Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.103314
Revised: February 6, 2025
Accepted: March 17, 2025
Published online: April 26, 2025
Processing time: 159 Days and 12.5 Hours
Mesenchymal stem cell (MSC)-based therapy may be a future treatment for myocardial infarction (MI). However, few studies have assessed the therapeutic efficacy of adipose tissue-derived MSCs (ADSCs) obtained from elderly patients in comparison to that of bone marrow-derived MSCs (BMSCs) from the same elderly patients. The metabolomics results revealed a significantly higher L-arginine excretion from aged ADSCs vs BMSCs in hypoxic conditions. This was hypothesized as the possible mechanism that ADSCs showed an improved angiogenic capacity and enhanced the therapeutic effect on ischemic heart diseases.
To investigate the role of L-arginine in enhancing angiogenesis and cardiac protection by comparing ADSCs and BMSCs in hypoxic conditions for MI therapy.
Metabolomic profiling of supernatants from ADSCs and BMSCs under hypoxic conditions were performed. Then, arginine succinate lyase (ASL) overexpression and short hairpin RNA plasmid were prepared and transfected into BMSCs. Subsequently, in vitro wound healing and Matrigel tube formation assays were used to verify the proangiogenetic effects of ADSC positive control, BMSCs, BMSCs ASL short hairpin RNA, BMSCs ASL overexpressed, and BMSC negative control on cocultured human umbilical vein endothelial cells. All sample sizes, which were determined to meet the statistical requirements and be greater than 3, were established on the basis of previously established literature standards. The protein levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor, etc. were detected. In vivo, the five types of cells were transplanted into the infarcted area of MI rat models, and the therapeutic effects of the transplanted cells were evaluated by echocardiography on cardiac function and by Masson’s staining/terminal-deoxynucleotidyl transferase mediated nick end labeling assay/immunofluorescence detection on the infarcted area.
Metabolomic analysis showed that L-arginine was increased. Using ASL gene transfection, we upregulated the production of L-arginine in aged patient-derived BMSCs in vitro, which in turn enhanced mitogen activated protein kinase and VEGF receptor 2 protein expression, VEGF and basic fibroblast growth factor secretion, and inductive angiogenesis to levels comparable to donor-matched ADSCs. After the cell transplantation in vivo, the modified BMSCs as well as ADSCs exhibited decreased apoptotic cells, enhanced vessel formation, reduced scar size, and improved cardiac function in the MI rat model. The therapeutic efficacy decreased by inhibiting L-arginine synthesis.
L-arginine is important for inducing therapeutic angiogenesis for ADSCs and BMSCs in hypoxic conditions. ADSCs have higher L-arginine secretion, which leads to better angiogenesis induction and cardiac protection. ADSC transplantation is a promising autologous cell therapy strategy in the context of the present aging society.
Core Tip: Adipose tissue-derived mesenchymal stem cells exhibited the advantages of angiogenesis compared to bone marrow-derived mesenchymal stem cells from elderly donors due to higher L-arginine secretion in serum-free and hypoxic conditions. In an animal model of MI, bone marrow-derived mesenchymal stem cells in which the arginine succinate lyase gene was integrated had a cardioprotective effect on angiogenesis. These results indicated that adipose tissue-derived mesenchymal stem cells from elderly patients with coronary heart disease and the introduction of L-arginine provide a promising strategy for stem cell therapy after MI.
- Citation: Li JZ, Zhan X, Sun HB, Chi C, Zhang GF, Liu DH, Zhang WX, Sun LH, Kang K. L-arginine from elder human mesenchymal stem cells induces angiogenesis and enhances therapeutic effects on ischemic heart diseases. World J Stem Cells 2025; 17(4): 103314
- URL: https://www.wjgnet.com/1948-0210/full/v17/i4/103314.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i4.103314
As the number of elderly individuals increases, coronary heart disease (CHD) is becoming the main cause of morbidity and mortality around the world[1], with myocardial infarction (MI) representing a more common CHD compared to other conditions. MI can result in ischemic heart damage and cardiac dysfunction that may be reversible or irreversible. Decreased cardiac function can lead to recurrence of heart failure as a result of ventricular tissue remodeling and scar tissue formation.
Many treatments, including coronary implantation of stents, thrombolysis, and coronary artery bypass grafting have been used in clinical therapy. Unfortunately, there is no effective treatment that is able to attenuate the damage of infarcted myocardium or restore heart geometry and enhance heart function. Therefore, alternative therapies for MI are needed.
In recent studies, mesenchymal stem cell (MSC) transplantation into the infarcted myocardium has been developed and widely regarded as a potential treatment for MI[2-4]. Adipose tissue-derived MSCs (ADSCs) and bone marrow MSCs (BMSCs) are the primary research focus among the various types of stem cells examined[5-7]. Some experiments have shown a satisfactory treatment effect of BMSCs from young donors. However, the effect of autologous MSC trans
The limitations of a lower cell number and the invasive nature of the harvesting procedures in elderly patients have affected the application of BMSCs in patients with MI even though BMSCs are the most commonly used type of MSCs in preclinical research on cell-based MI treatment. ADSCs in aged patients are more readily accessible and can be obtained repeatedly under local anesthesia with minimal patient discomfort. In addition, the quantities of ADSCs are greater than those of BMSCs[8,9]. Therefore, ADSCs are also considered to be a promising cell source for MI cell therapy[9].
Recently, some researchers have regarded ADSCs as a more promising candidate for MI than BMSCs because the former exhibit an enhanced capacity in preserving cardiac function after MI in terms of efficacy and accessibility[10]. The exact mechanisms of MSC-based therapy have not been entirely understood. Initially, the therapeutic mechanism was thought to be related to the number of MSCs migrating to damaged tissues and replacing dead cells[11]. With a growing understanding of transplanted MSCs, it has been recently identified that MSC integration into damaged tissues is too low to account for their therapeutic effects[12].
Some researchers have confirmed that generating a proregenerative microenvironment is now the most acceptable therapeutic mechanism associated with MSCs[13] and that their metabolic characteristics may be involved. This is consistent with our previous similar finding that metabolites play an important role in stem cell-based therapy[8,9]. ADSCs and BMSCs are the most commonly utilized cell types in current stem cell research and the clinic. Numerous comparative studies associated with therapeutic potential and corresponding mechanisms have been made.
For example, our previous studies demonstrated that there is a minimal difference in therapeutic outcomes between ADSCs and BMSCs derived from young individuals. ADSCs from elderly donors likely demonstrate enhanced therapeutic potential[14], with outcomes similar to others. To elucidate this phenomenon, researchers have explored various underlying mechanisms, including telomerase activity[15] and anti-inflammatory and antioxidant capacities[16], among others. Additionally, several teams have independently conducted metabolomic analyses and validation specifically focused on ADSCs[17,18]. To our knowledge, our study is the first to systematically compare the therapeutic efficacy and mechanistic basis of ADSCs and BMSCs derived from the same elderly patients from the perspective of metabolomic analysis.
The MI area microenvironment is a hypoxic or anaerobic niche. Therefore, transplanted stem cells have to live in a hypoxic or anaerobic environment. Therefore, it is important for the transplanted cells to possess an anti-hypoxic ability for MI treatment. On the other hand, hypoxic preconditioning is involved in protecting MSCs from hypoxia/reo
Therefore, the present work aimed to evaluate metabolite differences of ADSCs and BMSCs from the same elderly patients under hypoxic conditions and to explore a possible mechanism for the therapeutic efficacy in “aged” ADSCs compared with age-matched BMSCs. To simulate the cell transplantation microenvironment, ADSCs and BMSCs were cultured in serum-free and hypoxic conditions.
A total of 40 elderly patients (age: ≥ 60 years) underwent coronary artery bypass graft. They were hospitalized at the Second Affiliated Hospital of Harbin Medical University (Harbin, China) from January 2015 to December 2016 and were selected randomly and enrolled in the present study. To avoid the possible effects on the metabolomic results, patients with excessive obesity (body mass index ≥ 28 kg/m2) or metabolic disorders (e.g., diabetes, hyperlipidemia, gout, hypothyroidism etc.) were excluded from the study. The general clinical characteristics of the patients are listed in Table 1.
| Indicator | Value (n = 40) |
| Gender | |
| Male | 31 (77.5) |
| Female | 9 (22.5) |
| Age (years) | 68 ± 6.9 |
| BMI (kg/m2) | 24 ± 2.1 |
| Concomitant metabolic disorders | No |
To mitigate potential confounding effects of variables such as age and gender on experimental outcomes, we implemented an internal paired design across all experimental groups in this study. Specifically, ADSCs and BMSCs compared within the same experimental setup were sourced from the same donor, thereby minimizing inter-individual variability and enhancing methodological rigor. The study protocol has been approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University and informed consent was obtained from each patient.
ADSCs were obtained from the adipose tissue of thoracic subcutaneous fat in patients with CHD. The adipose tissues were washed with PBS that contained 1% penicillin and streptomycin and were subsequently digested with collagenase type I (1 mg/mL; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37 °C for 45-60 min. The suspension was then centrifuged to separate the floating adipocytes that were filtered through a 200-μm nylon mesh.
Bone marrow was obtained by sternal puncture from the same 40 patients with CHD to avoid other interference factors, collected in heparinized tubes, and transferred to the laboratory sterilely. Bone marrow was extracted using a Ficoll-Paque gradient (1.073 g/mL density; 17-5446-52, GE Healthcare, IL, United States). Mononuclear cells were seeded into 25-cm2 culture flasks (Corning, NY, United States) with Dulbecco’s modified Eagle medium (DMEM, 31600034, Gibco, NY, United States) supplemented with 10% fetal bovine serum (FBS; 0050, ScienCell, CA, United States) and cultured at 37 °C in an atmosphere containing 5% CO2. After 72 h, BMSCs were collected by removing the unattached cells.
The ADSCs and BMSCs were cultured in a humidified atmosphere containing 5% CO2 at 37 °C with the medium replaced every 3 days. DMEM supplemented with 10% FBS was used to culture BMSCs and ADSCs. A total of 105 cells at passage three were seeded in six-well plates and cultured in an incubator (Memmert incubator, Schwabach, Germany) containing 1% O2, 5% CO2, and 94% N2. The supernatants were then collected and preserved at -80 °C for subsequent analyses after 24 h of culture.
Metabolomic analyses of the supernatant samples from ADSCs and BMSCs cultured in serum-free and hypoxic conditions were performed using liquid chromatography quadrupole time-of-flight mass spectrometry (6530 series; Agilent Technologies, Inc., Santa Clara, CA, United States). The raw data were converted into mzData format files using Mass Hunter Qualitative Analysis Software (Agilent Technologies, CA, United States). There were 39 major metabolic compounds in this study, including L-arginine from various pathways that were selected for the metabolomics analysis. Previous studies have shown that L-arginine is an important functional amino acid that takes part in angiogenesis.
L-arginine is synthesized by the interaction of arginine succinate synthase and arginine succinate lyase (ASL). ASL is a key regulatory enzyme in L-arginine production in vivo[16]. Therefore, overexpression ASL or short hairpin RNA (shRNAs) ASL plasmids were designed by GenePharma (Genechem, Shanghai, China) in this study. The plasmids carrying green fluorescent protein (GFP) were used to identify the transfection efficiency of each kind of plasmid.
The transfection process was performed according to the manufacture’s instruction. Briefly, BMSCs were cultured in DMEM and 10% FBS in humidified air containing 5% CO2 at 37 °C until 60%-70% confluent. After 2 h of transfection, the transfection medium was removed. BMSCs were washed twice using transfection media and cultured in FBS-containing DMEM until further analysis 48 h after transfection. BMSCs expressing GFP were then observed under a fluorescence microscope, and their transfection efficiency was determined as the ratio of GFP-positive cells to all cells.
Protein expression levels of overexpression ASL and shRNA ASL-transfected BMSCs were assessed 48 h after transfection using western blot analysis. Briefly, BMSCs samples were subjected to western blot analyses using ASL (Abcam, Cambridge, United Kingdom) and β-actin as a loading control. The protein content was quantified with a bicinchoninic acid protein assay kit (Abcam, Cambridge, United Kingdom). After sodium-dodecyl sulfate gel electrophoresis and membrane transfer, the membranes were blocked using 5% (w/v) nonfat milk diluted with tris buffered saline with Tween. Then the membranes were incubated with ASL primary antibody (Abcam, Cambridge, United Kingdom) and HRP conjugated goat anti-rabbit IgG (H + L) secondary antibody (Abcam, Cambridge, United Kingdom) at room temperature for 1 h. Pierce ECL + substrate (Genscript, NJ, United States) was used to detect blot results.
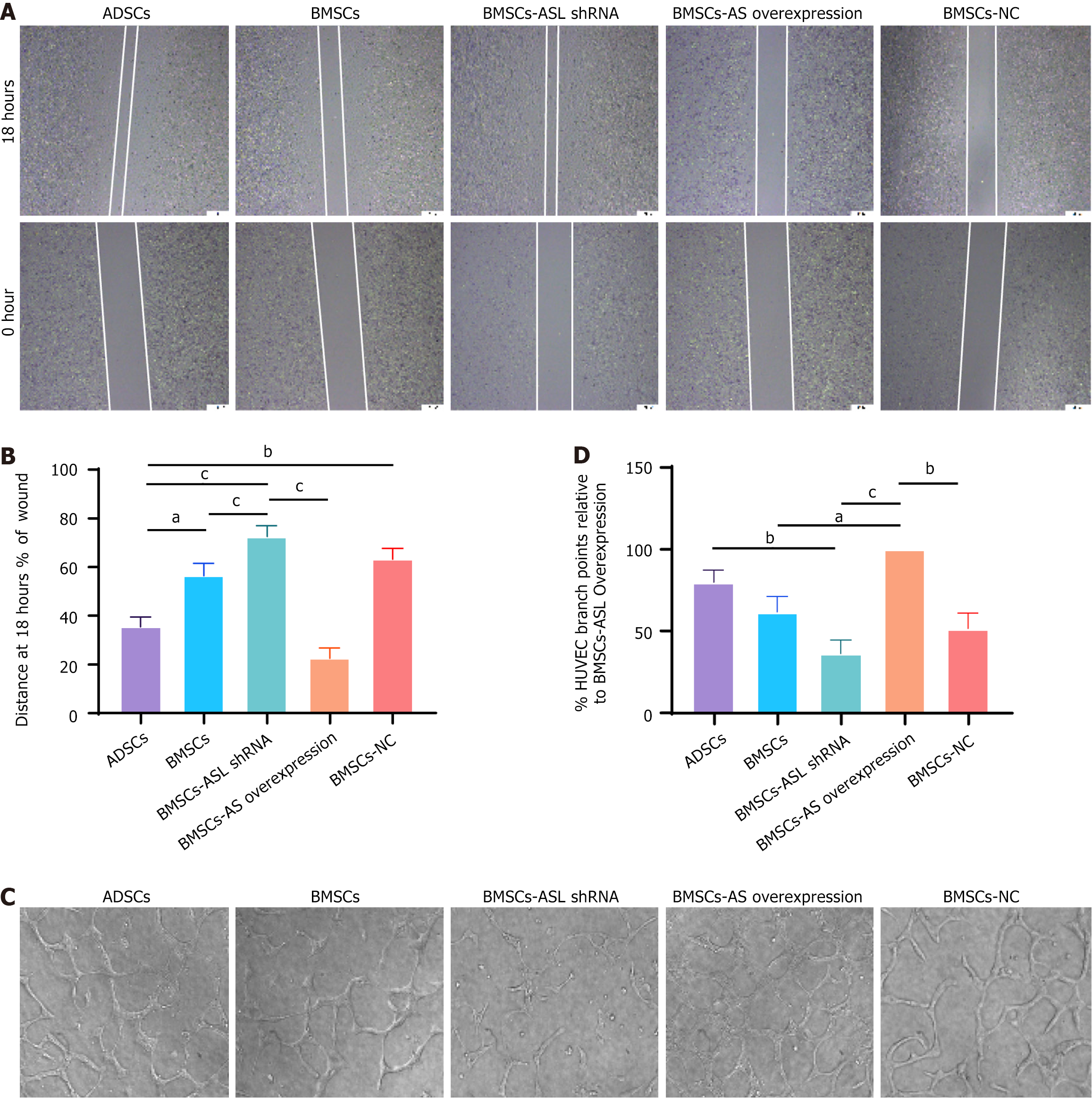
A total of 5 × 105 human umbilical vein endothelial cells (HUVECs, Cyagen, China) were seeded and incubated in hypoxic and serum-free conditions in the lower compartment of a six-well plate (Corning, NY, United States) overnight. MSCs from five groups (7.5 × 105/well in 1000 μL of DMEM) were added to the upper chamber inserts with a pore size of 0.4 μm. When the cells became 70%-80% confluent, cell monolayers were scratched in each well. The MSCs were then washed with PBS, and serum-free medium was added and incubated for 18 h in a hypoxic incubator. Microscopic images (× 40) were taken at 0 h and 18 h.
The 24-well plates were coated with 250 μL/well of chilled Matrigel solution (10 mg/mL) without air bubbles and incubated for 1 h at 37 °C to solidify. A total of 2.5 × 104 HUVECs were seeded in the lower compartment of 24-well Transwell plates for 24 h. ADSCs, BMSCs, BMSC-ASL shRNA, BMSC-ASL overexpressed, and BMSC-negative control (1 × 105) were seeded in the Transwell insert compartments. The co-cultures were incubated in a hypoxic incubator at 37 °C without a medium change for 24 h. The endothelial tubule-like vascular network formation was observed after 4 h, 8 h, 12 h, and 18 h of incubation under an inverted microscope. All of the experiments were performed as triplicate independent experiments.
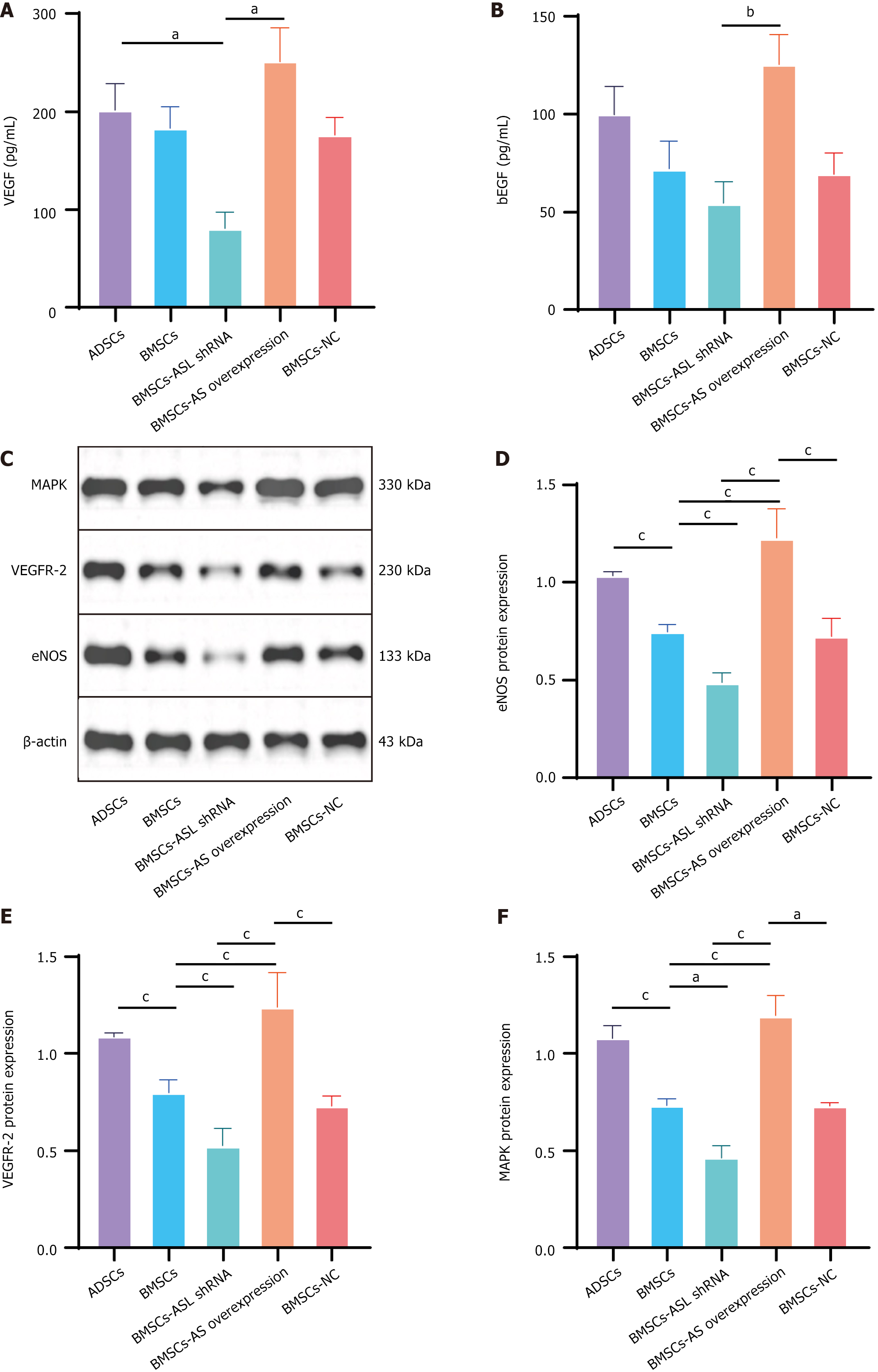
Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) protein levels in ADSCs, BMSCs, BMSC-ASL shRNA, BMSC-ASL overexpressed, and BMSC-negative control culture supernatants in hypoxic and serum-free conditions were assessed by ELISA using the manufacturer’s instructions (Sigma-Aldrich; Merck KGaA, Germany).
After the treatment as described above, endothelial nitric oxide synthase (eNOS), mitogen activated protein kinase (MAPK), and VEGF receptor 2 (VEGFR2) protein levels in five groups of MSCs were detected by western blot exami
Experimental animals: Adult Sprague Dawley male rats weighing 250-280 g were used in the study. The rat surgical procedures were compliant with the Guide for the Care and Use of Laboratory Animals (NIH Publication number 85-23, revised 1996) and were approved by the Animal Care and Use Committee of the Second Affiliated Hospital of Harbin Medical University.
Establishment of acute MI and MSC transplantation: MI was established as previously described[14]. In brief, adult Sprague Dawley male rats were anaesthetized and ventilated. Then, the hearts were exposed via a left thoracotomy and the left descending coronary artery was ligated with a 7-0 polypropylene suture. In this study, the MSCs were transplanted by intra-myocardial injection, as performed previously[22-24]. Briefly, 15 min after MI, the transplanted cells (2 × 106/100 μL) were resuspended in a 1-mL syringe and injected into one site in the center of the ischemic myocardium and four sites along the adjacent region of the infarcted and normal myocardium. In addition to injecting at the center, we carried out equidistant injections along the infarct boundary. First, we took the uppermost and lowermost points of the infarct boundary as the injection sites. Then, we selected the two injection sites as the axis and positioned the other two injection points perpendicular to this axis. Therefore, these four injection points were distributed at the 3-, 6-, 9-, and 12-o’clock positions around the infarct area. To avoid immune rejection, cyclosporin A (5 mg/kg; Novartis, Switzerland) was intraperitoneally administered every 3 days before and after MSC transplantation until postoperative day 28.
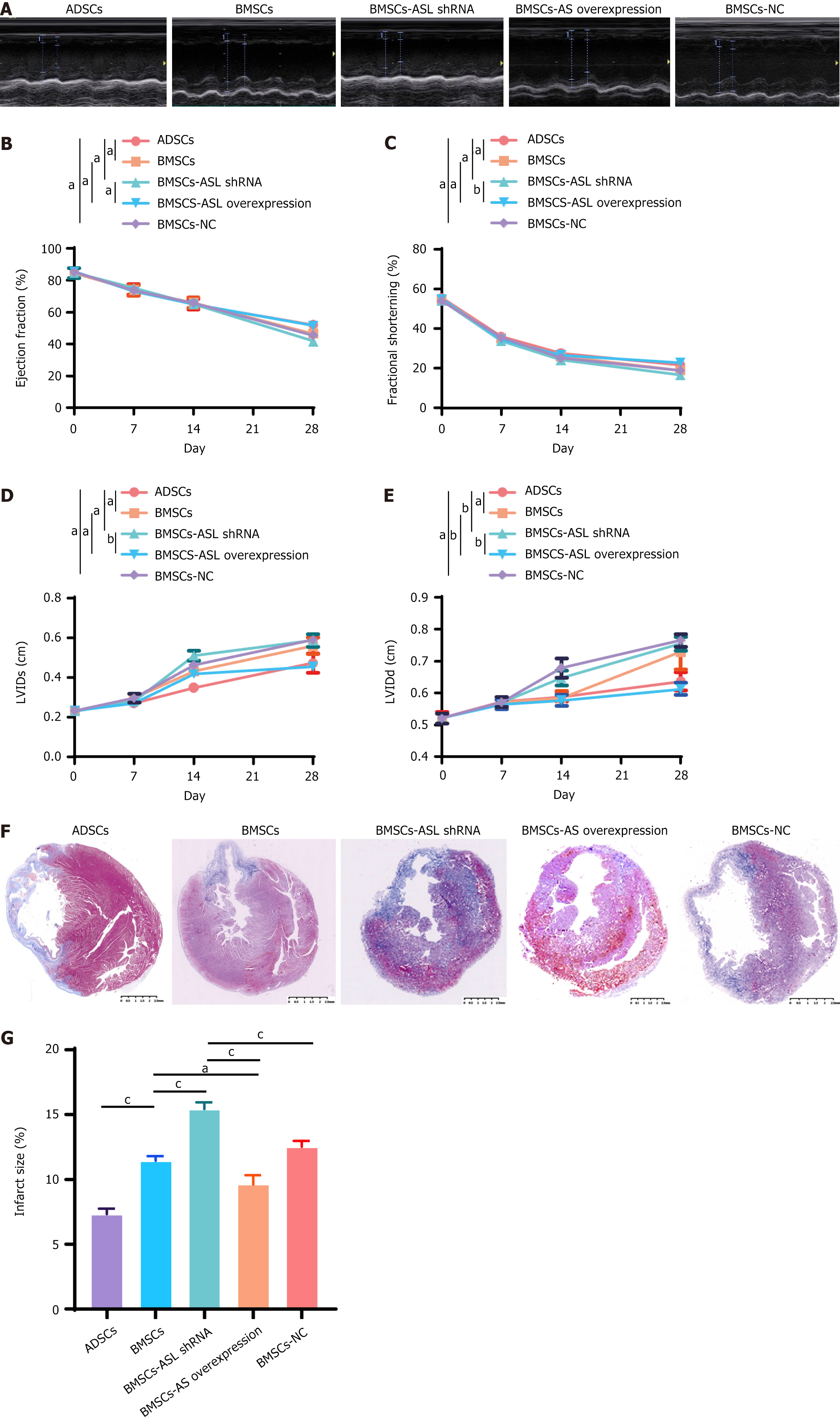
Echocardiography: Echocardiography was used to measure the cardiac function before the MI 1 week, 2 weeks, and 4 weeks after the MSC transplantation. Cardiac function and left ventricular (LV) volumes were evaluated. The ejection fraction (EF), fractional shortening (FS), LV internal systolic dimension (LVIDs), and LV internal diastolic dimension (LVIDd) were measured and recorded for further analysis.
Masson’s staining: At the endpoint all animals were euthanized by an injection of 10% KCl into the aorta. The rat heart was harvested and fixed with 4% paraformaldehyde. Tissue sections were stained according to the Masson’s staining protocol.
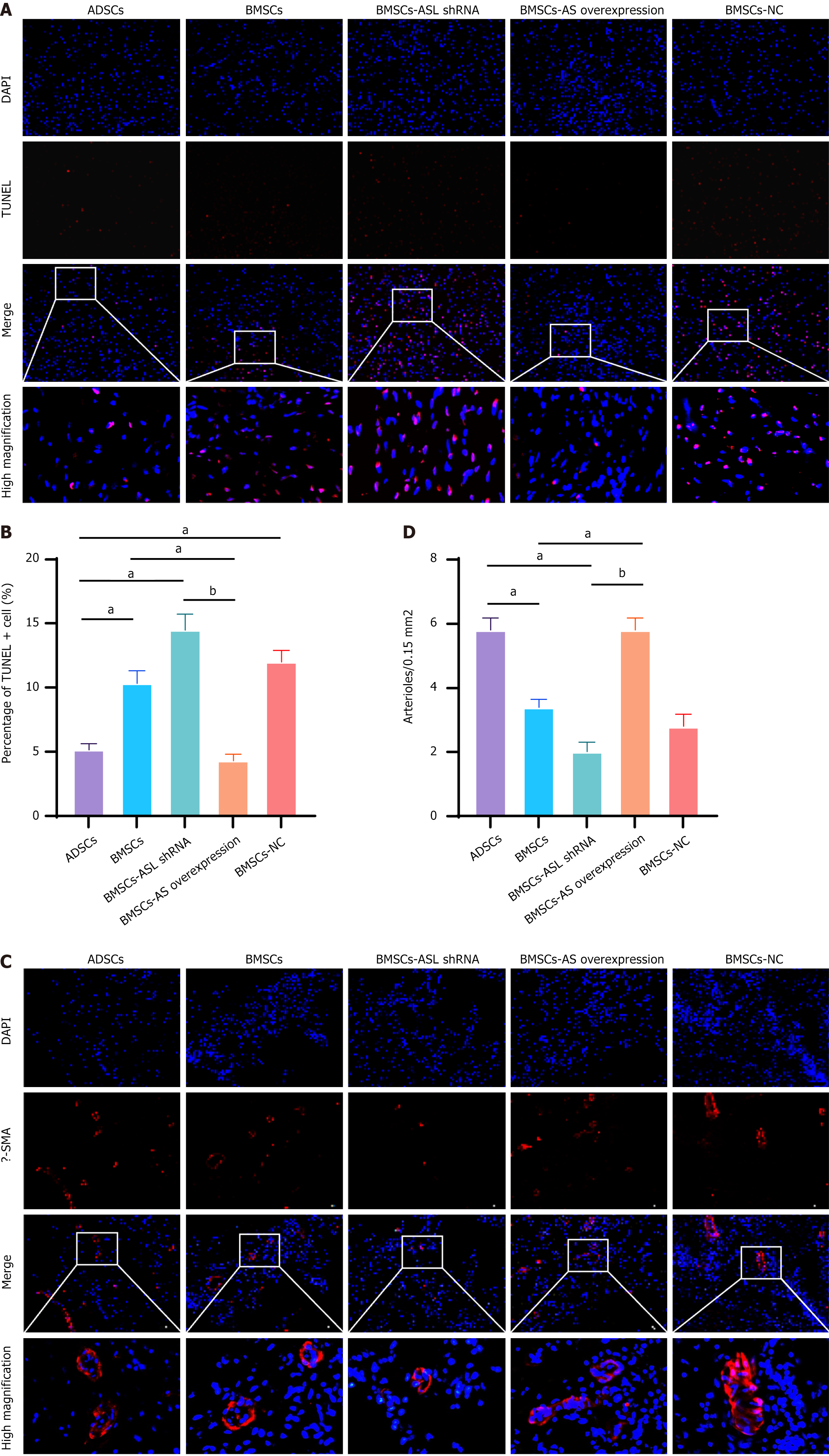
Terminal-deoxynucleotidyl transferase mediated nick end labeling assay: Tissue sections were stained according to the terminal-deoxynucleotidyl transferase mediated nick end labeling (TUNEL) assay instructions. 4,6-diamidino-2-pheny
Immunofluorescence examination: Immunofluorescent staining was performed according to a standard protocol. Briefly, the prepared frozen sections were washed three times for 15 min with PBS. Then, 0.1% Triton X-100 in tris buffered saline was used to permeabilize for 1 h. Furthermore, the slices were blocked with 5% bovine serum albumin for 30 min at 4 ºC overnight with mouse anti-human α-smooth muscle actin (α-SMA) (1:100, ab7817; SMA staining kit from Abcam Group, Cambridge, United Kingdom). Thereafter, slices were treated with goat anti-mouse IgG Texas Red secondary antibody for 1 h in the dark at room temperature. After the nuclei were counterstained with DAPI, the slices were mounted with antifading mounting medium. Vessels with a lumen larger than 10 μm were defined as mature, and the number was calculated and averaged[14].
The image analysis was conducted using ImageJ software (Version 1.8.0, Media Cybernetics, Silver Spring, MD, United States). For data analysis, GraphPad Prism 8 (GraphPad Software Inc, San Diego, CA, United States) was utilized. Data are presented as the mean ± SD. Statistical comparisons among three or more groups were analyzed using either one-way or two-way analysis of variance with repeated measures across time. If the F test was significant (P < 0.05), pairwise tests of individual group means were carried out using either the Newman-Keuls or Bonferroni post-hoc tests. A P value < 0.05 was considered statistically significant.
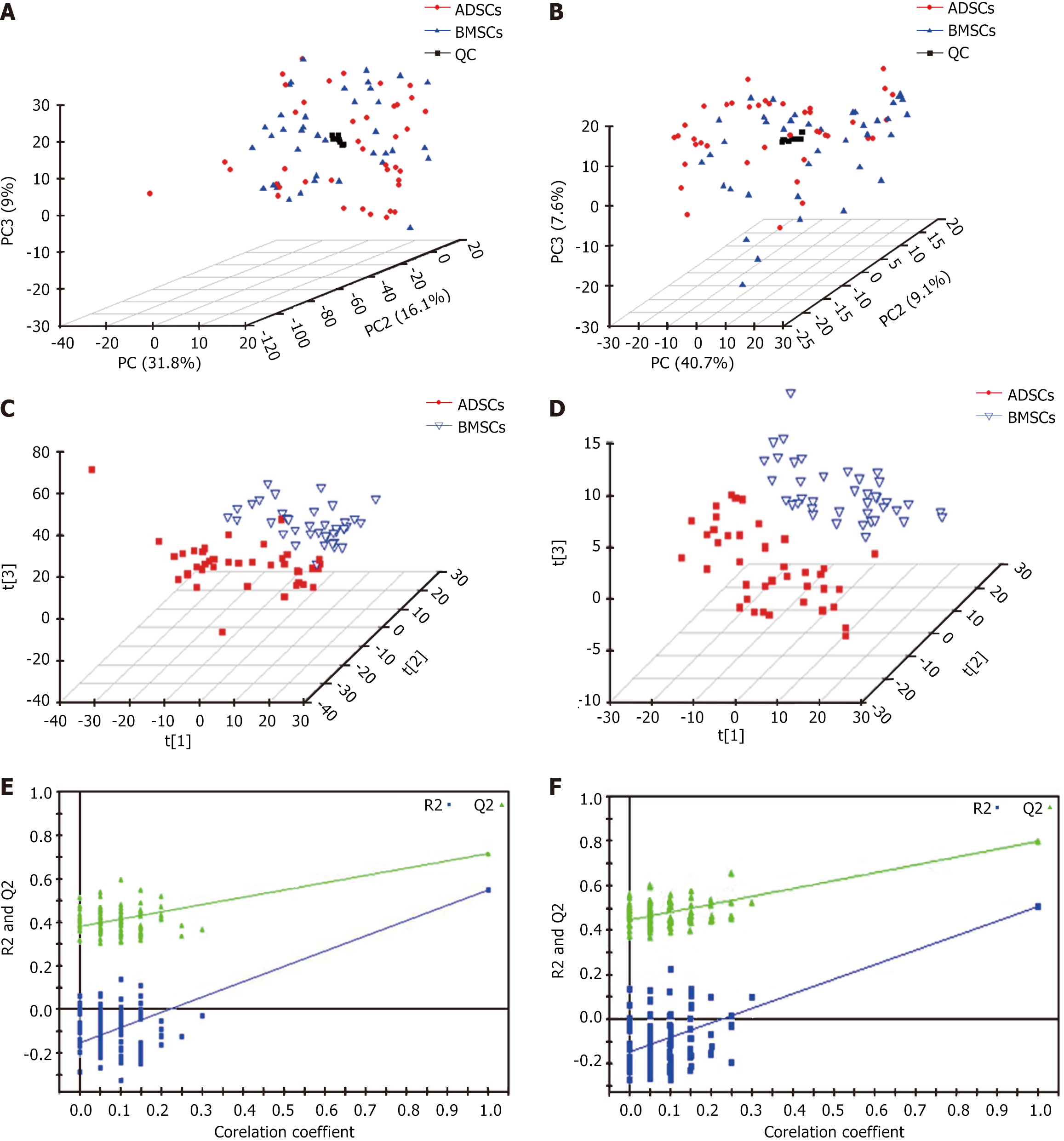
The baseline characteristics of the 40 patients with CHD are presented in Table 1. Metabolic analysis revealed many metabolic differences between ADSCs and BMSCs. The overall principal component analysis results based on all sample clustering in plots of the principal component analysis scores demonstrated that the metabolic profiling platform was robust. No outliers were present. Separation trends were observed between ADSCs and BMSCs (Figure 1A). A number of statistically significant ions were present between ADSCs and BMSCs (Figure 1A and B) as evidenced by the application of the positive electron spray ionization (ESI+) and negative (ESI-) modes. A notable separation was present in the partial least squares discriminant analysis score plot between ADSCs and BMSCs in the ESI+ (Figure 1C) and ESI- (Figure 1D) modes. The partial least squares discriminant analysis score models containing ESI+ and ESI- modes (Figure 1E and F) further confirmed the validity of the supervised models.
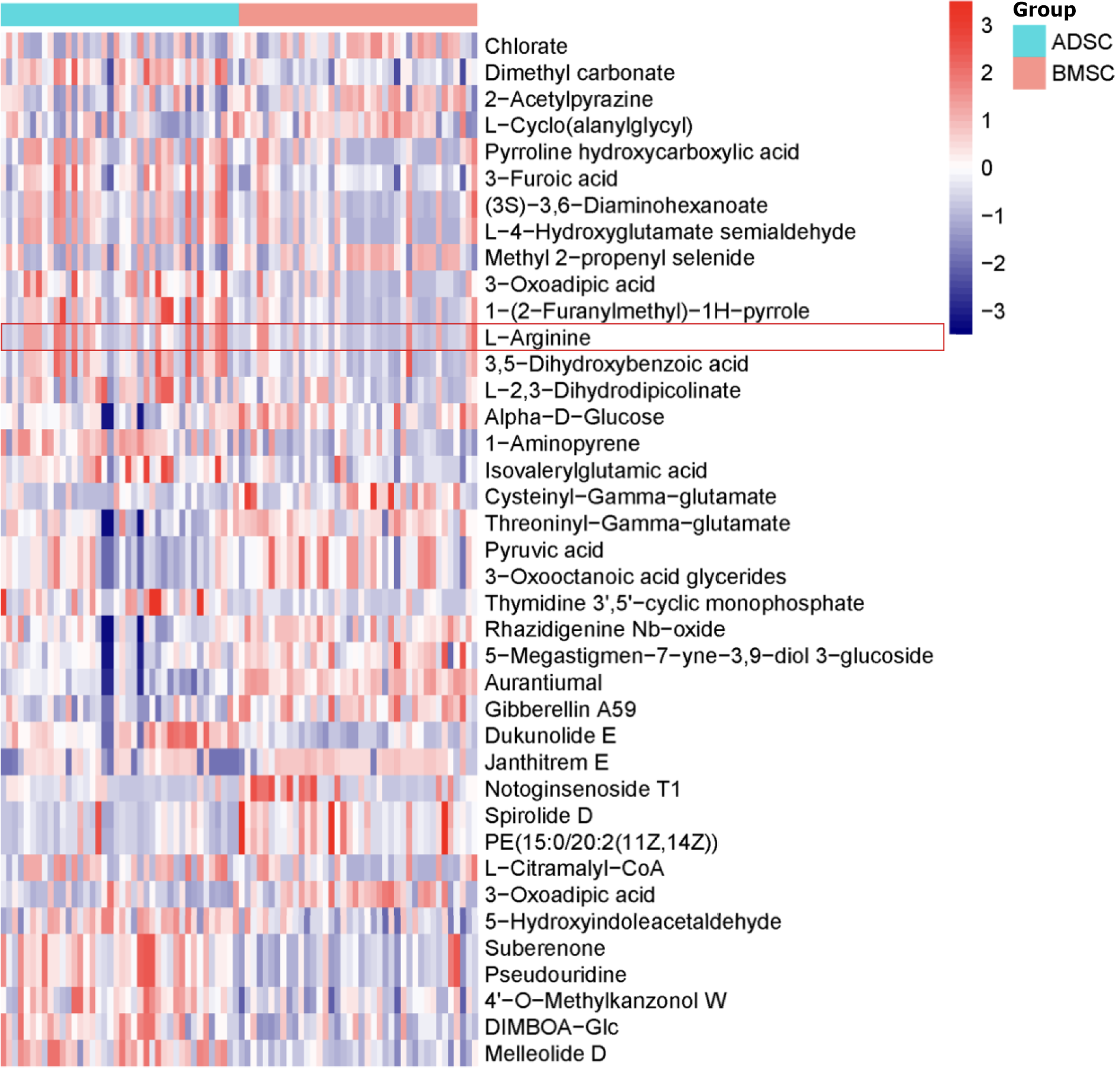
Differential ions were selected as biomarker candidates for subsequent metabolite identification according to the identification procedures[19]. There were 32 metabolites present in the ESI+ mode, including L-arginine, chlorate, dimethyl carbonate, 2-acetylpyrazine, and 3-furoic acid (Table 2, Figure 2). A total of seven metabolites in the ESI- mode were identified, including 3-oxoadipic acid, 5-hydroxyindoleacetaldehyde, suberenone, pseudouridine, 4’-O-methylkanzonol W, DIMBOA-glc, and melleolide D (Table 2, Figure 2). L-arginine was elevated in the ADSC and BMSC supernatants (Figure 3A).
| Num | Name | m/z | Rt(s) | P value | VIP |
| Positive mode (ESI+) | |||||
| P1 | Chlorate | 106.95 | 46.69 | 0.01211 | 1.18 |
| P2 | Dimethyl carbonate | 113.02 | 65.77 | 0.00001 | 2.13 |
| P3 | 2-acetylpyrazine | 123.05 | 62.40 | 0.00786 | 1.13 |
| P4 | L-cyclo(alanylglycyl) | 129.07 | 55.49 | 0.00850 | 1.19 |
| P5 | Pyrroline hydroxycarboxylic acid | 130.05 | 371.82 | 0.02397 | 1.11 |
| P6 | 3-furoic acid | 135.00 | 64.98 | 0.00144 | 1.27 |
| P7 | (3S)-3,6-diaminohexanoate | 147.11 | 44.84 | 0.02305 | 1.14 |
| P8 | L-4-hydroxyglutamate semialdehyde | 148.06 | 371.82 | 0.02329 | 1.12 |
| P9 | Methyl 2-propenyl selenide | 158.97 | 46.80 | 0.02157 | 1.17 |
| P10 | 3-oxoadipic acid | 161.05 | 426.39 | 0.00225 | 1.20 |
| P11 | 1-(2-furanylmethyl)-1H-pyrrole | 170.05 | 56.27 | 0.00071 | 1.28 |
| P12 | L-arginine | 175.12 | 54.32 | 0.04341 | 1.01 |
| P13 | 3,5-dihydroxybenzoic acid | 177.02 | 48.78 | 0.00178 | 1.17 |
| P14 | L-2,3-dihydrodipicolinate | 192.03 | 58.70 | 0.01307 | 1.07 |
| P15 | Alpha-D-glucose | 203.05 | 53.34 | 0.01773 | 1.40 |
| P16 | 1-aminopyrene | 218.10 | 65.96 | 0.00001 | 2.88 |
| P17 | Isovalerylglutamic acid | 232.12 | 80.44 | 0.00053 | 1.59 |
| P18 | Cysteinyl-gamma-glutamate | 250.08 | 367.38 | 0.00030 | 1.45 |
| P19 | Threoninyl-gamma-glutamate | 270.11 | 422.74 | 0.00134 | 1.70 |
| P20 | Pyruvic acid | 272.12 | 440.40 | 0.00153 | 1.75 |
| P21 | 3-oxooctanoic acid glycerides | 272.12 | 440.40 | 0.00153 | 1.75 |
| P22 | Thymidine 3’,5’-cyclic monophosphate | 305.05 | 437.12 | 0.00062 | 1.65 |
| P23 | Rhazidigenine Nb-oxide | 337.19 | 524.49 | 0.00155 | 1.49 |
| P24 | 5-megastigmen-7-yne-3,9-diol 3-glucoside | 371.20 | 478.05 | 0.00134 | 1.59 |
| P25 | Aurantiumal | 377.13 | 401.34 | 0.00000 | 3.58 |
| P26 | Gibberellin A59 | 383.11 | 53.34 | 0.00011 | 1.54 |
| P27 | Dukunolide E | 507.15 | 470.89 | 0.00000 | 3.03 |
| P28 | Janthitrem E | 604.35 | 424.91 | 0.00081 | 1.92 |
| P29 | Notoginsenoside T1 | 653.44 | 913.54 | 0.00174 | 2.31 |
| P30 | Spirolide D | 708.49 | 724.88 | 0.00184 | 1.75 |
| P31 | PE(15:0/20:2(11Z,14Z)) | 752.51 | 722.06 | 0.00158 | 1.78 |
| P32 | L-citramalyl-CoA | 920.14 | 54.81 | 0.04634 | 1.18 |
| Negative mode (ESI-) | |||||
| N1 | 3-oxoadipic acid | 159.03 | 65.65 | 0.00009 | 1.45 |
| N2 | 5-hydroxyindoleacetaldehyde | 174.06 | 470.87 | 0.00010 | 1.96 |
| N3 | Suberenone | 243.07 | 68.86 | 0.00000 | 2.64 |
| N4 | Pseudouridine | 243.07 | 68.86 | 0.00000 | 2.64 |
| N5 | 4’-o-methylkanzonol W | 349.10 | 64.34 | 0.00006 | 2.05 |
| N6 | DIMBOA-Glc | 372.10 | 68.31 | 0.00010 | 2.41 |
| N7 | Melleolide D | 481.16 | 68.85 | 0.00000 | 2.63 |
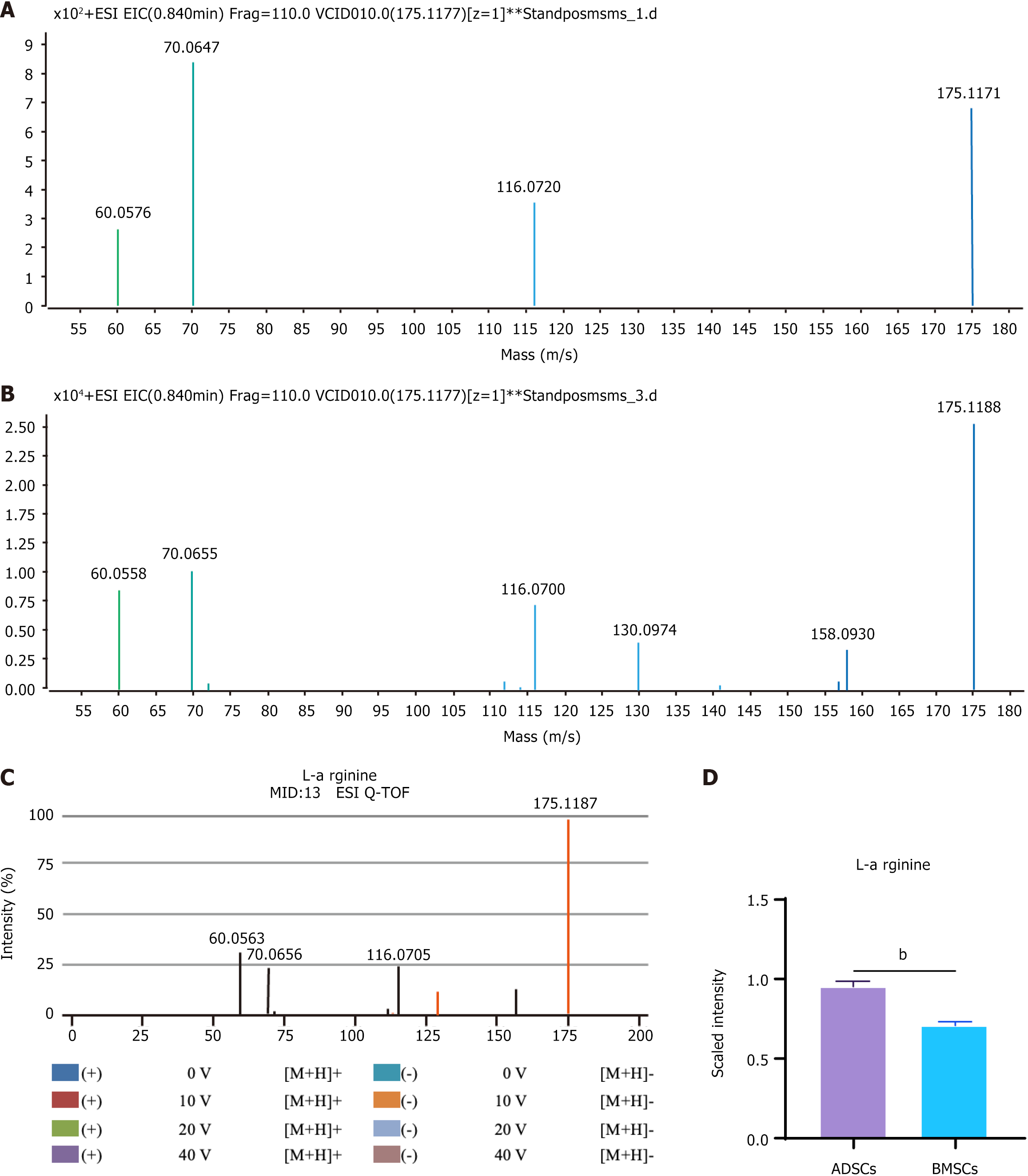
The identification procedure of L-arginine is shown in Figure 3. The extracted ion chromatographic peak and mass spectrum of mass-to-charge ratio (m/z) of 175.11 in the ESI+ mode were obtained at the retention time of 54.32 min (Figure 3B). The ion with an m/z of 175.11 was considered to be a fragment of L-arginine. This L-arginine compound was searched in the METLIN metabolite database. The L-arginine tandem mass spectrometry (MS/MS) spectrum in the ADSC supernatant was compared to the metabolite candidates in these databases (Figure 3C). The MS/MS spectrum of a quasimolecular ion was matched well with that of L-arginine from the METLIN database. For further identification, the MS/MS spectrum of standard L-arginine quasimolecular ion matched well with the METLIN database at m/z of 175.1177 at 54.32 min (Figure 3D).
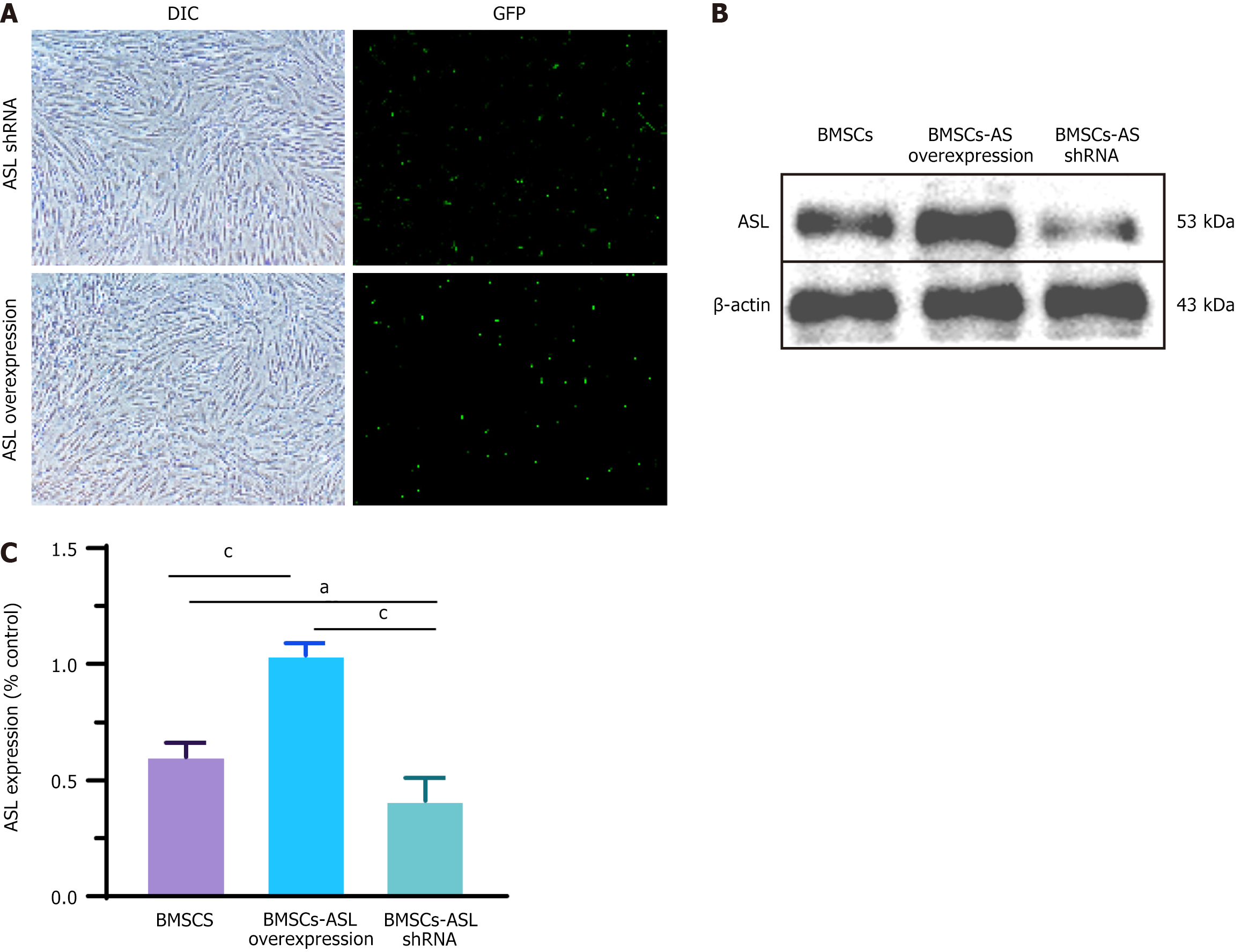
ASL is a key regulatory enzyme in L-arginine production in vivo. To study the effect of ASL on the synthesis of L-arginine in BMSCs, ASL was overexpressed or downregulated in BMSCs by plasmid transfection. The transfection efficiencies of ASL overexpression and shRNA plasmid in BMSCs were 23.0% ± 4.4% and 21.7% ± 3.7%, respectively, which was quantified by the ratio of GFP-positive cells to the total BMSC number (Figure 4A). After transfection, ASL expression of each group the protein level was determined by western blot. The total ASL protein expression was significantly upregulated or downregulated as compared with control group (Figure 4B and C).
Scratch wound healing assays were used to evaluate the indirect effect of ASL on cell migration in HUVECs (Figure 5A and B). After incubation for 18 h, HUVECs co-cultured with both BMSC-ASL overexpressed and ADSCs migrated faster than the BMSCs and BMSC-ASL shRNA groups. BMSCs migrated noticeably faster than the BMSC-ASL shRNA group (P < 0.05). Furthermore, the tube formation assay was performed to confirm that the increased secretion of L-arginine can promote vascular regeneration. Compared to the BMSCs and BMSC-ASL shRNA groups, MSCs from BMSC-ASL overexpressed and ADSCs groups promoted endothelial cells to form capillary-like structures. This experiment further exhibited that the BMSC-ASL overexpressed group had a greater proangiogenic capability than the other three BMSC-based groups (Figure 5C and D).
To evaluate proangiogenetic cytokines released by the MSCs in hypoxic and serum-free conditions, VEGF and bFGF secretion into culture supernatants was assessed by ELISA. VEGF and bFGF were increased in the BMSC-ASL overexpressed and ADSCs groups compared to the other groups (Figure 6A and B). This effect was significantly greater in the BMSC-ASL overexpressed group as compared to that in the BMSC-ASL shRNA group.
As expected, MAPK and VEGFR2 protein expression was higher in the BMSC-ASL overexpressed and ADSCs groups as compared with that of both the BMSCs and BMSC-ASL shRNA groups. There were no significant differences between the BMSC-ASL overexpressed and ADSCs groups. Protein expression level of eNOS in the BMSC-ASL overexpressed group was significantly higher than that in the BMSCs and BMSC-ASL shRNA groups. Additionally, eNOS protein expression in the ADSCs group was higher than that in the BMSCs group, though it was not significant (Figure 6C-F).
The present in vivo study was aimed at assessing cardiac function before, 1 week, and 4 weeks after the MI was treated by transplantation with MSCs. At 4 weeks after MI, EF and FS in the BMSC-ASL overexpressed and ADSCs groups were higher than those in other groups. The LVIDs and LVIDd in the BMSC-ASL overexpressed and ADSCs groups were significantly lower than those in the BMSCs and BMSC-ASL shRNA groups. These results demonstrate that transplantation of ASL-modified MSCs may enhance cardiac function to the level comparable to ADSCs after the MI (Figure 7A-E).
On the 28th day after implantation, computerized morphometric analysis results indicated that the infarct size was significantly smaller in the BMSC-ASL overexpressed and ADSCs groups compared to that in the BMSCs group (Figure 7F and G). As expected, the infarct size in the ADSCs group was smaller than that in both the BMSCs and BMSC-ASL shRNA groups. These results demonstrated that “aged” ADSCs could resist the expansion of LV more efficiently than “aged” BMSCs, and MI size was decreased after treatment with ASL-modified BMSCs.
To further assess the effect of L-arginine on the function of MSCs, ASL-modified BMSCs were injected into the infarcted area after the MI in vivo. As expected, the number of apoptotic cells (TUNEL+) was significantly lower in the border region in the BMSC-ASL overexpressed and ADSCs groups than in both the BMSCs and BMSC-ASL shRNA groups after BMSC transplantation (Figure 8A and B). To evaluate angiogenesis in the infarct area after MSC trans
The present study evaluated the effects of ADSCs and BMSCs from elderly patients with CHD as transplanted cells for MI after hypoxia and ischemia preconditioning. The major finding of this research was that ADSCs from elderly patients with CHD have the advantages of promoting angiogenesis and resisting apoptosis in a hypoxic and ischemic microenvironment due to more excretion of L-arginine (verified by metabolomics). These cells are more suitable as autotransplantation candidates for the treatment of MI patients.
Until now, the therapeutic efficacy of treatments on MI in aged patients has been limited[25]. Many studies have investigated BMSC transplantation, which has been recognized as an attractive therapy source for patients with MI[26] due to its angiogenic and antiapoptotic potential. However, MSC therapy is often inefficient because of low viability and poor therapeutic activity of the transplanted MSCs, especially in elderly patients with ischemic heart diseases (IHDs)[27].
Hypoxia and ischemia in the MI microenvironment are two important factors that affect MSC viability and therapeutic activity. In previous preclinical studies, hypoxic preconditioning has been demonstrated as an effective way to enhance therapeutic efficacy of MSCs[20,28-30]. Our previous studies have also found a higher protein expression of VEGFA when MSCs were cultured in hypoxic conditions vs in normoxic conditions[31]. MSCs had higher cytokine synthesis and secretion that may have resulted in better antiapoptotic and inductive angiogenic capacity in hypoxic conditions with serum deprivation[31].
Although MSCs are widely found in many tissues, BMSCs and ADSCs are cell sources that have been thoroughly investigated[32]. Generally, in comparison to ADSCs, BMSCs have their own inherent disadvantages, which include the relatively small numbers of obtainable cells and invasiveness of bone marrow aspiration. Furthermore, in comparison to that from young patients, the quantity of BMSCs from the bone marrow stroma of old patients is usually lower. Conversely, ADSCs can be obtained with less injury than BMSCs, and the amount of ADSCs is often greater in elderly patients because obesity is more common in older population around the world. More importantly, obesity acts as one of the risk factors to CHD, and MI often occurs in overweight and obese individuals, which means most patients with CHD have an adequate ADSC reservoir. Therefore, ADSCs have attracted attention, and many researchers have also demonstrated the efficacy and safety of autologous ADSCs[33,34]. However, the comparison of therapeutic efficacy of autologous ADSC and BMSC transplantation to the MI area from the same elderly patients and the possible mechanism have remained largely elusive.
In recent years, many studies focused on cellular metabolic states and metabolic functions and have uncovered a key role for metabolism in the generation of nucleotides, phospholipids, and amino acids[35]. This metabolism role in regulating the cell fate has represented a rapidly growing field of research and has been termed “metabolic reprogramming”. Establishing the metabolic signatures of ADSCs and BMSCs from the same elderly patients with CHD will be helpful for explaining the differences and development of clinical treatments.
To simulate the microenvironment in the infarcted tissue after transplantation and increase the survival and activity of therapeutic MSCs, ADSCs and BMSCs were cultured in serum-free and hypoxic conditions in the present study. As is shown, the compounds of metabolites demonstrated a significant difference in metabolites between ADSCs and BMSCs when serum and oxygen were deprived. Overall, 32 metabolites in the ESI+ mode and 7 metabolites in the ESI- mode were identified in the study (Table 2, Figure 2). Then, the metabolites were matched with known human metabolites in the Human Metabolome Database or Kyoto Encyclopedia of Genes and Genomes, which was helpful for understanding the differences between ADSCs and BMSCs after culture in serum-free and hypoxic conditions[9].
Interestingly, L-arginine from ADSCs was significantly increased compared with that from BMSCs. It was able to promote the process of angiogenesis. The MS/MS spectrum of L-arginine in the supernatant and quasimolecular ion of standard L-arginine was matched with the METLIN database, which further confirmed that L-arginine secretion by ADSCs was higher than that by BMSCs (Figure 3D). The L-arginine effect on angiogenesis has been well-documented by previous studies involving production of nitric oxide (NO)[36], which is generated in the vascular endothelium via the enzyme eNOS. It has also been reported that L-arginine levels within cells are key for both production and regulation of NO[37]. NO is a small short-lived free radical gas that plays an important role in different physiological processes, especially in those of the cardiovascular system. NO is crucial in promoting angiogenesis by enhancing endothelial cell proliferation and migration[38,39] due to an NO-mediated increase of VEGF and bFGF expression[40].
As is well-known, vascular endothelial cells play a crucial role in angiogenesis after MI. In this research, the effect of L-arginine on angiogenesis from ADSCs or BMSCs cultured in serum-free and hypoxic conditions was investigated using wound healing and tube formation assays in HUVECs co-cultured with MSCs. The results showed that L-arginine secretion by ADSCs and BMSCs was able to promote endothelial cell migration and tube formation on Matrigel (Figure 5). Furthermore, the increased secretion of L-arginine might accelerate the production and excretion of VEGF and bFGF in the study (Figure 6A and B).
VEGFR2 and MAPK were identified as the main mediators of MSCs in the present study. VEGFR2 has been recognized to have an important role in angiogenic and permeability-enhancing effects of VEGF on angiogenesis[41]. It has also been confirmed that VEGFR2 is essential for the proliferation of endothelial cells. VEGF can be upregulated by hypoxia-inducible factor α in hypoxic conditions[42]. MAPK signaling cascades are activated when VEGFR2 tyrosine kinase activity in vascular endothelial cells is able to release a series of cytokines and growth factors leading to angiogenesis[43]. L-arginine was also able to increase MAPK and VEGFR2 protein expression in HUVECs co-cultured with MSCs. Given the above results, it is evident that L-arginine-VEGFR2-MAPK signaling is involved in MSCs-mediated angiogenesis.
To assess the effect of MSCs on the diseased heart in vivo, adult Sprague Dawley male rats with surgically induced MI were injected with each group of MSCs into the infarction zone as described above. Rat heart function, including EF, FS, LVIDd, and LVIDs, was evaluated via echocardiography 28 days later. The BMSC-ASL overexpressed and ADSCs demonstrated a strong, positive treatment on the MI animals. Rats treated with the BMSC-ASL overexpressed plasmid exhibited an improved EF, FS, LVIDd, and LVIDs compared to the other BMSC-based groups as well as those treated with ADSCs (Figure 7A-E). Therefore, we have confirmed that the BMSC-ASL overexpressed and ADSCs groups had a protective effect on the MI heart.
To search for the possible reasons to the functional improvement, we performed Masson’s trichrome staining to find that the chamber geometry of the LV was more properly restored in the BMSC-ASL overexpressed and ADSCs groups in comparison to the other groups (Figure 7F and G). Additionally, it has been verified that the effect of MSC transplantation was proportional to the number of residual transplanted cells in the MI area[44]. The number of remaining MSCs is significantly higher in the ASL-overexpressed BMSCs and ADSCs transplantation (Figure 8B and D). We believe that the improvement may partially be attributed to their enhanced proangiogenic abilities associated with higher L-arginine secretion.
As shown in vitro, ASL-overexpressed BMSCs and ADSCs released more VEGF and bFGF cytokines via L-arginine that led to increased endothelial cell migration and capillary-like structure formation. In vivo, α-SMA staining demonstrated a higher vessel density in the ASL-overexpressed BMSCs and ADSCs group than that of the other groups. We believe that enhanced therapeutic effects due to improvement in angiogenesis may suggest two major mechanisms. On one hand, the increased VEGF and bFGF secretion due to increased L-arginine synthesis by MSCs can form a vessel network by recruiting more host endothelial progenitor cells, endothelial cells, and smooth muscle cells in the MI area[45,46]. On the other hand, ASL-overexpressed BMSCs and ADSCs were more resistant to post-transplantation hypoxia and ischemia. This increased angiogenesis could improve the microenvironment in the MI area and might also improve the viability of both transplanted and recruited host cells. Better MSC engraftment was associated with avoidance of ventricular thinning and dilation by neovascularization, which may enhance cardiac function.
At present, MSC-based therapy has emerged as a highly promising therapeutic approach for IHD. Autologous MSCs, in particular, present an attractive source selection owing to their effective avoidance of immune rejection and complex potential socioethical issues. However, in the present aging society, autologous stem cell therapy faces a significant challenge: Notably the inherent senescence of MSCs themselves. Two primary strategies have been proposed to address this dilemma: One involves implementing “rejuvenation” modifications to transplanted aged stem cells; and the other focuses on selecting stem cell types with superior bioactivity.
Multiple research teams have demonstrated that compared with BMSCs, ADSCs not only possess advantages such as convenient accessibility, abundant sources, and ease of clinical translation but also exhibit enhanced biological activity and therapeutic efficacy. The underlying mechanisms include reduced telomere attrition[15], diminished β-galactosidase activation[14], and increased resistance to oxidative stress and apoptosis[16], among others. In this study, we further validated the unique advantages of ADSCs over BMSCs derived from the same aging population through a metabolomics perspective, which is of great significance for the treatment of IHD in the context of aging and obesity.
MSCs from young patients were not assessed for donor limitations. In addition, this was a short-term study that used small animals. Therefore, long-term studies that assess the effect of MSCs from young patients and additional research on MI area in large animals will be needed in the future.
This study demonstrated that L-arginine secreted by ADSCs or BMSCs from elderly patients played an important role in inducing therapeutic angiogenesis under serum-free and hypoxic conditions. Comparatively, ADSCs had a higher L-arginine secretion, and in turn a better capacity for angiogenetic induction, ventricular chamber maintenance, and cardioprotection both in vitro and in vivo. ADSC transplantation represents a promising strategy for autologous cell-based therapy in the context of an aging society.
We thank Mrs. Hai-Yu Zhang for help with statistical analysis.
| 1. | GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3389] [Cited by in RCA: 3354] [Article Influence: 419.3] [Reference Citation Analysis (0)] |
| 2. | Goradel NH, Hour FG, Negahdari B, Malekshahi ZV, Hashemzehi M, Masoudifar A, Mirzaei H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J Cell Biochem. 2018;119:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 3. | Mirzaei H, Sahebkar A, Sichani LS, Moridikia A, Nazari S, Sadri Nahand J, Salehi H, Stenvang J, Masoudifar A, Mirzaei HR, Jaafari MR. Therapeutic application of multipotent stem cells. J Cell Physiol. 2018;233:2815-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Mirzaei H, Salehi H, Oskuee RK, Mohammadpour A, Mirzaei HR, Sharifi MR, Salarinia R, Darani HY, Mokhtari M, Masoudifar A, Sahebkar A, Salehi R, Jaafari MR. The therapeutic potential of human adipose-derived mesenchymal stem cells producing CXCL10 in a mouse melanoma lung metastasis model. Cancer Lett. 2018;419:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 5. | Moradian Tehrani R, Verdi J, Noureddini M, Salehi R, Salarinia R, Mosalaei M, Simonian M, Alani B, Ghiasi MR, Jaafari MR, Mirzaei HR, Mirzaei H. Mesenchymal stem cells: A new platform for targeting suicide genes in cancer. J Cell Physiol. 2018;233:3831-3845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Mianehsaz E, Mirzaei HR, Mahjoubin-Tehran M, Rezaee A, Sahebnasagh R, Pourhanifeh MH, Mirzaei H, Hamblin MR. Mesenchymal stem cell-derived exosomes: a new therapeutic approach to osteoarthritis? Stem Cell Res Ther. 2019;10:340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 7. | Asgarpour K, Shojaei Z, Amiri F, Ai J, Mahjoubin-Tehran M, Ghasemi F, ArefNezhad R, Hamblin MR, Mirzaei H. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 8. | Li JZ, Cao TH, Han JC, Qu H, Jiang SQ, Xie BD, Yan XL, Wu H, Liu XL, Zhang F, Leng XP, Kang K, Jiang SL. Comparison of adipose and bone marrowderived stem cells in protecting against oxLDLinduced inflammation in M1macrophagederived foam cells. Mol Med Rep. 2019;19:2660-2670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Li JZ, Qu H, Wu J, Zhang F, Jia ZB, Sun JY, Lv B, Kang Y, Jiang SL, Kang K. Metabolic profiles of adipose-derived and bone marrow-derived stromal cells from elderly coronary heart disease patients by capillary liquid chromatography quadrupole time-of-flight mass spectrometry. Int J Mol Med. 2018;41:184-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Rasmussen JG, Frøbert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, Fink T, Simonsen U. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2014;23:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 279] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1410] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 13. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1372] [Cited by in RCA: 1222] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 14. | Wu H, Li JZ, Xie BD, Tian H, Fang SH, Jiang SL, Kang K. Lower Senescence of Adipose-Derived Stem Cells than Donor-Matched Bone Marrow Stem Cells for Surgical Ventricular Restoration. Stem Cells Dev. 2018;27:612-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Iacomi DM, Rosca AM, Tutuianu R, Neagu TP, Pruna V, Simionescu M, Titorencu I. Generation of an Immortalized Human Adipose-Derived Mesenchymal Stromal Cell Line Suitable for Wound Healing Therapy. Int J Mol Sci. 2022;23:8925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 16. | Horie T, Hirata H, Sakamoto T, Kitajima H, Fuku A, Nakamura Y, Sunatani Y, Tanida I, Sunami H, Tachi Y, Ishigaki Y, Yamamoto N, Shimizu Y, Ichiseki T, Kaneuji A, Iwabuchi K, Osawa S, Kawahara N. Multiomics analyses reveal adipose-derived stem cells inhibit the inflammatory response of M1-like macrophages through secreting lactate. Stem Cell Res Ther. 2024;15:485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Xia Y, Xu X, Guo Y, Lin C, Xu X, Zhang F, Fan M, Qi T, Li C, Hu G, Peng L, Wang S, Zhang L, Hai C, Liu R, Yan W, Tao L. Mesenchymal Stromal Cells Overexpressing Farnesoid X Receptor Exert Cardioprotective Effects Against Acute Ischemic Heart Injury by Binding Endogenous Bile Acids. Adv Sci (Weinh). 2022;9:e2200431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Thandar M, Yang X, Zhu Y, Zhang X, Chen Z, Huang S, Chi P. Dysbiosis of gut microbiota and metabolites is associated with radiation-induced colorectal fibrosis and is restored by adipose-derived mesenchymal stem cell therapy. Life Sci. 2024;341:122502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Wang JA, Chen TL, Jiang J, Shi H, Gui C, Luo RH, Xie XJ, Xiang MX, Zhang X. Hypoxic preconditioning attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Acta Pharmacol Sin. 2008;29:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 466] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 21. | Brusilow SW, Batshaw ML. Arginine therapy of argininosuccinase deficiency. Lancet. 1979;1:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Zhang DY, Zhang CF, Fu BC, Sun L, Wang XQ, Chen W, Liu W, Liu KY, Du GQ, Ma CY, Jiang SL, Li RK, Tian H. Sirtuin3 protects aged human mesenchymal stem cells against oxidative stress and enhances efficacy of cell therapy for ischaemic heart diseases. J Cell Mol Med. 2018;22:5504-5517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Luo XL, Jiang Y, Li Q, Yu XJ, Ma T, Cao H, Ke MX, Zhang P, Tan JL, Gong YS, Wang L, Gao L, Yang HT. hESC-Derived Epicardial Cells Promote Repair of Infarcted Hearts in Mouse and Swine. Adv Sci (Weinh). 2023;10:e2300470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Armiñán A, Gandía C, García-Verdugo JM, Lledó E, Trigueros C, Ruiz-Saurí A, Miñana MD, Solves P, Payá R, Montero JA, Sepúlveda P. Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. J Am Coll Cardiol. 2010;55:2244-2253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Carrabba N, Parodi G, Valenti R, Migliorini A, Bellandi B, Antoniucci D. Prognostic value of reverse left ventricular remodeling after primary angioplasty for STEMI. Atherosclerosis. 2012;222:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Yang L, Yan F, Ma J, Zhang J, Liu L, Guan L, Zheng H, Li T, Liang D, Mu Y. Ultrasound-Targeted Microbubble Destruction-Mediated Co-Delivery of Cxcl12 (Sdf-1alpha) and Bmp2 Genes for Myocardial Repair. J Biomed Nanotechnol. 2019;15:1299-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Osterziel KJ. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1 year results of the REPAIR-AMI trial. Eur Heart J. 2007;28:638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Antebi B, Rodriguez LA 2nd, Walker KP 3rd, Asher AM, Kamucheka RM, Alvarado L, Mohammadipoor A, Cancio LC. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther. 2018;9:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Beegle J, Lakatos K, Kalomoiris S, Stewart H, Isseroff RR, Nolta JA, Fierro FA. Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells. 2015;33:1818-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 30. | Hu X, Xu Y, Zhong Z, Wu Y, Zhao J, Wang Y, Cheng H, Kong M, Zhang F, Chen Q, Sun J, Li Q, Jin J, Li Q, Chen L, Wang C, Zhan H, Fan Y, Yang Q, Yu L, Wu R, Liang J, Zhu J, Wang Y, Jin Y, Lin Y, Yang F, Jia L, Zhu W, Chen J, Yu H, Zhang J, Wang J. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity Without Remuscularization. Circ Res. 2016;118:970-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 31. | Kang K, Chuai JB, Xie BD, Li JZ, Qu H, Wu H, Fang SH, Cui JJ, Xiu LL, Han JC, Cao TH, Leng XP, Tian H, Li RK, Jiang SL. Mesenchymal Stromal Cells from Patients with Cyanotic Congenital Heart Disease are Optimal Candidate for Cardiac Tissue Engineering. Biomaterials. 2020;230:119574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1366] [Article Influence: 113.8] [Reference Citation Analysis (2)] |
| 33. | Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 34. | Lee HW, Lee HC, Park JH, Kim BW, Ahn J, Kim JH, Park JS, Oh JH, Choi JH, Cha KS, Hong TJ, Park TS, Kim SP, Song S, Kim JY, Park MH, Jung JS. Effects of Intracoronary Administration of Autologous Adipose Tissue-Derived Stem Cells on Acute Myocardial Infarction in a Porcine Model. Yonsei Med J. 2015;56:1522-1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 609] [Cited by in RCA: 751] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 36. | Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 37. | Cespuglio R, Amrouni D, Meiller A, Buguet A, Gautier-Sauvigné S. Nitric oxide in the regulation of the sleep-wake states. Sleep Med Rev. 2012;16:265-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131-3139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 891] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 39. | Dulak J, Józkowicz A, Dembinska-Kiec A, Guevara I, Zdzienicka A, Zmudzinska-Grochot D, Florek I, Wójtowicz A, Szuba A, Cooke JP. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 40. | Fiedler LR, Bachetti T, Leiper J, Zachary I, Chen L, Renné T, Wojciak-Stothard B. The ADMA/DDAH pathway regulates VEGF-mediated angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:2117-2124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6747] [Cited by in RCA: 6929] [Article Influence: 315.0] [Reference Citation Analysis (0)] |
| 42. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2182] [Article Influence: 103.9] [Reference Citation Analysis (0)] |
| 43. | Kim YM, Kim KE, Koh GY, Ho YS, Lee KJ. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. 2006;66:6167-6174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Liu Z, Wang H, Wang Y, Lin Q, Yao A, Cao F, Li D, Zhou J, Duan C, Du Z, Wang Y, Wang C. The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials. 2012;33:3093-3106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 45. | Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 46. | Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW. Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation. 1997;96:3555-3560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |