Published online Jul 26, 2025. doi: 10.4252/wjsc.v17.i7.104607
Revised: March 26, 2025
Accepted: June 18, 2025
Published online: July 26, 2025
Processing time: 210 Days and 0.3 Hours
Type 1 diabetes (T1D) results from the autoimmune-mediated loss of pancreatic β-cells. Current insulin therapies offer symptomatic relief but fall short of providing a definitive cure. Islet cell transplantation, while promising, faces limitations related to donor scarcity, procedural complexities, and the necessity for long-term immunosuppression. Consequently, there is an urgent need for innovative strategies aimed at β-cell regeneration. Patient-derived induced pluripotent stem cells (iPSCs), obtained from peripheral blood mononuclear cells (PBMCs) of T1D patients, hold great potential as a source of cells for therapeutic purposes. Therefore, the differentiation of T1D-iPSCs into functional pancreatic β-cells is a critical step toward effective β-cell replacement therapy.
To assess the potential of patient-derived T1D-β-like cells (differentiated from T1D-iPSCs reprogrammed from T1D-PBMCs) for restoring β-cell function in T1D.
T1D-iPSCs were reprogrammed from T1D-PBMCs using an episomal vector-based approach. Pluripotency was confirmed by flow cytometry (FCM), quanti
T1D-iPSCs were successfully generated from T1D-PBMCs. These cells exhibited the hallmark characteristics of pluripotent stem cells, including appropriate morphology, differentiation potential, genomic integrity, and expression of pluripotency-associated genes. Differentiation yielded insulin-positive (insulin+) pancreatic β-like cells that, at the mRNA level, expressed key β-cell markers such as pancreatic duodenal homeobox-1, Ngn3, MafA, NeuroD, glucagon-like peptide-1 receptor, Nkx6.1, glucose transporter 2, and Kir6.2. Notably, the T3 + Vc group displayed the lowest Ngn3 expression (1.31 ± 0.38 vs 1.96 ± 0.25 vs 2.51 ± 0.24, P < 0.01), while the M3C + T3 + Vc group exhibited the highest MafA expression (0.49 ± 0.11 vs 0.32 ± 0.06 vs 0.29 ± 0.08, P < 0.05). Both in vitro and in vivo assessments confirmed the insulin secretion ability of the generated β-like cells; however, they did not demonstrate appropriate modulation of insulin release in response to variations in extracellular glucose concentrations.
T1D-iPSCs derived from T1D-PBMCs can be differentiated into insulin+ β-like cells, albeit with functional immaturity. These cells represent a potential source of seed cells for β-cell replacement therapy in T1D.
Core Tip: This study successfully employed an episomal plasmid system to reprogram patient peripheral blood mononuclear cells into type 1 diabetes (T1D)-induced pluripotent stem cells (iPSCs). Optimization of the differentiation protocol, utilizing triiodothyronine (T3), vitamin C (Vc), and adenovirus-M3C, facilitated the directed differentiation of T1D-iPSCs toward pancreatic β-cells. The T3 + Vc group exhibited minimal neurogenin 3 expression, while the M3C + T3 + Vc group demonstrated maximal MAF bZIP transcription factor A expression. While insulin⁺ β-like cells differentiated from T1D-iPSCs displayed basal insulin secretion, they lacked the ability to respond to glucose stimulation, indicating functional immaturity compared to fully mature β-cells. These results underscore their potential as seed cells for β-cell replacement therapy in T1D.
- Citation: Wang K, Lin W, Han JY, Chen JY, Liu RH, Yu Z, Jin JJ. Differentiation of patient-specific induced pluripotent stem cells derived from type 1 diabetes peripheral blood mononuclear cells into pancreatic β-like cells. World J Stem Cells 2025; 17(7): 104607
- URL: https://www.wjgnet.com/1948-0210/full/v17/i7/104607.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i7.104607
Diabetes mellitus is a metabolic condition marked by chronic hyperglycemia resulting from various underlying factors, and is a major health concern that affects a significant number of individuals globally. The International Diabetes Federation[1] projects that by 2045, approximately one in eight adults will live with diabetes, a 46% increase from 2021, potentially affecting approximately 700 million people.
Individuals with T1D require lifelong insulin therapy. Although islet transplantation can improve blood glucose control[2,3], its widespread application is hindered by the limited availability of donor organs, technical difficulties in efficient islet isolation, and the requirement for prolonged immunosuppressant therapy. The underlying factor in diabetes pathogenesis[4,5] is the reduction in functional pancreatic β-cell capacity, leading to insufficient insulin secretion to maintain blood glucose balance. Current investigations into β-cell replacement therapy focus on exploring the regenerative capabilities of the pancreas and identifying cell sources for generating functional β-cells[6-8]. These efforts primarily involve promoting the proliferation of existing pancreatic β-cells, promoting the maturation of pancreatic progenitors into insulin-secreting β-cells, transdifferentiating pancreatic ductal cells, α-cells, and acinar cells into functional β-cells, and facilitating the maturation of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) into fully functional β-cells. Consequently, identifying novel seed cells has emerged as a prominent area of research[9-11].
ESCs and iPSCs can differentiate into mature β-cells. However, ethical considerations and potential cancer risks remain barriers to their clinical use[12-17]. In this study, patient-specific type 1 diabetes (T1D)-iPSCs were generated by inducing peripheral blood mononuclear cells (PBMCs) in vitro from a T1D patient using a less invasive and ethically sound approach to obtain donor cells. This approach ensured that the resulting T1D-iPSCs resisted immune rejection, unlike other iPSC sources. Furthermore, the non-integrating, highly efficient episomal vector system used in this study offers a feasible source of patient-specific iPSCs for β-cell replacement therapy[18].
Triiodothyronine (T3) and vitamin C (Vc) were employed to modulate the expression of mature and immature cell markers during β-cell differentiation to improve insulin secretion, enhance the differentiation of T1D-iPSCs into β-cells, and promote cell maturation[14,19-23]. However, β-cell maturation involves intricate gene regulation, requiring the coordinated action of various β-cell-specific transcription factors, including pancreatic duodenal homeobox-1 (Pdx1), neurogenin 3 (Ngn3), MAF bZIP transcription factor A (MafA), NeuroD, glucagon-like peptide-1 receptor (GLP1R), Nkx6.1, glucose transporter 2 (Glut2), and Kir6.2. In this study, M3C adenoviruses expressing Pdx1, Ngn3, and MafA were used to enhance the expression of mature β-cell surface markers[24,25].
Pdx1, a key transcription factor in islet endocrine cell development, functions as a master regulator of pancreatic differentiation[25]. Ngn3 plays a critical role in early pancreatic development and endocrine differentiation by activating downstream genes and promoting the expression of related transcription factors to facilitate directional islet cell differentiation. MafA, a β-cell-specific insulin transcription factor, is critical in pancreatic development and regulates insulin synthesis[23]. During early pancreatic development, Pdx1 and Ngn3 are expressed in various pancreatic islet cells, while MafA expression is restricted to the mass development of insulin-producing cells. Specific genes in pancreatic development regulate β-cell neogenesis in streptozotocin (STZ)-induced diabetic mice[23-25]. Therefore, inducing the differentiation of T1D-iPSCs into insulin+ β-like cells represents a potential advancement in diabetes treatment.
This study adhered to the Helsinki Declaration principles and was approved by the Ethics Committee of Fujian Provincial Hospital (approval number: K2021-04-020). Blood samples were collected from patients with T1D at Fujian Provincial Hospital after obtaining informed consent in compliance with ethical guidelines. Blood (8 mL) was drawn via veni
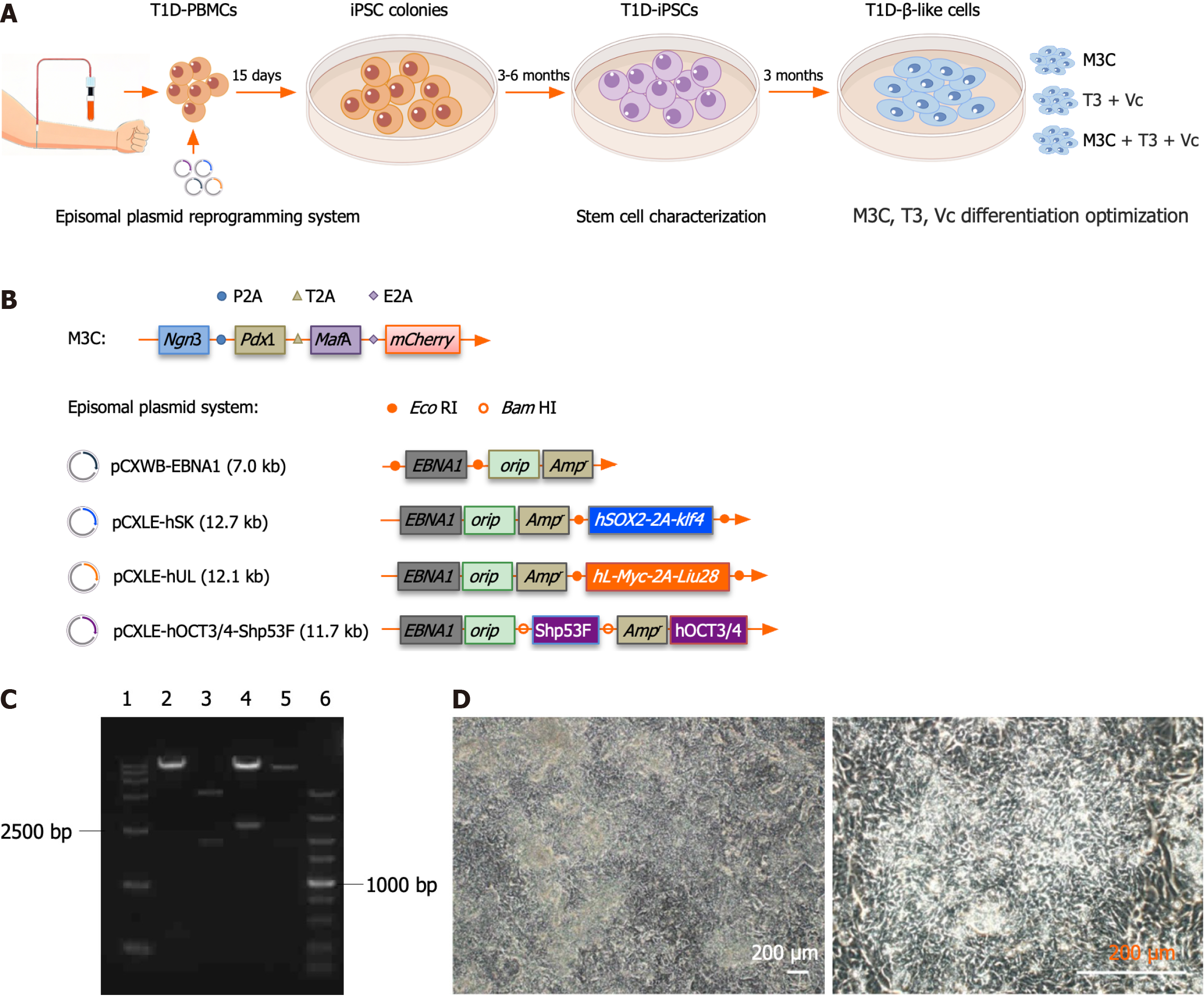
Adenovirus M3C (Figure 1B; #61041, Addgene, MA, United States), designed to express Pdx1, Ngn3, MafA, and mCherry, was introduced into 293A cells using Lipofectamine™ 3000 (Invitrogen, CA, United States), and amplified at a multiplicity of infection (MOI) between 10 and 20. When cytopathological effects were observed in most cells, the cells and culture medium were harvested in a centrifuge tube. Adherent cells were detached using a cell scraper and centrifuged at 3500 × g for 15 minutes to pellet them. The virus was concentrated using the Vivapure® AdenoPACK™ kit (SARTORIUSVS, Lower Saxony, Germany), aliquoted into 100 μL aliquots, according to the manufacturer’s instructions, and preserved at -80 °C.
Reprogramming was achieved via electroporation using an episomal system[26-30], comprising four plasmids (#27078, #37624, #27080, #27077; Figure 1B; Addgene, MA, United States): PCXLE-hSK (12.7 kb), pCXWB-EBNA1 (7.0 kb), pCXLE-hUL (12.1 kb), and pCXLE-hOCT3/4-Shp53F (11.7 kb). The plasmids were extracted in large quantities and were ensured to be free of endotoxins using a plasmid purification kit (QIAGEN, Hilden, Germany). Before electroporation, T1D-PBMCs were thawed and cultured for 4-5 days. To enhance the survival rate of transfected cells[27], six-well plates were pre-coated with fibronectin (ThermoFisher Scientific, MA, United States) and CD34+ cells (Nuwacell Biotech, Hefei, China) the day before electroporation. Cryopreserved CD34+ cells were thawed and cultured in an expansion medium on fibronectin-coated plates: StemSpan SFEM (STEMCELL Technologies, Vancouver, Canada) supplemented with EX-CYTE (1:1000, Merck Millipore, Billerica, MA, United States), 2 mmol/L GlutaMAX, and a cytokine cocktail containing 250 ng/mL stem cell factor, 250 ng/mL fms-like tyrosine kinase 3 ligand, 100 ng/mL thrombopoietin, 20 ng/mL interleukin-3 (IL-3), 50 ng/mL IL-6, and 10 ng/mL IL-6 receptor (Eprotech, NJ, United States).
After 7 days of expansion, T1D-PBMCs and the episomal system were used for electroporation. Each 100 μL electroporation mixture included 82 μL of solution and 18 μL of supplement (containing up to 10 μL DNA and 3 × 106 to 1 × 107 T1D-PBMCs). This mixture was transferred to an electroporation cuvette and electroporated using a Nucleofector™ 2b device (Lonza, Basel, Switzerland) with program U-014. After electroporation, cells were incubated in X-vivo™10 medium (Lonza, Basel, Switzerland) at 37 °C with 5% CO2 for 5 hours, followed by adding medium containing 30 IU/mL IL-2 (PeproTech, NJ, United States) and 25 μL/mL CD3/CD28 Dynabeads™ (ThermoFisher Scientific, MA, United States). The T1D-PBMCs (1 × 106/mL) were seeded into fibronectin and CD34+ cell-coated well plates for 43 hours. Subsequently, DMEM/F12 medium (ThermoFisher Scientific, MA, United States) containing 10 μmol/L Y27632 (StemCell Technologies, British Columbia, Canada), 1 × N-2 (ThermoFisher Scientific, MA, United States), 1 × B-27 (ThermoFisher Scientific, MA, United States), 100 ng/mL basic fibroblast growth factor (PeproTech, NJ, United States), 0.5 μmol/L PD0325901 (Stemgent, MA, United States), 3 μmol/L CHIR99021 (Stemgent, MA, United States), 0.5 μmol/L A-83-01 (Stemgent, MA, United States), 1000 U/mL hLIF (ThermoFisher Scientific, MA, United States), and 10 μmol/L HA-100 (Santa Cruz Biotechnology, TX, United States) were added. The medium was entirely refreshed with Essential 8 Medium (Thermo
To assess SSEA4+/TRA-1-81+ expression in T1D-iPSCs and insulin+/mCherry+ in T1D-β-like cells transplanted under the renal capsule using flow cytometry (FCM), T1D-iPSCs were dissociated into single-cell suspensions. Kidney transplant site tissues were processed into single-cell suspensions via homogenization and sieving for FCM analysis. Isotype antibody controls served as negative controls. The cell pellet was fixed with 0.5 mL of 4% paraformaldehyde after screening. The mixture was gently agitated and incubated at 4 °C for 15 minutes in the dark. The supernatant was removed after centrifugation at 500 × g for 5 minutes at 4 °C. Subsequently, the cells were permeabilized with 0.5 mL of 0.1% Triton X-100 and incubated at 4 °C for 10 minutes in the dark. Fluorescently conjugated antibodies, including mouse anti-SSEA-4 PE (1:100, Cat. No. 560128; BD Biosciences, CA, United States), mouse anti-human TRA-1-81 PE (1:100, Cat. No. 560161; BD Biosciences, CA, United States), insulin (C27C9) rabbit mAb PE (1:50, Cat. No. 8508S, Cell Signaling Technology, MA, United States), and rabbit (DA1E) mAb IgG XP® Isotype Control (PE) (Cat. No. 5742, Cell Signaling Technology, MA, United States), diluted in blocking solution, was added and maintained at room temperature (20-28 °C) for 30 minutes in the dark. The cells were rinsed with 2 mL phosphate buffered saline (PBS), centrifuged at 500 × g for 5 minutes at 4 °C, and the supernatant was removed. Finally, the cell pellet was resuspended in 0.5 mL of 1% paraformaldehyde. After fixation and sieve filtration, the cell suspension was analyzed using FCM.
Adherent cells were harvested, and total RNA was extracted and quantified. RNA integrity was verified using electrophoresis (Supplementary Figure 1A). The cDNA was synthesized using quantitative real-time polymerase chain reaction (qRT-PCR), and relative qPCR was performed in triplicate for each sample, using GAPDH as a reference gene. The stable expression of GAPDH cDNA was confirmed across all groups (Supplementary Figure 1B). The primer sequences used are listed in Supplementary Table 1. A 20-μL reaction mixture was prepared in a PCR tube containing 10 μL of 2 × SYBR Green Master Mix, 0.4 μL each of Primer 1 and 10 μmol/L Primer 2, 0.4 μL of 50 × ROX Reference Dye, N μL of cDNA, and (8.8 - N) μL of ddH2O. The following steps were performed: Denaturation at 95 °C for 5 minutes, followed by 40 cycles of denaturation at 95 °C for 10 seconds, annealing/extension at 60 °C for 34 seconds, and final extension with melting curve analysis at 95 °C for 15 seconds, 60 °C for 60 seconds, and 95 °C for 15 seconds.
The Ct values of octamer-binding transcription factor 4 (OCT4), gEBNA1, and gOrip were determined to evaluate exogenous gene loss in T1D-iPSCs. The ratio of gEBNA1 to gOrip, termed X(n), was calculated for each sample. X(n) was used to generate a standard curve to determine Y(n). Complete loss of the exogenous gene was defined as Y(n) < Y(0.1), where Y(0.1) represents 0.1 copies of the foreign gene in the standard curve. Otherwise, the gene was considered to be retained. The mRNA expression levels of pluripotency markers (OCT4 and NANOG) in T1D-iPSCs and pancreatic β-cell markers [insulin 1 (Ins1), Ins2, NeuroD, GLP1R, Nkx6.1, Glut2, Kir6.2, Pdx1, Ngn3, and MafA] in T1D-β-like cells were determined. Relative quantification was conducted using the comparative Ct method, calculated as 2-ΔΔCt.
T1D-iPSCs were seeded into six-well plates. Upon reaching 60% confluence, the cells were incubated with 50 ng/mL colchicine at 37 °C for 2 hours, followed by dissociation, centrifugation at 300 × g for 5 minutes, and a single wash with PBS. Preheated hypotonic potassium chloride (KCl) at 37 °C was added, and the cells were transferred to 15 mL centrifuge tubes. Hypotonic KCl was added to reach a final volume of 10 mL, and the cells were incubated at 37 °C for 20-40 minutes. Methanol:glacial acetic acid (3:1) fixing solution was added, and the mixture was centrifuged at 200 × g for 10 minutes. After retaining 1 mL of the supernatant, the suspension was gently swirled while adding more fixing solution until the tube was full. The mixture was allowed to stand for 15 minutes and centrifuged at 500 × g for 5 minutes, and the supernatant was discarded. The fixing solution was slowly added while gently oscillating the tube until it was full, and the tubes were incubated at 4 °C overnight. The following day, the supernatant was discarded, and the cell pellet was immediately dropped onto pre-cooled glass slides (-20 °C). The slides were dried at 75 °C for 1-2 hours, numbered, and treated with trypsin and normal saline solution. After staining with Giemsa dye and drying at room temperature (20-28 °C), the slides were scanned to determine the karyotype.
The presence of bacteria and fungi was determined using standard culture methods. Syphilis was detected using agglutination tests. Hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were screened using enzyme-linked immunosorbent assay (ELISA). Mycoplasma species were identified using a two-step PCR with the cell culture supernatant as the template. PCR products were examined using agarose gel electrophoresis and compared with controls to verify the presence of mycoplasma.
All animal procedures were conducted in specific pathogen-free (SPF) animal barrier facilities and were approved by the Ethics Committee of the Fujian Academy of Medical Sciences (approval number: DL2021-05). T1D-iPSCs in the log phase were dissociated into single-cell suspensions and counted. Cells (2 × 106) were subcutaneously injected into the left lower abdominal region of severe combined immunodeficiency (SCID) mice (n = 5, males, 8-10 weeks, 18-22 g, SLAC Laboratory Animal, Shanghai, China). After 60 days, the mice were euthanized by intraperitoneal administration of sodium pentobarbital (150 mg/kg, Sigma-Aldrich, MA, United States). Death was confirmed by the absence of respiration, heartbeat, and pupillary reflex, followed by cervical dislocation. Teratomas were excised, fixed with paraffin, stained with hematoxylin and eosin, and examined microscopically to identify differentiation across the three germ layers and assess the differentiation potential of T1D-iPSCs.
T1D-iPSCs were cultured in a feeder-free system using Matrigel. Upon reaching approximately 85% confluence, the cells were passaged with EDTA. The cells were rinsed once with calcium- and magnesium-free Dulbecco’s PBS. The EDTA (0.5 mmol/L) was added, and the cells were incubated at 37 °C for 8 minutes. The EDTA solution was removed, and 2 mL of medium was slowly added to dissociate the cell clusters. Following gentle agitation, most cells were detached from the matrix. Dissociated cell clusters were promptly transferred to Matrigel-coated plates in a fresh medium. To enhance cell attachment and viability, 2.5 μmol/L of the ROCK inhibitor, blebbistatin (674289-55-5, KKLmed Inc., VA, United States), was added during the initial passage. Cells were passaged at a 1:8 to 1:10 ratio and cryopreserved every 3-4 days using this approach.
After confirming the identity of T1D-iPSCs, a four-step differentiation protocol (R protocol) was adopted[31-33]. Step 1: Cells were cultured with activin A (100 ng/mL; Peprotech, NJ, United States), wortmannin (1 μmol/L; Sigma-Aldrich, MA, United States), and SB-216763 (10 μmol/L; Santa Cruz Biotechnology, TX, United States) for 3 days. Step 2: The cells were cultured with PNU-74654 (5 μmol/L; Santa Cruz Biotechnology, TX, United States) for 3 days. Step 3: The cells were cultured with 5 ng/mL basic fibroblast growth factor and 5 μM forskolin (Merck-Calbiochem, NJ, United States) for 3 days. Step 4: Cells were cultured with retinoic acid (2 μM; Sigma-Aldrich, MA, United States), and 1 × 107 cells/tube were collected to promote cell differentiation into mature T1D-β-like cells in the direction of the pancreatic endocrine cells. T3 (20 ng; Sigma-Aldrich, MA, United States), Vc (10 μM; Sigma-Aldrich, MA, United States), and adenovirus M3C at an MOI of 20 were added to each milliliter of cell medium to enhance the expression of mature markers and reduce naive marker expression. Then, cells were differentiated into T1D-β-like cells.
T1D-β-like cells (1 × 105) were immunofluorescence-stained 24 hours post-viral infection (MOI = 20). Cells were fixed with 4% paraformaldehyde for 10 minutes and permeabilized with 0.5% Triton X-100 for 10 minutes at room temperature. The cells were blocked with 10% goat serum for 30 minutes to reduce non-specific binding. Cells were treated with an insulin-specific antibody (1:1000; Cat. No. 8138, Cell Signaling Technology, MA, United States) overnight at 4 °C. Subsequently, the cells were treated with diluted secondary antibodies (1:1000), specifically goat anti-rabbit IgG (Alexa Fluor® 488; Cat. No. 4412S, Cell Signaling Technology, MA, United States), for 2 hours at room temperature in dark. The nuclei were counterstained with DAPI solution for 5 minutes at room temperature. Images were captured using an inverted Leica DMi8 microscope (Leica, Wetzlar, Germany).
T1D-β-like cells were plated in 12-well plates and incubated overnight. On the day of the experiment, the culture medium was removed, and the wells were blotted with absorbent paper. Each experimental group (low-glucose, high-glucose, and KCl) was treated with 1 mL of Krebs-Ringer bicarbonate buffer with 1.7 mmol/L glucose, 20 mmol/L glucose, or 30 mmol/L KCl and added gently to the sides of the wells. After incubation at 37 °C in 5% CO2 for 1 hour, the supernatant was harvested, centrifuged at 1500 × g for 5 minutes at 4 °C, and 25 μL was stored at -20 °C for subsequent analysis. The remaining cells in each well were scraped into 0.18 M ethanol (35%) and disrupted by ultrasonication (10 seconds per cycle, three cycles, with 1 minute intervals between cycles). Then, the plates were shaken horizontally at 4 °C overnight. The next day, the lysates were centrifuged at 10000 × g for 30 minutes at 4 °C, and the supernatant was collected and stored at -20 °C. The cell pellet was reconstituted in 0.1 M NaOH, and the overall protein concentration was quantified using an enhanced bicinchoninic acid protein assay kit (Beyotime Biotechnology, Shanghai, China). Secreted and intracellular insulin levels were assessed using a human insulin ELISA kit (Merck Millipore, Darmstadt, Germany) and normalized to the overall protein concentrations.
After sonication, the culture supernatant and cell precipitates were collected and secreted, and intracellular insulin levels were measured. A parallel blank control was included for total cellular protein extraction and quantified using the bicinchoninic acid assay. The total protein content (μg) of the cells in parallel wells served as a standardized benchmark. Insulin content (pg or ng) was normalized to total protein and expressed as pg/μg or ng/μg for comparative analyses. The total insulin to total cellular protein ratio was calculated to standardize the results. ELISA was used to detect secreted and intracellular insulin, according to the manufacturer’s instructions.
All animal procedures complied with the regulations of the Fujian Academy of Medical Sciences Animal Care and Use Committee, China. Experiments were performed in SPF facilities, following strict biosafety and ethical protocols. The sample size was determined based on prior studies demonstrating statistical significance in functional assessments[34]. Randomization was not used due to the nature of the experimental design. All procedures were performed by blinded investigators. Male SCID mice (n = 5 per group, 8-10 weeks, 18-22 g, SLAC Laboratory Animal, Shanghai, China) were used in this study. Diabetes was induced by intraperitoneal administration of STZ (S0130, Sigma-Aldrich, MA, United States) at 160 mg/kg, prepared as a 20 g/L solution[32-34]. Blood glucose levels were monitored daily at 16:00 using tail vein blood and an ACCU-CHEK® Performa glucometer (Roche Diabetes Care GmbH, Mannheim, Germany). Diabetes was confirmed if blood glucose levels were > 19.4 mmol/L for 2 consecutive days.
The renal capsule was chosen as a model due to its high vascularization and immune-privileged status, which supports cell survival and function. The site also allows for graft monitoring, and its efficacy has been demonstrated in previous studies[32-34]. Based on the differentiation protocols for T1D-β-like cells, STZ-diabetic mice were divided into three groups (n = 5 per group): M3C, M3C + T3 + Vc, and control (sham-operated, receiving saline injection at the right kidney transplantation site). Following a 4-hour fasting period (with free access to water), renal subcapsular transplantation was performed in an SPF-grade sterile operating room. Given the potential for impaired peritoneal absorption, SCID diabetic mice were anesthetized via intramuscular administration of sodium pentobarbital (30 mg/kg) into the hind limb muscles[32-34]. A 1.5-cm surgical incision was made in the left flank to exteriorize the kidney, allowing for precise transplantation of 1 × 107 T1D-β-like cells (in 100 μL suspension) into the subcapsular space using a 30G catheter needle (BD Biosciences, NJ, United States), with careful control of the injection rate (2 μL/second) and post-injection dwell time (60 seconds) to prevent reflux. Before injection, the cells were aggregated overnight to form clusters. The control group underwent sham surgery.
After closing the incision, the mice were subcutaneously administered ketoprofen (5 mg/kg; Aladdin Biochemical Technology, Shanghai, China) for postoperative analgesia, followed by a second dose 24 hours later. Postoperatively, the mice were housed in a warm and quiet environment and monitored twice weekly. Two weeks post-transplantation, cell functionality was evaluated using an in vivo intraperitoneal glucose tolerance test (IGTT) and insulin tolerance test (ITT). Blood glucose levels were monitored daily from the tail vein at a consistent time for 20 days following transplantation. Mice were euthanized via intraperitoneal administration of sodium pentobarbital (150 mg/kg). Death was verified by the cessation of respiration, heartbeat, and pupillary reflexes. The right kidney, containing the transplanted T1D-β-like cells, was excised, homogenized, and processed into a cell suspension for FCM to assess insulin expression.
Before both tests, the bedding cage was replaced at 21:00 to prevent interference from food residue, and the mice were fasted for 12 hours with unrestricted access to water. At 9:00, the mice were weighed and assigned numbers, and fasting glucose levels were measured using tail vein blood samples. For the IGTT, mice were administered an intraperitoneal injection of 10 μL of 200 g/L glucose per gram of body weight, and blood glucose levels were assessed 15, 30, 60, 90, and 120 minutes after injection. Insulin was administered to treat hyperglycemia if needed. ITT was performed 3 days after the IGTT. Mice were administered an intraperitoneal injection of 10 μL of 0.05 IU/mL insulin per gram of body weight, and blood glucose levels were assessed 15, 30, 45, and 60 minutes after injection. Glucose was administered to prevent hypoglycemia, if necessary. After each test, the mice were provided with food and water ad libitum.
Statistical analysis was performed using Statistical Package for the Social Sciences software (version 21.0; IBM, Chicago, IL, United States). Statistical comparisons between two groups were performed using Student’s t-test, and multiple groups were analyzed using one-way analysis of variance. Data are presented as mean ± SD, with significance levels of aP < 0.05, bP < 0.01.
T1D-PBMC samples exhibited a high viability of 97.4% and a density of 1 × 106 cells/mL, fulfilling the requirements to initiate reprogramming. The cells displayed typical morphologies and robust activities. T1D-PBMCs were reprogrammed into T1D-iPSCs via electroporation using an episomal plasmid system. This plasmid system was prepared in large quantities and confirmed to be endotoxin-free. The spectrophotometric analysis yielded an A260/280 ratio of 1.8, indicating high DNA purity and a DNA concentration of > 0.5 μg/μL. The episomal plasmids pCXLE-hSK, pCXWB-EBNA1, and pCXLE-hUL were digested with the EcoRI restriction enzyme, while pCXLE-hOCT3/4-Shp53-F was digested with BamHI[18]. The resulting small DNA fragments (0.4-2.5 kbp) are visualized in Figure 1C. After electroporation, approximately 10% of the cells were lost. However, the positive control group consistently demonstrated a high trans
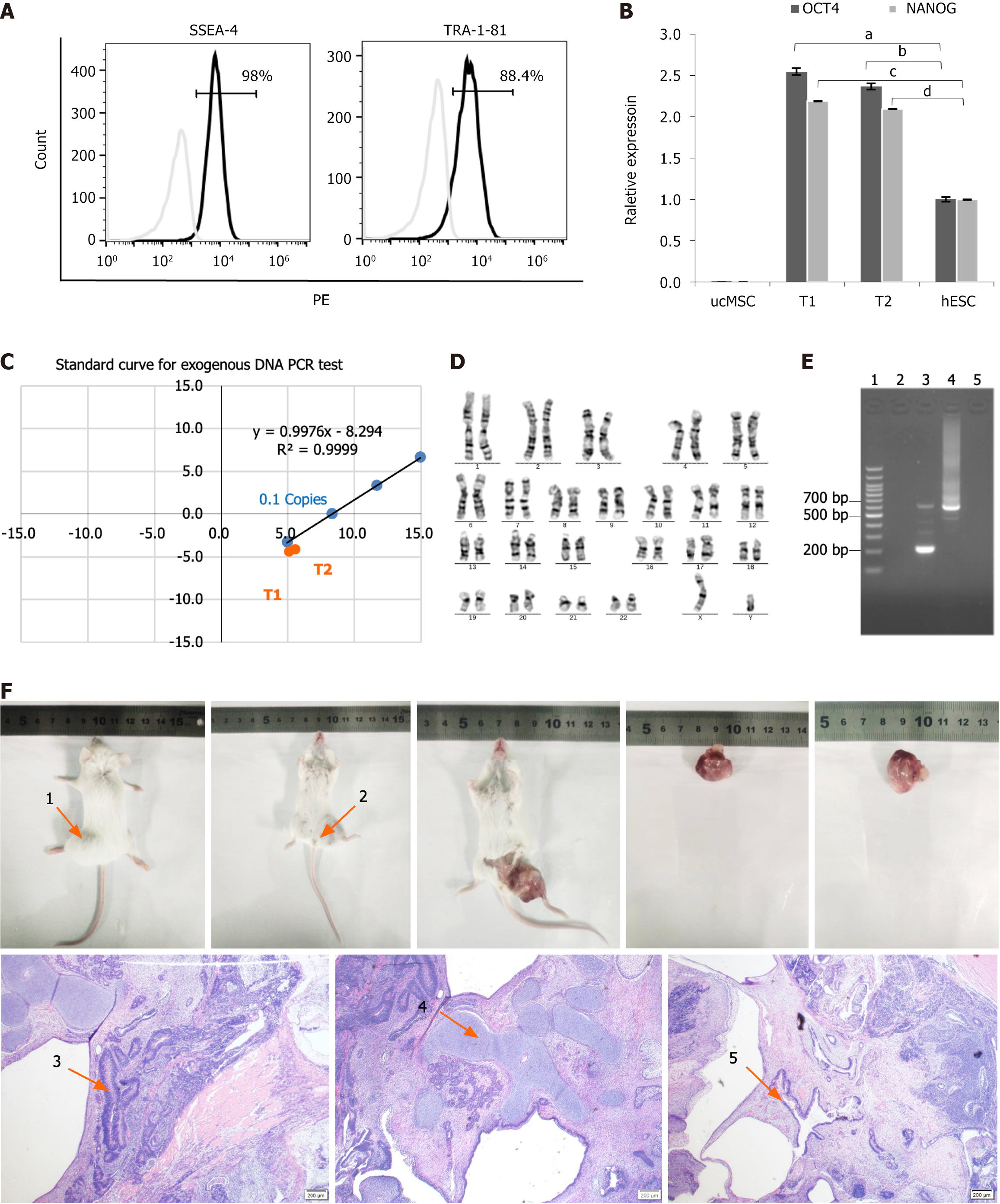
Approximately 14 days after electroporation of T1D-PBMCs with the episomal plasmid system, T1D-iPSC colonies were microscopically observed. By day 17, these colonies exhibited increased density, well-defined borders, closely packed cells, and a high nucleus-to-cytoplasm ratio, all characteristic of iPSCs. Visible colonies were selected around day 20 via EDTA-mediated dissociation and subcultured in fibronectin-coated dishes to establish stable, actively growing human iPSC colonies. FCM was employed to assess the expression of essential stem cell-specific markers, SSEA4 and TRA-1-81. Figure 2A depicts a significant proportion of cells (98%) expressed SSEA-4, with 88.4% demonstrating TRA-1-81 expression. The qRT-PCR was conducted to measure the relative expression levels of OCT4 and NANOG in different cell types. Figure 2B illustrates that T1D-iPSCs-1 (T1) and T1D-iPSCs-2 (T2) exhibited elevated expression levels of OCT4 and NANOG when normalized to those of human ESCs (n = 1). In contrast, umbilical cord mesenchymal stem cells displayed the lowest expression levels of OCT4 and NANOG. Umbilical cord mesenchymal stem cells and human ESCs were sourced from Nuwacell Biotech (Hefei, China). Furthermore, qRT-PCR analysis using Ct values for OCT4, gEBNA1, and gOrip confirmed the absence of episomal plasmid integration in T1D-iPSCs (Figure 2C). The positioning of T1 and T2 on the exogenous gene standard curve confirmed the complete elimination of exogenous genetic material.
The genomic stability of T1D-iPSCs was rigorously evaluated using chromosomal karyotyping to ensure the integrity of the reprogrammed cells. The analysis revealed a normal chromosomal karyotype devoid of numerical or structural aberrations (Figure 2D). Mycoplasma detection using PCR yielded negative results, indicating the absence of contamination, whereas the infected positive control exhibited the expected positive result (Figure 2E). Bacterial and fungal cultures were also negative, confirming the absence of microbial contamination. The pluripotency of T1D-iPSCs, indicating their ability to differentiate into various cell types, was further validated by in vivo teratoma formation. Teratoma formation assays confirmed the capacity of T1D-iPSCs to differentiate into cell types representative of all three primary germ layers (Figure 2F). The resulting tumor was 2.3 cm long, 2.1 cm wide, and weighed 1.91 g. Histological examination with hematoxylin and eosin staining revealed the presence of cells characteristic of ectodermal neural tissue, mesodermal cartilage tissue, and endodermal intestinal epithelial tissue, as indicated by the arrows in the figure.
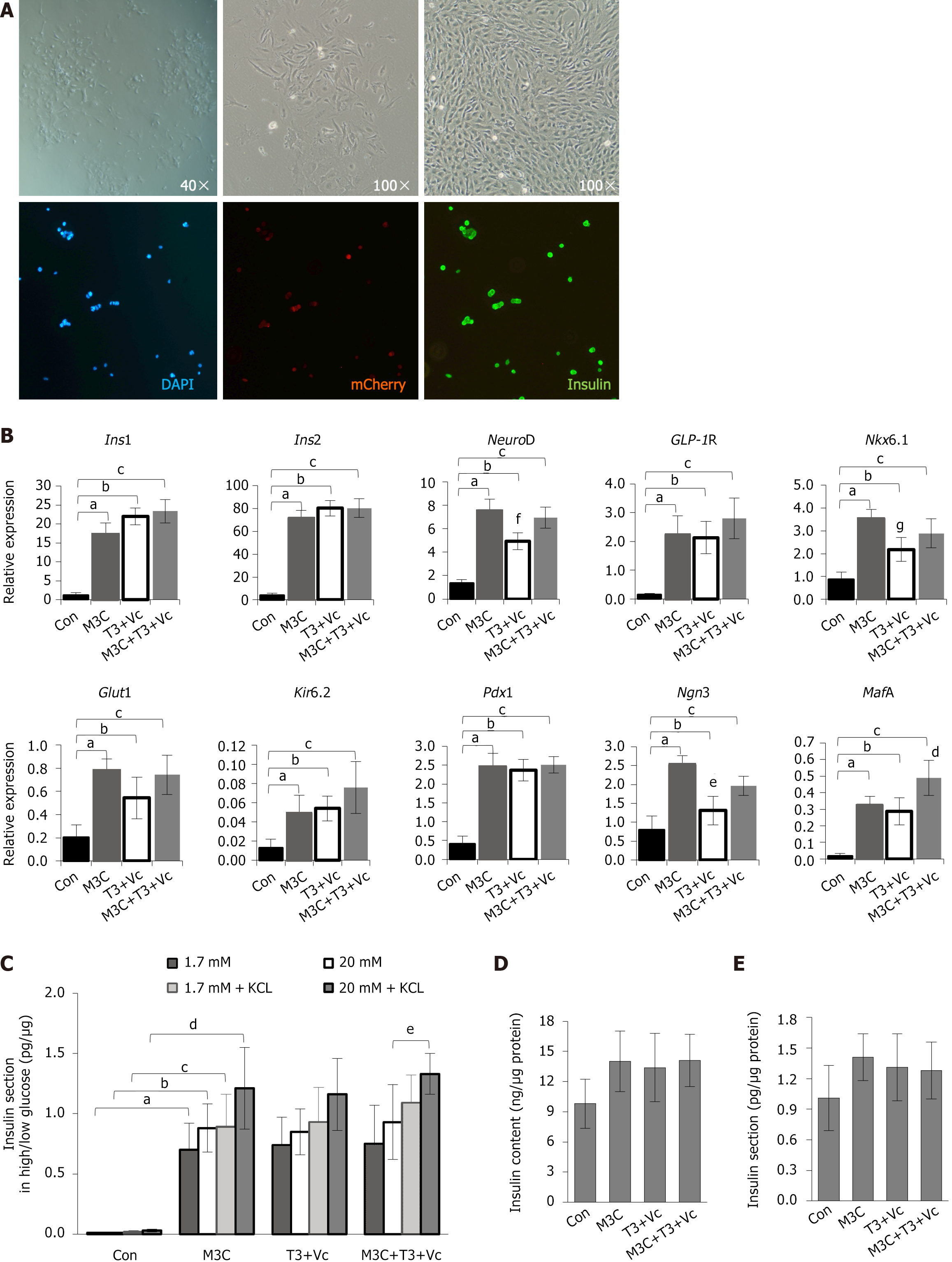
The T1D-iPSCs were differentiated into pancreatic β-cells using a meticulously optimized protocol incorporating T3, Vc, and M3C. Following 4 days of induction and culture, the original clonal adherent cells progressively differentiated into spindle- or rod-shaped monolayer cells, reaching approximately 50% confluence by day 15. These cells displayed an oval or stone-like morphology, distinctly different in size and shape from mature pancreatic β-cells. Immunofluorescence staining revealed the presence of insulin+ cells within the mCherry+ cells. However, the co-expression levels were not exceptionally high (Figure 3A).
On day 15 post-induction, qRT-PCR analysis demonstrated that the expression levels of key β-cell markers, including Ins1, Ins2, NeuroD, GLP1R, Nkx6.1, Glut2, Kir6.2, Pdx1, Ngn3, and MafA, was significantly increased in the M3C, T3 + Vc, and M3C + T3 + Vc groups compared to the undifferentiated T1D-iPSCs control group (Ins1, 17.13 ± 3.06, 21.96 ± 2.22, 23.36 ± 3.12, vs 1.14 ± 0.70, aP < 0.01, bP < 0.01, cP < 0.01; Ins2, 71.19 ± 7.06, 80.14 ± 6.64, 80.31 ± 8.11, vs 4.01 ± 2.00, aP < 0.01, bP < 0.01, cP < 0.01; NeuroD, 7.50 ± 1.02, 4.93 ± 0.73, 6.93 ± 0.89, vs 1.37 ± 0.30, aP < 0.01, bP < 0.01, cP < 0.01; GLP1R, 2.23 ± 0.65, 2.13 ± 0.56, 2.80 ± 0.71, vs 0.16 ± 0.04, aP < 0.01, bP < 0.01, cP < 0.01; Nkx6.1, 3.53 ± 0.42, 2.19 ± 0.53, 2.89 ± 0.63, vs 0.89 ± 0.31, aP < 0.01, bP < 0.01, cP < 0.01; Glut2, 0.78 ± 0.10, 0.54 ± 0.18, 0.74 ± 0.17, vs 0.21 ± 0.11, aP < 0.01, bP < 0.01, cP < 0.01; Kir6.2, 0.05 ± 0.02, 0.05 ± 0.01, 0.08 ± 0.03, vs 0.01 ± 0.01, aP < 0.01, bP < 0.01, cP < 0.01; Pdx1, 2.46 ± 0.35, 2.37 ± 0.28, 2.51 ± 0.22, vs 0.41 ± 0.21, aP < 0.01, bP < 0.01, cP < 0.01; Ngn3, 2.51 ± 0.25, 1.31 ± 0.38, 1.96 ± 0.25, vs 0.80 ± 0.36, aP < 0.01, bP < 0.05, cP < 0.05; and MafA, 0.32 ± 0.05, 0.29 ± 0.08, 0.49 ± 0.11, vs 0.02 ± 0.01, aP < 0.01, bP < 0.01, cP < 0.01). However, statistical analysis revealed no significant differences among the three experimental groups (M3C, T3 + Vc, and M3C + T3 + Vc) in the expression of Ins1, Ins2, GLP1R, Glut2, Kir6.2, and Pdx1. The highest expression of MafA was detected in the M3C + T3 + Vc group (0.49 ± 0.11 vs 0.32 ± 0.06 vs 0.29 ± 0.08, dP < 0.05). Conversely, Ngn3 expression was the lowest in the T3 + Vc group among the three experimental groups (1.31 ± 0.38 vs 1.96 ± 0.25 vs 2.51 ± 0.24, eP < 0.01). Consistent with these findings, NeuroD and Nkx6.1 expression, which are transcription factors downstream of Ngn3, was also significantly reduced in the T3 + Vc group compared to M3C and M3C + T3 + Vc groups (NeuroD, 4.93 ± 0.73 vs 7.49 ± 1.02 vs 6.93 ± 0.89, fP < 0.05; Nkx6.1, 2.19 ± 0.53 vs 3.53 ± 0.42 vs 2.89 ± 0.63, gP < 0.05; Figure 3B).
The cells were stimulated with low (1.7 mmol/L) and high (20 mmol/L) glucose concentrations, with and without adding 30 mmol/L KCl to assess insulin secretion. The glucose-stimulated insulin secretion (GSIS) assay demonstrated that insulin release in all experimental groups was significantly elevated M3C (0.70 ± 0.22, 0.88 ± 0.20, 0.89 ± 0.27, 1.21 ± 0.34, aP < 0.01, bP < 0.01, cP < 0.01, dP < 0.01), T3 + Vc ( 0.74 ± 0.23, 0.85 ± 0.19, 0.93 ± 0.29, 1.16 ± 0.30), M3C + T3 + Vc (0.75 ± 0.32, 0.93 ± 0.31, 1.09 ± 0.23, 1.33 ± 0.17) than in the control group (0.01 ± 0.01, 0.01 ± 0.01, 0.02 ± 0.01, 0.03 ± 0.01; Figure 3C). Notably, KCl enhances cell membrane depolarization in response to varying glucose concentrations by increasing extracellular potassium ion concentration and potentiating insulin secretion. The M3C + T3 + Vc group exhibited the highest insulin secretion under high glucose and 30 mmol/L KCl, significantly higher than in the same group without KCl (1.33 ± 0.17 vs 0.93 ± 0.31, eP < 0.05). However, the stimulatory responses of M3C, T3 + Vc, and M3C + T3 + Vc groups to low and high glucose concentrations were not significantly different, and the total increment in insulin secretion remained rather meager. These results suggest that adding T3, Vc, and M3C did not significantly enhance insulin secretion. Measuring secreted and intracellular insulin levels did not reveal a significant increase in any groups (Figure 3D and E).
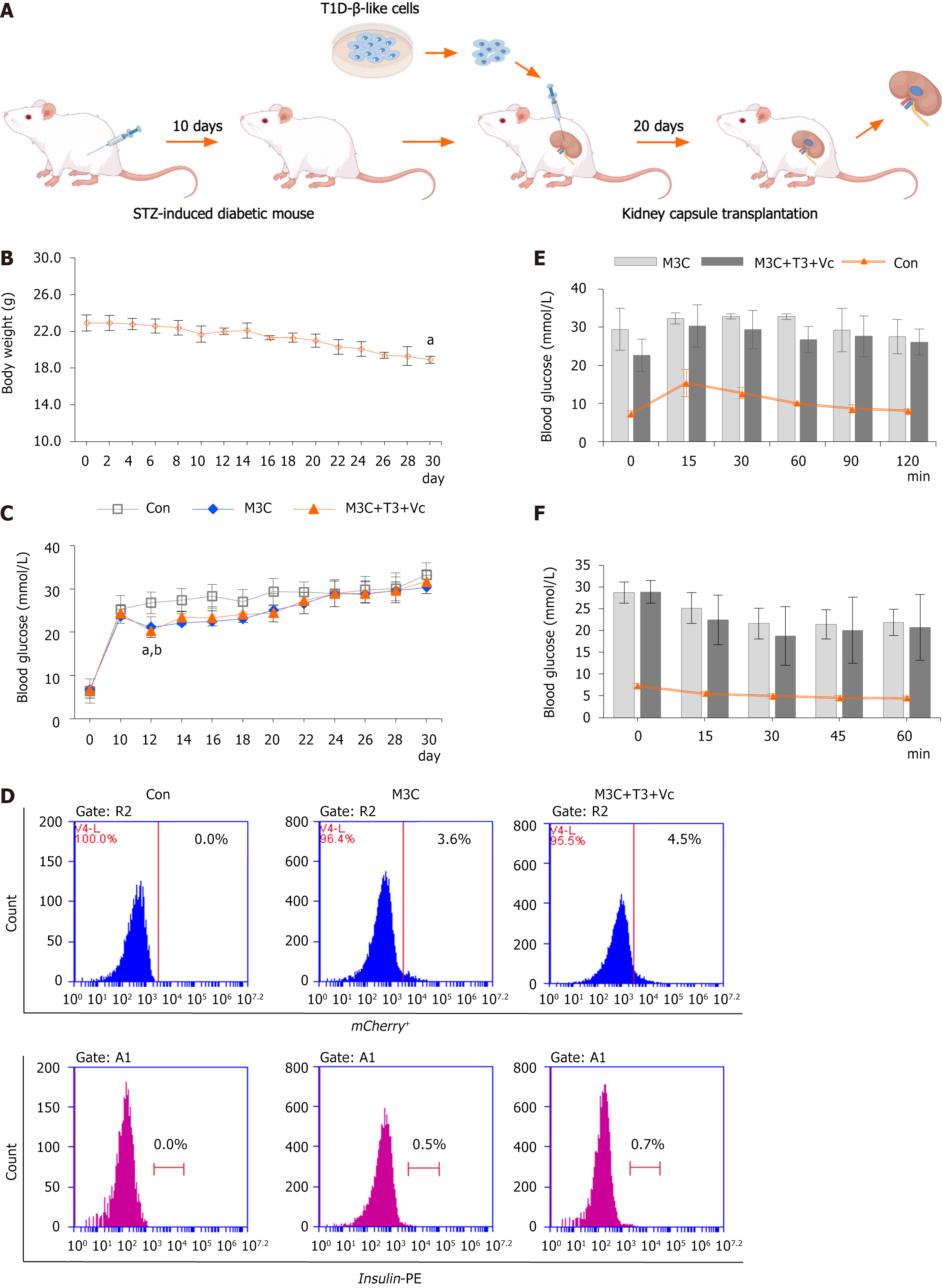
SCID mice were used to establish a diabetic model by intraperitoneally injecting STZ to evaluate the therapeutic potential of the differentiated cells (Figure 4A). In the immediate aftermath of STZ administration, transiently reduced glucose levels are observed due to the initial release of insulin from the damaged pancreatic islets, occasionally resulting in fatal hypoglycemia. By day 2, blood glucose levels began to increase, indicating the progressive destruction of endogenous islets. Random blood glucose levels were monitored daily at 16:00 to mitigate the risk of severe hypoglycemia. Symptoms indicative of diabetic hyperglycemia (random blood glucose > 19.4 mmol/L) were typically observed on day 10. Following sustained hyperglycemia, the mice exhibited characteristic diabetic symptoms, including dietary alterations, increased water consumption, and frequent urination. The animals’ fur acquired a greasy texture, and the cage bedding emitted a characteristic “glycosuria” odor. Consistent with disease progression, daily weight monitoring demonstrated a gradual decline in body weight that began concurrently with the rise in blood glucose levels and continued until the terminal experimental period (n = 15, 18.90 ± 0.40 vs 22.9 ± 0.89, aP < 0.01). M3C, M3C + T3 + Vc, and STZ-induced diabetic control groups, subjected to sham operations (n = 5 per group), were concurrently monitored for random blood glucose levels for 30 days (Figure 4B). The two experimental groups displayed a transient decrease in random blood glucose levels for 2 days post-surgery compared to STZ-diabetic control groups (21.16 ± 2.13 vs 26.8 ± 2.51, aP < 0.01, M3C group compared with the control group; 20.3 ± 1.50 vs 26.8 ± 2.51, bP < 0.01, M3C + T3 + Vc group compared with the control group), after which the blood glucose levels continued to increase. By day 30, although blood glucose levels were modestly lower than those in the sham operation group, the mice remained hyperglycemic (Figure 4C). After 20 days of transplantation of T1D-β-like cells under the renal capsule of hyperglycemic mice, the injection site was dissected, and single cells were isolated via enzymatic digestion and labeled with insulin-PE antibody. Flow cytometric analysis of the experimental groups revealed that the proportions of mCherry+ (3.8%, 4.5%) and insulin+ cells (0.5%, 0.7%) were elevated relative to those in the isotype-negative control groups. However, no statistically significant differences were observed between M3C and M3C + T3 + Vc groups (Figure 4C). After intraperitoneal glucose administration, the IGTTs revealed elevated blood glucose levels in M3C and M3C + T3 + Vc groups (Figure 4D). Blood glucose levels displayed a preli
This investigation revealed that patient-derived T1D-iPSCs, obtained from peripheral blood samples of individuals with T1D, displayed key attributes of pluripotent stem cells. These characteristics include typical cellular morphology, capacity for differentiation, and presentation of specific surface markers. Furthermore, the cells exhibited genetic stability, expressed pluripotency-associated genes, and lacked evidence of exogenous gene integration. These cells can develop into insulin-secreting T1D-β-like cells that exhibit insulin secretion capabilities. However, in vitro and in vivo analyses have demonstrated that these cells cannot modulate insulin release in response to increased glucose levels. Generating patient-specific T1D-iPSCs from T1D-PBMCs provides a valuable resource for investigating disease mechanisms and developing potential therapeutic agents. Additionally, the relative ease of access and reduced invasiveness of blood collection compared to procedures such as skin biopsies position blood cells as a promising source for iPSC derivation.
In this study, non-integrating episomal plasmids were used as gene delivery vehicles to induce iPSCs that did not incorporate foreign DNA. The episomal plasmid system included coding sequences for hSK, EBNA1, hUL, and hOCT3/4, designed to enhance the efficiency of T1D-iPSC induction. This approach facilitates the production of patient-specific, non-integrated iPSCs suitable for clinical applications[29,30]. The findings demonstrated that T1D-iPSCs generated via episomal electroporation-mediated reprogramming of T1D-PBMCs possessed pluripotent stem cell characteristics, including appropriate clonal morphology, differentiation potential, and expression of stem cell surface markers. These cells also displayed genomic stability, expressed pluripotency genes, and eliminated foreign genetic materials. Moreover, they can differentiate into pancreatic β-cells.
A recombinant adenoviral vector, M3C, was employed to deliver Pdx1, Ngn3, and MafA transcription factors to enhance differentiation efficiency and promote cellular maturation. Pdx1 is essential for developing the pancreas and its β-cells, Ngn3 plays a critical role in islet cell specification, and MafA is vital in the final stages of β-cell maturation. The combined action of these three factors is required for reprogramming exocrine cells into β-like cells that produce insulin[24,31-34]. M3C uses a polycistronic design, allowing a single adenovirus vector to simultaneously express these three transcription factors. The vector employs a 2A peptide sequence to mediate “skipping” during protein translation, enabling the production of multiple proteins from a single transcript, thereby significantly enhancing the transduction efficiency and overall reprogramming effect. Studies on transcription factors and markers that maintain β-cell functional maturation have revealed that embryonic β-cells exhibit limited glucose responsiveness, with functional maturation largely occurring within the first 2 weeks after birth. Concurrently, T3, transforming growth factor-β, and estrogen-related receptor gamma regulate the mechanistic target of rapamycin complex 1 (mTORC1) cell signaling pathway[35]. T3 upregulates insulin-like growth factor-1 expression, activating mTORC1 via the phosphatidylinositol-3-kinase/protein kinase B signaling pathway and stimulating gene expression associated with ribosome biogenesis and protein synthesis, thereby enhancing protein production within the cell. Conversely, transforming growth factor-β inhibits mTORC1 activity by activating the Smad signaling pathway and enhancing the expression of inhibitors that regulate the cyclin-dependent kinases. This inhibition prevents the transition of the cell cycle from the G1 phase to the S phase, thereby suppressing cell proliferation. Estrogen-related receptor gamma influences mTORC1 pathway activity by regulating the expression and function of metabolic enzymes. Vc has been demonstrated to reduce Ngn3 mRNA expression during the early stages of β-cell differentiation and secretion of multiple hormones induced by Ngn3 in immature pancreatic β-cells[23,24]. Therefore, in this study, the experimental groups treated with M3C, T3 + Vc, and M3C + T3 + Vc were subjected to the R induction regimen, and differences in differentiation efficiency were assessed by measuring transcription levels. The mRNA expression levels of Ins1, Ins2, NeuroD, GLP1R, Nkx6.1, Glut2, and Kir6.2 were detected in all three treatment groups. These expression levels were significantly different from those in the uninduced control group. The β-like cells induced using the R regimen optimized with M3C, T3, and Vc expressed Pdx1, Ngn3, MafA, NeuroD, GLP1R, Nkx6.1, Glut2, and Kir6.2. The increased expression of these β-cell-specific transcription factors suggests their potential to transform into β-like cells following transduction. M3C, T3, and Vc did not significantly alter the general β-cell marker expression. The mRNA expression levels of Ins1 and Ins2 were elevated in M3C and M3C + T3 + Vc groups. However, the differences between the groups were not statistically significant. These findings suggest that T3 and Vc may be important supplements to enhance M3C transduction efficiency during in vitro culture. Whether T3 and Vc influence T1D-β-like cells and promote their maturation within the complex in vivo environment warrants further investigation. Ngn3 expression was lower in the T3 + Vc group than in M3C and M3C + T3 + Vc groups. NeuroD and Nkx6.1 expressions, downstream targets of Ngn3, were also reduced in the T3 + Vc group compared to the other two experimental groups. T3 + Vc did not enhance the expression of typical β-cell markers, including Ins1, Ins2, NeuroD, GLP1R, Nkx6.1, Glut2, Kir6.2, Pdx1, and MafA. The Pdx1, Ngn3, and MafA expression levels were increased in M3C-treated cells. Furthermore, MafA expression aligned with the pattern observed during postnatal β-cell maturation, with the highest MafA expression detected in the M3C + T3 + Vc group. MafA is a key regulator of β-cell maturation and function, considering its function in promoting insulin secretion in response to glucose in islets derived from neonatal mice[36,37].
The primary physiological role of mature β-cells is to synthesize and secrete insulin. Although T1D-β-like cells can secrete insulin, they fail to respond to increases in glucose concentration, indicating functional immaturity and potentially impaired mitochondrial function. These cells exhibited limited responses to glucose stimulation. Despite the upregulation of genes encoding β-cell membrane channel proteins, Glut2 and Kir6.2, other elements required for functional glucose sensing and insulin secretion were not adequately induced. This suggests that incomplete reprogramming results in reduced expression of mature β-cell markers. Similarly, although the experimental groups exhibited elevated insulin levels compared to the control group, the magnitude of this increase was lower than that typically observed in mature β-cells, reflecting the functional immaturity of the transduced cells and highlighting significant functional differences between these cells and fully mature β-cells.
Immunodeficient SCID mice were chosen for this study because they can tolerate exogenous grafts and their improved immune tolerance, which minimizes the impact of non-experimental factors and increases the feasibility of the experiments. T1D was induced in these mice by intraperitoneal injection of STZ, which selectively destroys β-cells. On the first day following STZ injection, blood glucose levels were reduced due to the destruction of β-cells and the release of pre-stored insulin from the damaged islets. This effect can sometimes lead to fatal hypoglycemia. On the second day after the STZ injection, blood glucose levels increased, indicating impaired pancreatic function and reduced endogenous insulin production. The normal blood glucose level could not be maintained, and hyperglycemia eventually reached the threshold for diagnosing diabetes, confirming the establishment of a diabetes model. Diabetic mice exhibited typical diabetic symptoms, including continued weight loss. The expression of mCherry+ insulin+ cells, as detected by FCM 10 days after transplantation, was elevated in M3C and M3C + T3 + Vc groups. However, no significant differences were identified among these groups. The fluorescence intensity of mCherry+ at the transplantation site gradually decreased 20 days post-transplantation, potentially due to a decline in the transient expression of the non-integrated viral vector. During the first 2 weeks following surgery, blood glucose levels in the experimental groups improved compared to those in the STZ control group; however, the mean blood glucose levels gradually increased. Additionally, glucose tolerance tests indicated a degree of glucose-handling capacity in the experimental animals, which correlated with increased serum insulin levels, likely resulting from insulin-producing cells. However, the induced β-like cells differ from mature β-cells in terms of number and function, potentially limiting their capacity to maintain glucose homeostasis in vivo. Alternatively, the lack of complete islet structure formation by transplanted cells may have contributed to their reduced effectiveness. Furthermore, there is no evidence to support the spontaneous conversion of exocrine cells to β-cells in STZ-treated animals. These findings demonstrate that iPSCs derived from the peripheral blood of patients with T1D can generate insulin-expressing pancreatic β-like cells capable of producing and secreting insulin in vitro and in vivo. Although the function of these iPSCs in maintaining blood glucose homeostasis is insufficient to counteract STZ-induced hyperglycemia, these observations confirm the efficacy of the chosen induction protocol.
In this study, T1D-PBMCs were reprogrammed into patient-specific T1D-iPSCs using an episomal system that served as an electroporation-based plasmid vector containing hSK, EBNA1, hUL, and hOCT3/4 coding sequences. The protocol for generating iPSCs from peripheral blood cells, initially reported by Loh et al[38], involved the use of a retroviral vector for gene delivery and infection of CD34+ cells obtained from donors who had been injected with granulocyte colony-stimulating factor for 3 days. However, this method is relatively cumbersome, complex, and difficult to implement. The donor requires granulocyte-stimulating factor injections, adding to the burden and potential risks. Moreover, incorporating retroviral vectors into the host cell genome can interfere with the native organization of the genome, potentially compromising the integrity of in vitro disease models. In 2013, Okita et al[28] used a combination of plasmids encoding short hairpin RNAs targeting OCT3/4, Krüppel-like factor 4, sex-determining region Y-box 2, LIN28, L-MYC, and TP53 to induce iPSCs from adult fibroblasts; however, this approach yielded low efficiency[18,26-29,39].
The episomal system employed in this study incorporated EBNA1, derived from human herpesvirus type 4, into the Yamanaka induction strategy. This system relies on two key components: Orip and EBNA1, which are essential for expanding episomal vectors. The EBNA1 protein enables the Orip sequence to facilitate plasmid replication in synchrony with the DNA replication of the host cell, thereby enhancing the efficiency of reprogramming PBMCs into iPSCs, particularly for inducing αβT cells. This improvement allowed the establishment of blood-derived iPSCs, making it feasible and efficient to generate iPSCs from blood cells. Following the four-step R protocol for β-cell differentiation, M3C, T3, and Vc were used to improve cell differentiation. The recombinant adenovirus M3C, a precisely defined combination of Pdx1, Ngn3, and MafA, is critical for reprogramming exocrine cells into insulin-expressing β-like cells[18]. T3 promotes the maturation of rat pancreatic β-cells, increasing the number of insulin-producing cells that express mature β-cell markers, such as MafA, NKX6.1, and Pdx1, thereby improving the insulin secretion function of these cells[33]. Vc can reduce Ngn3 mRNA expression, modulating the differentiation of pancreatic endocrine cells while leaving downstream targets of Ngn3, including NeuroD1 and Nkx6.1, and other markers, including Pdx1, unaffected. The results of this study are consistent with these reported effects. However, enhancing insulin secretion in T1D β-like cells remains challenging, as no significant differences in differentiation efficiency were observed between experimental groups. M3C, T3, and Vc did not significantly impact cell differentiation, indicating that further optimization of culture conditions is necessary to improve trans-differentiation efficiency, representing an area for future research.
Transplanting insulin-producing cells derived from pancreatic progenitor cells or ESCs is a promising therapeutic strategy for diabetes[33,40]. These cells express essential markers associated with mature β-cells, such as MafA, and exhibit similarities to human islets during static in vitro incubation, while differences in cell morphology and dynamic functional tests have been observed compared to primary human β-cells. However, these transplanted cells could rapidly reverse diabetes in a mouse model within 40 days, nearly four times faster than that observed with pancreatic progenitor cells.
The failure of β-cells to secrete insulin under high glucose concentrations is a significant issue that may arise from multiple factors, including functional immaturity and limitations inherent to the experimental environment. The findings of this study suggest that the induced β-like cells, despite expressing key functional genes associated with β-cells, exhibit impaired insulin secretion in response to high glucose levels, underscoring their incomplete maturation. Insufficient activation of late-stage maturation markers, such as urocortin 3 and MafA, may cause functional immaturity of β-cells. Even with optimized differentiation protocols, cells may not reach the level of maturity required to respond appropriately to glucose stimuli.
Furthermore, insulin secretion is a highly regulated process that involves intricate signaling pathways. Disruptions in critical components of these pathways, such as Kir6.2 (KATP channel) or Glut2 (glucose transporter), can cause metabolic defects or ion channel dysfunction, impairing the ability of cells to sense and respond to elevated glucose levels. These signaling irregularities may cause a deficiency in insulin release from β-like cells when exposed to high glucose levels. Beyond intrinsic cellular factors, the microenvironment surrounding β-cells is critical for shaping their function. In vivo, β-cells interact with neighboring cells and tissues, receiving regulatory signals challenging to replicate in vitro. The absence of key microenvironmental factors, such as specific cytokines and cell-cell contacts, in the culture system may have further compromised the capacity of the cells to respond to glucose. Moreover, the experimental limitations inherent to in vitro models, including variations in culture medium composition and substrate properties, may introduce confounding factors that interfere with glucose sensing and insulin secretion.
In conclusion, the inability of β-like cells to secrete insulin under high glucose concentrations may result from a combination of functional immaturity, disruptions in signaling pathways, and non-optimal culture conditions. Future research should focus on refining differentiation protocols to fully mature β-cells and optimizing culture systems to mimic the in vivo microenvironment more accurately. Addressing these challenges is critical for developing functional β-cell replacement therapies for diabetes.
In subsequent studies designed to further validate β-cell functionality, we will focus on personalized protocols for T1D-iPSCs and the synergistic effects of combining T3, Vc, and adenovirus-M3C. Optimized approaches will be compared to traditional protocols to identify potential upstream regulators of key markers, including Nkx6.1 and Glut2, using RNA sequencing and CRISPR screening. Furthermore, mechanistic experiments, including gene knockout and overexpression studies, will be performed to validate the functional roles of the identified targets. Key experiments, such as GSIS assays and in vivo transplantation studies, will be refined to provide deeper insights into the molecular basis of functional deficiencies in β-cells. The goal was to demonstrate that reprogramming patient-specific T1D-PBMCs into patient-specific T1D-iPSCs can improve blood glucose homeostasis, which is feasible and effective for diabetes treatment. Future research should identify additional factors that enhance the differentiation efficiency and functional capacity of T1D-β-cells[40].
Patient-specific T1D-iPSCs derived from individuals with T1D can be effectively induced to generate insulin-expressing T1D-β-like cells. The results indicate that the combined use of M3C, T3, and Vc effectively regulates the expression of pancreatic β-cell markers in vitro and in vivo. Specifically, the administration of T3 + Vc significantly inhibited Ngn3 expression, reducing the formation of immature multi-hormonal endocrine cells and promoting their differentiation into mature insulin-expressing cells. In contrast, the M3C + T3 + Vc treatment group displayed the highest MafA expression, underscoring the synergistic impact of these factors on β-cell maturation.
However, T1D-β-like cells lacked glucose responsiveness, suggesting functional differences compared to mature β-cells. The observed changes in marker expression provide valuable insights for future research. The limitations of this study include the small sample size and the need for further assessment of the long-term functionality and stability of induced cells in vivo. Future studies should include expanding the sample size, investigating long-term cell survival and immune compatibility, exploring upstream regulatory mechanisms of key markers (Nkx6.1 and Glut2) using RNA sequencing or CRISPR screening, and optimizing differentiation protocols to enhance the efficiency and scalability of T1D-β-like cell production for clinical applications, with the ultimate goal of advancing cell-based therapies for T1D.
We thank Professor Gang Chen for scientific discussions; Ze-Hua Zheng, Yun-Lu Liu, Ji-Lin Shi, and Jin-Feng Wei for technical assistance; and Shu-Yan Chen, Chen Lin, Jing-Jing Yu, Hong-Mou Zheng, and Shun-Min Guo for administrative assistance.
| 1. | Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 4813] [Article Influence: 1604.3] [Reference Citation Analysis (36)] |
| 2. | Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, O'Connell PJ, Greenbaum C, Kudva YC, Brayman KL, Aull MJ, Larsen C, Kay TW, Fernandez LA, Vantyghem MC, Bellin M, Shapiro AM. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35:1436-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 620] [Cited by in RCA: 537] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 3. | Pinheiro-Machado E, de Haan BJ, Engelse MA, Smink AM. Secretome Analysis of Human and Rat Pancreatic Islets Co-Cultured with Adipose-Derived Stromal Cells Reveals a Signature with Enhanced Regenerative Capacities. Cells. 2025;14:302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Zhang H, Wei Y, Wang Y, Liang J, Hou Y, Nie X, Hou J. Emerging Diabetes Therapies: Regenerating Pancreatic β Cells. Tissue Eng Part B Rev. 2024;30:644-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Forgioni A, Watanabe M, Goto R, Harada T, Ota T, Shimamura T, Taketomi A. Anti-Inflammatory Effects of Ex Vivo-Generated Donor Antigen-Specific Immunomodulatory Cells on Pancreatic Islet Transplantation. Cell Transplant. 2025;34:9636897251317887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Feng X, Zhang H, Yang S, Cui D, Wu Y, Qi X, Su Z. From stem cells to pancreatic β-cells: strategies, applications, and potential treatments for diabetes. Mol Cell Biochem. 2025;480:173-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Aguayo-Mazzucato C, Bonner-Weir S. Pancreatic β Cell Regeneration as a Possible Therapy for Diabetes. Cell Metab. 2018;27:57-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 175] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 8. | Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Neumann M, Arnould T, Su BL. Encapsulation of stem-cell derived β-cells: A promising approach for the treatment for type 1 diabetes mellitus. J Colloid Interface Sci. 2023;636:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Csobonyeiova M, Polak S, Danisovic L. Generation of Pancreatic β-cells From iPSCs and their Potential for Type 1 Diabetes Mellitus Replacement Therapy and Modelling. Exp Clin Endocrinol Diabetes. 2020;128:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Mu-U-Min RBA, Diane A, Allouch A, Al-Siddiqi HH. Immune Evasion in Stem Cell-Based Diabetes Therapy-Current Strategies and Their Application in Clinical Trials. Biomedicines. 2025;13:383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao Z, Warnock GL, Kieffer TJ. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes. 2011;60:239-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Campo F, Neroni A, Pignatelli C, Pellegrini S, Marzinotto I, Valla L, Manenti F, Policardi M, Lampasona V, Piemonti L, Citro A. Bioengineering of a human iPSC-derived vascularized endocrine pancreas for type 1 diabetes. Cell Rep Med. 2025;6:101938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O'Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1163] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 15. | Champeris Tsaniras S, Jones PM. Generating pancreatic beta-cells from embryonic stem cells by manipulating signaling pathways. J Endocrinol. 2010;206:13-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Farhat C, Xega V, Liu JL. A stepwise approach to deriving functional β-cells from human embryonic or induced pluripotent stem cells. Med Rev (2021). 2025;5:23-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol. 2021;22:142-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 368] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 18. | Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1544] [Article Influence: 110.3] [Reference Citation Analysis (0)] |
| 19. | Aguayo-Mazzucato C, Lee TB Jr, Matzko M, DiIenno A, Rezanejad H, Ramadoss P, Scanlan T, Zavacki AM, Larsen PR, Hollenberg A, Colton C, Sharma A, Bonner-Weir S. T(3) Induces Both Markers of Maturation and Aging in Pancreatic β-Cells. Diabetes. 2018;67:1322-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Aguayo-Mazzucato C, DiIenno A, Hollister-Lock J, Cahill C, Sharma A, Weir G, Colton C, Bonner-Weir S. MAFA and T3 Drive Maturation of Both Fetal Human Islets and Insulin-Producing Cells Differentiated From hESC. J Clin Endocrinol Metab. 2015;100:3651-3659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Hidalgo-Álvarez J, Salas-Lucia F, Vera Cruz D, Fonseca TL, Bianco AC. Localized T3 production modifies the transcriptome and promotes the hepatocyte-like lineage in iPSC-derived hepatic organoids. JCI Insight. 2023;8:e173780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, Hollister-Lock J, El Khattabi I, Marsili A, Weir GC, Sharma A, Larsen PR, Bonner-Weir S. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes. 2013;62:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1544] [Cited by in RCA: 1523] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 24. | Akinci E, Banga A, Greder LV, Dutton JR, Slack JM. Reprogramming of pancreatic exocrine cells towards a beta (β) cell character using Pdx1, Ngn3 and MafA. Biochem J. 2012;442:539-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Cavelti-Weder C, Zumsteg A, Li W, Zhou Q. Reprogramming of Pancreatic Acinar Cells to Functional Beta Cells by In Vivo Transduction of a Polycistronic Construct Containing Pdx1, Ngn3, MafA in Mice. Curr Protoc Stem Cell Biol. 2017;40:4A.10.1-4A.10.12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1896] [Cited by in RCA: 1692] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 27. | Yu J, Chau KF, Vodyanik MA, Jiang J, Jiang Y. Efficient feeder-free episomal reprogramming with small molecules. PLoS One. 2011;6:e17557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 28. | Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 562] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 29. | Roig-Merino A, Urban M, Bozza M, Peterson JD, Bullen L, Büchler-Schäff M, Stäble S, van der Hoeven F, Müller-Decker K, McKay TR, Milsom MD, Harbottle RP. An episomal DNA vector platform for the persistent genetic modification of pluripotent stem cells and their differentiated progeny. Stem Cell Reports. 2022;17:143-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Pratumkaew P, Luanpitpong S, Klaihmon P, Lorthongpanich C, Laowtammathron C, Meesa S, Suksomboon W, Issaragrisil S. Episomal vector-based generation of human induced pluripotent stem cell line MUSIi020-A from peripheral blood T-cells. Stem Cell Res. 2022;64:102929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Corritore E, Dugnani E, Pasquale V, Misawa R, Witkowski P, Lei J, Markmann J, Piemonti L, Sokal EM, Bonner-Weir S, Lysy PA. β-Cell differentiation of human pancreatic duct-derived cells after in vitro expansion. Cell Reprogram. 2014;16:456-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Cavelti-Weder C, Li W, Weir GC, Zhou Q. Direct lineage conversion of pancreatic exocrine to endocrine Beta cells in vivo with defined factors. Methods Mol Biol. 2014;1150:247-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun. 2016;7:11463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 34. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1528] [Article Influence: 152.8] [Reference Citation Analysis (1)] |
| 35. | Rajak S, Xie S, Tewari A, Raza S, Wu Y, Bay BH, Yen PM, Sinha RA. MTORC1 inhibition drives crinophagic degradation of glucagon. Mol Metab. 2021;53:101286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Kerper N, Ashe S, Hebrok M. Pancreatic β-Cell Development and Regeneration. Cold Spring Harb Perspect Biol. 2022;14:a040741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Li W, Cavelti-Weder C, Zhang Y, Clement K, Donovan S, Gonzalez G, Zhu J, Stemann M, Xu K, Hashimoto T, Yamada T, Nakanishi M, Zhang Y, Zeng S, Gifford D, Meissner A, Weir G, Zhou Q. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nat Biotechnol. 2014;32:1223-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, Kim K, Miller JD, Ng K, Daley GQ. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476-5479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 434] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 39. | Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4072] [Cited by in RCA: 4372] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 40. | Cierpka-Kmiec K, Wronska A, Kmiec Z. In vitro generation of pancreatic β-cells for diabetes treatment. I. β-like cells derived from human pluripotent stem cells. Folia Histochem Cytobiol. 2019;57:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |