Published online Feb 26, 2025. doi: 10.4252/wjsc.v17.i2.96893
Revised: November 19, 2024
Accepted: January 16, 2025
Published online: February 26, 2025
Processing time: 282 Days and 20.4 Hours
Sepsis is a severe illness characterized by systemic and multiorgan reactive res
To investigate the effects of sepsis on BMSCs and the underlying mechanisms.
BMSCs were obtained from healthy donors and patients with sepsis. We com
Septic BMSCs showed decreased proliferation and self-renewal, bias towards adipogenic differentiation, and weakened osteogenic differentiation. Additionally, hematopoietic supportive capacity declines in sepsis. The levels of aging markers were significantly higher in the septic BMSCs. After NAD treatment, the proliferation capacity of septic BMSCs showed a recovery trend, with increased osteogenic and hematopoietic supportive capacities. Sepsis resulted in decreased expression of sirtuin 3 (SIRT3) in BMSCs, whereas NAD treatment restored SIRT3 expression, enhanced superoxide dismutase enzyme activity, reduced intracellular reactive oxygen species levels, maintained mitochondrial stability and function, and ultimately rejuvenated septic BMSCs.
Sepsis accelerates the aging of BMSCs, as evidenced by a decline in self-renewal and osteogenic capabilities, as well as weakened hematopoietic support functions. These deficiencies can be effectively reversed via the
Core Tip: Sepsis often leads to multiorgan dysfunction, including damage to bone marrow mesenchymal stem cells (BMSCs). This damage accelerates the aging of BMSCs, resulting in impaired self-renewal and differentiation abilities and weakened hematopoietic support functions. These changes disrupt hematopoiesis and may even cause long-term immunosuppression and bone loss. Nicotinamide adenine dinucleotide, which protects mitochondrial function, can rejuvenate septic BMSCs and provide a new target for adjunctive therapy to control post-sepsis complications.
- Citation: Xia X, Zhou K, An LY, Zhao M, Tian BL, Zhao JY, Zhou ZG, Tong Y. Nicotinamide adenine dinucleotide rejuvenates septic bone marrow mesenchymal stem cells. World J Stem Cells 2025; 17(2): 96893
- URL: https://www.wjgnet.com/1948-0210/full/v17/i2/96893.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i2.96893
Sepsis is a common systemic reactive disease that can damage multiple organs and tissues[1,2]. Current research has predominantly focused on acute organ damage, such as acute lung injury and acute kidney injury, to save lives. However, the impact of sepsis on the bone marrow is often chronic and does not immediately threaten life. Few studies have focused on the damage caused by sepsis in the bone marrow. In our long-term clinical observation, patients still experienced long-term physiological abnormalities, such as persistent lymphocytopenia after discharge, causing unplanned readmissions[3], indicating the presence of prolonged damage to the hematopoietic system. Lymphocytopenia is often accompanied by granulocytosis, which is similar to the hematopoietic composition observed in elderly individuals. Hematopoietic stem cells (HSCs), which are located in the bone marrow at the headquarters of the hema
Sepsis continuously consumes immune cells and damages the bone marrow, thereby inhibiting immune system recovery[8]. Although previous studies have shown that sepsis causes osteoblast ablation[9], it is unknown whether this is due to advanced defects in BMSCs. In recent years, increasing attention has been paid to the aging of BMSCs and the resulting bone health issues. Four types of senescence have been proposed: Replicative senescence, oncogene-induced senescence, stress-induced premature senescence, and developmental senescence. Patients with sepsis may be more prone to stress-induced premature senescence due to an inflammatory cytokine storm caused by infection. We compared BMSC differences between healthy individuals and septic patients and found that sepsis-affected BMSCs exhibited a more senescent state, manifested in decreased proliferation and self-renewal capabilities, biased differentiation towards adipogenesis over osteogenesis, and an inability to provide hematopoietic supportive capabilities matching those of healthy individuals. Additionally, we identified senescence markers in septic BMSCs, including increased levels of reactive oxygen species (ROS), expression of the senescence-associated secretory phenotype (SASP), and markers related to aging P16 and P21.
Nicotinamide adenine dinucleotide (NAD) is essential for activating acetylases, rapidly consuming oxidative free radicals in cells, and aiding in cellular resistance to aging[10-12]. We found that in septic BMSCs, NAD can restore NAD-dependent sirtuin 3 (SIRT3) expression in the mitochondria, which can enhance the activity of superoxide dismutase (SOD) and reduce harmful oxidative free radicals inside the cells[13,14]. Therefore, NAD treatment rejuvenates septic BMSCs and restores their function. It should be noted that our research provides an important reference for other similar adverse conditions, such as radiation and chemical injuries.
All samples were collected from septic patients and healthy donors (HD) aged 26 to 67 years after obtaining informed consent and approval from Shanghai General Hospital. Detailed information on the samples is provided in Supplementary Table 1. To isolate bone marrow mononuclear cells (BMNCs), we used LymphoprepTM (StemCell Technologies, 07801, Canada) following the indicated procedure. Isolated cells were cryopreserved in liquid nitrogen until they were seeded into 12-well plates. BMSCs were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) (Gibco, A3161001C, MA, United States), 1% penicillin/streptomycin (Gibco, 15070063, MA, United States), 1% insulin transferrin selenium sodium pyruvate additive (Gibco, 51300044, MA, United States), 10 ng/mL epidermal growth factor (PeproTech, AF-100-15, NJ, United States), and 10 ng/mL platelet-derived growth factor BB (PeproTech, AF-100-14B, NJ, United States)[15] at 37 °C in a humidified atmosphere with 5% CO2. Non-adherent cells were removed after one week, and the remaining cells were continuously expanded. Septic BMSCs were maintained in the above basic medium with an additional 1 mmol/L NAD for 7 days before the experiments began under certain circumstances. Cells from passages 1 to 4 were used in all experiments.
BMNCs were seeded at a density of one million cells per well in 6-well plates and incubated for 14 days with a weekly medium exchange. The colony formation units were stained with crystal violet (Beyotime, C0121, Shanghai, China) for 10 minutes and counted manually.
A total of 5000 BMSCs derived from both HD and septic samples were seeded per well in 96-well plates. After allowing the cells to adhere for 4 hours, 10 μL of CCK-8 reagent (Yeasen, 40203ES60, Shanghai, China) was added to each well. After incubation for 2 hours, the OD at 450 nm was measured and recorded daily. Growth curves were plotted using GraphPad Prism software.
HD and septic BMSCs (2 × 104) were seeded in 96-well plates, and after 24 hours of adhesion, the medium was supplemented with 50 μM F-ara-EdU (Sigma, T511293, Japan). The cells were fixed 12 hours later. For F-ara-EdU staining, cells were incubated with an EdU staining working solution containing 10 μM Cy3 AZIDE (Thermo, A10266, MA, United States), 1 mmol/L CuSO4 (Sigma, 908940, Japan), and 100 mmol/L (+)-sodium-L-ascorbic (Sigma, A7631, Japan) for 30 minutes at room temperature, followed by washing three times with phosphate-buffered saline (PBS). DAPI was used for nuclear staining. Images were acquired using Operetta.
Total RNA was extracted from cultured BMSCs using TRI Reagent (Sigma, T9424, Japan), following the manufacturer’s instructions. cDNA was synthesized using a HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, R312-01, Nanjing, China). Quantitative real-time polymerase chain reaction (qPCR) was performed as previously described using SYBR qPCR SuperMix plus (Novoprotein, E096, Suzhou, China). Primer sequences used in these reactions are listed in Supplementary Table 2. The relative fold-change in expression was calculated using the comparative threshold cycle (2-ΔΔCt) method, as described in previous studies.
Single digestive cells were collected and washed twice with cold PBS. Subsequently, they were blocked for 10 minutes on ice (BD Pharmingen, 564220, CA, United States) to prevent nonspecific antibody binding. Mixed antibodies were then added, and the cells were incubated at 4 °C for 30 minutes in the dark. The corresponding isotype controls were prepared using the same procedure. Finally, the stained cells were washed and resuspended in an appropriate volume of PBS containing 0.4% bovine serum albumin before evaluation using a flow cytometer (CYTOFLEX, Beckman). Data analysis was performed using FlowJo_V10 software. The antibodies used in these experiments are listed in Supplementary
BMSCs at passage three were cultured in an appropriate induction medium for osteogenic and adipogenic differentiation[16,17]. Osteogenic medium was prepared using the MesenCultTM Osteogenic Differentiation Kit (StemCell Technologies, 05465, Canada) supplemented with 2 mmol/L L-glutamine (Gibco, 21051024, MA, United States) and 1% penicillin/streptomycin. The adipogenic medium consisted of DMEM/F12, 10% FBS, 1% penicillin/streptomycin, 1% insulin transferrin selenium sodium pyruvate additive, 60 μM indomethacin (Sigma, I7378, Japan), 10-6 M dexamethasone (Sigma, D4902, Japan) and 0.5 mmol/L 3-isobutyl-1-methylxanthine (Sigma, I5879, Japan). Osteogenic differentiation was evaluated after 21 days using Alizarin Red S staining and qPCR, whereas adipogenic differentiation was evaluated using Oil Red O staining and qPCR.
Cell layers were fixed using methanol-acetone fixation for 30 minutes and rinsed twice with PBS, followed by 30 minutes of incubation with a 2% Alizarin Red S staining solution (Beyotime, C0138, Shanghai, China). The samples were then washed four times with distilled water, and images were captured under a light microscope. For quantification, a 10% cetylpyridinium chloride solution was used to re-dissolve the dye.
Cells were fixed in 4% formaldehyde for 30 minutes. A stock solution of 0.5% Oil Red O saturated in isopropanol (Sigma, O0625, Japan) was prepared beforehand, and a working solution was created by diluting the stock solution with water in a ratio of 3:2. After fixation, the cells were washed once with isopropanol and incubated in the working solution for 30 minutes. Subsequently, they were washed four times with distilled water, and images were captured under a light microscope. For quantification, 100% isopropanol was used to re-dissolve the dye.
BMSCs were seeded in 24-well plates and expanded for 48 hours. The purified hematopoietic stem/progenitor cells (HSPCs) from HD were seeded at a density of 1 × 104 in transwell chambers (Corning, CLS3413, NY, United States) and cultured in StemSpan SFEM Medium (StemCell Technologies, 09600, Canada) supplemented with 1% penicillin/streptomycin, 100 ng/mL stem cell factor (PeproTech, 300-07, NJ, United States), 100 ng/mL Flt3 L (PeproTech, 300-19, NJ, United States), 20 ng/mL thyroid peroxidase (PeproTech, 300-18, NJ, United States) and 20 ng/mL interleukin 6 (PeproTech, 200-06, NJ, United States)[18]. After 3 days of co-culture, HSPCs were collected and counted using trypan blue. Cellular immunofluorescence was performed to assess proliferative or apoptotic CD34+ HSPC stained with Ki67 or caspase 3 antibodies. The antibodies used in the experiment are listed in Supplementary Table 3. CD34- and Ki67- or caspase-positive cells were counted as observational cells. At least 50 cells per sample were quantified for each random view.
Immunostaining was performed as previously described. Briefly, cells were fixed for 5 minutes at room temperature with 4% formaldehyde. Permeabilization of cell membranes was achieved by treatment with PBS containing 0.2% Triton X-100. Subsequently, the cells underwent a 30-minute blocking step with 0.5% FBS in PBS before incubation with the prepared antibodies at room temperature for 3 hours on a shaker. After incubation with the primary antibody, the cells were washed three times with PBS containing 0.1% Tween-20. They were then incubated with secondary fluorescent antibodies for 1 hour at room temperature. DAPI was utilized for nuclear staining, and images were captured using Operetta.
CD34+ HSPCs were isolated using the EasySep Human CD34 Positive Selection Kit II protocol (StemCell Technologies, 17856, Canada)[19]. In summary, EasySep Human CD34 Positive Selection Cocktail (StemCell Technologies, 17856C, Canada) was added to BMNCs from a pool of HD at a concentration of approximately 108 cells/mL in EasySep buffer, as recommended. The samples were mixed and incubated at room temperature for 10 minutes. RapidSpheres were added and mixed with the samples, followed by incubation at room temperature for 5 minutes. The tube was then placed on an EasySep magnet (StemCell Technologies, 18000, Canada) and incubated at room temperature for 3 minutes. Cells were washed four times, and enriched CD34+ cells were collected in PBS with 2% FBS. For further purification of CD34+ cells, fluorescence-activated cell sorting was performed to obtain pure CD34+ cells. Briefly, antibodies were added to the primary enriched cells and incubated for 30 minutes in the dark at 4 °C. The detailed cell sorting strategy is plotted in Supplementary Figure 1. SONY MA900 was used for sorting.
The cell layers were fixed at room temperature for 15 minutes. After removing the fixative solution, the cells were washed twice with PBS. Subsequently, the cells were incubated at 37 °C for 12 hours with a working solution prepared according to the manufacturer’s instructions (Beyotime, C0602, Shanghai, China). Images were captured under a light microscope.
After cell digestion under different treatments, cells were collected and resuspended in cold PBS, and then the cell homogenate was prepared using non-contact automatic ultrasonic liquid processors (Q-sonic, United States). The supernatants were collected after centrifugation, and protein quantification was performed using the BCA Protein Quantification Kit (Vazyme, E112, Nanjing, China). Subsequently, SOD activity for every 20 μg of protein was measured using Cu/Zn-SOD and Mn-SOD Assay Kit with WST-8 (Beyotime, S0103, Shanghai, China) according to the manufacturer’s instructions.
BMSCs were seeded in 24-well plates at a density of 5 × 104 cells per well and cultured for 24 hours. Subsequently, these cells were utilized for mitochondrial membrane potential (MMP) measurement using the Mitochondrial Membrane Potential Assay Kit with TMRE (Beyotime, C2001S, Shanghai, China), according to the manufacturer’s instructions. After rinsing with PBS, the BMSCs were incubated with TMRE working solution at 37 °C for 30 minutes. Nuclear staining was performed using Hoechst 33342. Images were acquired using an inverted fluorescence microscope (Olympus IX73, Japan), and the mean intensity of each sample was calculated using Image J software.
All data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism software. An unpaired two-tailed Student’s t-test was used to compare two groups. One-way analysis of variance (ANOVA) was used to compare the three groups. Statistical significance was set at P < 0.05.
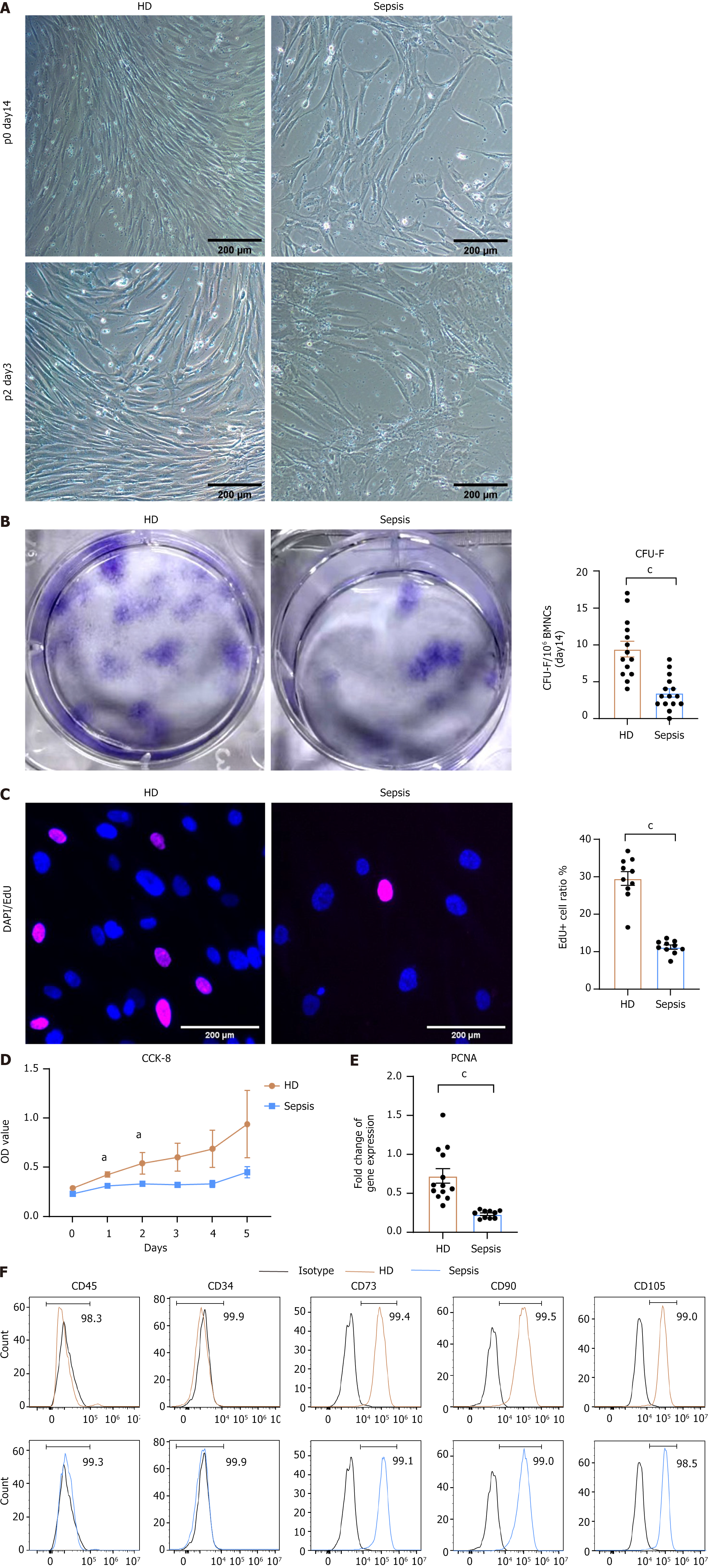
Purified BMSCs were isolated from bone marrow aspirates of septic patients and HD according to standard protocols[20]. After culturing for 14 days, HD BMSCs exhibited robust growth with a characteristic fibroblastic appearance, whereas septic BMSCs showed significantly altered growth despite maintaining a similar morphology. Upon passage to the second generation, septic BMSCs appeared as disorganized and vesicular structures (Figure 1A) as replicative aging BMSCs. To assess the self-renewal capacity of BMSC in sepsis, we compared the colony formation capacity of BMNC from septic patients and HD (Figure 1B). A markedly reduced number of colony-forming units-fibroblast was observed in septic samples. Regarding replication capacity, a lower EdU-positive rate suggested a decreased capacity for cell reproduction (Figure 1C), and the results of the CCK-8 assay indicated a slower proliferative rate in septic BMSCs (Figure 1D). Furthermore, reduced expression of proliferating cell nuclear antigen was detected in septic BMSCs (Figure 1E). Moreover, we confirmed the immunophenotypes of BMSCs by flow cytometry to determine whether the characteristics of septic BMSCs had undergone any changes. Immunophenotypic analysis showed that both expressed markers were associated with stromal cells (CD73, CD90, and CD105) and were negative for hematopoietic markers (CD45 and CD34) (Figure 1F)[21]. Collectively, these findings demonstrated that sepsis inhibits the proliferation and self-renewal of BMSCs.
To confirm their differentiation potential, we induced adipogenic and osteogenic differentiation of BMSCs in vitro. The BMSCs obtained from the septic samples showed reduced osteogenesis capacity (Figure 2A) and stronger adipogenesis capacity (Figure 2B). Quantification of Alizarin Red S and Oil Red O staining revealed a more than 50% decrease in calcium deposition (Figure 2C) and an approximately 12% increase in lipid droplet accumulation (Figure 2D) in septic BMSCs. Furthermore, the lower expression of runt-related transcription factor 2, alkaline phosphatase, and Sp7 transcription factor 7 (osteogenic genes)[22-24] (Figure 2E) and the higher expression of adipogenic genes such as peroxisome proliferator-activated receptor gamma, fatty acid binding protein 4, and perilipin-1[25,26] (Figure 2F) during lineage determination further corroborated our findings.
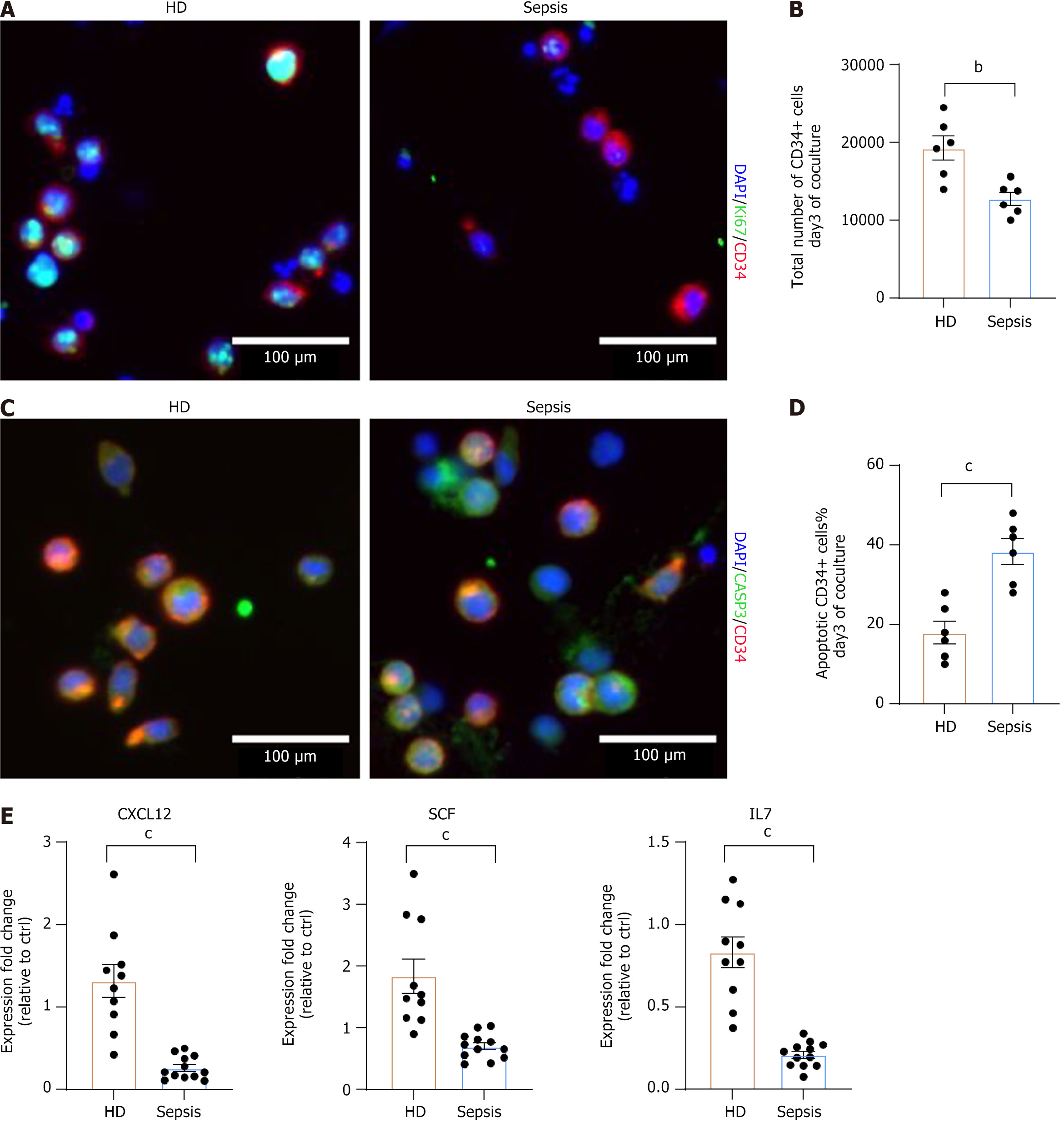
As one of the most crucial functions of BMSCs is to support homeostasis, we aimed to determine whether the defects mentioned above impaired their ability to support HSPCs. We cultured BMSC layers from HD and septic patients, and the same number of purified CD34+ HSPCs (Supplementary Figure 1) was seeded in transwell chambers[27]. Favorable BMSCs can produce supportive cytokines to maintain HSPC expansion and fitness. After three days of coculture, the frequency of Ki67-positive CD34+ cells (Figure 3A) and the total number of CD34+ cells were significantly lower in the septic BMSC group compared to HD group (Figure 3B). In particular, a higher number of apoptotic CD34+ cells was observed in septic BMSCs (Figure 3C and D). Subsequently, we evaluated the production of critical HSC factors such as stem cell factor, C-X-C chemokine ligand 12, and interleukin 7[28,29] in two groups of BMSCs, and the results indicated that the production of supportive cytokines was impaired in septic BMSCs (Figure 3E). These findings suggest that sepsis affects the hematopoietic support capacity of BMSCs.
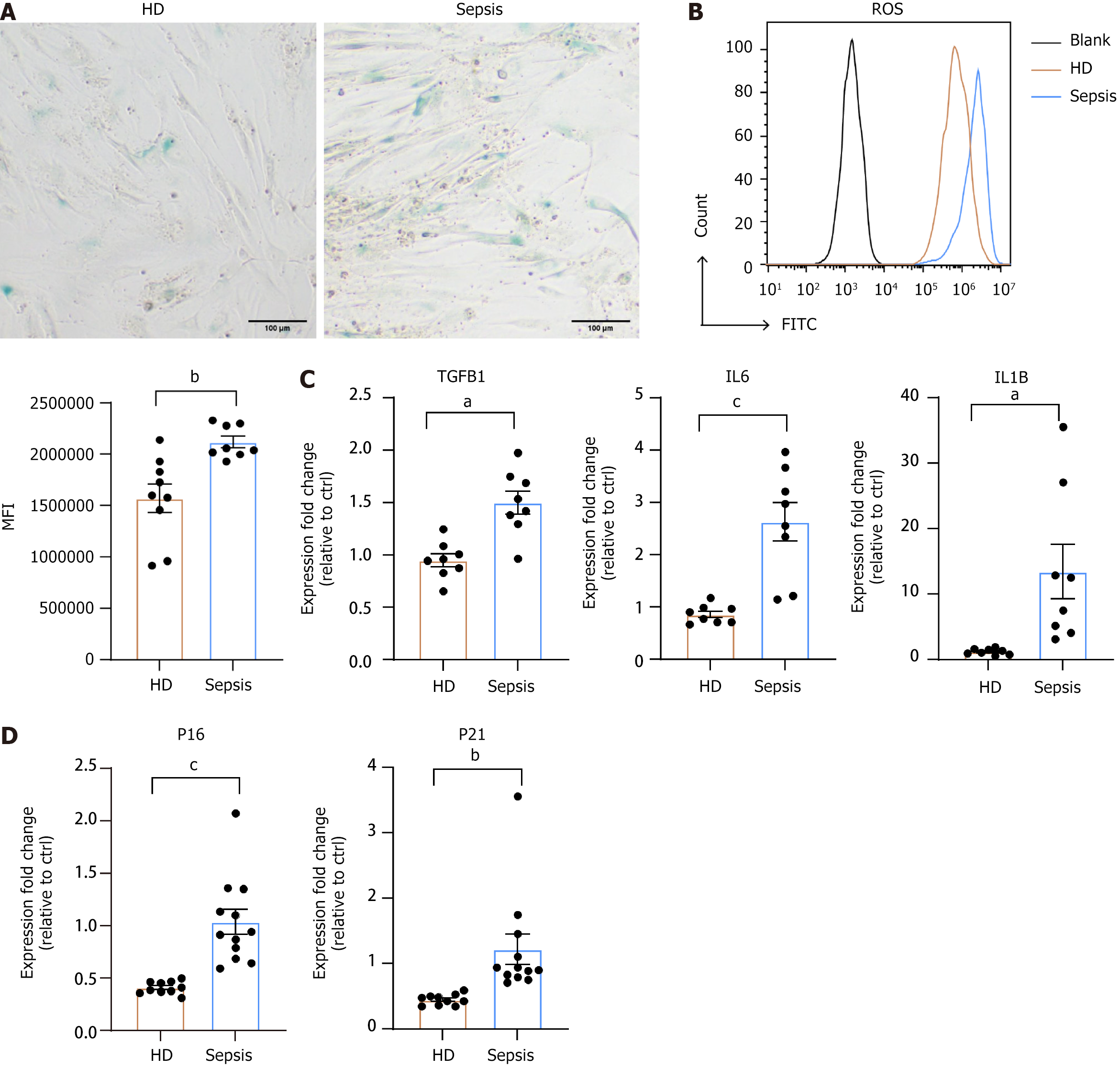
Slow proliferation, change in differentiation potency from osteoblasts to adipocytes, and impaired hematopoietic supportive capacity are all characteristic features of aging BMSCs[25]. Therefore, we conducted beta-galactosidase detection, which is a commonly used indicator of senescence. Septic BMSCs exhibited significantly higher staining compared to HD BMSCs (Figure 4A). Furthermore, we measured ROS levels and SASP expression, including transforming growth factor-β, interleukin-6, and interleukin-1β. As depicted in Figure 4B, the mean fluorescence intensity of intracellular ROS in septic BMSCs was higher than that in HD BMSCs. Elevated expressions of SASP were observed in septic BMSCs (Figure 4C). In addition, classical markers of aging, P16 and P21, were upregulated in septic BMSCs (Figure 4D). Therefore, senescence may be the basis for the functional defects observed in septic BMSCs.
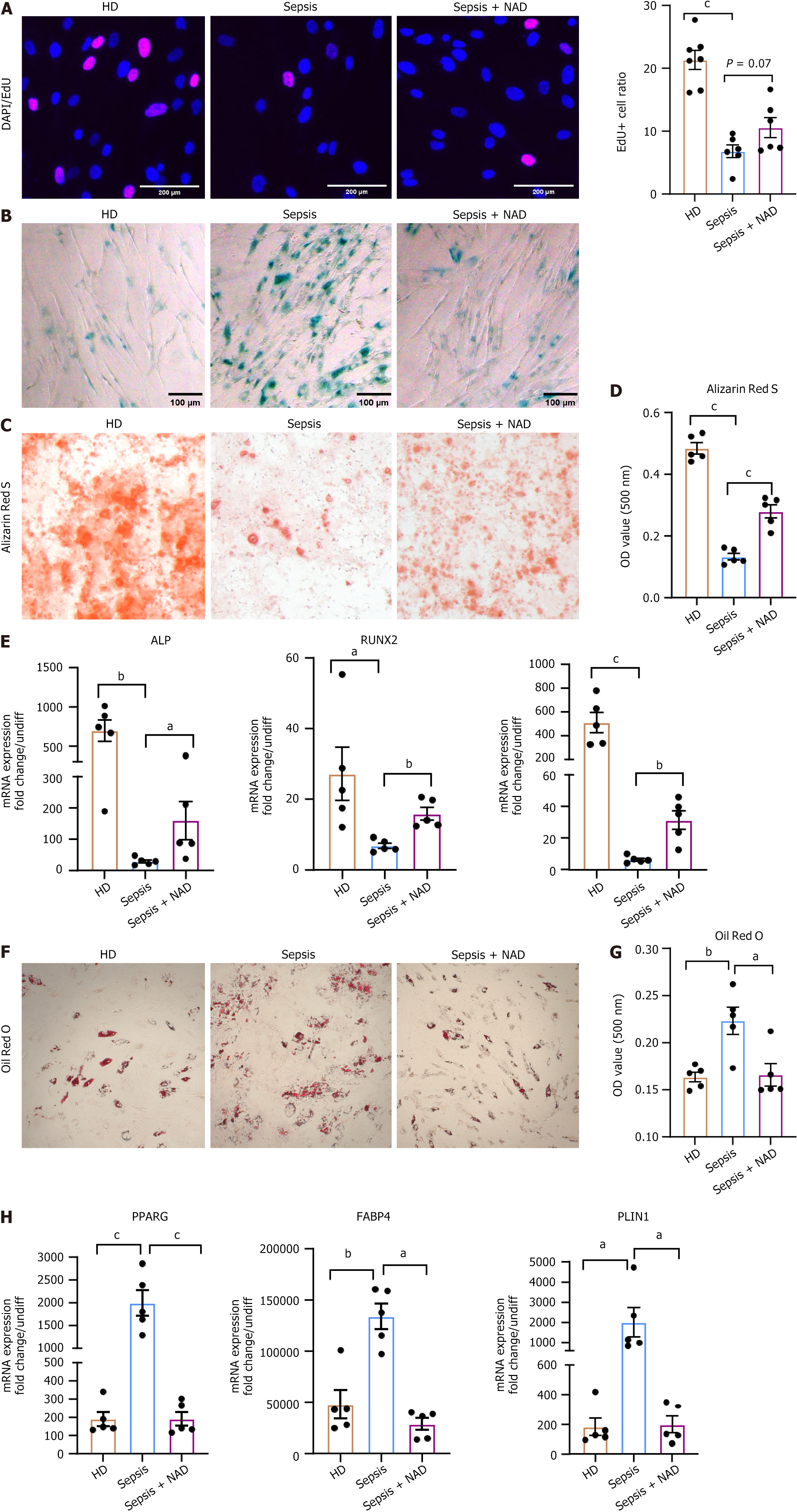
As it is well known that NAD interacts with several biological hallmarks of aging[30,31], we aimed to investigate whether septic BMSCs could benefit from NAD treatment. Firstly, we observed a restoration of the proliferation capacity of septic BMSCs after NAD treatment, as evidenced by an increased EdU-positive rate (Figure 5A). Furthermore, we found that NAD treatment contributed to reduced senescence, as demonstrated by a decrease in beta-galactosidase staining (Figure 5B). Thus, we were curious to know whether the differentiation potency had been restored. Thus, we induced osteogenic and adipogenic differentiation in NAD-treated septic BMSCs. Importantly, NAD treatment partially preserved the osteogenesis of septic BMSCs, as indicated by Alizarin Red S staining (Figure 5C and D) and upregulation of osteogenic genes (Figure 5E). On the contrary, the capacity for adipogenesis was inhibited after exposure to NAD, as verified by the decreased accumulation of lipid droplets (Figure 5F and G) and decreased expression of adipogenesis genes (Figure 5H). Additionally, NAD treatment rescued the hematopoietic support capacity of septic BMSCs. We observed better expansion of HSPCs (Supplementary Figure 2A and B) and fewer apoptotic HSPCs co-cultured with NAD-treated septic BMSCs than those with raw septic BMSCs (Supplementary Figure 2C and D). In summary, NAD represents an option to restore the intrinsic characteristics and stromal functions of septic BMSCs in vitro.
NAD plays a crucial role in mitochondrial function within cells. As mentioned above, the decline in mitochondrial free radical clearance capacity and increase in ROS levels indicate cellular aging. Therefore, we were curious whether NAD counteracted the aging BMSC by restoring mitochondrial function. First, we examined the expression levels of the important NAD-dependent deacetylase SIRT3 in different groups of BMSCs. We found a significant decrease in SIRT3 expression in septic BMSCs, which was restored after NAD treatment (Figure 6A). Furthermore, intracellular ROS levels decreased after NAD treatment (Figure 6B). SIRT3 deacetylates SOD enzymes in mitochondria to activate them and improve mitochondrial antioxidant capacity. As depicted in Figure 6C, SOD enzyme activity decreased in septic BMSC but was restored after NAD treatment. On the other hand, abnormal mitochondrial function partially affected mitochondrial integrity, so we detected MMP to evaluate whether mitochondrial function had been damaged. We observed a significant reduction in MMP in septic BMSCs, which was rescued after NAD treatment (Figure 6D). Based on these results, we demonstrated that NAD effectively reversed impaired mitochondrial function in septic BMSCs through the NAD/SIRT3/SOD pathway (Figure 6E).
Under natural circumstances, the functionality of BMSCs decreases with age, leading to organ failure and age-related diseases, such as osteoporosis, bone marrow fibrosis, and even HSC aging. Our research indicates that sepsis induces senescence in BMSCs, characterized by inhibited proliferation and self-renewal capabilities and biased differentiation toward adipogenesis rather than osteogenesis, leading to an increased incidence of diseases such as osteoporosis. Furthermore, sepsis-affected senescent BMSCs exhibit reduced hematopoietic support capacity, particularly lymphoid progenitor cells near the endosteum, which impedes the recovery of the immune system in patients with sepsis. Long-term lymphocytopenia weakens immune function and increases the risk of secondary infection and hospitalization.
Our experiments also confirmed a significant increase in SASP in septic BMSCs. These elevated levels of inflammatory factors induce HSC aging. Transforming growth factor-β has been shown to slow the progression of the HSC cell cycle and inhibit self-renewal potential[32]. IL-1β is a crucial cytokine driving HSC in elderly mice[33]. Therefore, aging BMSCs not only do not provide good maintenance capability of HSC but also promote HSC aging. In our study, treatment with NAD effectively improved the hematopoietic supportive capacities of septic BMSCs, achieving the goal of sepsis treatment with a longer-lasting effect and avoiding side effects. In particular, our research suggests that similar phenotypes may exist in other related diseases, such as tendinopathies, tumors, drug-induced injuries, and other conditions in which BMSC damage is possible. Focusing on the aging of BMSCs provides new insights and suggestions for the research and treatment of these diseases.
MSCs are widely distributed throughout the body, and their therapeutic purpose has been proven to be efficient with few side effects in many diseases[34,35]. However, the long-standing challenge is to conduct a good expansion of MSCs in vitro[36], mainly because of the difficulty in maintaining the stemness of MSCs under culture conditions[37]. Our research suggests that anti-aging measures contribute to maintaining the stemness of MSCs, potentially enhancing the quality and stability of cultured MSCs.
Our limitation lies in needing an in-depth investigation of the exact substances that cause BMSC aging under septic conditions. According to previous reports, aging mechanisms include DNA damage, mitochondrial dysfunction, exosomes, and other intracellular signaling pathways. It remains unclear whether these mechanisms exist in the bone marrow of patients with sepsis and to what extent they contribute to aging. In addition, there are many anti-aging drugs, including natural compounds such as quercetin, resveratrol, spermidine, curcumin, and sulforaphane, and other compounds such as rapamycin, metformin, and NAD enhancers[38], which may have better and more suitable effects on septic BMSC. Determining how to combine and administer these drugs and refining treatment regimens through animal and clinical trials will be the focus of future research.
We demonstrated that sepsis induces senescence of BMSCs, manifested by decreased proliferative capacity, self-renewal capacity, osteogenic differentiation, and impaired hematopoietic supportive effects. Treatment with the anti-aging drug NAD effectively reversed this damage. These findings improve our understanding of bone marrow complications of sepsis and provide a new treatment option for associated complications.
The authors thank Mr. An Zeng for technical support during the experiment.
| 1. | Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 2315] [Article Influence: 257.2] [Reference Citation Analysis (0)] |
| 2. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17221] [Article Influence: 1913.4] [Reference Citation Analysis (2)] |
| 3. | Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, Li L, Cao J, Xu F, Zhou Y, Guan CX, Jin SW, Deng J, Fang XM, Jiang JX, Zeng L. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 223] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 4. | Comazzetto S, Shen B, Morrison SJ. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev Cell. 2021;56:1848-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 5. | Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 915] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 6. | Beerman I, Luis TC, Singbrant S, Lo Celso C, Méndez-Ferrer S. The evolving view of the hematopoietic stem cell niche. Exp Hematol. 2017;50:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Leimkühler NB, Gleitz HFE, Ronghui L, Snoeren IAM, Fuchs SNR, Nagai JS, Banjanin B, Lam KH, Vogl T, Kuppe C, Stalmann USA, Büsche G, Kreipe H, Gütgemann I, Krebs P, Banz Y, Boor P, Tai EW, Brümmendorf TH, Koschmieder S, Crysandt M, Bindels E, Kramann R, Costa IG, Schneider RK. Heterogeneous bone-marrow stromal progenitors drive myelofibrosis via a druggable alarmin axis. Cell Stem Cell. 2021;28:637-652.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Takizawa H, Fritsch K, Kovtonyuk LV, Saito Y, Yakkala C, Jacobs K, Ahuja AK, Lopes M, Hausmann A, Hardt WD, Gomariz Á, Nombela-Arrieta C, Manz MG. Pathogen-Induced TLR4-TRIF Innate Immune Signaling in Hematopoietic Stem Cells Promotes Proliferation but Reduces Competitive Fitness. Cell Stem Cell. 2017;21:225-240.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 9. | Terashima A, Okamoto K, Nakashima T, Akira S, Ikuta K, Takayanagi H. Sepsis-Induced Osteoblast Ablation Causes Immunodeficiency. Immunity. 2016;44:1434-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019;30:630-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 498] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 11. | Reiten OK, Wilvang MA, Mitchell SJ, Hu Z, Fang EF. Preclinical and clinical evidence of NAD(+) precursors in health, disease, and ageing. Mech Ageing Dev. 2021;199:111567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 12. | Chini CCS, Cordeiro HS, Tran NLK, Chini EN. NAD metabolism: Role in senescence regulation and aging. Aging Cell. 2024;23:e13920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 13. | Wang T, Cao Y, Zheng Q, Tu J, Zhou W, He J, Zhong J, Chen Y, Wang J, Cai R, Zuo Y, Wei B, Fan Q, Yang J, Wu Y, Yi J, Li D, Liu M, Wang C, Zhou A, Li Y, Wu X, Yang W, Chin YE, Chen G, Cheng J. SENP1-Sirt3 Signaling Controls Mitochondrial Protein Acetylation and Metabolism. Mol Cell. 2019;75:823-834.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 159] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 14. | Mitchell S, Zhang P, Cannon M, Beaver L, Lehman A, Harrington B, Sampath D, Byrd JC, Lapalombella R. Anti-tumor NAMPT inhibitor, KPT-9274, mediates gender-dependent murine anemia and nephrotoxicity by regulating SIRT3-mediated SOD deacetylation. J Hematol Oncol. 2021;14:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Li R, Zhou Y, Cao Z, Liu L, Wang J, Chen Z, Xing W, Chen S, Bai J, Yuan W, Cheng T, Xu M, Yang FC, Zhao Z. TET2 Loss Dysregulates the Behavior of Bone Marrow Mesenchymal Stromal Cells and Accelerates Tet2(-/-)-Driven Myeloid Malignancy Progression. Stem Cell Reports. 2018;10:166-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Ciuffreda MC, Malpasso G, Musarò P, Turco V, Gnecchi M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Methods Mol Biol. 2016;1416:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 17. | Moseley KF, Doyle ME, Jan De Beur SM. Diabetic serum from older women increases adipogenic differentiation in mesenchymal stem cells. Endocr Res. 2018;43:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Wei W, Xu C, Ye ZY, Huang XJ, Yuan JE, Ma TB, Lin HB, Chen XQ. [Biological characteristics of mesenchymal stem cell and hematopoietic stem cell in the co-culture system]. Sheng Li Xue Bao. 2016;68:691-698. [PubMed] |
| 19. | Van Egeren D, Escabi J, Nguyen M, Liu S, Reilly CR, Patel S, Kamaz B, Kalyva M, DeAngelo DJ, Galinsky I, Wadleigh M, Winer ES, Luskin MR, Stone RM, Garcia JS, Hobbs GS, Camargo FD, Michor F, Mullally A, Cortes-Ciriano I, Hormoz S. Reconstructing the Lineage Histories and Differentiation Trajectories of Individual Cancer Cells in Myeloproliferative Neoplasms. Cell Stem Cell. 2021;28:514-523.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 20. | Ferrin I, Beloqui I, Zabaleta L, Salcedo JM, Trigueros C, Martin AG. Isolation, Culture, and Expansion of Mesenchymal Stem Cells. Methods Mol Biol. 2017;1590:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Vieira CP, McCarrel TM, Grant MB. Novel Methods to Mobilize, Isolate, and Expand Mesenchymal Stem Cells. Int J Mol Sci. 2021;22:5728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kanawa M, Igarashi A, Fujimoto K, Saskianti T, Nakashima A, Higashi Y, Kurihara H, Kato Y, Kawamoto T. The Identification of Marker Genes for Predicting the Osteogenic Differentiation Potential of Mesenchymal Stromal Cells. Curr Issues Mol Biol. 2021;43:2157-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 24. | Zhou CC, Xiong QC, Zhu XX, Du W, Deng P, Li XB, Jiang YZ, Zou SJ, Wang CY, Yuan Q. AFF1 and AFF4 differentially regulate the osteogenic differentiation of human MSCs. Bone Res. 2017;5:17044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 26. | Stefańska K, Nemcova L, Blatkiewicz M, Żok A, Kaczmarek M, Pieńkowski W, Mozdziak P, Piotrowska-Kempisty H, Kempisty B. Expression Profile of New Marker Genes Involved in Differentiation of Human Wharton's Jelly-Derived Mesenchymal Stem Cells into Chondrocytes, Osteoblasts, Adipocytes and Neural-like Cells. Int J Mol Sci. 2023;24:12939. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Bucar S, Branco ADM, Mata MF, Milhano JC, Caramalho Í, Cabral JMS, Fernandes-Platzgummer A, da Silva CL. Influence of the mesenchymal stromal cell source on the hematopoietic supportive capacity of umbilical cord blood-derived CD34(+)-enriched cells. Stem Cell Res Ther. 2021;12:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Mattiucci D, Maurizi G, Izzi V, Cenci L, Ciarlantini M, Mancini S, Mensà E, Pascarella R, Vivarelli M, Olivieri A, Leoni P, Poloni A. Bone marrow adipocytes support hematopoietic stem cell survival. J Cell Physiol. 2018;233:1500-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 29. | Cordeiro Gomes A, Hara T, Lim VY, Herndler-Brandstetter D, Nevius E, Sugiyama T, Tani-Ichi S, Schlenner S, Richie E, Rodewald HR, Flavell RA, Nagasawa T, Ikuta K, Pereira JP. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity. 2016;45:1219-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 260] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 30. | Arslan NP, Taskin M, Keles ON. Nicotinamide Mononucleotide and Nicotinamide Riboside Reverse Ovarian Aging in Rats Via Rebalancing Mitochondrial Fission and Fusion Mechanisms. Pharm Res. 2024;41:921-935. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Wu QJ, Zhang TN, Chen HH, Yu XF, Lv JL, Liu YY, Liu YS, Zheng G, Zhao JQ, Wei YF, Guo JY, Liu FH, Chang Q, Zhang YX, Liu CG, Zhao YH. The sirtuin family in health and disease. Signal Transduct Target Ther. 2022;7:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 392] [Reference Citation Analysis (1)] |
| 32. | Wang X, Dong F, Zhang S, Yang W, Yu W, Wang Z, Zhang S, Wang J, Ma S, Wu P, Gao Y, Dong J, Tang F, Cheng T, Ema H. TGF-β1 Negatively Regulates the Number and Function of Hematopoietic Stem Cells. Stem Cell Reports. 2018;11:274-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Kovtonyuk LV, Caiado F, Garcia-Martin S, Manz EM, Helbling P, Takizawa H, Boettcher S, Al-Shahrour F, Nombela-Arrieta C, Slack E, Manz MG. IL-1 mediates microbiome-induced inflammaging of hematopoietic stem cells in mice. Blood. 2022;139:44-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 34. | Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 197] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 35. | Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 202] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 36. | Tsai AC, Jeske R, Chen X, Yuan X, Li Y. Influence of Microenvironment on Mesenchymal Stem Cell Therapeutic Potency: From Planar Culture to Microcarriers. Front Bioeng Biotechnol. 2020;8:640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 37. | Yin JQ, Zhu J, Ankrum JA. Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng. 2019;3:90-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 38. | Moskalev A, Guvatova Z, Lopes IA, Beckett CW, Kennedy BK, De Magalhaes JP, Makarov AA. Targeting aging mechanisms: pharmacological perspectives. Trends Endocrinol Metab. 2022;33:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |