-derived exosomes is a safe, effective, and simple method. The results of those studies indicate that EVs can exert their effects in the lungs through nebulization, potentially becoming a new therapeutic approach for pulmonary diseases. It is worth mentioning that, as previously discussed, nasal administration can also lead to the accumulation of EVs in the lungs.
As a rising star in cell-free therapy, EVs have demonstrated great potential in the treatment of various diseases. In this review, we presented strategies for both systemic and localized applications of EVs. Researchers select EVs from appropriate sources and employ engineering approaches to enhance their therapeutic potential, immune evasion, and targeting capabilities. Building on this foundation, the integration of suitable in vitro systems has led to significant progress in improving EV immune evasion, targeting efficiency, sustained release, and adverse effect control. Numerous studies in animal models have yielded promising results, addressing some of these challenges to a certain extent.
However, the clinical translation of EV-based therapies still has a long way to go. Large-scale EV production, preservation, and transportation remain major challenges that require further technological advancements and more standardized protocols. Additionally, clinical trials for EV-based therapies are still relatively scarce. Many clinical studies related to EVs are still in the safety verification stage. Even among those that have achieved therapeutic effects, relatively simple administration protocols are often used. At the same time, although there are research guidelines for various types of studies[86], a complete set of clinical treatment guidelines has not yet been established. The good news is that we are seeing an increasing number of EV-related studies being conducted, as seen in the Clinical Trials database. We hope that in the near future, EV-based treatments will become a reality in clinical settings, offering new hope to patients worldwide. We also hope that researchers from all fields can work together to develop clinical research and treatment guidelines for EVs as soon as possible, establishing standardized treatment and research protocols.
| 1. | Nowak M, Górczyńska J, Kołodzińska K, Rubin J, Choromańska A. Extracellular Vesicles as Drug Transporters. Int J Mol Sci. 2023;24:10267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 2. | Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1470] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Harding C, Heuser J, Stahl P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol. 1984;35:256-263. [PubMed] |
| 4. | Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2293] [Cited by in RCA: 2691] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 5. | Schuh CMAP, Cuenca J, Alcayaga-Miranda F, Khoury M. Exosomes on the border of species and kingdom intercommunication. Transl Res. 2019;210:80-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2579] [Article Influence: 214.9] [Reference Citation Analysis (0)] |
| 7. | Tang Y, Zhou Y, Li HJ. Advances in mesenchymal stem cell exosomes: a review. Stem Cell Res Ther. 2021;12:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 8. | Yang C, Xue Y, Duan Y, Mao C, Wan M. Extracellular vesicles and their engineering strategies, delivery systems, and biomedical applications. J Control Release. 2024;365:1089-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Kang M, Jordan V, Blenkiron C, Chamley LW. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J Extracell Vesicles. 2021;10:e12085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 10. | Parada N, Romero-Trujillo A, Georges N, Alcayaga-Miranda F. Camouflage strategies for therapeutic exosomes evasion from phagocytosis. J Adv Res. 2021;31:61-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 11. | Sadeghi S, Tehrani FR, Tahmasebi S, Shafiee A, Hashemi SM. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology. 2023;31:145-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 12. | Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 904] [Article Influence: 226.0] [Reference Citation Analysis (0)] |
| 13. | Gu Z, Yin Z, Song P, Wu Y, He Y, Zhu M, Wu Z, Zhao S, Huang H, Wang H, Tong C, Qi Z. Safety and biodistribution of exosomes derived from human induced pluripotent stem cells. Front Bioeng Biotechnol. 2022;10:949724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 14. | Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S, Hashemi SM. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 2021;236:5906-5920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 15. | Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, Saji H, Takakura Y. Accelerated growth of B16BL6 tumor in mice through efficient uptake of their own exosomes by B16BL6 cells. Cancer Sci. 2017;108:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 16. | Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC. Milk-derived exosomes for oral delivery of paclitaxel. Nanomedicine. 2017;13:1627-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 395] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 17. | Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 552] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 18. | Lightner AL, Sengupta V, Qian S, Ransom JT, Suzuki S, Park DJ, Melson TI, Williams BP, Walsh JJ, Awili M. Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicle Infusion for the Treatment of Respiratory Failure From COVID-19: A Randomized, Placebo-Controlled Dosing Clinical Trial. Chest. 2023;164:1444-1453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 19. | Zamanian MH, Norooznezhad AH, Hosseinkhani Z, Hassaninia D, Mansouri F, Vaziri S, Payandeh M, Heydarpour F, Kiani S, Shirvani M, Rajati M, Bakhtiari M, Esmaili F, Yarani R, Mansouri K. Human placental mesenchymal stromal cell-derived small extracellular vesicles as a treatment for severe COVID-19: A double-blind randomized controlled clinical trial. J Extracell Vesicles. 2024;13:e12492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Liu S, Xiao X, Zhang L, Wang J, Zhao W, Liu H, Liao R, Li Z, Xu M, Guo J, Zhou B, Du C, Peng Q, Jiang N. Reprogramming Exosomes to Escape from Immune Surveillance for Mitochondrial Protection in Hepatic Ischemia-Reperfusion Injury. Theranostics. 2024;14:116-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Reference Citation Analysis (0)] |
| 21. | Creeden JF, Sevier J, Zhang JT, Lapitsky Y, Brunicardi FC, Jin G, Nemunaitis J, Liu JY, Kalinoski A, Rao D, Liu SH. Smart exosomes enhance PDAC targeted therapy. J Control Release. 2024;368:413-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Reference Citation Analysis (0)] |
| 22. | Qiao L, Hu S, Huang K, Su T, Li Z, Vandergriff A, Cores J, Dinh PU, Allen T, Shen D, Liang H, Li Y, Cheng K. Tumor cell-derived exosomes home to their cells of origin and can be used as Trojan horses to deliver cancer drugs. Theranostics. 2020;10:3474-3487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 23. | Ji P, Yang Z, Li H, Wei M, Yang G, Xing H, Li Q. Smart exosomes with lymph node homing and immune-amplifying capacities for enhanced immunotherapy of metastatic breast cancer. Mol Ther Nucleic Acids. 2021;26:987-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Gebeyehu A, Kommineni N, Meckes DG Jr, Sachdeva MS. Role of Exosomes for Delivery of Chemotherapeutic Drugs. Crit Rev Ther Drug Carrier Syst. 2021;38:53-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Wang Y, Huo Y, Zhao C, Liu H, Shao Y, Zhu C, An L, Chen X, Chen Z. Engineered exosomes with enhanced stability and delivery efficiency for glioblastoma therapy. J Control Release. 2024;368:170-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 26. | Rädler J, Gupta D, Zickler A, Andaloussi SE. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol Ther. 2023;31:1231-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 27. | Lin Z, Xu G, Lu X, Liu S, Zou F, Ma X, Jiang J, Wang H, Song J. Chondrocyte-targeted exosome-mediated delivery of Nrf2 alleviates cartilaginous endplate degeneration by modulating mitochondrial fission. J Nanobiotechnology. 2024;22:281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 28. | Zhang C, Pathrikar TV, Baby HM, Li J, Zhang H, Selvadoss A, Ovchinnikova A, Ionescu A, Chubinskaya S, Miller RE, Bajpayee AG. Charge-Reversed Exosomes for Targeted Gene Delivery to Cartilage for Osteoarthritis Treatment. Small Methods. 2024;8:e2301443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 29. | Yu F, Zhao X, Wang Q, Fang PH, Liu L, Du X, Li W, He D, Zhang T, Bai Y, Liu L, Li S, Yuan J. Engineered Mesenchymal Stromal Cell Exosomes-Loaded Microneedles Improve Corneal Healing after Chemical Injury. ACS Nano. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 30. | Liu Y, Lin S, Xu Z, Wu Y, Wang G, Yang G, Cao L, Chang H, Zhou M, Jiang X. High-Performance Hydrogel-Encapsulated Engineered Exosomes for Supporting Endoplasmic Reticulum Homeostasis and Boosting Diabetic Bone Regeneration. Adv Sci (Weinh). 2024;11:e2309491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 31. | Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98:290-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 330] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 32. | Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 271] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Chen J, Qiu S, Liu Y, Sun W, Zhou T, Zhao L, Li Z, Duan Y. Ultrasound targeted microbubble destruction assisted exosomal delivery of siHmox1 effectively inhibits doxorubicin-induced cardiomyocyte ferroptosis. J Nanobiotechnology. 2024;22:531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 34. | Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313-7352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2102] [Cited by in RCA: 1856] [Article Influence: 123.7] [Reference Citation Analysis (0)] |
| 35. | Sileika TS, Barrett DG, Zhang R, Lau KH, Messersmith PB. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew Chem Int Ed Engl. 2013;52:10766-10770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 701] [Cited by in RCA: 550] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 36. | Kumar S, Michael IJ, Park J, Granick S, Cho YK. Cloaked Exosomes: Biocompatible, Durable, and Degradable Encapsulation. Small. 2018;14:e1802052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Shin M, Lee HA, Lee M, Shin Y, Song JJ, Kang SW, Nam DH, Jeon EJ, Cho M, Do M, Park S, Lee MS, Jang JH, Cho SW, Kim KS, Lee H. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat Biomed Eng. 2018;2:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 38. | Lin Y, Zhang K, Zhang R, She Z, Tan R, Fan Y, Li X. Magnetic nanoparticles applied in targeted therapy and magnetic resonance imaging: crucial preparation parameters, indispensable pre-treatments, updated research advancements and future perspectives. J Mater Chem B. 2020;8:5973-5991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Li X, Wang Y, Shi L, Li B, Li J, Wei Z, Lv H, Wu L, Zhang H, Yang B, Xu X, Jiang J. Magnetic targeting enhances the cutaneous wound healing effects of human mesenchymal stem cell-derived iron oxide exosomes. J Nanobiotechnology. 2020;18:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 40. | Lee JR, Park BW, Kim J, Choo YW, Kim HY, Yoon JK, Kim H, Hwang JW, Kang M, Kwon SP, Song SY, Ko IO, Park JA, Ban K, Hyeon T, Park HJ, Kim BS. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020;6:eaaz0952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 41. | Zhang J, Ji C, Zhang H, Shi H, Mao F, Qian H, Xu W, Wang D, Pan J, Fang X, Santos HA, Zhang X. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci Adv. 2022;8:eabj8207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 42. | Liu S, Chen X, Bao L, Liu T, Yuan P, Yang X, Qiu X, Gooding JJ, Bai Y, Xiao J, Pu F, Jin Y. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng. 2020;4:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 184] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 43. | Li B, Chen X, Qiu W, Zhao R, Duan J, Zhang S, Pan Z, Zhao S, Guo Q, Qi Y, Wang W, Deng L, Ni S, Sang Y, Xue H, Liu H, Li G. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv Sci (Weinh). 2022;9:e2105451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 44. | Chen Y, Hou S. Recent progress in the effect of magnetic iron oxide nanoparticles on cells and extracellular vesicles. Cell Death Discov. 2023;9:195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 45. | Zhong J, Xia B, Shan S, Zheng A, Zhang S, Chen J, Liang XJ. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277:121126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 46. | Melnik BC, Stremmel W, Weiskirchen R, John SM, Schmitz G. Exosome-Derived MicroRNAs of Human Milk and Their Effects on Infant Health and Development. Biomolecules. 2021;11:851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 47. | Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 686] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 48. | Esfahani SK, Dehghani S, Hosseinzadeh H, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. An exosomal approach for oral delivery of resveratrol: Implications for inflammatory bowel disease treatment in rat model. Life Sci. 2024;346:122638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Hwang JH, Park YS, Kim HS, Kim DH, Lee SH, Lee CH, Lee SH, Kim JE, Lee S, Kim HM, Kim HW, Kim J, Seo W, Kwon HJ, Song BJ, Kim DK, Baek MC, Cho YE. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 100] [Reference Citation Analysis (0)] |
| 50. | Chen Q, Zu M, Gong H, Ma Y, Sun J, Ran S, Shi X, Zhang J, Xiao B. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. J Nanobiotechnology. 2023;21:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 74] [Reference Citation Analysis (0)] |
| 51. | Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1083] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 52. | Huang M, Zheng M, Song Q, Ma X, Zhang Q, Chen H, Jiang G, Zhou S, Chen H, Wang G, Dai C, Li S, Li P, Wang H, Zhang A, Huang Y, Chen J, Gao X. Comparative Proteomics Inspired Self-Stimulated Release Hydrogel Reinforces the Therapeutic Effects of MSC-EVs on Alzheimer's Disease. Adv Mater. 2024;36:e2311420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 53. | Ho TC, Chang CC, Chan HP, Chung TW, Shu CW, Chuang KP, Duh TH, Yang MH, Tyan YC. Hydrogels: Properties and Applications in Biomedicine. Molecules. 2022;27:2902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 332] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 54. | Nishimoto S, Takagi M, Wakitani S, Nihira T, Yoshida T. Effect of chondroitin sulfate and hyaluronic acid on gene expression in a three-dimensional culture of chondrocytes. J Biosci Bioeng. 2005;100:123-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Jeon YH, Choi JH, Sung JK, Kim TK, Cho BC, Chung HY. Different effects of PLGA and chitosan scaffolds on human cartilage tissue engineering. J Craniofac Surg. 2007;18:1249-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Xue X, Hu Y, Deng Y, Su J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv Funct Mater. 2021;31:2009432. [RCA] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 251] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 57. | Yuan N, Shao K, Huang S, Chen C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int J Biol Macromol. 2023;240:124321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 144] [Reference Citation Analysis (0)] |
| 58. | Tan S, Liu Z, Cong M, Zhong X, Mao Y, Fan M, Jiao F, Qiao H. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing Staphylococcus aureus exotoxins for the care of invasive wounds. J Control Release. 2024;368:355-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 59. | Shang S, Zhuang K, Chen J, Zhang M, Jiang S, Li W. A bioactive composite hydrogel dressing that promotes healing of both acute and chronic diabetic skin wounds. Bioact Mater. 2024;34:298-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 60. | Han X, Saengow C, Ju L, Ren W, Ewoldt RH, Irudayaraj J. Exosome-coated oxygen nanobubble-laden hydrogel augments intracellular delivery of exosomes for enhanced wound healing. Nat Commun. 2024;15:3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 75] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 61. | Yang Y, Zhang J, Wu S, Deng Y, Wang S, Xie L, Li X, Yang L. Exosome/antimicrobial peptide laden hydrogel wound dressings promote scarless wound healing through miR-21-5p-mediated multiple functions. Biomaterials. 2024;308:122558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 62. | Liu X, Chen H, Lei L, Yang P, Ju Y, Fan X, Fang B. Exosomes-carried curcumin based on polysaccharide hydrogel promote flap survival. Int J Biol Macromol. 2024;270:132367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 63. | Liang Y, Shuai Q, Zhang X, Jin S, Guo Y, Yu Z, Xu X, Ao R, Peng Z, Lv H, He S, Wang C, Song G, Liu Z, Zhao H, Feng Q, Du R, Zheng B, Chen Z, Xie J. Incorporation of Decidual Stromal Cells Derived Exosomes in Sodium Alginate Hydrogel as an Innovative Therapeutic Strategy for Advancing Endometrial Regeneration and Reinstating Fertility. Adv Healthc Mater. 2024;13:e2303674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 64. | Wang G, Li Q, Liu S, Li M, Liu B, Zhao T, Liu B, Chen Z. An injectable decellularized extracellular matrix hydrogel with cortical neuron-derived exosomes enhances tissue repair following traumatic spinal cord injury. Mater Today Bio. 2024;28:101250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 65. | Xiong J, Liu Z, Jia L, Sun Y, Guo R, Xi T, Li Z, Wu M, Jiang H, Li Y. Bioinspired engineering ADSC nanovesicles thermosensitive hydrogel enhance autophagy of dermal papilla cells for androgenetic alopecia treatment. Bioact Mater. 2024;36:112-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 66. | Chen X, Tao J, Zhang M, Lu Z, Yu Y, Song P, Wang T, Jiang T, Zhao X. Iota carrageenan gold-silver NPs photothermal hydrogel for tumor postsurgical anti-recurrence and wound healing. Carbohydr Polym. 2022;298:120123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 67. | Liao Z, Ke W, Liu H, Tong B, Wang K, Feng X, Hua W, Wang B, Song Y, Luo R, Liang H, Zhang W, Zhao K, Li S, Yang C. Vasorin-containing small extracellular vesicles retard intervertebral disc degeneration utilizing an injectable thermoresponsive delivery system. J Nanobiotechnology. 2022;20:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 68. | Pan S, Yin Z, Shi C, Xiu H, Wu G, Heng Y, Zhu Z, Zhang J, Gui J, Yu Z, Liang B. Multifunctional Injectable Hydrogel Microparticles Loaded with miR-29a Abundant BMSCs Derived Exosomes Enhanced Bone Regeneration by Regulating Osteogenesis and Angiogenesis. Small. 2024;20:e2306721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 69. | Chen M, Lu Y, Liu Y, Liu Q, Deng S, Liu Y, Cui X, Liang J, Zhang X, Fan Y, Wang Q. Injectable Microgels with Hybrid Exosomes of Chondrocyte-Targeted FGF18 Gene-Editing and Self-Renewable Lubrication for Osteoarthritis Therapy. Adv Mater. 2024;36:e2312559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 70. | Rzhevskiy AS, Singh TRR, Donnelly RF, Anissimov YG. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J Control Release. 2018;270:184-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 71. | Avcil M, Çelik A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines (Basel). 2021;12:1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 72. | Park GH, Kwon HH, Seok J, Yang SH, Lee J, Park BC, Shin E, Park KY. Efficacy of combined treatment with human adipose tissue stem cell-derived exosome-containing solution and microneedling for facial skin aging: A 12-week prospective, randomized, split-face study. J Cosmet Dermatol. 2023;22:3418-3426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 73. | Yao WD, Zhou JN, Tang C, Zhang JL, Chen ZY, Li Y, Gong XJ, Qu MY, Zeng Q, Jia YL, Wang HY, Fan T, Ren J, Guo LL, Xi JF, Pei XT, Han Y, Yue W. Hydrogel Microneedle Patches Loaded with Stem Cell Mitochondria-Enriched Microvesicles Boost the Chronic Wound Healing. ACS Nano. 2024;18:26733-26750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 74. | Liu W, Zhai X, Zhao X, Cai Y, Zhang X, Xu K, Weng J, Li J, Chen X. Multifunctional Double-Layer and Dual Drug-Loaded Microneedle Patch Promotes Diabetic Wound Healing. Adv Healthc Mater. 2023;12:e2300297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 75. | Bui VD, Jeon J, Duong VH, Shin S, Lee J, Ghahari F, Kim CH, Jo YJ, Jung WK, Um W, Park JH. Chondroitin sulfate-based microneedles for transdermal delivery of stem cell-derived extracellular vesicles to treat rheumatoid arthritis. J Control Release. 2024;375:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 76. | Ge W, Gao Y, Zeng Y, Yu Y, Xie X, Liu L. Silk Fibroin Microneedles Loaded with Lipopolysaccharide-Pretreated Bone Marrow Mesenchymal Stem Cell-Derived Exosomes for Oral Ulcer Treatment. ACS Appl Mater Interfaces. 2024;16:37486-37496. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 77. | Zhang Q, Liu T, Li Y, Fan Y, Shang H, Zhao H, Sun H, Yu Z, Han M, Wan C. Gelatin methacryloyl microneedle loaded with 3D-MSC-Exosomes for the protection of ischemia-reperfusion. Int J Biol Macromol. 2024;275:133336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 78. | Chen Z, Hu F, Xiang J, Zhou X, Wu B, Fan B, Tang H, Liu B, Chen L. Mesoporous Microneedles Enabled Localized Controllable Delivery of Stimulator of Interferon Gene Agonist Nanoexosomes for FLASH Radioimmunotherapy against Breast Cancer. ACS Appl Mater Interfaces. 2024;16:58180-58190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 79. | Cao Y, Chen B, Liu Q, Mao Y, He Y, Liu X, Zhao X, Chen Y, Li X, Li Y, Liu L, Guo C, Liu S, Tan F, Lu H, Liu J, Chen C. Dissolvable microneedle-based wound dressing transdermally and continuously delivers anti-inflammatory and pro-angiogenic exosomes for diabetic wound treatment. Bioact Mater. 2024;42:32-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 80. | Mu S, Chang H, Qu F. Fabrication and Characterization of Microneedle Patches for Loading and Delivery of Exosomes. J Vis Exp. 2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 81. | Lyu S, Liu Q, Yuen HY, Xie H, Yang Y, Yeung KW, Tang CY, Wang S, Liu Y, Li B, He Y, Zhao X. Correction: A differential-targeting core-shell microneedle patch with coordinated and prolonged release of mangiferin and MSC-derived exosomes for scarless skin regeneration. Mater Horiz. 2024;11:2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Li Z, Lu H, Fan L, Ma X, Duan Z, Zhang Y, Fu Y, Wang S, Guan Y, Yang D, Chen Q, Xu T, Yang Y. Microneedle-Delivered PDA@Exo for Multifaceted Osteoarthritis Treatment via PI3K-Akt-mTOR Pathway. Adv Sci (Weinh). 2024;11:e2406942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 83. | Zheng W, Zhu T, Tang L, Li Z, Jiang G, Huang X. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer. J Transl Med. 2023;21:383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 84. | Shi MM, Yang QY, Monsel A, Yan JY, Dai CX, Zhao JY, Shi GC, Zhou M, Zhu XM, Li SK, Li P, Wang J, Li M, Lei JG, Xu D, Zhu YG, Qu JM. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J Extracell Vesicles. 2021;10:e12134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 85. | Chu M, Wang H, Bian L, Huang J, Wu D, Zhang R, Fei F, Chen Y, Xia J. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev Rep. 2022;18:2152-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 86. | Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, Cai H, Di Vizio D, Driedonks TAP, Erdbrügger U, Falcon-Perez JM, Fu QL, Hill AF, Lenassi M, Lim SK, Mahoney MG, Mohanty S, Möller A, Nieuwland R, Ochiya T, Sahoo S, Torrecilhas AC, Zheng L, Zijlstra A, Abuelreich S, Bagabas R, Bergese P, Bridges EM, Brucale M, Burger D, Carney RP, Cocucci E, Crescitelli R, Hanser E, Harris AL, Haughey NJ, Hendrix A, Ivanov AR, Jovanovic-Talisman T, Kruh-Garcia NA, Ku'ulei-Lyn Faustino V, Kyburz D, Lässer C, Lennon KM, Lötvall J, Maddox AL, Martens-Uzunova ES, Mizenko RR, Newman LA, Ridolfi A, Rohde E, Rojalin T, Rowland A, Saftics A, Sandau US, Saugstad JA, Shekari F, Swift S, Ter-Ovanesyan D, Tosar JP, Useckaite Z, Valle F, Varga Z, van der Pol E, van Herwijnen MJC, Wauben MHM, Wehman AM, Williams S, Zendrini A, Zimmerman AJ; MISEV Consortium, Théry C, Witwer KW. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024;13:e12404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1603] [Cited by in RCA: 1393] [Article Influence: 1393.0] [Reference Citation Analysis (0)] |