Published online Apr 26, 2025. doi: 10.4252/wjsc.v17.i4.101290
Revised: December 13, 2024
Accepted: March 21, 2025
Published online: April 26, 2025
Processing time: 225 Days and 0.1 Hours
In vivo degradation of bone scaffolds is significantly influenced by osteoclast (OC) activity, which is orchestrated by the interplay between receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin (OPG). The ratio of RANKL/OPG is a crucial determinant of OC-mediated bone resorption, which plays an integral role in bone remodeling and scaffold degradation. Elevated levels of RANKL relative to OPG enhance osteoclastogenesis, thereby accelerating the degradation process essential for integrating bone scaffolds into the host tissue.
To elucidate the effects of OPG gene silencing on osteoclastogenesis within rat bone marrow-derived mesenchymal stem cells (BMSCs). By investigating these effects, the study aimed to provide deeper insights into the regulatory mecha
We employed recombinant lentiviral plasmids to silence the OPG gene in rat BMSCs to achieve the aims. The efficacy of gene silencing was assessed using quantitative reverse transcription polymerase chain reaction and western blot analysis to measure the expression levels of OPG and RANKL. Tartrate-resistant acid phosphatase staining was utilized to evaluate the formation of OCs. Additionally, co-immunoprecipitation assays were conducted to explore the interactions between RANKL and OPG proteins, further assessing the biochemical pathways involved in osteoclastogenesis.
The silencing of the OPG gene in BMSCs resulted in a significant increase in the RANKL/OPG ratio, evidenced by decreased expression levels of OPG and increased levels of RANKL. Enhanced osteoclastogenesis was observed through tartrate-resistant acid phosphatase staining, which indicated a substantial rise in OC formation in response to the altered RANKL/OPG balance. The co-immunoprecipitation assays provided concrete evidence of the direct interaction between RANKL and OPG proteins, substantiating their pivotal roles in regulating OC activity.
The findings from this study underscore the critical role of the RANKL/OPG axis in osteoclastogenesis. Silencing of the OPG gene in BMSCs effectively increases the RANKL/OPG ratio, promoting OC activity and potentially enhancing bone scaffold degradation. This regulatory mechanism offers a promising avenue for modulating bone remodeling processes, which is essential for effective bone repair and the successful integration of bone scaffolds into damaged sites. Future research might focus on optimizing the control of this axis to better facilitate bone tissue engineering and regenerative therapies.
Core Tip: This study reports, for the first time, that enhancing the receptor activator of nuclear factor-kappa B ligand/osteoprotegerin (RANKL/OPG) ratio through RNA interference promotes osteoclastogenesis. Our findings reveal a significant upregulation of RANKL mRNA levels after OPG gene silencing. The study demonstrates a significant downregulation of OPG mRNA and protein levels. The increase in the RANKL/OPG ratio significantly promotes osteoclastogenesis. This study provides a new theoretical basis and molecular targets for degrading bone scaffolds and bone tissue repair.
- Citation: Wei SG, Chen HH, Xie LR, Qin Y, Mai YY, Huang LH, Liao HB. RNA interference-mediated osteoprotegerin silencing increases the receptor activator of nuclear factor-kappa B ligand/osteoprotegerin ratio and promotes osteoclastogenesis. World J Stem Cells 2025; 17(4): 101290
- URL: https://www.wjgnet.com/1948-0210/full/v17/i4/101290.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i4.101290
Bone scaffolds play a crucial role in clinical bone tissue repair and are widely used in bone regeneration and repair. Bone scaffolds aid bone tissue regeneration by providing physical support and promoting new bone formation. With advan
Currently, clinical treatment of fractures primarily relies on the implantation and fixation of exogenous bone scaffold materials. However, this surgical approach requires an additional operation to remove the fixation materials. If porous structures can be developed, allowing bone tissue or stem cells to grow within, bioactive bone scaffolds could degrade after being implanted into the body. At this stage, osteoclasts (OCs) activate and begin regulatory functions, prompting bone growth. Over time, the body would completely absorb the bioactive scaffold, and the bone would largely heal. Such bone materials could save patients half the time and costs, enabling faster recovery without the risk of a second surgery.
OCs play a central role in the degradation of bone scaffold materials, primarily regulating degradation rates through the bone resorption[2]. Studies have demonstrated that the receptor activator of nuclear factor-kappa B ligand (RANKL)/receptor activator of nuclear factor-kappa B (RANK)/osteoprotegerin (OPG) system is crucial for bone remodeling and OC function regulation[3]. OCs, as multinucleated giant cells, are key mediators of bone resorption and remodeling[4]. Their formation and activity are regulated by RANKL and OPG[5,6].
Existing research has established the decisive role of the RANKL/OPG balance in OC differentiation and bone remodeling, forming the theoretical foundation of this signaling axis[7]. RANKL binds to the RANK receptor on OC precursor cells, promoting OC differentiation and maturation[5]. Conversely, OPG acts as a decoy receptor for RANKL, competitively binding and preventing its interaction with RANK, thereby inhibiting osteoclastogenesis and activity[8]. Thus, the RANKL/OPG ratio is a key factor in OC activity regulation[9,10]. Further studies indicate that this signaling axis regulates bone homeostasis and plays a critical role in osteoimmunology[11]. An increased RANKL/OPG ratio is closely associated with enhanced OC activity, suggesting that modulating this ratio could effectively regulate OC function[3,7]. Additionally, under RANKL stimulation, OCs can undergo cyclic renewal through osteomorphs, providing new insights into RANKL-mediated bone resorption mechanisms[12]. Specific peptide interventions have been used to modulate RANKL-mediated OC activation, further verifying the promoting effect of OPG inhibition on osteoclastogenesis[13].
Advancements in osteoimmunology are driving the development of interventions targeting abnormal osteoclastogenesis[14]. For instance, anti-RANKL antibodies have been clinically validated as an effective treatment for postmenopausal osteoporosis[11,15,16]. Meanwhile, targeted OC drug delivery systems are also considered promising for clinical applications[13].
OCs play a central role in the degradation process of bone scaffolds, regulating the degradation rate of the bone matrix through bone resorption[2]. Therefore, studying the enhancement of OC-mediated bone resorption activity regulation is of great significance for optimizing the performance of bone scaffold materials. OC are multinucleated giant cells responsible for bone resorption and remodeling[4]. Their generation and activity are regulated by RANKL and OPG[5,6]. RANKL binds to the RANK receptor on the surface of OC precursor cells, promoting OC differentiation and maturation[5]. As a decoy receptor for RANKL, OPG binds to RANKL, preventing its binding to the RANK receptor, thereby inhibiting OC generation and activity[8]. Hence, the RANKL/OPG ratio is a key factor in regulating OC activity[9,10]. Studies have found that an increase in the RANKL/OPG ratio is closely related to the gradual enhancement of OC activity, suggesting that regulating the RANKL/OPG ratio may be an effective approach to control OC activity.
RNA interference (RNAi) technology is a technique that silences target gene expression specifically using small interfering RNA or short hairpin RNA (shRNA) and is widely applied in gene function and disease mechanism studies[17,18]. In recent years, RNAi technology has shown great potential in regulating gene expression, exploring gene function, and developing new therapeutic approaches. In this study, we utilized recombinant lentiviral plasmids to introduce shRNA into rat bone marrow-derived mesenchymal stem cells (BMSCs) to specifically interfere with the expression of the OPG gene. Through this method, we could stably and efficiently silence the OPG gene, enabling the study of its effects on the RANKL/OPG ratio and OC generation.
This study investigates the effect of RNAi-mediated silencing of the OPG gene in BMSCs on the RANKL/OPG ratio and OC generation. By modulating this signaling axis, we seek to explore its potential to enhance the degradation properties of OCs on bone scaffold materials, thereby improving bone tissue repair. The findings of this study may provide a theoretical basis for gene-regulated bone tissue engineering and contribute to the development of novel therapeutic strategies. Future research will focus on expanding the application of RNAi technology to regulate other genes and evaluating its effects on different bone scaffolds to further advance bone tissue engineering.
BMSCs were isolated from the bone marrow of Sprague-Dawley (SD) rats (Beijing Vital River Laboratory Animal Technology Co., Ltd., Cat. No: 101) and cultured and passaged in vitro using a cell adhesion culture method. One-week-old specific pathogen-free SD rats (weighing 80-100 g) were intraperitoneally injected with 2% pentobarbital sodium and euthanized. The femurs and tibias were collected aseptically, all soft tissues were removed, and the bones were washed three times with phosphate-buffered saline (PBS) and sterilized by soaking in 75% ethanol for 7-10 minutes. The bones were dissected to remove the epiphyses, and bone marrow cells were collected by washing the marrow cavity with culture medium (DMEM/F12; Gibco, Nanjing, China) containing 10% fetal bovine serum (Gibco, Nanning, China) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). Cells isolated from each rat were used to prepare single-cell suspensions. The suspensions were centrifuged at 1000 rpm for 5 minutes, and then the cell pellets were re-suspended and seeded into a 25 cm2 culture flask. BMSCs were cultured in a 37 °C, 5% CO2 humidified incubator. Initially, the medium was replaced 24 hours after seeding to remove non-adherent cells. Subsequently, the medium was changed twice a week until confluence was reached, followed by passaging. The 3rd passage BMSCs were differentiated into adipocytes and osteoblasts (OBs) under specific induction conditions: When the BMSCs reached 80%-90% confluence, they were cultured in induction media containing specific inducers (adipocytes: 1 μM dexamethasone, 0.5 mmol/L isobutyl-1-methylxanthine, 10 μg/mL insulin, 200 μM indomethacin; OBs: 50 μg/mL ascorbic acid, 10 mmol/L β-glycerophosphate, 100 nM dexamethasone) for 21 days, with medium changes every 3 days. Oil Red O and Alizarin Red staining assessed adipogenic and osteogenic differentiation.
Cell morphology was observed daily under an inverted phase-contrast microscope. When cell confluency reached approximately 90%, cells were washed with PBS, fixed with 4% paraformaldehyde for 10 minutes, stained with Giemsa for 2 minutes, rinsed with distilled water, and observed under a microscope equipped with a camera. The cells used in the experiments were from the 3rd passage. On day 21, Oil Red O staining was used to monitor adipocyte differentiation, and Alizarin Red staining was used to monitor OB differentiation.
Flow cytometry was used to identify surface markers of rat BMSCs. E BMSCs were incubated at 4 °C in the dark for 45 minutes with PE-labeled CD45 (12-0461-80), FITC-labeled CD11B (11-0112-82), FITC-labeled CD73 (11-0739-42), and FITC-labeled CD90 (11-0900-81) antibodies (all from eBioscience, San Diego, CA, USA). After incubation, the cells were washed twice with PBS to remove unbound antibodies and resuspended in 500 μL of FACS buffer. Equal amounts of cell preparations were incubated with the corresponding isotype control antibodies to determine nonspecific fluorescence signals. Flow cytometry analysis was performed using a BD Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, United States), and data were acquired and analyzed using BD Accuri C6 software.
Invitrogen Life Technologies (Carlsbad, CA, United States) synthesized the shRNA coding DNA sequences and cons
During the logarithmic growth phase of the 3rd passage, rat BMSCs were seeded in a 6-well cell culture plate for 12-16 hours (during this period, cells did not exceed 50% confluence). Subsequently, the cells were treated with shOPG or shScr (scrambled) for 24 hours, then replaced with fresh medium (containing 2 μg/mL puromycin). Untreated cells were used as controls. RNA and protein were isolated on days 3, 7, 14, and 21. Detailed information regarding the shRNA sequences can be found in Table 1.
| Name | shRNA primer sequence (5’-3’) |
| shOPG (F) | 5’-CCGGACTTGCATTATGACCCAGAACTCGAGTTTCTGGGTCATAATGCAAGTTTTTTG-3’ |
| shOPG (R) | 5’-AATTCAAAAAACTTGCATTATGACCCAGAAACTCGAGTTTCTGGGTCATAATGCAAGT-3’ |
The experimental group seeded 8 × 103 cells per well into a 96-well culture plate and cultured them in 200 μL of medium per well for 1, 3, 5, 7, and 9 days. 20 μL of 5% MTT solution (Abcam, Cat. No: Ab211091) was added to each well and incubated for 4 hours. After incubation, the supernatant was discarded, and 100 μL of DMSO was added to each well. After shaking for 10 minutes on a microplate reader, the absorbance was measured at a wavelength of 570 nanometers (A value). The time points and absorbance were plotted to create a cell growth curve.
Non-adherent bone marrow stromal cells were obtained from 4-week-old SD rats. In the co-culture experiment, shOPG, shScr, and control group cells were cultured at a density of 1 × 106 cells/mL in six-well plates, followed by adding 1 mL of medium containing 1 × 106 non-adherent rat bone marrow stromal cells (as OC precursor cells) to each group. The co-culture was maintained for 7 days. The old medium was replaced with a fresh medium every 3 days. TRAP staining was used to quantify the presence of TRAP-positive multinucleated cells (more than three nuclei) through cytochemical staining, thereby measuring OC formation.
Cells from the shOPG, shScr, and control groups are seeded onto bovine bone slices in 6-well plates at 1 × 106 cells/mL density. Each well is supplemented with 1 mL of culture medium containing 1 × 106 non-adherent rat bone marrow stromal cells (serving as OC precursor cells). The co-culture is maintained for 7 days. OCs are detached from the bone slices using mechanical agitation and ultrasonic treatment. After collection, the cells are stained with toluidine blue, and resorption pits are visualized and imaged under a microscope (ZEISS, Jena, Germany). The percentage of resorption pit area is quantified. Three random fields of view are selected from each bone slice for further statistical analysis[19].
According to the manufacturer’s instructions, total RNA was extracted from cells using TRIzol (Takara, Dalian, Liaoning, China). The optical density of RNA was assessed at 260 nanometers (OD260) and 280 nanometers (OD280) using a Nanodrop 2000 spectrophotometer (Thermo Scientific, MA, United States) to evaluate RNA concentration. The OD260:
| Gene | Primer sequence (5’-3’) | PCR product (bp) |
| RANKL | 5’-TTACCTGTACGCCAACATTTGC-3’ (F) | 283 |
| 5’-AAGTACGTCGCATCTTGATCC-3’ (R) | ||
| OPG | 5’-TGTTCTGGTGGACAGTTTGC-3’ (F) | 167 |
| 5’-AGAGGTCAATGTCTTGGAT-3’ (R) | ||
| CTSK | 5’-ATATGTGCAGCAGAATGGAGG-3’ (F) | 195 |
| 5’-CTTGCATCGATGGACACAGAG-3’ (R) | ||
| TRAP | 5’-CCACAACCTGCAGTATCTTC-3’ (F) | 200 |
| 5’-CCACATACGTGATGCTCATTTC-3’ (R) | ||
| MMP-9 | 5’-TTGCCTGCAAAGTTGAACTCAG-3’ (F) | 264 |
| 5’-CAAGCGAGTAACGCTCTGG-3’ (R) | ||
| CTR | 5’-AGCTGGTGTAATGTCCTATCAG-3’ (F) | 236 |
| 5’-CAATCAGAGCAGCAATTGACATGG-3’ (R) | ||
| β-actin | 5’-GGAGATTACTGCCCTGGCTCCTA-3’ (F) | 150 |
| 5’-GACTCATCGTACTCCTGCTTGCTG-3’ (R) |
Culture cells in a six-well culture plate with DMEM/F12 containing 10% fetal bovine serum until reaching nearly 90% confluence. Subsequently, the cells were washed twice with ice-cold PBS and harvested using cell RIPA buffer (Fudebio-tech, Hangzhou, China). After centrifugation at 14000 × g and 4 °C for 15 minutes, collect the supernatant and determine the total protein concentration using a BCA protein assay kit (Fudebio-tech, Hangzhou, China) following the manu
CoIP is a commonly used experimental technique for detecting protein-protein interactions in vivo. Firstly, cells are lysed using RIPA lysis buffer (Thermo Fisher Scientific, Cat. No. 89900), and the lysates are centrifuged at 15000 × g for 10 minutes at 4 °C to collect the supernatant, referred to as the Input. The Input represents the BMSC protein lysates before antibody addition or immunoprecipitation. Next, specific antibodies against RANKL (R&D Systems, Cat. No. AF462) or OPG (R&D Systems, Cat. No. AF805), as well as control IgG, are pre-incubated with protein A/G agarose beads (Santa Cruz Biotechnology, Cat. No. sc-2003) to form antibody-protein A/G bead complexes. Then, the cell lysate supernatants are mixed with the antibody-protein A/G bead complexes and incubated overnight at 4 °C to allow the antibodies to capture the target proteins and their interacting partners. The residues are washed multiple times with wash buffer (Thermo Fisher Scientific, Cat. No: 28360) to remove non-specifically bound proteins and impurities. Finally, the target protein complexes (RANKL, OPG, and IgG) are eluted from the beads using elution buffer, separated by sodium-dodecyl sulfate gel electrophoresis electrophoresis (Bio-Rad, Cat. No: 456-1093), transferred to a polyvinylidene fluoride membrane (Millipore, Cat. No: IPVH00010), and detected using western blot to analyze the target proteins (such as RANKL and OPG) and their interacting proteins. This method successfully validates the protein-protein interaction between RANKL and OPG, providing a reference for studying other protein interactions.
Statistical analysis was conducted using SPSS 17.0 software. Data are expressed as the mean ± SD of all experiments. Significant differences between the study groups were determined using statistical methods such as one-way analysis of variance (ANOVA) or Student’s t-test as appropriate. A P-value less than 0.05 was considered statistically significant.
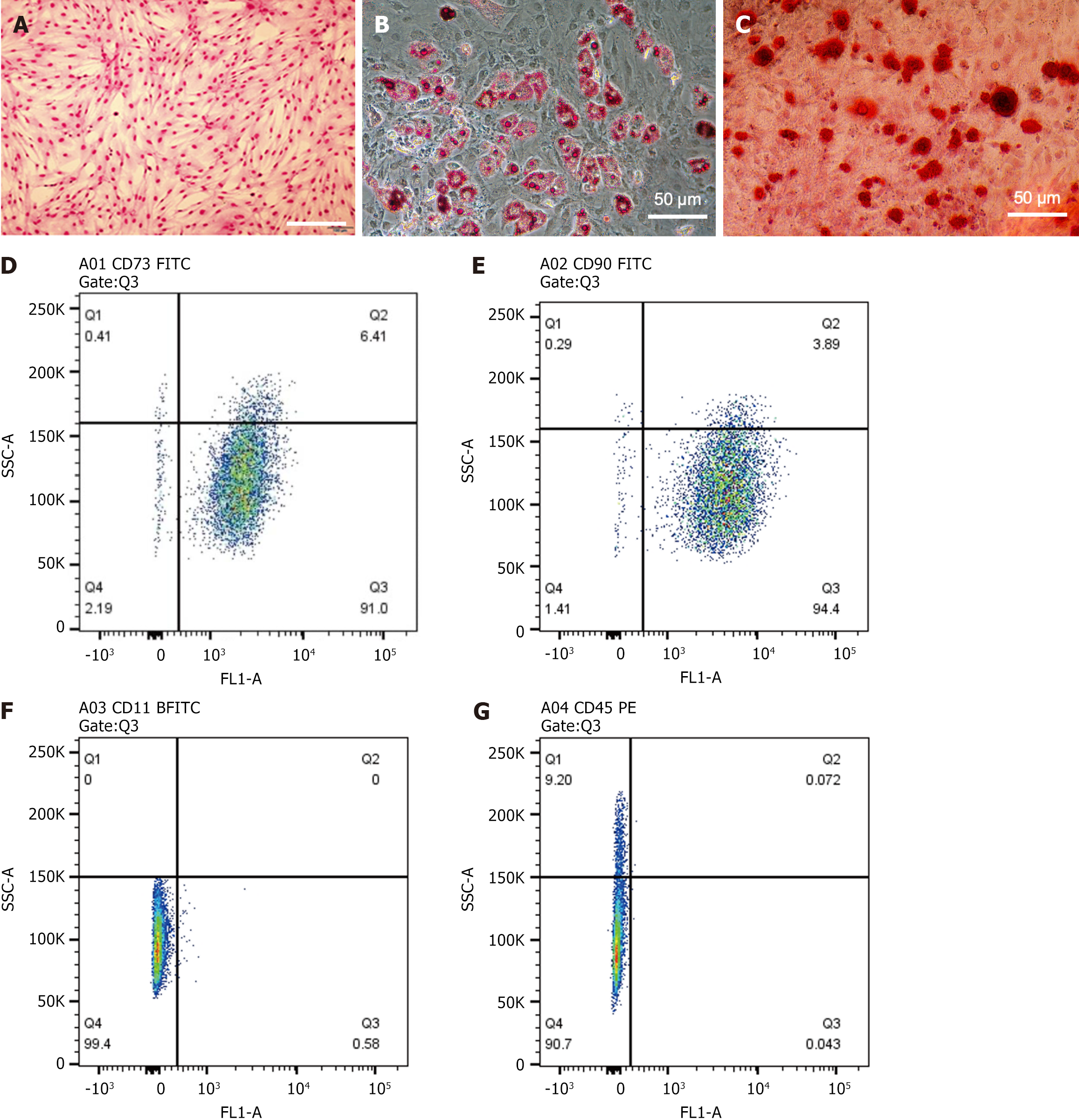
The morphological and phenotypic characteristics of third-generation rat BMSCs were identified. Giemsa staining revealed a typical spindle-shaped morphology (Figure 1A). Under specific induction conditions, these cells were successfully differentiated into adipocytes (Figure 1B) and OBs (Figure 1C), confirmed by Oil Red O and Alizarin Red staining, respectively. Flow cytometry analysis showed that over 90% of the cells expressed the mesenchymal stem cell markers CD73 (91.0%) and CD90 (94.4%) (Figure 1D and E), while the expression of hematopoietic markers CD11B (0.58%) and CD45 (0.043%) was negligible (Figure 1F and G).
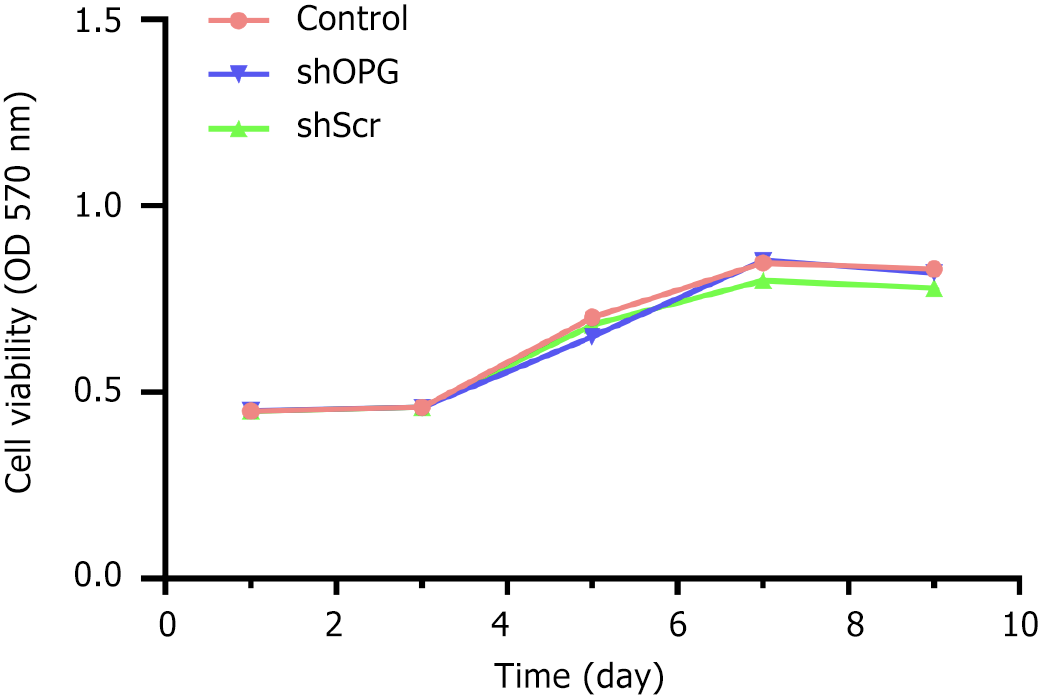
In this study, we evaluated the effect of OPG gene silencing on the proliferation of BMSCs using the MTT cell proliferation assay. The experimental results showed that the growth of cells in the shOPG, shScr, and control groups was similar throughout the experiment. Specifically, all groups of cells were in an adaptation period during the first three days after seeding, followed by rapid growth, reaching a peak proliferation on the 7th day. Subsequently, the proliferation rate of the cells gradually stabilized (Figure 2). At each time point from day 1 to day 9, the absorbance values (A values) of the three groups of cells were measured to assess their proliferation activity. The results indicated that the proliferation curve of the shOPG group almost overlapped with that of the shScr group and the control group, suggesting that the shOPG treatment did not significantly affect the proliferation capacity of BMSCs. Specific statistical data showed no significant differences in absorbance values between the groups on day 1, day 3, day 5, day 7, and day 9 (P > 0.05). These results indicate that although the OPG gene in BMSCs was successfully silenced using RNAi technology, this gene silencing did not significantly affect the proliferation activity of the cells.
To evaluate the effect of silencing the OPG gene using RNAi technology, we transduced BMSCs with a lentiviral vector encoding shRNA targeting OPG (OPG shRNA). We then assessed the mRNA expression levels of OPG and RANKL genes at different time points post-transfection using RT-qPCR. We compared the shOPG group with a mock group (shScr) and a control group (non-transfected) to validate the specificity and efficiency of gene silencing.
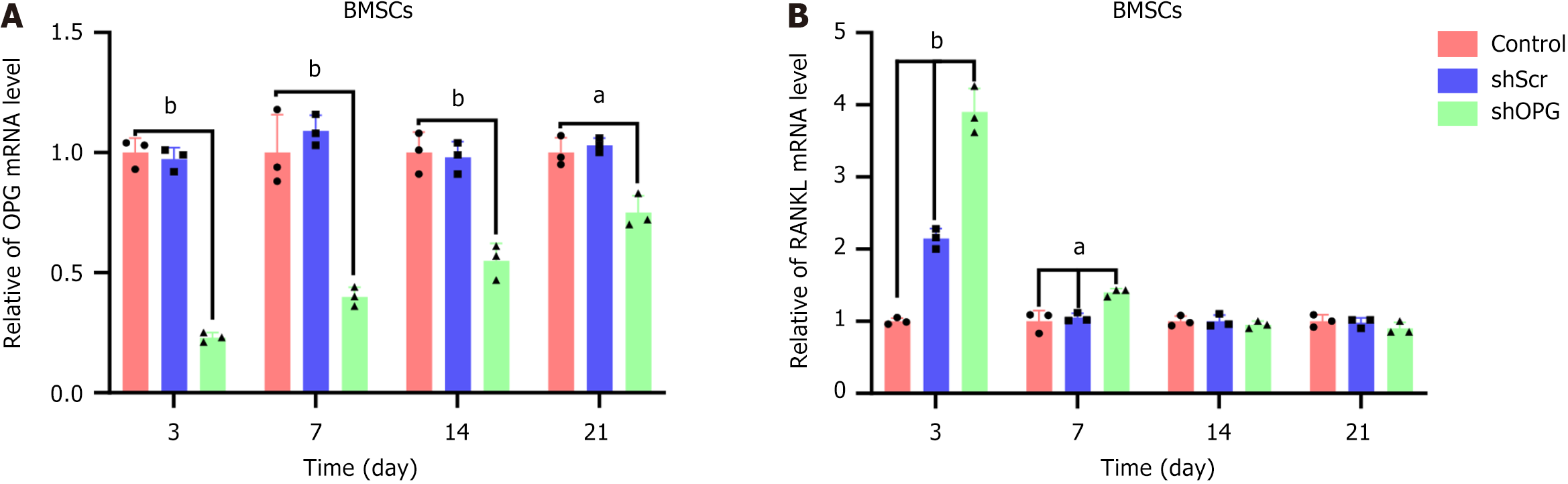
At days 3, 7, 14, and 21 post-transfection, we measured the expression levels of OPG mRNA in the cells of each group. The results showed that the OPG mRNA expression in the shOPG group was significantly lower than in the control group at all time points post-transfection. On day 3 post-transfection, the OPG mRNA expression in the shOPG group was only 23% of the control group; on days 7 and 14, it was 37% and 54% of the control group, respectively; and on day 21, it recovered to 74% of the control group (Figure 3A). In comparison, the expression of OPG mRNA in the shScr group did not show significant differences from the control group at all time points, indicating that the gene silencing effect of shOPG on the OPG gene is highly specific, with the inhibition reaching its peak at day 3 post-transfection and gradually diminishing thereafter.
Furthermore, we also measured the mRNA expression levels of RANKL in the cells of each group. The results showed that the RANKL mRNA in the shOPG group was significantly upregulated at day 3 post-transfection, reaching 390% of the control group; on days 7 and 14, the expression levels were 135% and 97% of the control group, respectively; by day 21, the expression level recovered to 84% of the control group (Figure 3B). It indicates that in BMSCs transduced with shOPG, the expression of RANKL mRNA peaked briefly at day 3 post-transfection and gradually returned to normal levels. In contrast, the expression of RANKL mRNA in the shScr group was 212% of the control group on day 3 but did not differ significantly from the control group at other time points.
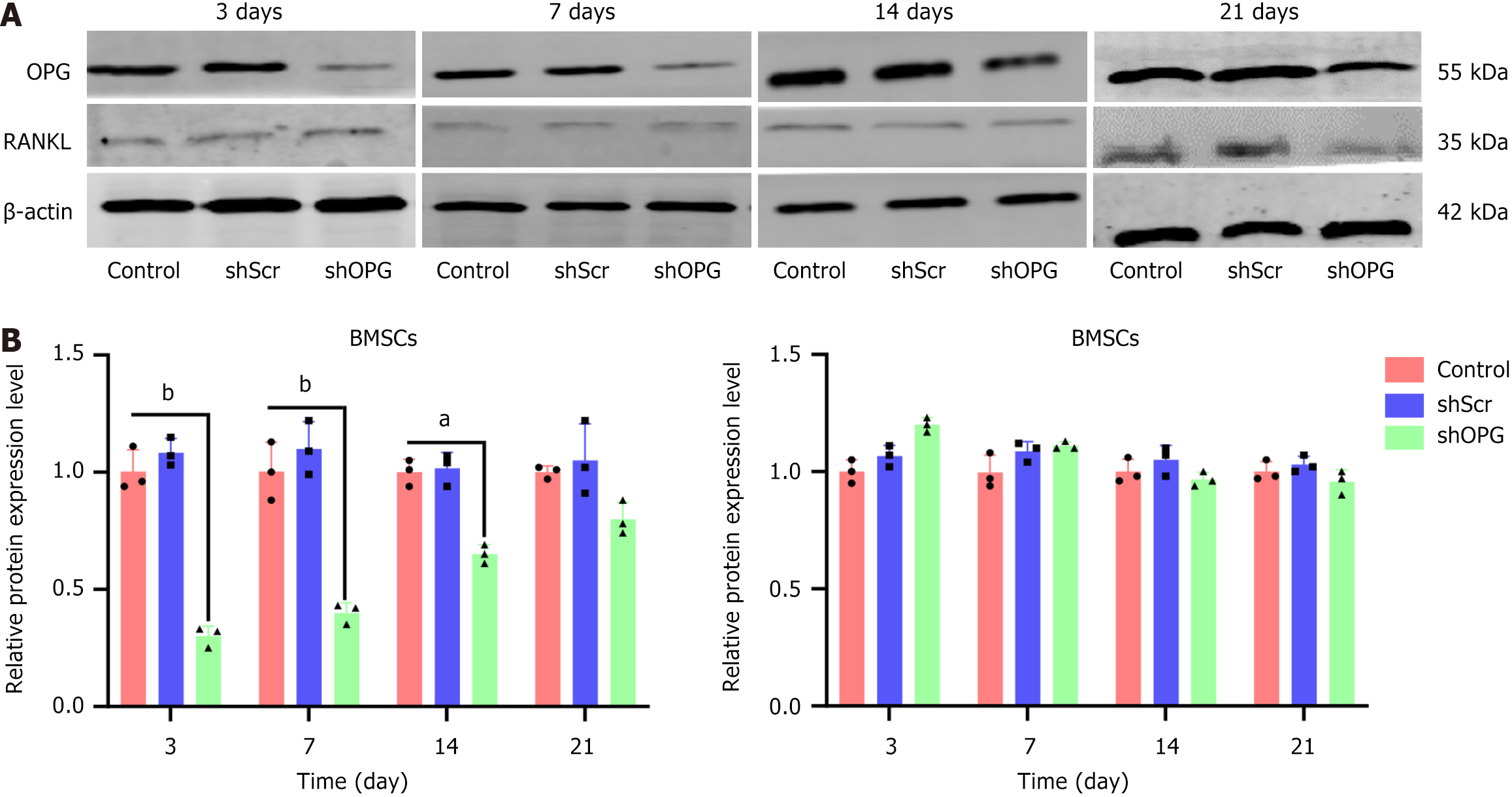
To further validate the effects of shOPG on silencing the OPG gene, we performed western blot analysis to examine the expression levels of OPG and RANKL proteins in cells from different groups at different time points post-transfection (3rd, 7th, 14th, and 21st days) (Figure 4). The results showed that the expression of OPG protein in the shOPG group significantly decreased to 31% of the control group on the 3rd day post-transfection, gradually increasing to 45% and 68% of the control group on the 7th and 14th days, respectively, and eventually returning to 87% on the 21st day. In contrast, the expression of OPG protein in the shScr group did not show significant differences from the control group at all time points (Figure 4A). These results indicate that the inhibitory effect of shOPG on OPG protein expression was most significant on the 3rd day post-transfection, gradually weakening but still maintaining a significant inhibitory effect thereafter. Additionally, we also measured the expression levels of RANKL protein in the cells from each group. The results demonstrated no significant differences in the expression levels of RANKL protein among the shOPG group, shScr group, and control group at all time points post-transfection (Figure 4B). It indicates that while shOPG significantly inhibited the expression of OPG protein, it did not affect the expression level of RANKL protein.
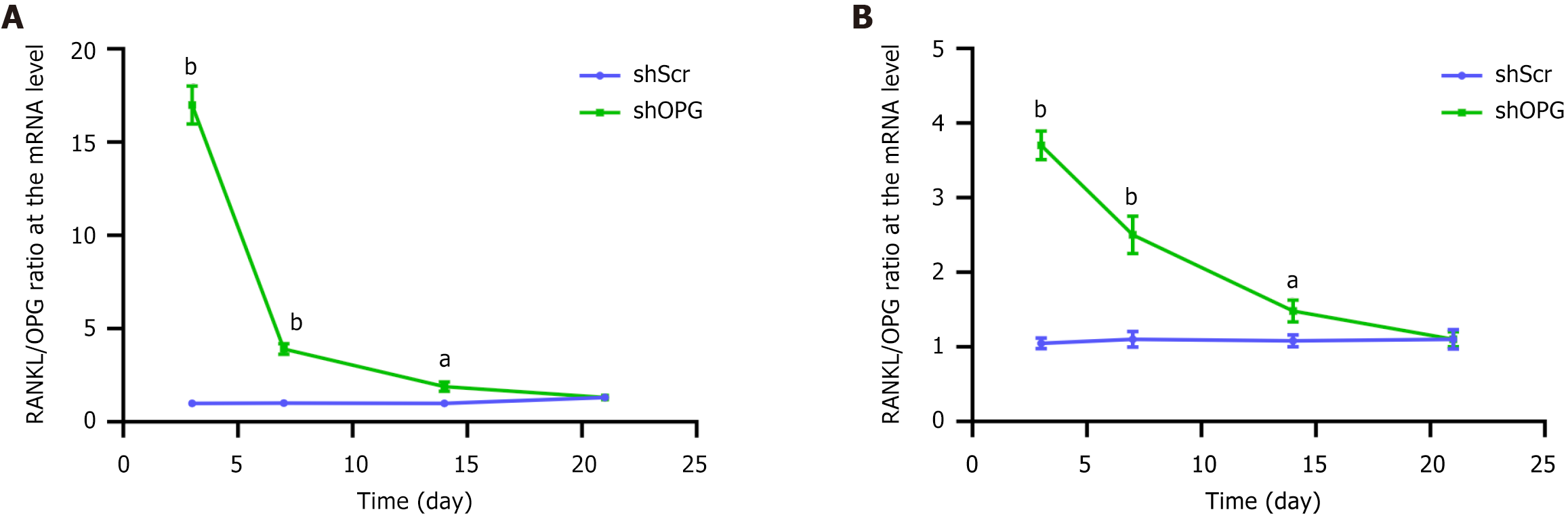
We evaluated the impact of shOPG on the RANKL/OPG ratio, analyzing it at both mRNA and protein levels. At the mRNA level, the RANKL/OPG ratio in the shOPG group significantly increased on the 3rd, 7th, and 14th days post-transfection (Figure 5A). Specifically, the RANKL/OPG ratio in the shOPG group was nearly 20-fold higher than that in the control groups on the 3rd day; on the 7th and 14th days, while the ratio decreased slightly, it remained significantly higher than the control group. However, by the 21st day, the RANKL/OPG ratio decreased to a level similar to the control group. At the protein level, the RANKL/OPG ratio in the shOPG group also significantly increased on the 3rd, 7th, and 14th days post-transfection (Figure 5B). On the 3rd day, the ratio in the shOPG group increased by 3.7-fold; on the 7th day, the ratio increased by 2.5-fold. Although the ratio decreased on the 7th and 14th days, it remained higher than the control group at these time points. These results indicate that shOPG significantly elevated the RANKL/OPG ratio in the first 14 days post-transfection, with this elevation observed at both the mRNA and protein levels, and the ratio returned to normal levels by the 21st day. The silencing of OPG increased the RANKL/OPG ratio and promoted the generation of OC-like cells.
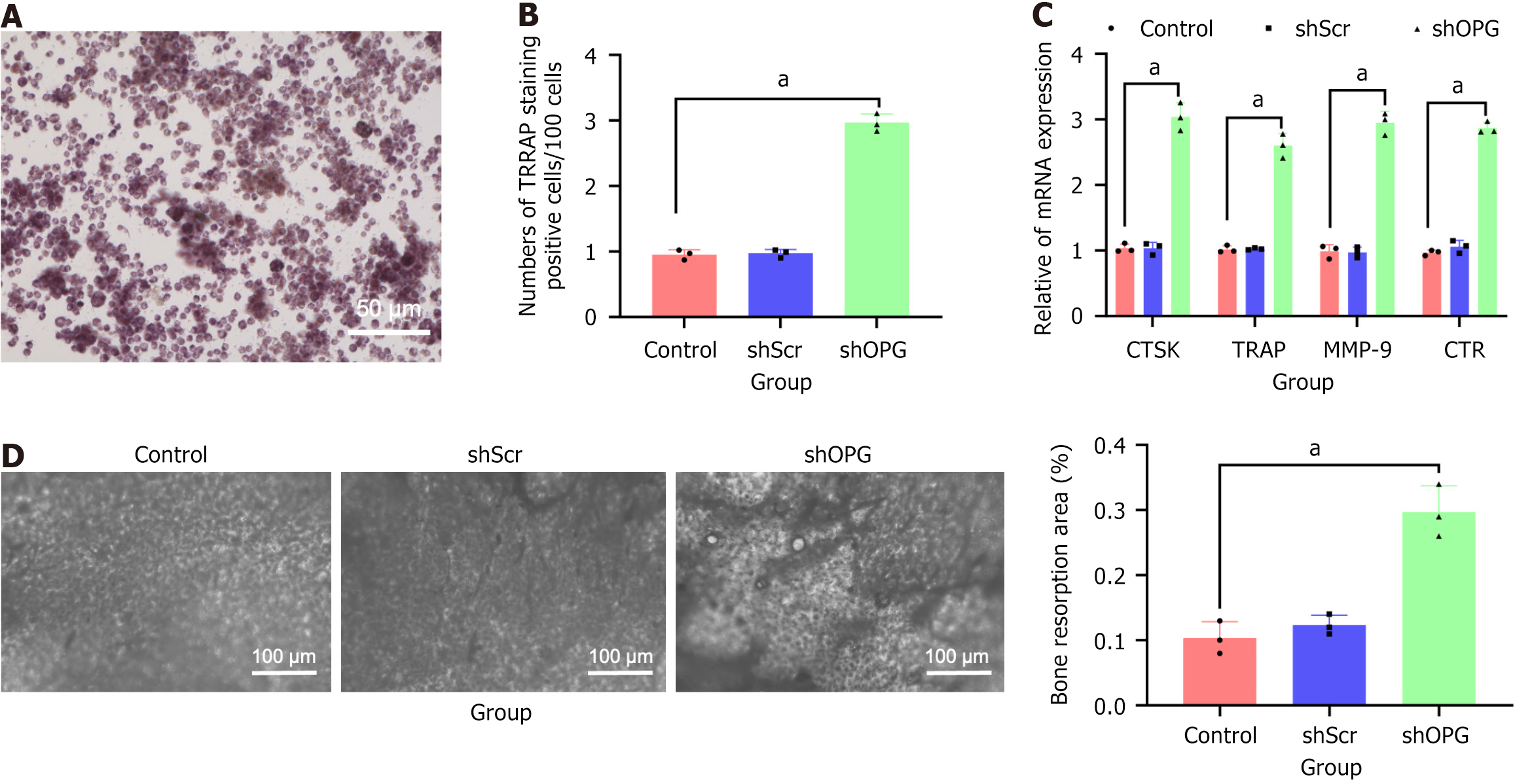
To assess the impact of OPG gene silencing on osteoclastogenesis, we co-cultured BMSCs transfected with shOPG with non-adherent bone marrow stromal cells and evaluated the generation of TRAP-positive multinucleated cells. The experimental results showed that, compared to the shScr and the control groups, the shOPG group exhibited a significant increase in TRAP-positive multinucleated cells (Figure 6A and B). Specifically, during the co-culture process with shOPG BMSCs, where the OPG gene was knocked out, there was a significant increase in TRAP-positive multinucleated cells in non-adherent bone marrow stromal cells. In contrast, there was no significant difference in the co-culture results between the shScr and the control groups (P > 0.05), further confirming the specific effect of shOPG. These results suggest inhibiting the OPG gene in BMSCs can significantly enhance OC generation by increasing the RANKL/OPG ratio.
Additionally, we further validated the levels of OC marker genes cathepsin K (CTSK), TRAP, matrix metalloproteinase-9, and receptors for calcitonin through quantitative reverse transcription polymerase chain reaction experiments. The results showed that during co-culture with OPG-silenced shOPG BMSCs, the CTSK, TRAP, matrix metalloproteinase-9, and receptors for calcitonin mRNA levels were significantly elevated in non-adherent bone marrow stromal cells (Figure 6C). Simultaneously, bone resorption assays revealed that during co-culture with OPG-silenced shOPG BMSCs, bone resorption by non-adherent bone marrow stromal cells was significantly increased, whereas no significant differences were observed in the shScr and control groups (P > 0.05). These findings further confirmed that OPG gene silencing promotes OC-mediated bone resorption (Figure 6D).
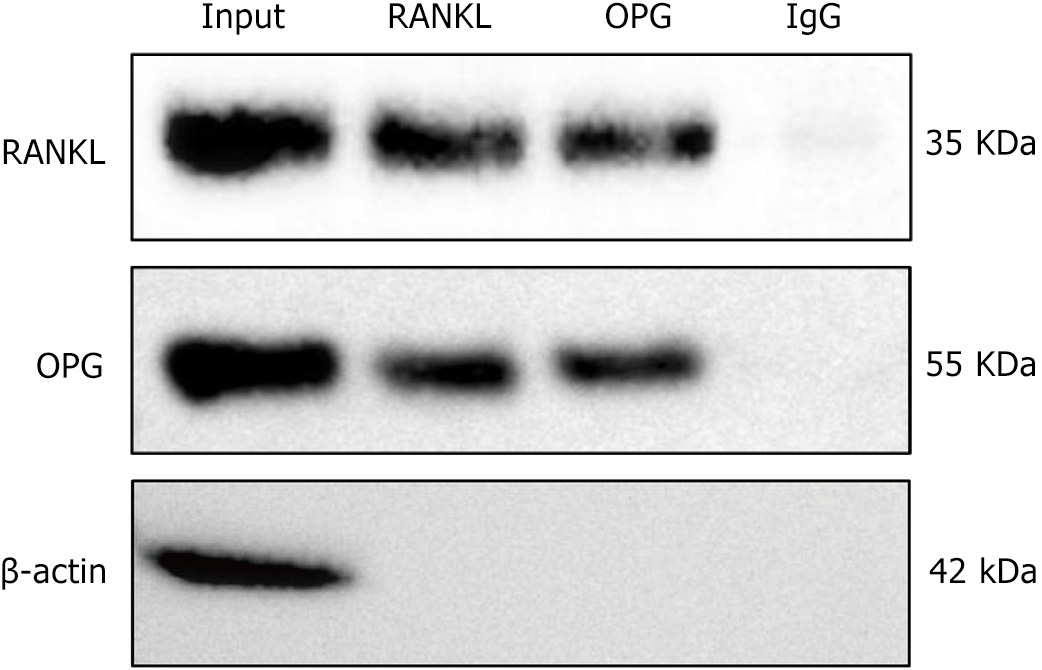
The CoIP experiment results showed that RANKL and OPG exhibited clear protein bands at 55 and 35 kDa, indicating that RANKL can directly bind to OPG. In the negative control group, no significant signals were observed, validating the specificity of the experimental results (Figure 7). These findings reveal a direct protein-protein interaction between RANKL and OPG, supporting their important bone metabolism and immune regulation functions.
The bone scaffold is crucial in bone tissue repair, especially in treating fractures, bone defect repair, and bone rege
This study employed RNAi technology to achieve stable silencing of the OPG gene in BMSCs using a recombinant lentiviral plasmid vector for efficient shRNA delivery. RT-qPCR and western blot analyses confirmed effective OPG silencing, significantly reducing its mRNA and protein levels. TRAP staining revealed increased OC numbers following OPG downregulation, suggesting its inhibitory role in OC differentiation. Additionally, CoIP assays validated the protein interaction between RANKL and OPG, consistent with previous studies, indicating that OPG may indirectly influence osteoclastogenesis by modulating RANKL availability. Although OPG silencing led to an upregulation of RANKL mRNA, no significant change was observed at the protein level, suggesting that the increased RANKL/OPG ratio primarily resulted from reduced OPG-mediated inhibition rather than direct RANKL upregulation. These findings provide a foundation for further investigation into the regulatory role of OPG in OC function and offer a potential strategy for modulating bone scaffold degradation and bone tissue repair.
This study demonstrates significant innovation in applying RNAi compared with previous studies[24,25]. While RNAi has been widely used for gene expression regulation and disease mechanism research[26-28] and has been reported in osteogenesis studies[25], specific silencing of the OPG gene remains limited. Previous studies primarily targeted genes such as S100 calcium-binding protein A4[29], guanine nucleotide-binding protein alpha-stimulating activity polypeptide 1[30], and Noggin[31], whereas precise regulation of OPG in osteoclastogenesis has not been thoroughly explored. By optimizing the RNAi vector system and employing a recombinant lentiviral plasmid vector for efficient shRNA delivery, this study successfully achieved stable OPG silencing in BMSCs. This strategy enhances the stability and specificity of gene silencing and ensures experimental reproducibility and reliability.
In terms of RANKL/OPG ratio modulation, this study differs from previous research in key aspects. Prior studies have primarily focused on the independent roles of RANKL or OPG in osteoclastogenesis and activity regulation, whereas this study specifically examines the overall impact of RANKL/OPG ratio changes on OC formation[3,32-34]. The findings provide new evidence supporting RNAi-mediated OPG inhibition to promote osteoclastogenesis by increasing the RANKL/OPG ratio. Compared with previous studies[35-37], this study further confirms the critical role of RANKL/OPG ratio regulation in osteoclastogenesis.
Additionally, while previous research has explored various regulatory factors in osteoclastogenesis, such as gene expression[38], signaling pathways[39], and microenvironmental changes, systematic investigations on RNAi-mediated OPG suppression and its direct impact on osteoclastogenesis remain limited. By stably silencing OPG through RNAi, this study significantly reduced OPG mRNA and protein levels while maintaining stable RANKL expression, thereby specifically increasing the RANKL/OPG ratio and promoting osteoclastogenesis. This mechanism aligns with prior findings on the RANKL/OPG axis in OC regulation[40-43]. Although RNAi has been explored in bone regeneration[25], its application in enhancing OC activity to optimize bone scaffold degradation remains underdeveloped. This study confirms that OPG silencing increases the RANKL/OPG ratio, enhancing osteoclastogenesis and accelerating scaffold degradation, providing a new strategy for developing highly degradable bone scaffolds. Furthermore, this approach offers potential for designing personalized bone repair materials, such as RNAi-based precision-engineered scaffolds, contributing to the clinical translation of RNAi technology in bone tissue repair.
This study introduces methodological innovations compared with previous research. A recombinant lentiviral plasmid vector delivered shRNA into rat BMSCs, effectively silencing OPG expression. Unlike other studies, this study validated the reliability and specificity of gene silencing through RT-qPCR and western blot, assessed OC formation via TRAP staining, and examined the RANKL-OPG interaction using co-immunoprecipitation[44-46]. TRAP, a classic OC marker, was used to evaluate osteoclastogenesis, while additional markers such as cathepsin K and Occlusion of Stomatal Pore 1 will be explored in future studies for a more comprehensive assessment of OC formation and differentiation.
This research provides new scientific evidence for promoting osteoclastogenesis by regulating the RANKL/OPG ratio, which holds significant application value in the degradation of bone scaffold and bone tissue repair. By controlling the expression of the OPG gene, the degradation rate of the bone scaffold can be precisely controlled, thereby improving the efficiency and effectiveness of bone regeneration. Moreover, this study’s findings have potential clinical implications in treating bone-related diseases such as osteoporosis, offering new theoretical support and molecular targets for related therapeutic strategies.
Although this study demonstrates that RNAi-mediated OPG silencing promotes the differentiation of non-adherent bone marrow stromal cells into OCs, its main limitation is that the experiments were conducted in vitro, lacking in vivo evidence for validation. Currently, many studies have employed vesicles or exosomes derived from BMSCs in expe
Further research must validate the findings across different animal models and clinical samples. Multi-faceted data collection, including studies on various animal models, fracture types, materials, and intervention durations, should be conducted to ensure the reliability and reproducibility of the results. Future research should focus on in vivo experiments to verify the efficacy and safety of RNAi technology in regulating osteoclastogenesis. Expanding into multiple directions, such as RNAi regulation of ABCC1 and CDC37 expression to promote OC differentiation or RNAi intervention in the RANKL signaling pathway to regulate OC function, could provide further insights. Moreover, exploring the effects of different doses and time points of RNAi on the RANKL/OPG ratio and osteoclastogenesis could help optimize intervention strategies.
Related applications, such as C-176, are already reported, which inhibits the STING-mediated nuclear factor-kappa B pathway activation and can downregulate OC formation. C-176, as a STING inhibitor, also promotes M1 macrophage polarization to the M2 phenotype, suggesting that the cGAS-STING pathway may suppress osteoclastogenesis and differentiation by inhibiting M1 macrophage polarization[49-51]. Further research should explore the potential application of this mechanism in other bone-related diseases and develop corresponding therapeutic approaches to provide more options for clinical treatment.
Currently, the clinical treatment of fractures primarily relies on the implantation and fixation of exogenous bone scaffold materials. However, this approach necessitates a second surgery to remove the fixation materials. If porous-structured materials can be developed to allow bone tissue or stem cells to grow within, bioactive bone scaffolds could begin degrading within 1-2 months after implantation, during which OCs play a critical role in activation and regulation, promoting bone growth. Within 5-6 months, the body would completely absorb the scaffold, and the bone would largely regenerate. OCs are central to the degradation of bone scaffold materials, as they regulate the degradation rate through bone resorption processes[2]. It could save patients half the time and costs, facilitating faster recovery. Therefore, researching ways to enhance the regulation of osteoclastic bone resorption activity is crucial for optimizing the performance of bone scaffold materials.
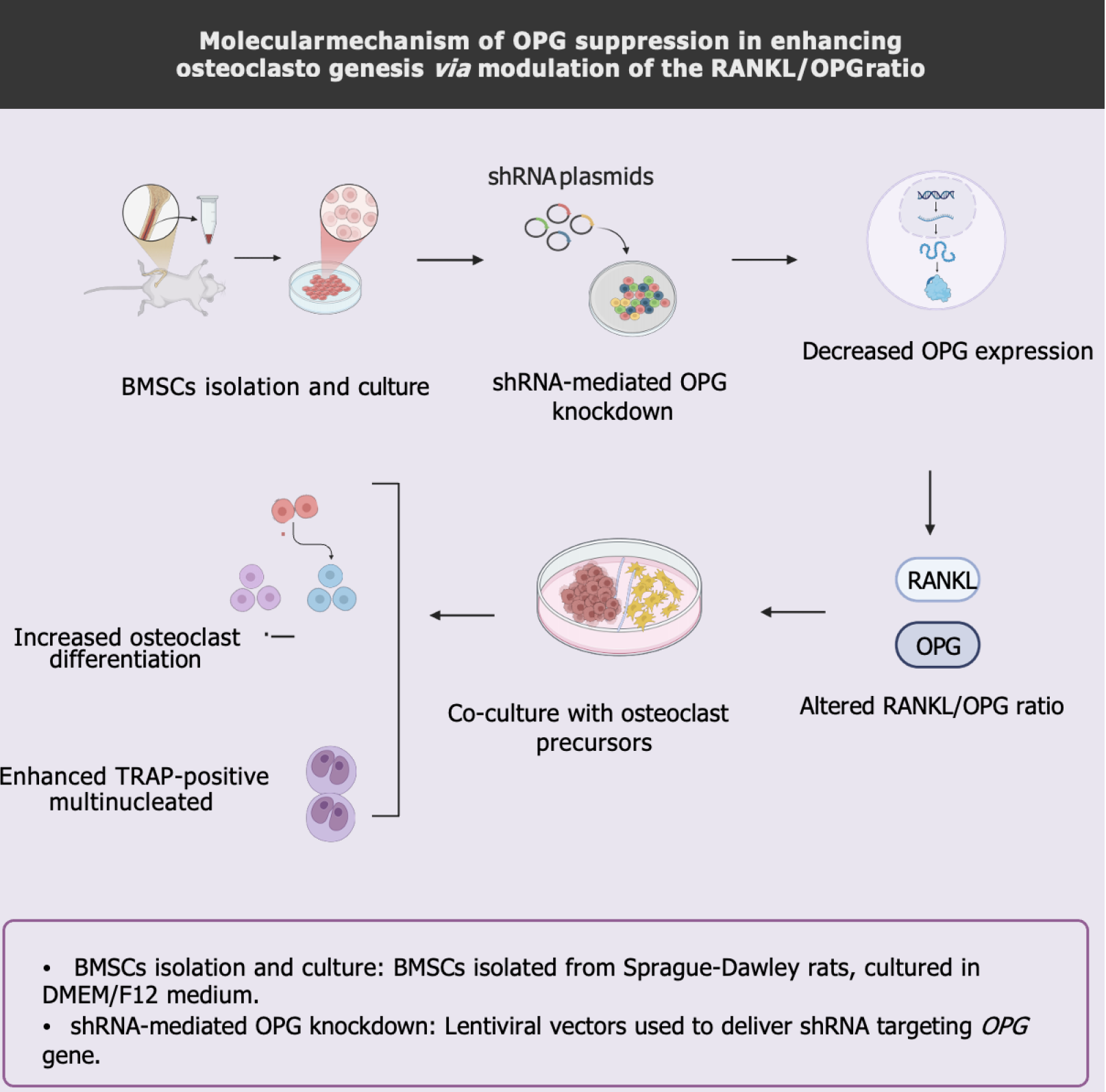
This study utilized recombinant lentiviral plasmids to interfere with the expression of the OPG gene in BMSCs in rats. It was observed that the mRNA level of RANKL was significantly upregulated while the mRNA and protein levels of OPG were downregulated in the BMSCs, leading to an increased ratio of RANKL/OPG and promotion of osteoclastogenesis. It indicates that inhibiting the expression of the OPG gene in BMSCs through RNAi can effectively regulate OC generation and activity, revealing the RANKL/OPG ratio’s critical role in bone resorption (Figure 8).
In conclusion, our study revealed the effects of RNAi on the RANKL/OPG ratio and osteoclastogenesis. These novel findings hold significant potential for advancing bone tissue engineering and regenerative medicine. They are particularly important for the degradation of bone scaffold materials and bone tissue repair, highlighting the critical role of RANKL/OPG regulation in osteoclastogenesis and scaffold degradation. This research provides a new direction for the clinical development of advanced scaffold materials and therapeutic strategies.
| 1. | Dos Santos Jorge Sousa K, de Souza A, de Almeida Cruz M, de Lima LE, do Espirito Santo G, Amaral GO, Granito RN, Renno AC. 3D printed scaffolds of biosilica and spongin from marine sponges: analysis of genotoxicity and cytotoxicity for bone tissue repair. Bioprocess Biosyst Eng. 2024;47:1483-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Müller WEG, Neufurth M, Wang S, Ackermann M, Muñoz-Espí R, Feng Q, Lu Q, Schröder HC, Wang X. Amorphous, Smart, and Bioinspired Polyphosphate Nano/Microparticles: A Biomaterial for Regeneration and Repair of Osteo-Articular Impairments In-Situ. Int J Mol Sci. 2018;19:427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Yasuda H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. 2021;39:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 4. | Lee YJ, Ahn JC, Oh CH. Oxyresveratrol attenuates bone resorption by inhibiting the mitogen-activated protein kinase pathway in ovariectomized rats. Nutr Metab (Lond). 2024;21:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Chen K, Ng PY, Chen R, Hu D, Berry S, Baron R, Gori F. Sfrp4 repression of the Ror2/Jnk cascade in osteoclasts protects cortical bone from excessive endosteal resorption. Proc Natl Acad Sci U S A. 2019;116:14138-14143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, Kyaw W, Pathmanandavel K, Grootveld AK, Moran I, Butt D, Nguyen A, Corr A, Warren S, Biro M, Butterfield NC, Guilfoyle SE, Komla-Ebri D, Dack MRG, Dewhurst HF, Logan JG, Li Y, Mohanty ST, Byrne N, Terry RL, Simic MK, Chai R, Quinn JMW, Youlten SE, Pettitt JA, Abi-Hanna D, Jain R, Weninger W, Lundberg M, Sun S, Ebetino FH, Timpson P, Lee WM, Baldock PA, Rogers MJ, Brink R, Williams GR, Bassett JHD, Kemp JP, Pavlos NJ, Croucher PI, Phan TG. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021;184:1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 7. | Murata T, Sugatani T, Shimizu K, Manganiello VC, Tagawa T. Effect of the phosphodiesterase 4 inhibitor, rolipram, on retinoic acid-increased alkaline phosphatase activity in the mouse fibroblastic C3H10T1/2 cell line. Arch Oral Biol. 2003;48:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Vachliotis ID, Polyzos SA. Osteoprotegerin/Receptor Activator of Nuclear Factor-Kappa B Ligand/Receptor Activator of Nuclear Factor-Kappa B Axis in Obesity, Type 2 Diabetes Mellitus, and Nonalcoholic Fatty Liver Disease. Curr Obes Rep. 2023;12:147-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 9. | Greisen SR, Kragstrup TW, Thomsen JS, Hørslev-Pedersen K, Hetland ML, Stengaard-Pedersen K, Østergaard M, Ørnbjerg L, Junker P, Sharpe AH, Freeman GJ, Hvid M, Moestrup SK, Hauge EM, Deleuran B. The Programmed Death-1 Pathway Counter-Regulates Inflammation-Induced Osteoclast Activity in Clinical and Experimental Settings. Front Immunol. 2022;13:773946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Niu J, Bi F, Tian Q, Tian K. Melittin Treats Periprosthetic Osteolysis in a Rat Model by Inhibiting the NF-kB Pathway and Regulating the Ratio of Receptor Activator of Nuclear Factor Kappa B Ligand/Osteoprotegerin. J Arthroplasty. 2024;39:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hashimoto T, Arakawa K, Ohta Y, Suehiro T, Uesugi N, Nakayama M, Tsuchihashi T. Acquired fanconi syndrome with osteomalacia secondary to monoclonal gammopathy of undetermined significance. Intern Med. 2007;46:241-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | McDonald MM, Khoo WH, Ng PY, Xiao Y, Zamerli J, Thatcher P, Kyaw W, Pathmanandavel K, Grootveld AK, Moran I, Butt D, Nguyen A, Corr A, Warren S, Biro M, Butterfield NC, Guilfoyle SE, Komla-Ebri D, Dack MRG, Dewhurst HF, Logan JG, Li Y, Mohanty ST, Byrne N, Terry RL, Simic MK, Chai R, Quinn JMW, Youlten SE, Pettitt JA, Abi-Hanna D, Jain R, Weninger W, Lundberg M, Sun S, Ebetino FH, Timpson P, Lee WM, Baldock PA, Rogers MJ, Brink R, Williams GR, Bassett JHD, Kemp JP, Pavlos NJ, Croucher PI, Phan TG. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell. 2021;184:1330-1347.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 229] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 13. | Liang QL, Xu HG, Yu L, Ding MR, Li YT, Qi GF, Zhang K, Wang L, Wang H, Cui X. Binding-induced fibrillogenesis peptide inhibits RANKL-mediated osteoclast activation against osteoporosis. Biomaterials. 2023;302:122331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 14. | Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1355] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 15. | Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 359] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 16. | Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1069] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 17. | Aquino-Jarquin G. CircRNA knockdown based on antisense strategies. Drug Discov Today. 2024;29:104066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Bao WL, Wu Q, Hu B, Sun D, Zhao S, Shen X, Cheng H, Shen W. Oral Nanoparticles of SNX10-shRNA Plasmids Ameliorate Mouse Colitis. Int J Nanomedicine. 2021;16:345-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Zhao S, Ge C, Li Y, Chang L, Dan Z, Tu Y, Deng L, Kang H, Li C. Desferrioxamine alleviates UHMWPE particle-induced osteoclastic osteolysis by inhibiting caspase-1-dependent pyroptosis in osteocytes. J Biol Eng. 2022;16:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Wang H, Wang Q, Zhang H, Shi W, Lai Z, Cui Y, Li Q, Wang Z. [Repair of segmental bone defects in rabbits' radius with domestic porous tantalum encapsulated with pedicled fascial flap]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31:1200-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Qiu D, Cao C, Prasopthum A, Sun Z, Zhang S, Yang H, Xu Z, Tao J, Ai F, Yang J. Elucidating osseointegration in vivo in 3D printed scaffolds eliciting different foreign body responses. Mater Today Bio. 2023;22:100771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Pesce P, Zubery Y, Goldlust A, Bayer T, Abundo R, Canullo L. Ossification and Bone Regeneration in a Canine GBR Model, Part 2: Glycated Cross-Linked Collagenated Alloplastic Hydroxyapatite Scaffold vs Non-Cross-Linked Collagenated Xenographic Bone Hydroxyapatite. Int J Oral Maxillofac Implants. 2023;38:923-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Yao Z, Getting SJ, Locke IC. Regulation of TNF-Induced Osteoclast Differentiation. Cells. 2021;11:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 150] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 24. | Ji X, Chen X, Yu X. MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis. Int J Mol Sci. 2016;17:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Damiati LA, El-Messeiry S. An Overview of RNA-Based Scaffolds for Osteogenesis. Front Mol Biosci. 2021;8:682581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Alshaer W, Zureigat H, Al Karaki A, Al-Kadash A, Gharaibeh L, Hatmal MM, Aljabali AAA, Awidi A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur J Pharmacol. 2021;905:174178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 27. | Maxwell MM. RNAi applications in therapy development for neurodegenerative disease. Curr Pharm Des. 2009;15:3977-3991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Tang Q, Khvorova A. RNAi-based drug design: considerations and future directions. Nat Rev Drug Discov. 2024;23:341-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 85] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 29. | Kato C, Kojima T, Komaki M, Mimori K, Duarte WR, Takenaga K, Ishikawa I. S100A4 inhibition by RNAi up-regulates osteoblast related genes in periodontal ligament cells. Biochem Biophys Res Commun. 2005;326:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Zhao Y, Ding S. A high-throughput siRNA library screen identifies osteogenic suppressors in human mesenchymal stem cells. Proc Natl Acad Sci U S A. 2007;104:9673-9678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Takayama K, Suzuki A, Manaka T, Taguchi S, Hashimoto Y, Imai Y, Wakitani S, Takaoka K. RNA interference for noggin enhances the biological activity of bone morphogenetic proteins in vivo and in vitro. J Bone Miner Metab. 2009;27:402-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, Kobayashi Y, Furuya Y, Yasuda H, Fukuda C, Tsuda E. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 2021;39:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 406] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 33. | Takayanagi H. RANKL as the master regulator of osteoclast differentiation. J Bone Miner Metab. 2021;39:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 34. | El-Masri BM, Andreasen CM, Laursen KS, Kofod VB, Dahl XG, Nielsen MH, Thomsen JS, Brüel A, Sørensen MS, Hansen LJ, Kim AS, Taylor VE, Massarotti C, McDonald MM, You X, Charles JF, Delaisse JM, Andersen TL. Mapping RANKL- and OPG-expressing cells in bone tissue: the bone surface cells as activators of osteoclastogenesis and promoters of the denosumab rebound effect. Bone Res. 2024;12:62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Vulf M, Khlusov I, Yurova K, Todosenko N, Komar A, Kozlov I, Malashchenko V, Shunkina D, Khaziakhmatova O, Litvinova L. MicroRNA Regulation of Bone Marrow Mesenchymal Stem Cells in the Development of Osteoporosis in Obesity. Front Biosci (Schol Ed). 2022;14:17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Lai G, Zhao R, Zhuang W, Hou Z, Yang Z, He P, Wu J, Sang H. BMSC-derived exosomal miR-27a-3p and miR-196b-5p regulate bone remodeling in ovariectomized rats. PeerJ. 2022;10:e13744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Liu L, Guo S, Shi W, Liu Q, Huo F, Wu Y, Tian W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng Part A. 2021;27:962-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | Diemar SS, Dahl SS, West AS, Simonsen SA, Iversen HK, Jørgensen NR. A Systematic Review of the Circadian Rhythm of Bone Markers in Blood. Calcif Tissue Int. 2023;112:126-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 39. | Takegahara N, Kim H, Choi Y. Unraveling the intricacies of osteoclast differentiation and maturation: insight into novel therapeutic strategies for bone-destructive diseases. Exp Mol Med. 2024;56:264-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 40. | Hofbauer LC, Kühne CA, Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact. 4:268-275. [PubMed] |
| 41. | López Roldán A, García Giménez JL, Alpiste Illueca F. Impact of periodontal treatment on the RANKL/OPG ratio in crevicular fluid. PLoS One. 2020;15:e0227757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Lee DW, Kwon JY, Kim HK, Lee HJ, Kim ES, Kim HJ, Kim HJ, Lee HB. Propofol attenuates osteoclastogenesis by lowering RANKL/OPG ratio in mouse osteoblasts. Int J Med Sci. 2018;15:723-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Wang XF, Zhang YK, Yu ZS, Zhou JL. The role of the serum RANKL/OPG ratio in the healing of intertrochanteric fractures in elderly patients. Mol Med Rep. 2013;7:1169-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Bolzoni M, Storti P, Bonomini S, Todoerti K, Guasco D, Toscani D, Agnelli L, Neri A, Rizzoli V, Giuliani N. Immunomodulatory drugs lenalidomide and pomalidomide inhibit multiple myeloma-induced osteoclast formation and the RANKL/OPG ratio in the myeloma microenvironment targeting the expression of adhesion molecules. Exp Hematol. 2013;41:387-97.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 45. | Yahiro Y, Maeda S, Morikawa M, Koinuma D, Jokoji G, Ijuin T, Komiya S, Kageyama R, Miyazono K, Taniguchi N. BMP-induced Atoh8 attenuates osteoclastogenesis by suppressing Runx2 transcriptional activity and reducing the Rankl/Opg expression ratio in osteoblasts. Bone Res. 2020;8:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Rucci N, Rufo A, Alamanou M, Teti A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem. 2007;100:464-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Wang D, Guo Y, Heng BC, Zhang X, Wei Y, He Y, Xu M, Xia B, Deng X. Cell membrane vesicles derived from hBMSCs and hUVECs enhance bone regeneration. Bone Res. 2024;12:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 48. | Zhang Y, Bai J, Xiao B, Li C. BMSC-derived exosomes promote osteoporosis alleviation via M2 macrophage polarization. Mol Med. 2024;30:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Yu ZC, Fu R, Li Y, Zhao DY, Jiang H, Han D. The STING inhibitor C-176 attenuates osteoclast-related osteolytic diseases by inhibiting osteoclast differentiation. FASEB J. 2023;37:e22867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 50. | Muire PJ, Lofgren AL, Shiels SM, Wenke JC. Fracture healing in a polytrauma rat model is influenced by mtDNA:cGAS complex mediated pro-inflammation. J Exp Orthop. 2023;10:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 51. | Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 1281] [Article Influence: 320.3] [Reference Citation Analysis (0)] |