INTRODUCTION
Knee osteoarthritis (KOA) is characterized by the progressive loss of articular cartilage and the disruption of the joint matrix[1]. Clinically, patients with KOA typically present with gradually worsening joint pain and stiffness, which can progress to irreversible disability in the advanced stage[2]. Current treatments for KOA are extremely limited. Conservative treatments, such as non-steroidal anti-inflammatory drugs or hyaluronic acid injections, as well as arthroscopic surgery, often provide symptomatic relief but do not alter the progression of KOA, ultimately leading to the need for knee replacement surgery[3]. However, the limited lifespan of prosthetic implants makes knee replacement unsuitable for young patients with KOA. The field of regenerative medicine and stem cell technology provides cutting-edge solutions for the management of KOA.
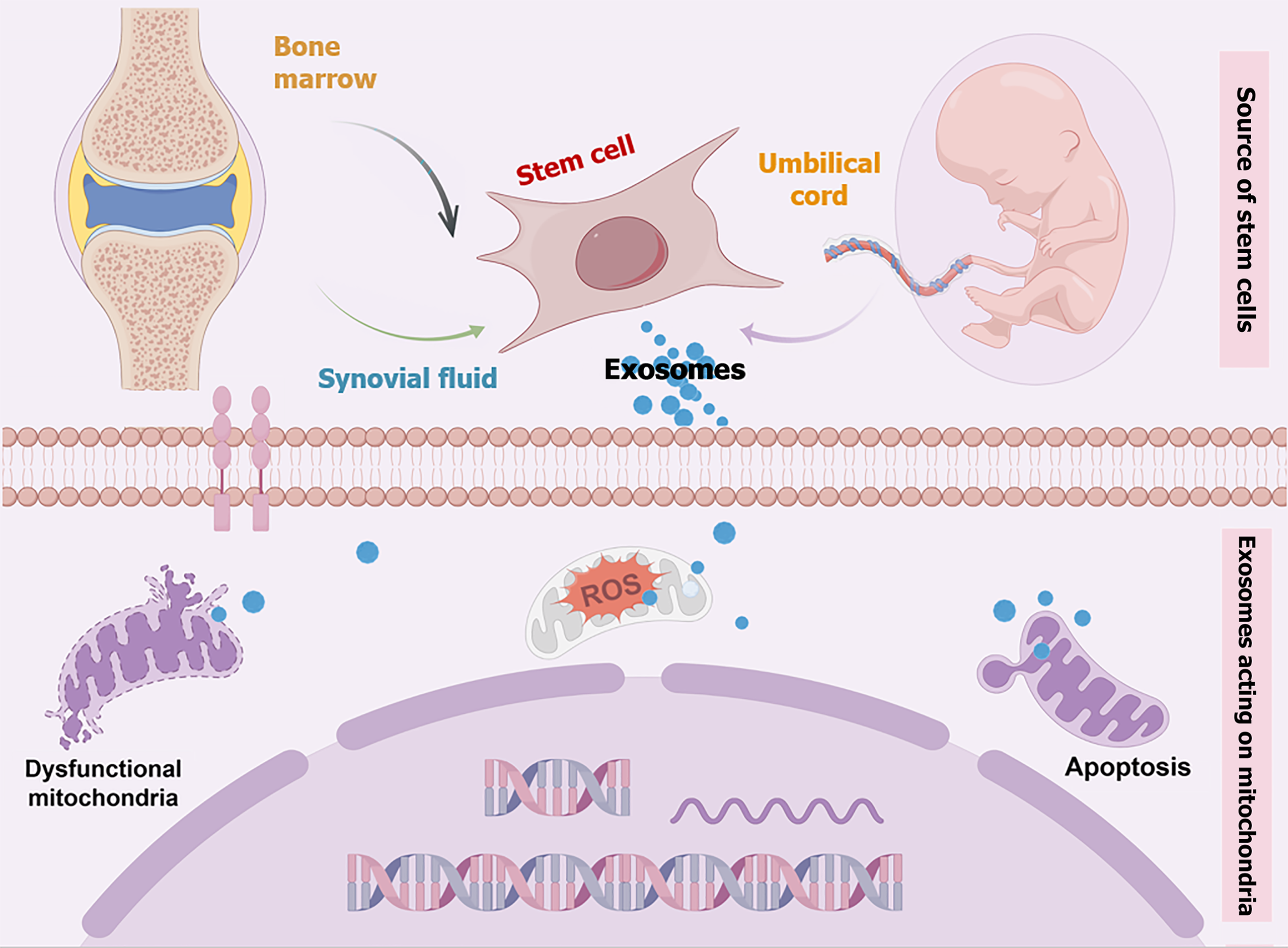
Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) have the ability to proliferate extensively in vitro and differentiate into bone, cartilage, and ligaments, making them promising candidates for applications in regenerative medicine[4]. It has been reported that hUC-MSCs can be safely expanded in vitro and are resistant to malignant transformation in serum-free media, making them suitable for cell therapy[5]. The functions of hUC-MSCs include promoting immune regulation, inhibiting the release of inflammatory factors, and facilitating tissue repair, thereby demonstrating significant potential for application in the treatment of KOA. The use of serum-free hUC-MSCs (N-hUCMSCs) eliminates cross-species effects and minimizes the risk of infection. Therefore, it is necessary to evaluate the efficacy of N-hUCMSCs in KOA. The commented paper has effectively demonstrated that N-hUCMSCs exhibited therapeutic effects in mice with KOA[6]. Moreover, the therapeutic efficacy of N-hUCMSCs was found to be comparable to that of serum-containing hUC-MSCs and hyaluronic acid. However, the mechanisms underlying MSC-based treatment of osteoarthritis (OA) remain intricate and warrant further investigation (Figure 1).
Figure 1 Potential mechanisms of mesenchymal stem cell-based therapy for osteoarthritis.
Mesenchymal stem cells mitigate oxidative stress, cellular senescence, and apoptosis by restoring dysfunctional mitochondria in chondrocytes, thereby facilitating cartilage regeneration. Exosomes play a pivotal role in mediating the therapeutic effects of mesenchymal stem cells. MSC: Mesenchymal stem cell; ROS: Reactive oxygen species.
THERAPEUTIC MECHANISMS AND CHALLENGES OF MESENCHYMAL STEM CELLS IN OA
In recent years, numerous clinical studies have demonstrated the significant efficacy of MSC-based therapies in treating KOA. The primary therapeutic mechanism may involve direct differentiation of MSCs into cartilage tissue and their paracrine effects[7]. Additionally, some studies suggest that MSCs facilitate cartilage repair or regeneration by stimulating articular chondrocyte proliferation or promoting stem cell recruitment[8,9].
The persistence of chronic inflammation is a crucial factor in the progression of OA. Recent studies have revealed the involvement of multiple signaling pathways in the development of chronic inflammation in KOA[10]. Notably, pro-inflammatory cytokines such as interleukin (IL)-1β and IL-18, produced by macrophages, play a pivotal role in the pathogenesis of KOA. The specific mechanism involves the inhibition of chondrocyte proliferation and migration, as well as the induction of chondrocyte apoptosis by proinflammatory factors. Additionally, IL-1β can disrupt chondrocyte metabolism by accelerating extracellular matrix degradation. Recent research has revealed that intraarticular injections of MSCs exhibit significant therapeutic efficacy in animal models of OA[8]. The important role of exosome (Exo)-mediated paracrine effect in the regeneration of cartilage tissue by MSCs has been increasingly emphasized by numerous studies. The mechanism of action of MSC-derived Exos (MSC-Exos) in inflammatory bone diseases has also garnered significant attention[11]. For instance, a study demonstrated that the combination of the infrapatellar fat pad (IPFP)-derived MSCs (MSCIPFP) with chitosan/hyaluronic acid nanoparticles effectively facilitated chondrogenic differentiation. Moreover, MSCIPFP-Exos were shown to augment autophagy in chondrocytes through the inhibition of the mammalian target of rapamycin signaling pathway. The transfer of exosomal miR-100-5p by MSCIPFP-Exos may serve as a crucial mechanism for safeguarding articular cartilage against injury[12]. Another study found that hUCMSCs-Exos can reduce m6A levels in RNA by inhibiting the expression of methyltransferase-like 3. m6A is an important marker involved in the regulation of cellular aging and inflammation. By downregulating m6A, hUCMSCs-Exos can inhibit cartilage tissue inflammation and degradation of collagen type II alpha-1 and aggrecan to significantly slow the progression of OA in mice[13].
Pain is typically the earliest and most common symptom of KOA. A study demonstrated that treatment with MSCs-Exos can effectively diminish the expression levels of calcitonin gene-related peptide and inducible nitric oxide synthase in the dorsal root ganglion, thereby alleviating pain symptoms in rat models of OA[14].
The pathogenesis of KOA involves the progressive loss of articular cartilage, formation of osteophytes, subchondral osteosclerosis, and varying degrees of synovial inflammation[1]. The mitochondrial dysfunction of MSCs is a crucial factor in the progression of OA, which deserves special attention. Mounting evidence indicates that mitophagy plays a pivotal role in the progression of OA. For instance, inflammatory factors such as IL-1β stimulate OA chondrocytes, elevating reactive oxygen species levels and activating the PRKN-mediated mitophagy pathway, ultimately triggering mitochondrial loss and apoptosis[15]. Additionally, the autophagy regulator spermidine has been shown to mitigate oxidative stress and inflammation by modulating mitophagy, thereby delaying OA progression and highlighting a novel therapeutic avenue[16]. The excessive influx of Ca2+ into mitochondria has been identified as a significant contributor to the imbalance in mitochondrial oxidative stress homeostasis and impairment of oxidative capacity[17]. The impairment of mitochondria also hinders the differentiation process of MSCs. To address this, researchers have developed a nano-repair material, which is a nanoparticle that responds to intracellular microenvironment (esterase and low pH), capable of selectively absorbing Ca2+ around MSC mitochondria to regulate excessive Ca2+ intake and alleviate mitochondrial dysfunction. These findings suggest that the restoration of mitochondrial function in MSCs may also serve as one of the mechanisms through which MSCs alleviate OA.
MSC senescence is a pivotal driver in OA, an age-related degenerative joint disease. Targeting MSC senescence thus represents a critical therapeutic strategy. Ye et al[18] identified a novel anti-senescence mechanism involving ALKBH5, an m6A demethylase essential for cellular metabolic regulation. Their study demonstrated that ALKBH5 knockdown accelerates MSC senescence and induces mitochondrial dysfunction. Notably, ALKBH5 knockout exacerbated OA progression in murine models. These findings underscore that mitigating MSC senescence directly influences the pathogenesis of OA.
The interaction between MSCs and biomaterials appears to be a promising avenue for cartilage repair and promotion of cartilage regeneration. For example, the utilization of novel PLGA-p188-PLGA microspheres in certain studies has successfully impeded the differentiation process of stem cells into chondrocytes, thereby demonstrating that PLGA-based microcarriers can effectively establish a favorable microenvironment for MSCs to facilitate cartilage repair, offering renewed hope for the treatment of OA[19]. Another study revealed that kartogenin, an inductive small molecule, alleviates OA by enhancing the chondrogenic differentiation of synovial fluid-derived MSCs[20]. To address the low water solubility of kartogenin, researchers engineered Exos surface-functionalized with E7 peptides to improve its delivery efficiency. This innovative strategy—transplanting synovial fluid-derived MSCs in combination with Exos surface-functionalized with E7 peptide delivering kartogenin to induce in situ chondrogenesis— holds significant potential as an advanced stem cell-based therapeutic strategy for OA. Furthermore, the potential of MSCs-Exos in cartilage repair is worth exploring. One study investigated the use of MSC-Exos to enhance the therapeutic efficacy of acellular cartilage extracellular matrix scaffolds for osteochondral regeneration[21]. The findings highlighted the ability of exosomes derived from human umbilical cord Wharton’s jelly MSCs to significantly improve extracellular matrix-mediated regeneration through immunomodulation and microRNA-driven mechanisms.
Biomaterials play a pivotal role in the field of regenerative medicine, serving as carriers for cells or Exos and providing an optimal mechanical environment necessary for effective tissue repair. When transplanted effectively into injured cartilage, these materials can substantially promote chondrocyte repair. Collectively, the synergistic combination of biomaterials and MSC-based therapies holds great promise for advancing the treatment of OA.