INTRODUCTION
Wound healing is a complex process that encompasses various physiological and biochemical mechanisms. It involves dynamic interactions among multiple cell types, the extracellular matrix (ECM), and cytokines. Research has delineated that wound healing progresses through four interrelated phases: Haemostasis, inflammation, proliferation, and remodelling. These phases are highly coordinated and synergistic, facilitating tissue repair[1]. Wounds from trauma, acute illnesses, or major surgeries often exhibit impaired or incomplete healing due to a complex pathological environment characterized by ischemia, hypoxia, increased oxidative stress, and prolonged inflammatory responses, with diabetic wounds being especially susceptible[2-4]. Statistically, patients with diabetic foot ulcer (DFU) face a 5-year mortality rate of up to 30%, and the rate for those requiring above-foot amputations exceeds 70%[5]. Up to 20% of DFUs result in lower extremity amputation[6]. Current therapies include conventional surgical debridement, weight-bearing reduction, dermal regeneration templates, flap grafts, growth factors, and platelet gels[7-10]. However, these methods present numerous limitations, including prolonged hospitalization, high costs, and complex management requirements[11]. A uniform, standardized treatment protocol has yet to be established, and new therapeutic strategies are continually being investigated.
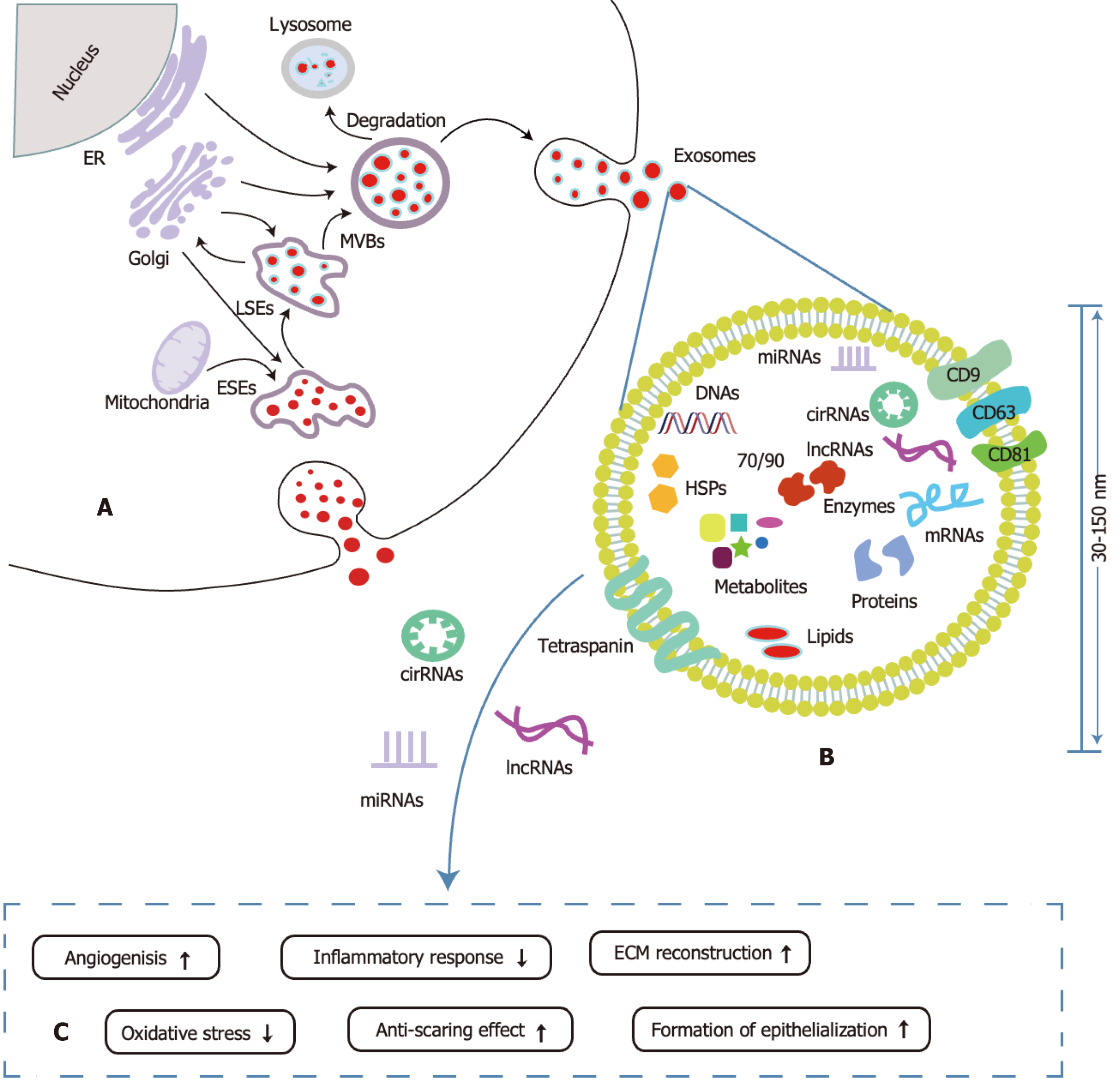
Recently, stem cell-based therapies and their exosomes have shown considerable promise in wound healing and tissue repair[12-14]. Stem cell-derived exosomes are characterized by their progenitor cells’ inherent abilities to self-renew and differentiate, offering substantial potential for tissue regeneration and immunomodulation[15]. Adipose-derived stem cell exosomes (ADSC-Exos) can replicate the biological functions of their parent cells by enhancing cellular proliferation, mitigating oxidative stress, modulating inflammation, and promoting tissue regeneration[13,16]. Compared to traditional non-coding delivery vectors such as viral vectors, liposomes, and synthetic nanoparticles, exosomes offer numerous advantages, including enhanced safety, superior biocompatibility, low immunogenicity, efficient targeting, effective cell penetration, and the capacity to protect RNA from degradation[17-19]. Exosomes, a subtype of extracellular vesicles, are typically 30-150 nm in diameter and are released through the fusion of multivesicular bodies (MVBs) with the plasma membrane[20]. ADSC-Exos play a pivotal role in regulating gene expression, modulating protein function, facilitating intercellular communication, and mediating molecular exchange by transporting a diverse array of bioactive molecules, including proteins, lipids, nucleic acids, and non-coding RNAs - primarily microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNA (circRNAs)[17,18]. Notably, non-coding RNAs derived from ADSC-Exos act on endothelial cells (ECs), endothelial progenitor cells (EPCs), immune cells, fibroblasts, and keratinocytes to modulate cellular activities, enhancing angiogenesis, combating oxidative stress, regulating inflammation, stimulating skin cell proliferation and epithelialization, and facilitating collagen remodeling, thereby minimizing scarring and accelerating wound healing. This study reviews the mechanisms, functions, and challenges of miRNAs, lncRNAs, and circRNAs derived from ADSC-Exos in wound healing, offering a theoretical foundation and proposing directions for advancing both basic research and clinical applications of ADSC-Exos in wound healing.
ROLES AND MECHANISMS OF NON-CODING RNAS IN ADSC-EXOS IN WOUND HEALING
Current research primarily focuses on miRNAs, circRNAs, and lncRNAs derived from ADSC-Exos, which are crucial mediators of intercellular signaling. Their unique double-layered membrane structure protects internal non-coding RNAs from degradation by external nucleases, ensuring efficient transport to target cells[17] (Figure 1). Studies suggest these non-coding RNAs significantly enhance oxidative stress resistance, modulate immune responses, promote neovascularization, accelerate epithelialization, facilitate collagen remodeling, and reduce keloid hyperplasia.
MiRNAs
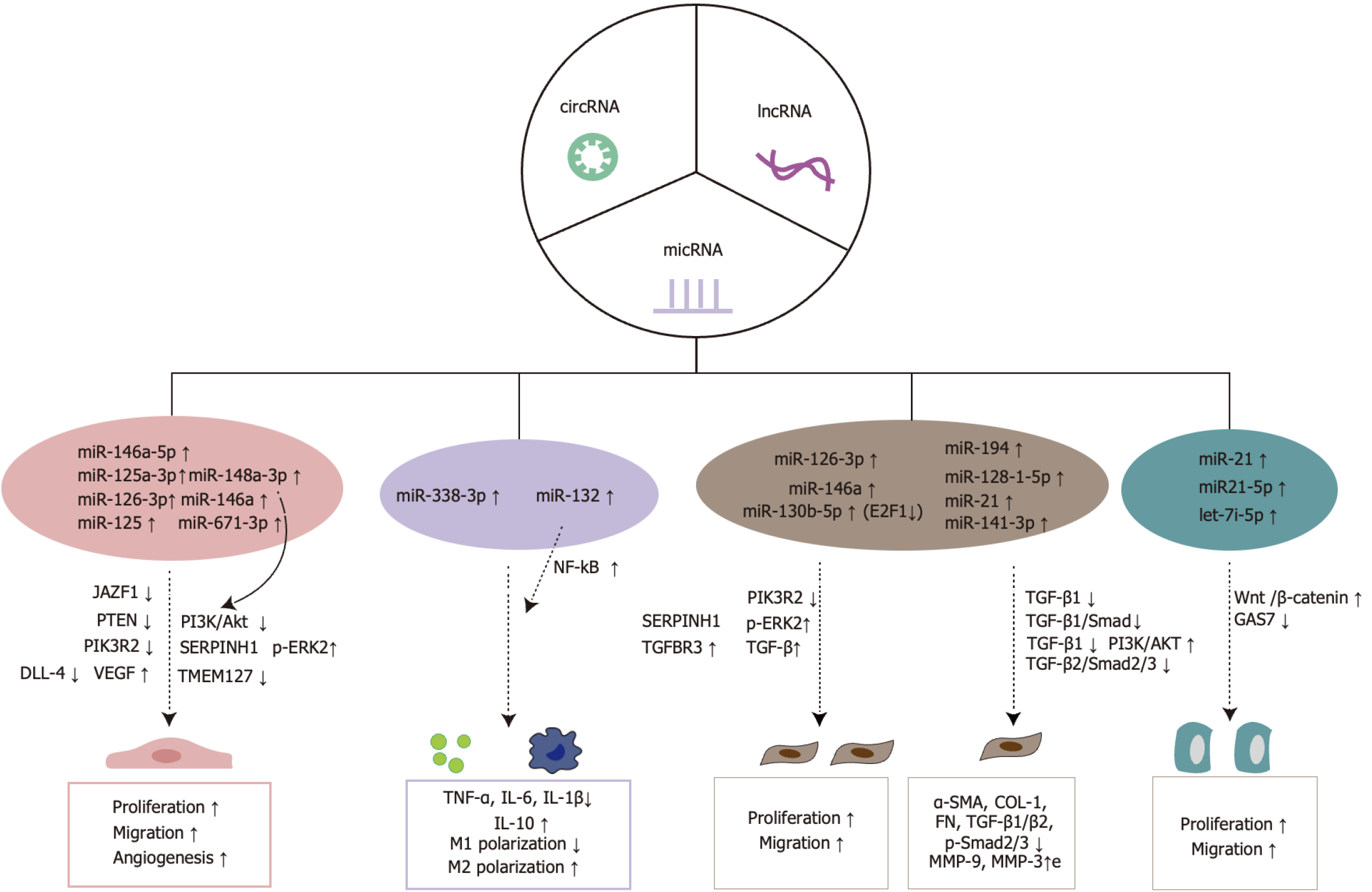
MiRNAs are evolutionarily conserved, non-coding, single-stranded RNA molecules that regulate gene and protein expression by binding to the 3’ untranslated region of target mRNAs, thereby inhibiting their translation process[32]. High-throughput sequencing by Wang et al[33] revealed that in hypoxia-pretreated ADSC-Exos, 215 miRNAs were upregulated and 369 downregulated compared to untreated ADSC-Exos. Notably, miR-21-3p, miR-126-5p, and miR-31-5p associated with wound healing were upregulated, while miR-99b and miR-146-a, linked to the phosphoinositide-3-kinase (PI3K)/protein kinase B (Akt) signaling pathway, were significantly downregulated. The specific regulatory mechanisms and potential interactions between these miRNAs require further investigation to fully understand their roles in promoting angiogenesis. MiRNAs from ADSC-Exos play crucial roles in wound healing by modulating inflammatory responses, promoting angiogenesis, facilitating re-epithelialization, and enhancing ECM generation (Figure 2).
Figure 2 Mechanisms by which microRNAs from adipose-derived stem cell exosomes promote wound healing.
miRNAs: MicroRNAs; cirRNAs: Circular RNAs; lncRNAs: Long non-coding RNAs; JAZF1: Juxtaposed with another zinc finger protein 1; PTEN: Phosphatase and tensin homolog deleted on chromosome ten; PIK3R2: Phosphoinositide-3-kinase regulatory subunit 2; DLL-4: Delta-like ligand-4; VEGF: Vascular endothelial growth factor; PI3K: Phosphoinositide-3-kinase; Akt: Protein kinase B; SERPINH1: Serine peptidase inhibitor 1; p-ERK2: Phosphorylated extracellular signal-regulated kinase 2; TMEM127: Transmembrane protein 127; NF-κB: Nuclear factor-kappaB; TGFBR3: Transforming growth factor-beta type III receptor; TGF-β: Transforming growth factor beta; E2F1: Transcription factor 1; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; α-SMA: Alpha-smooth muscle actin; COL-1: Collagen I; FN: Fibronectin; MMP-9: Matrix metalloproteinase-9.
Promoting angiogenesis: Neovascularization is crucial to the wound healing process. The adequate re-establishment of blood flow ensures sufficient oxygen and nutrient supply to peri-wound tissues, while facilitating the removal of metabolic wastes, thus creating a favorable microenvironment for wound healing[34,35]. Recently, miRNAs from ADSC-Exos have attracted significant attention for their role in promoting vascular regeneration in wounds through the regulation of multiple signaling pathways. Revascularization is a complex, multifactorial process that involves the coordinated action of various cell types and cytokines, with vascular endothelial growth factor (VEGF) serving as a principal regulator. ADSC-Exos directly enhance VEGF activity through miRNA delivery. For instance, miR-132 and miR-146a, delivered via ADSC-Exos, promote the proliferation and vascularization of human umbilical vein ECs (HUVECs)[36]. Additionally, ADSC-Exos miRNAs accelerate vascular regeneration by modulating the expression of downstream pro-angiogenic genes. MiR-146a-5p in ADSC-Exos targets the human pre-designed siRNA Set A (Juxtaposed with another zinc finger protein 1) to upregulate VEGFA secretion, thereby promoting vascular regeneration. MiR-126a-5p suppresses Juxtaposed with another zinc finger protein 1 expression, increasing VEGFA secretion, enhancing vascular EC (VEC) proliferation and migration, and ultimately improving angiogenesis and tissue perfusion in diabetic mice[37]. MiR-125a-3p further enhances angiogenesis in diabetic rat wounds by inhibiting phosphatase and tensin homolog deleted on chromosome ten expression in VECs and granulation tissue[38]. Recent studies indicate that ADSC-Exos miR-126-3p targets the PI3K regulatory subunit 2 gene, promoting the proliferation and migration of VECs[39]. Furthermore, human ADSC-Exos with high expression of miR-671-3p significantly enhance the proliferation, migration, and invasion of HUVECs by modulating the transmembrane protein 127 gene. Consequently, these exosomes facilitate angiogenesis and adipogenic differentiation following fat grafting, providing a critical foundation for the recirculation and generation of ADSC-Exos[40]. These ADSC-Exos contribute to the necessary material for continued exosome production, supporting regenerative processes.
To enhance the pro-angiogenic capabilities of ADSC-Exos equipped with miRNAs, scientists have utilized lentiviral transfection and additional bioengineering techniques to alter ADSC-Exos. Transfected ADSC-Exos with miRNA-146a effectively stimulated periwound angiogenesis by altering serine peptidase inhibitor 1 and phosphorylated extracellular signal-regulated kinase levels in rat dorsal wound tissues[41]. The targeting of homeodomain interacting protein kinase 2, a serine/threonine kinase and tumor suppressor active in angiogenesis, by miR-125-engineered ADSC-Exos proved substantially more beneficial than their non-modified counterparts, as shown by Guo et al[42]. These ADSC-Exos markedly alleviated the negative impacts of a high-glucose environment on HUVECs, by increasing CD34, VEGF, Ki-67, and transforming growth factor beta 1 (TGFβ-1) expression, and decreasing delta-like ligand-4 and inflammation-related proteins in wound tissues of DFU rats. This decrease in inflammation-related proteins, such as Toll-like receptor-4 and interleukin (IL)-6, further supported wound healing in DFU rats[42]. For practical applications, Zhang et al[43] demonstrated that human ADSC-Exos loaded with miR-148a-3p could inhibit PI3K/Akt signaling, thus boosting HUVEC proliferation and fostering angiogenesis. These exosomes were then integrated into a Pluronic F-127 hydrogel to forge an innovative exosome-hydrogel treatment for full-thickness rat wounds. The outcomes revealed that, relative to treatment with exosomes alone, the exosome-hydrogel group noticeably improved angiogenesis in rat wounds. Recently, Ge et al[44] created ADSC-Exos expressing miR-132 and applied them to a diabetic rat dorsal random skin flap model. The analysis confirmed that these modified exosomes significantly increased the formation of new microvessels within the random skin flap, elevated blood perfusion, and enhanced the survival rate of the diabetic rat skin flap model.
Regulation of immune response and oxidative stress: Adequate levels of reactive oxygen species (ROS) at the wound site, combined with low immunologic antibody concentrations, are essential for supporting physiological wound healing. Contributions to excessive ROS production at wound sites include local hypoxia and elevated glucose levels[45]. Additionally, the high-glucose environment and accumulation of advanced glycation end-products at the wound site result in excessive infiltration of immune cells (macrophages, neutrophils, and mast cells), resulting in an overproduction of tumor necrosis factor-alpha (TNF-α), IL-6, and IL-1β, and a decrease in IL-10 levels, thus impeding wound healing. Earlier research has shown that miR-338-3p-modified ADSC-Exos diminish IL-6, IL-1β, and TNF-α levels by targeting Runt-related transcription factor 2 expression, underscoring the influence of miRNAs sourced from ADSC-Exos on immune response modulation[46]. Although significant, there have been limited investigations into miRNAs sourced from ADSC-Exos in the realm of wound healing. In a recent study, Ge et al[44] exhibited notable success in a diabetic rat model by deploying engineered exosomes overexpressing miR-132. This strategy promoted macrophage M2 polarization and reduced the proportion of M1 macrophages through nuclear factor-kappaB pathway activation. Consequently, this method considerably lowered TNF-α and IL-6 levels at the wound site, improved the local inflammatory response, promoted vascular regeneration, and accelerated wound healing.
Promotion of epithelial cell proliferation: Granulation tissue development is crucial for the re-epithelialization process. This tissue covers the wound, providing nutrients and synthesizing ECM to serve as attachment points for keratinocytes[47]. Its formation depends heavily on fibroblast proliferation, migration, differentiation into myofibroblasts, and ECM synthesis. MiRNA-derived ADSC-Exos reduce DNA damage in fibroblasts, boost migration and proliferation, suppress apoptosis-related proteins (Bax and caspase-3), diminish matrix metalloproteinase-1 (MMP-1) and MMP-3 expression, and elevate collagen I and III synthesis, while optimizing collagen distribution through specific signaling pathways to enhance wound healing. For example, Ma et al[39] showed that ADSC-Exos carrying miR-126-3p improved fibroblast proliferation and migration rates, increased collagen deposition, and facilitated epithelialization in rat wounds by targeting PI3K regulatory subunit 2 expression, thereby aiding the repair of full-thickness skin defects. Additionally, ADSC-Exos modified by miR-146a transfection proved more effective than unmodified exosomes in encouraging fibroblast migration and proliferation by upregulating serine peptidase inhibitor 1 and phosphorylated extracellular signal-regulated kinase expression, thus aiding wound healing in rats[41]. Gene editing to boost ADSC-Exos cargo loading has become an innovative strategy to utilize cellular exosomes to speed up wound healing. Transcription factor 1 (E2F1), a key player in the E2F transcription factor family involved in cell growth and apoptosis, regulates both the transcriptional and paracrine activities of various miRNAs. Yu et al[48] discovered that E2F1 knockdown promoted miR-130b-5p expression in ADSC-Exos. In vitro, this modification boosted fibroblast proliferation and migration through the TGF-β type III receptor axis and TGF-β activation. In vivo, it enhanced collagen deposition, accelerated epithelialization, and promoted wound healing.
The epithelialization process is sustained throughout much of wound healing, playing a vital role in wound coverage, skin barrier integrity, and resistance to infection. It is driven by the proliferation and migration of keratinocytes toward the wound center. ADSC-Exos have demonstrated a positive impact on keratinocyte migration and proliferation. ADSC-Exos containing miR-21 directly boost HaCaT cell migration and proliferation, accelerating full-thickness wound healing in murine models. Additionally, Lv et al[49] used miR-21-5p mimics introduced into human ADSC-Exos through electroporation, achieving elevated miR-21-5p levels within these exosomes. They established that exosomes engineered with high levels of miR-21-5p, relative to their unmodified counterparts, triggered the Wnt/β-catenin signaling pathway, significantly enhancing keratinocyte proliferation and migration. This enhancement facilitated epithelial reformation and collagen remodeling in diabetic wound models, thus speeding up wound healing. Advancements in engineered exosomes have significantly boosted the therapeutic efficacy of extracellular miRNA-carrying exosomes. Recently, Liu et al[50] demonstrated that overexpression of growth arrest-specific 7 (GAS7) negated the impact of let-7i-5p on HaCaT cell viability and migration in vitro. The presence of the miRNA let-7i-5p in ADSC-Exos enhances HaCaT cell viability and migration by reducing GAS7 expression. Lowering GAS7 levels encourages proliferation, migration, and viability of HaCaT cells exposed to H2O2[50], positioning let-7i-5p as a potential therapeutic target for promoting oxidative stress resistance and wound healing.
Remodeling collagen to reduce scarring: ECM synthesis is vital not only during initial wound healing phases but also for determining the severity of wound scarring in subsequent stages. However, excessive fibroblast proliferation, extensive ECM deposition, and chaotic collagen synthesis during advanced stages of wound healing lead to scar formation. Earlier studies have shown that ADSC-Exos administered intravenously are drawn to the wound site, where they are absorbed by fibroblasts. These exosomes then exert differentiated effects on wound healing, boosting collagen I and III production to aid wound closure initially, while later reducing collagen synthesis to curtail scar development[51]. Subsequent studies have also confirmed the dual function of ADSC-Exos in promoting repair of diabetic wounds in murine models[52]. TGF-β, a key pro-fibrotic factor, along with the TGF-β/Smad signaling pathway, suppresses protease and stromelysin activity while promoting ECM buildup, such as collagen accumulation. Xu et al[53] showed that ADSC-Exos could limit TGF-β1 expression by delivering miR-194, and also decrease IL-1β and TNF-α levels around the scar, collectively contributing to improved pathological scars in rabbit ear models. Additionally, ADSC-Exos supported wound healing in diabetic rats through miR-128-1-5p by blocking the TGF-β1/Smad pathway, reducing scar fibrosis by lowering TGF-β1 and alpha-smooth muscle actin expression, collagen I deposition, and Smad2/3 phosphorylation at the wound site[54]. A recent study showed that miRNA-21-loaded ADSC-Exos enhanced MMP-9 and MMP-3 expression in wounds by inhibiting TGF-β1 protein expression, and suppressed tissue inhibitors of metalloproteinase 1 and tissue inhibitors of metalloproteinase 2 proteins via the PI3K/Akt signaling pathway to diminish scar formation. Interestingly, an overabundance of miR-21 was found to inhibit TGF-β1 expression, while increased TGF-β1 levels initiated a negative feedback loop affecting miR-21, regulated through the PI3K/Akt pathway[55]. Traditional injection methods often do not provide the necessary depth and uniformity for effective exosome delivery, compromising the therapeutic potential of exosome treatments on scar tissue. Meng et al[56] devised ADSC-Exos with elevated miR-141-3p expression embedded in soluble microneedle arrays, enabling continuous release of miR-141-3p to optimize fibroblast distribution and collagen fiber arrangement. This method also reduced the levels of scar fibroblast markers (alpha-smooth muscle actin, collagen I, fibronectin, TGF-β2, and p-Smad2/3) at the wound site, effectively reducing hypertrophic scar thickness. This novel injection technique not only ensures a more consistent distribution of exosomes but also minimizes injection discomfort and controls the depth of exosome delivery. The innovative microneedle drug delivery system has proven highly effective in exosome therapy, offering substantial benefits for therapeutic applications.
LncRNAs
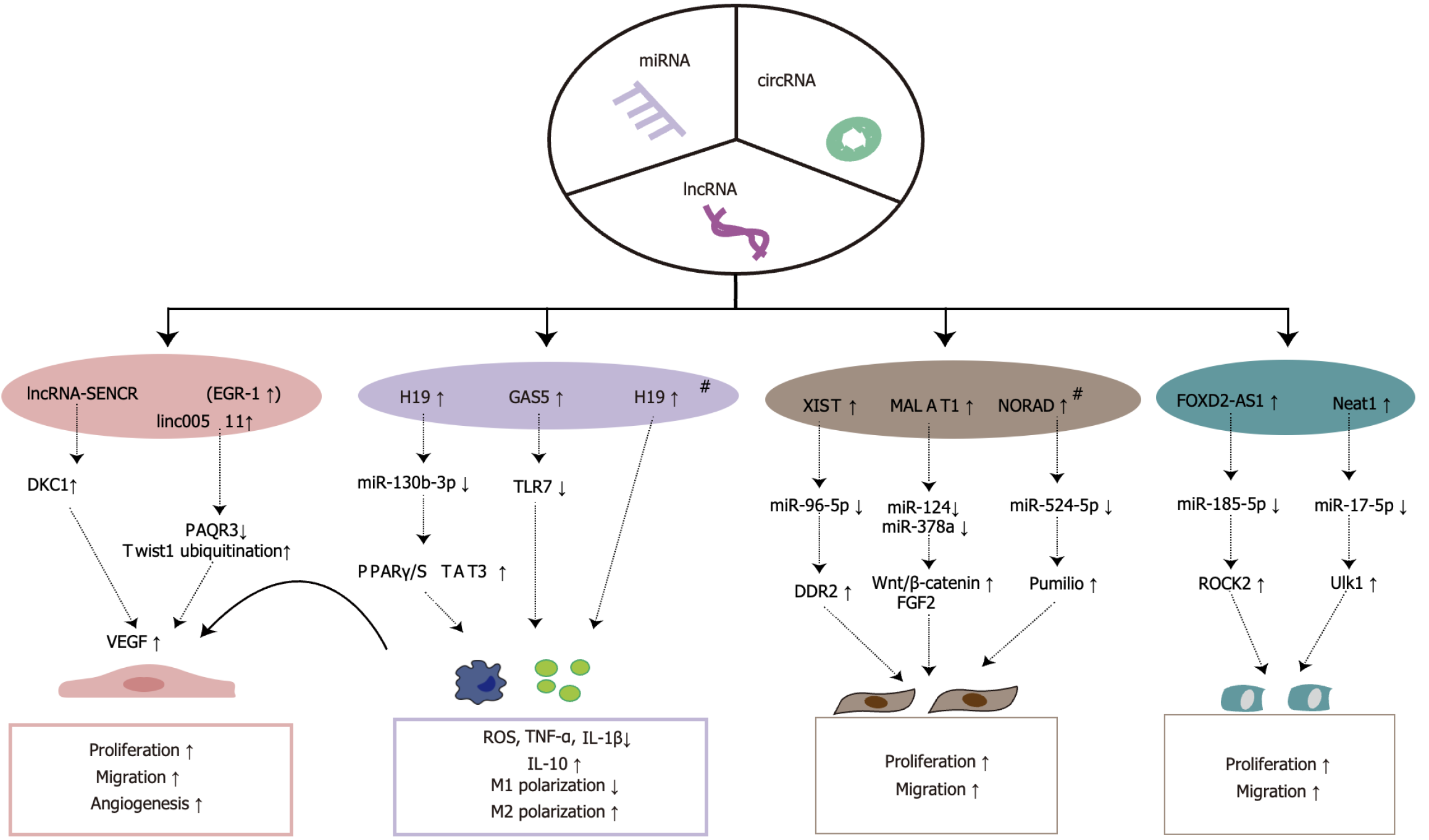
LncRNAs, defined as RNA molecules exceeding 200 nucleotides in length, are categorized as non-coding RNAs[57]. Recognized as critical regulators of gene expression, lncRNAs modulate cellular activities such as proliferation, migration, and immune responses through epigenetic, transcriptional, and post-transcriptional mechanisms[58]. These molecules also influence cellular dynamics by mitigating negative regulation of target genes by miRNAs through their binding to 3’ untranslated regions of miRNA-targeted mRNAs, acting as competitive endogenous RNAs and functioning as miRNA sponges to curb miRNA activity[59-61]. Prominent lncRNAs include GAS5, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), and H19[62-64]. Emerging evidence suggests that lncRNAs from ADSC-Exos significantly bolster wound healing by regulating signaling pathways that promote angiogenesis and reduce local inflammation at the wound site, thereby aiding wound epithelialization (Figure 3).
Figure 3 Mechanisms by which long non-coding RNAs from adipose-derived stem cell exosomes promote wound healing.
# represents pretreated with hypoxia. miRNAs: MicroRNAs; cirRNAs: Circular RNAs; lncRNAs: Long non-coding RNAs; EGR-1: Early growth response factor-1; MALAT1: Metastasis-associated lung adenocarcinoma transcript 1; NORAD: Non-coding RNA activated by DNA damage; FOXD2-AS1: FOXD2 antisense RNA 1; DKC1: Dyskeratosis Congenita 1; PAQR3: Progestin and adipoQ receptor family member 3; TLR7: Toll-like receptor 7; VEGF: Vascular endothelial growth factor; PPAR: Peroxisome proliferator-activated receptor; STAT3: Signal transducer and activator of transcription 3; DDR2: Discoidin domain receptor tyrosine kinase 2; FGF2: Fibroblast growth factor 2; ROCK2: Rho-associated coiled-coil containing protein kinase 2; Ulk1: Unc-51 like autophagy activating kinase 1; ROS: Reactive oxygen species; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin.
Promoting angiogenesis: VGEF is essential for angiogenesis, particularly VEGFA and its associated tyrosine kinase receptors, VEGFR1 and VEGFR2, which are key in mediating angiogenic processes. LncRNAs from ADSC-Exos have been demonstrated to promote wound angiogenesis by regulating downstream targets and enhancing VEGF secretion. Sun et al[65] demonstrated that ADSC-Exos increased the proliferation and migration of HUVECs, observing a rise in VEGFA secretion and improved trabecular angiogenesis, through the upregulation of lncRNA-SENCR via early growth response factor-1 in human ADSC-Exos, which subsequently activated dyskeratosis congenita 1 to stabilize VEGF-A. Additionally, lncRNAs can control ubiquitination modifications of angiogenic genes crucial to the angiogenic process[66]. Qiu et al[67] reported reduced expression levels of CD31, VEGFA, and phosphorylated VEGFR2 proteins in EPCs from diabetic sources, severely limiting their angiogenic capabilities. Notably, an increase in progestin and adipoQ receptor family member 3 expression was observed along with a decrease in Twist family BHLH transcription factor 1 expression. In both in vitro and in vivo experiments, it was demonstrated that ADSC-Exos overexpressing linc00511 directly downregulated progestin and adipoQ receptor family member 3 expression, inhibited the ubiquitination and degradation of Twist family BHLH transcription factor 1 in EPCs, and enhanced the expression levels of CD31, VEGFA, VEGFR2, and phosphorylated VEGFR2 under high glucose conditions. This intervention led to increased proliferation and migration of EPCs, thus accelerating angiogenesis in DFU.
Regulation of immune response and oxidative stress: An imbalance in macrophage polarization is recognized as a significant factor affecting wound healing. Promoting IL-10 secretion through the enhancement of M2 macrophage polarization is vital in the wound healing process. LncRNAs in ADSC-Exos have been shown to effectively reduce inflammatory factors around injury sites by modulating the inflammatory response, thus aiding tissue repair. For instance, ADSC-Exos-derived lncRNA DLEU2 fosters M2 polarization of macrophages by regulating the miR-106a-5p/LXN axis, thereby mitigating sepsis-induced lung injury[68]. Peroxisome proliferator-activated receptor gamma, widely present in the monocyte-macrophage system, facilitates the differentiation of monocytes towards an M2 macrophage phenotype[69]. H19, a long non-coding RNA encoded by the H19 gene, plays a significant role in immune response regulation[70]. Recently, Li et al[63] demonstrated that H19 in ADSC-Exos, by sponging miR-130b-3p, upregulated peroxisome proliferator-activated receptor gamma/signal transducer and activator of transcription 3 expression. This mechanism promoted macrophage M2 polarization, reduced the expression of TNF-α and IL-1β, increased the levels of IL-10, accelerated the secretion of VEGFA, and enhanced the proliferation and migration of VECs and fibroblasts, thereby facilitating wound healing. Additionally, hypoxia-treated H19, highly expressed in ADSC-Exos, has been shown to mitigate oxidative stress in HUVECs exposed to H2O2 by deubiquitinating and stabilizing hypoxia-inducible factor 1-alpha (HIF-1α), thereby rescuing their proliferation, migration, and angiogenic capabilities[71]. Moderate levels of oxidative stress can aid wound healing by combating harmful microorganism invasion and activating key signaling pathways, such as mitogen-activated protein kinases and PI3K/Akt, thereby enhancing cellular proliferation, migration, and tissue repair[72]. However, excessive oxidative stress, especially from ROS, may hinder healing, leading to chronic wounds. GAS5, a novel lncRNA, is crucial in regulating cell growth arrest and the cellular stress response[73]. Patel et al[64] demonstrated that human ADSCs-Exos with high GAS5 expression could counteract H2O2-induced oxidative stress, reversing impairments in the basal oxygen consumption rate and mitochondrial dysfunction in human dermal fibroblasts, ultimately fostering their proliferation and migration. Furthermore, in a lipopolysaccharide-induced inflammatory environment, ADSCs-Exos with elevated GAS5 expression reduced the gene expression of key pro-inflammatory cytokines, including interferon-α, IL1-β, and TNF-α, while restoring cellular activity, viability, and migratory capacity in human dermal fibroblasts. Mechanistically, this effect is likely mediated through the regulation of Toll-like receptor 7 expression. These findings position GAS5 derived from ADSC-Exos as a promising therapeutic agent for chronic wound repair by modulating oxidative stress and inflammatory responses.
Promotion of epithelial cell proliferation: The epithelialization phase is critical in wound healing, depending on the proliferation and migration of fibroblasts and keratinocytes to achieve wound closure. Numerous studies have shown that lncRNAs from ADSC-Exos facilitate skin regeneration at the wound site by influencing the proliferation and migration of these cells through various signaling pathways. For instance, ADSC-Exos containing XIST boost discoidin domain receptor tyrosine kinase 2 expression by suppressing miR-96-5p, thus enhancing the proliferation and migration of mouse dermal fibroblasts, increasing collagen deposition, and expediting wound healing[74]. MALAT1, originally identified in non-small cell lung cancer, is involved in crucial physiological processes such as alternative mRNA splicing, competitive endogenous RNA activity, and miRNA recruitment[58]. Cooper et al[75] demonstrated that MALAT1 from human ADSC-Exos stimulated fibroblast proliferation and migration, thereby speeding up wound healing in rats. Mechanistically, MALAT1 promotes epithelial cell proliferation and migration, facilitating re-epithelialization by targeting miR-124 to activate the Wnt/β-catenin pathway and regulating miR-378a to elevate fibroblast growth factor 2 (FGF2) levels[62,76]. Subsequently, Kong et al[77] discovered that ceramide regulates the biosynthesis of MALAT1 in ADSC-Exos, thereby enhancing the proliferative and migratory effects of ADSC-Exos on human fibroblasts, as well as improving mitochondrial function. This insight provides new avenues for optimizing exosomal encapsulation of lncRNAs to boost the therapeutic efficacy of wound healing treatments. Similarly, hypoxia leads to greater lncRNA enrichment in ADSC-Exos. Under hypoxic conditions, ADSC exosomes with high MALAT1 expression mediate autophagy related 4A activation through miR-92a-3p sponge adsorption, significantly enhancing mitochondrial metabolism in cardiomyocytes[78]. Additionally, hypoxia-induced ADSC-Exos with elevated levels of non-coding RNA activated by DNA damage regulated miR-524-5p expression, upregulated Pumilio protein, enhanced fibroblast proliferation and migration, and expedited wound healing[79].
While research on the regulation of keratinocyte biological activity by lncRNAs in ADSC-Exos is less frequent, evidence suggests a strong link between them. Previous studies have identified significant differential expression of 119 LncRNAs in rat keratinocytes treated with ADSC-Exos compared to controls. Additionally, 95 miRNAs were markedly upregulated, while 123 were downregulated[80]. Nevertheless, the dynamics within the lncRNA-miRNA regulatory network are yet to be fully explored. Chang et al[81] demonstrated that the highly expressed lncRNA FOXD2 antisense RNA 1 in ADSC-Exos enhanced HaCaT cell migration and proliferation, also elevating the protein levels of MMP-2 and MMP-9. This effect was mediated by the downregulation of miR-185-5p, which subsequently increased Rho-associated coiled-coil containing protein kinase 2 expression. Autophagy, a cellular self-protection mechanism, is pivotal in the wound healing process. This process is largely governed by autophagy-related genes and involves the lysosomal degradation of cytoplasmic proteins and damaged organelles. An et al[80] demonstrated that lncRNA Neat1 from ADSC-Exos could be internalized by rat keratinocytes and function as a miR-17-5p sponge, which in turn upregulated miR-185-5p expression in keratinocytes. This increased expression subsequently elevated the levels of keratinocyte autophagy-related Unc-51 like autophagy activating kinase 1, as well as the autophagy markers LC3I/II and p-Unc-51 like autophagy activating kinase 1, thereby promoting keratinocyte proliferation and migration, and ultimately accelerating wound healing in a murine model.
CircRNAs
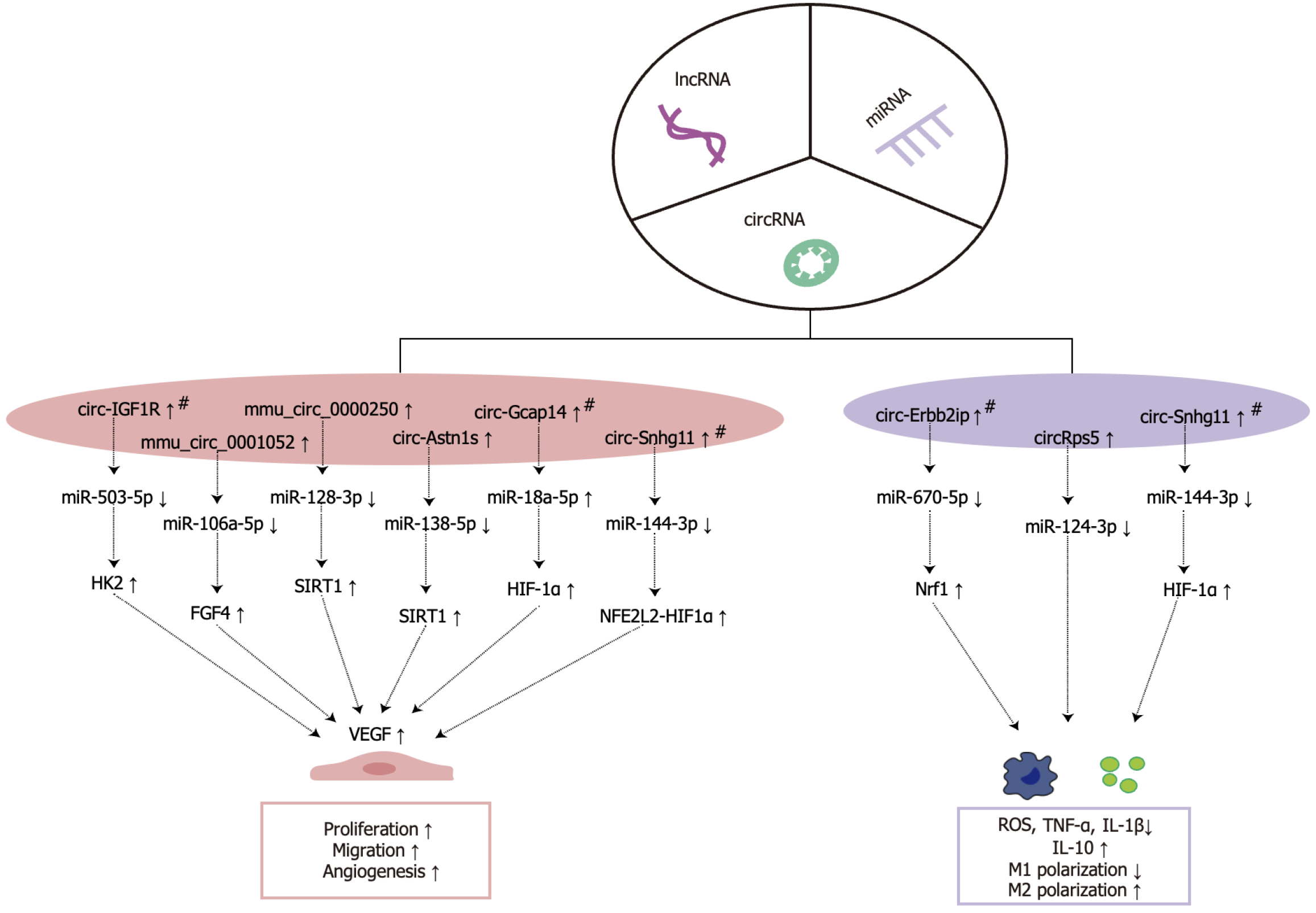
CircRNAs, a novel group of non-coding RNAs, are characterized by their covalently closed ring structure, synthesized via the back-splicing of precursor mRNA[82]. Unlike typical RNA molecules, which possess a 5’ cap and 3’ poly(A) tail and interact with various cellular proteins, circRNAs lack these modifications and are thus highly resistant to nuclease-mediated degradation within the cellular environment, contributing to their remarkable stability[83]. CircRNAs serve as miRNA sponges or competing endogenous RNAs, effectively sequestering target miRNAs in a base-complementary pairing manner, thereby modulating the negative regulatory effects of miRNAs on target mRNAs[84]. The expression levels of circRNA are closely linked with wound healing processes. Utilizing high-throughput gene sequencing, Li et al[85] observed differential expression patterns of circRNAs and miRNAs in chronic refractory wounds compared to normal skin tissues, revealing that 157 out of 356 differentially expressed circRNAs were upregulated, while 199 were downregulated, and 2 out of 42 differentially expressed circRNAs were upregulated, while 40 were downregulated. This provides essential data for further investigation into the role of circRNAs in wound healing. In current research on ADSC-Exos-derived circRNAs and their role in promoting wound healing, the primary focus lies on enhancing angiogenesis and modulating immune responses; however, further studies are required to elucidate these mechanisms comprehensively (Figure 4).
Figure 4 Mechanisms by which circular RNA from adipose-derived stem cell exosomes promote wound healing.
# represents pretreated with hypoxia. miRNAs: MicroRNAs; cirRNAs: Circular RNAs; lncRNAs: Long non-coding RNAs; HK2: Hexokinase 2; FGF4: Fibroblast growth factor 4; SIRT1: Silent information regulator 1; HIF-1α: Hypoxia-inducible factor 1-alpha; NFE2L2: Nuclear factor, erythroid 2 like 2; VEGF: Vascular endothelial growth factor; Nrf1: Nuclear respiratory factor 1; ROS: Reactive oxygen species; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin.
Promoting angiogenesis: Research on ADSC-Exos circRNAs in wound healing highlights their regulatory “sponge effect” on miRNAs, which facilitates the upregulation of pro-angiogenic genes downstream, increases VEGF secretion, improves VECs proliferation, and expedites wound repair. Shi et al[86] demonstrated that hypoxia-preconditioned ADSC-Exos, overexpressing circ-IGF1R, sponged miR-503-5p to modulate hexokinase 2/VEGFA, thereby enhancing VEGFA secretion, preventing hyperglycemia-induced ECs injury, and enhancing vascular regeneration in diabetic wounds. Furthermore, ADSC-Exos modified by mmu_circ_0001052 enhanced the proliferation, migration, and angiogenesis of HUVECs through miR-106a-5p-mediated regulation of FGF4, elevating FGF4, VEGF, and p-p38/p38 expression while reducing HUVECs apoptosis to support angiogenesis in diabetic wounds[87]. EPCs differentiate into ECs or stimulate angiogenesis via paracrine signaling, acting as key agents in wound revascularization. The silent information regulator 1 (SIRT1), a histone deacetylase, is known to shield cells from external stressors and bolster metabolic processes[88]. In EPCs challenged by high glucose levels, SIRT1 expression is typically reduced, impairing the biological function of VECs. Shi et al demonstrated that ADSC-Exos enriched with mmu_circ_0000250 restored the proliferative and migratory capacities of high-glucose-induced EPCs through miR-128-3p adsorption and SIRT1 upregulation, while autophagy activation curbed apoptosis in EPCs, speeding up vascular regeneration in diabetic wounds[89]. Additionally, ADSC-Exos overexpressing circ-Astn1 inhibited EPCs apoptosis and enhanced angiogenesis in a diabetic mouse wound model via miR-138-5p sponge adsorption, with SIRT1 upregulation and decreased forkhead box O1 expression[90].
To replicate the hypoxic microenvironment typical of diabetic wounds, recent studies have concentrated on hypoxic prestimulation and its impact on the secretory functions of ADSC-Exos. These findings reveal that hypoxic conditions not only increase circRNA content in ADSC-Exos but also significantly enhance their effectiveness in promoting vascular regeneration and wound repair. Wang et al[91] demonstrated that, compared to non-hypoxia-pretreated ADSC-Exos, hypoxia-pretreated ADSC-Exos suppressed miR-18a-5p expression at wound sites by delivering circ-Gcap14, thereby upregulating HIF-1α, promoting VEGF secretion, enhancing angiogenesis, and accelerating wound healing in diabetic rat models. To overcome the limitations of ADSC-Exos non-coding RNAs in the wound microenvironment, such as rapid degradation, unstable release, and limited duration of action, a combined hydrogel-exosome delivery system has been explored, showing promise in enhancing the therapeutic efficacy of exosomal circRNAs. Integrating hydrogels with exosome delivery systems has become a key research focus, ensuring stable, prolonged release of exosomal circRNAs to optimize therapeutic potential in wound settings. Hu et al[92] encapsulated ADSC-Exos with high circ-Snhg11 expression, following hypoxic pretreatment, into GelMA hydrogel to create a novel GelMA-HExo formulation. In diabetic rat models, this GelMA-human ADSC-Exos sustained EC migration and proliferation under hyperglycemic conditions by activating miR-144-3p/nuclear factor erythroid 2 like 2/HIF1α signaling pathways, facilitating vascular regeneration and accelerating wound healing.
Regulation of immune response and oxidative stress: ADSC-Exos-derived circRNAs show significant potential in alleviating organ damage through the modulation of inflammatory factors and immune responses. Noteworthy examples include mmu_circ_0001295[93] and circ-Fryl[94]. Early wound stages, characterized by ischemia and hypoxia, induce excessive oxidative stress, leading to elevated levels of ROS and compromising peripheral cellular functions. ADSC-Exos-derived circRNAs contribute to tissue repair by modulating inflammatory responses in the periwound area. Using high-throughput sequencing, Tang et al[95] identified that circ-Erbb2ip from hypoxia-preconditioned ADSC-Exos decreased ROS and inflammatory factors TNF-α, IL-6, and IL-1β in vascular EPCs by targeting miR-670-5p/nuclear respiratory factor 1-regulated pathways, thereby diminishing oxidative stress, modulating inflammation, and expediting wound repair in diabetic mice. Ferroptosis, a cell death modality marked by lipid peroxidation, is integral to wound healing processes. Recent studies indicate that ADSC-Exos-derived circRNAs facilitate tissue injury repair by modulating ferroptosis initiation in cells. Liu et al[96] demonstrated that hypoxia-preconditioned ADSC-Exos overexpressing circ-Stt3b reduced ROS levels and inflammatory factor expression in mouse atrial myocardial HL-1 cells under hypoxic conditions by activating miR-15a-5p/GPX4 signaling pathways. This pathway effectively lowered ROS levels and inflammatory factor expression, alleviating cardiac injury post-myocardial infarction.
Disruption of the M1/M2 macrophage polarization balance is identified as a crucial factor contributing to delayed wound healing[97], particularly in diabetic wounds. Modulating macrophage polarization states has emerged as a promising therapeutic target in promoting wound healing. Recent studies suggest that ADSC-Exos regulate macrophage polarization through encapsulated circRNAs, playing a vital role in tissue repair[98]. Studies indicate that ADSC-Exos can regulate miR-124-3p-induced M2 macrophage polarization via circRps5 sponge adsorption, inhibiting diabetic wound inflammatory factors, including TNF-α and IL-6, while enhancing the secretion of IL-10 and IL-4, thereby promoting wound healing[99]. Furthermore, overexpression of circRps5 loaded with ADSC-Exos inhibited high-glucose-induced macrophage autophagy proteins, p27, and LC3II/LC3I, further elucidating its regulatory role in cellular autophagy and inflammatory response modulation. Shi et al[100] observed that hypoxia-pretreated ADSC-Exos exhibited significantly increased circ-Snhg11 expression, which enhanced M2 macrophage polarization in hyperglycemic environments through miR-144-3p adsorption, promoting HIF-1α expression, decreasing the levels of TNF-α, IL-6, and IL-1β, and speeding up wound healing. In conclusion, circRNAs in ADSC-Exos are pivotal in wound healing, particularly in diabetic contexts, by modulating macrophage polarization. However, more research is needed to completely understand these mechanisms.
CONCLUSION
This review synthesizes recent advancements over the past three years concerning miRNAs, circRNAs, and lncRNAs from ADSC-Exos, highlighting their roles in enhancing wound healing through various mechanisms. Extensive preclinical studies have shown that non-coding RNAs from ADSC-Exos can expedite wound healing by promoting neovascularization, modulating oxidative stress and inflammation, enhancing epithelialization, and regulating collagen remodeling. These findings underscore the potential of ADSC-Exos as a novel non-cellular therapeutic option for clinical wound management, especially in diabetic wounds. Nonetheless, numerous challenges hinder their translation into clinical practice. Techniques for isolating non-coding RNAs from ADSC-Exos need standardization to ensure quality control, particularly for individuals with chronic conditions, and methods for large-scale production and safety evaluations are still lacking, which restricts their clinical use. Additionally, non-coding RNAs from ADSC-Exos achieve therapeutic outcomes by synergistically regulating multiple healing processes, including immune responses, angiogenesis, and epithelialization. The precise mechanisms that orchestrate this intricate regulatory network of miRNAs, lncRNAs, and circRNAs, especially the selective encapsulation of non-coding RNAs into exosomes, require further exploration. Although research has shown promise in integrating ADSC-Exos-derived non-coding RNAs with hydrogels and microneedles, the biocompatibility, degradation, elimination pathways, and potential toxicity of these combinations necessitate comprehensive investigation. Initially, research should focus on developing efficient, scalable, and cost-effective methods for purifying exosomes to enhance their purity and yield, thus improving the safety and practicability of treatments. Secondly, employing high-throughput screening and multi-omics approaches could facilitate a personalized and systematic exploration of the regulatory networks involving miRNAs, circRNAs, and lncRNAs from ADSC-Exos, identifying new regulatory targets and therapeutic strategies. Third, the development of engineered exosomal RNA constructs using nanotechnology could improve RNA stability, enhance targeting accuracy, and thus amplify therapeutic outcomes by overcoming issues related to rapid degradation and limited release duration. In summary, research on ADSC-Exos-derived non-coding RNAs in wound healing is predominantly at the preclinical stage. Additional clinical data are essential to substantiate the efficacy and safety of ADSC-Exos in therapeutic applications, indicating that significant advancements are still required.