Published online Feb 26, 2025. doi: 10.4252/wjsc.v17.i2.102702
Revised: December 18, 2024
Accepted: January 18, 2025
Published online: February 26, 2025
Processing time: 121 Days and 10.8 Hours
Peripheral nerve injuries are clinical conditions that often result in functional deficits, compromising patient quality of life. Given the relevance of these injuries, new treatment strategies are constantly being investigated. Although mesenchymal stem cells already demonstrate therapeutic potential due to their paracrine action, the transdifferentiation of these cells into Schwann-like cells (SLCs) represents a significant advancement in nerve injury therapy. Recent studies indicate that SLCs can mimic the functions of Schwann cells, with promising results in animal models. However, challenges remain, such as the diversity of transdifferentiation protocols and the scalability of these therapies for clinical applications. A recent study by Zou et al provided a comprehensive overview of the role of bone marrow-derived mesenchymal stem cells in the treatment of peripheral nerve injuries. Therefore, we would like to discuss and explore the use of SLCs derived from bone marrow-derived mesenchymal stem cells in more detail as a promising alternative in the field of nerve regeneration.
Core Tip: Schwann-like cells (SLCs) derived from bone marrow-mesenchymal stem cells have emerged as a promising therapeutic approach for peripheral nerve regeneration. However, further in vitro and in vivo studies are needed to optimize transdifferentiation and transplantation methodologies, as well as to explore the efficacy of SLCs in different injury models. The development of strategies that integrate SLCs could enhance neuroregeneration, promoting cell survival and therapeutic success.
- Citation: Ferreira LVO, Amorim RM. Perspectives on Schwann-like cells derived from bone marrow-mesenchymal stem cells: Advancing peripheral nerve injury therapies. World J Stem Cells 2025; 17(2): 102702
- URL: https://www.wjgnet.com/1948-0210/full/v17/i2/102702.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i2.102702
We would like to express our sincere appreciation for the publication of the review entitled “Bone marrow-mesenchymal stem cells in treatment of peripheral nerve injury” by Zou et al[1], which provides a comprehensive overview of the role of bone marrow-derived mesenchymal stem cells (BM-MSCs) in the treatment of peripheral nerve injuries (PNI). In addition to highlighting the relevance of BM-MSCs, we would like to commend the authors and offer a more in-depth perspective on the use of Schwann-like cells (SLCs) derived from BM-MSCs as a promising alternative in the field of nerve regeneration.
PNI represents a significant clinical challenge in both humans and animals[2,3], due to the complexity of the neural microenvironment and the limited regenerative capacity, especially in severe injuries[4-6]. In long-gap injury, autograft is the gold standard treatment; however, it presents several limitations, including incomplete functional recovery, donor nerve morbidity, scar tissue formation, and the risk of neuroma formation[7,8]. Given these challenges, new therapeutic strategies are constantly being investigated.
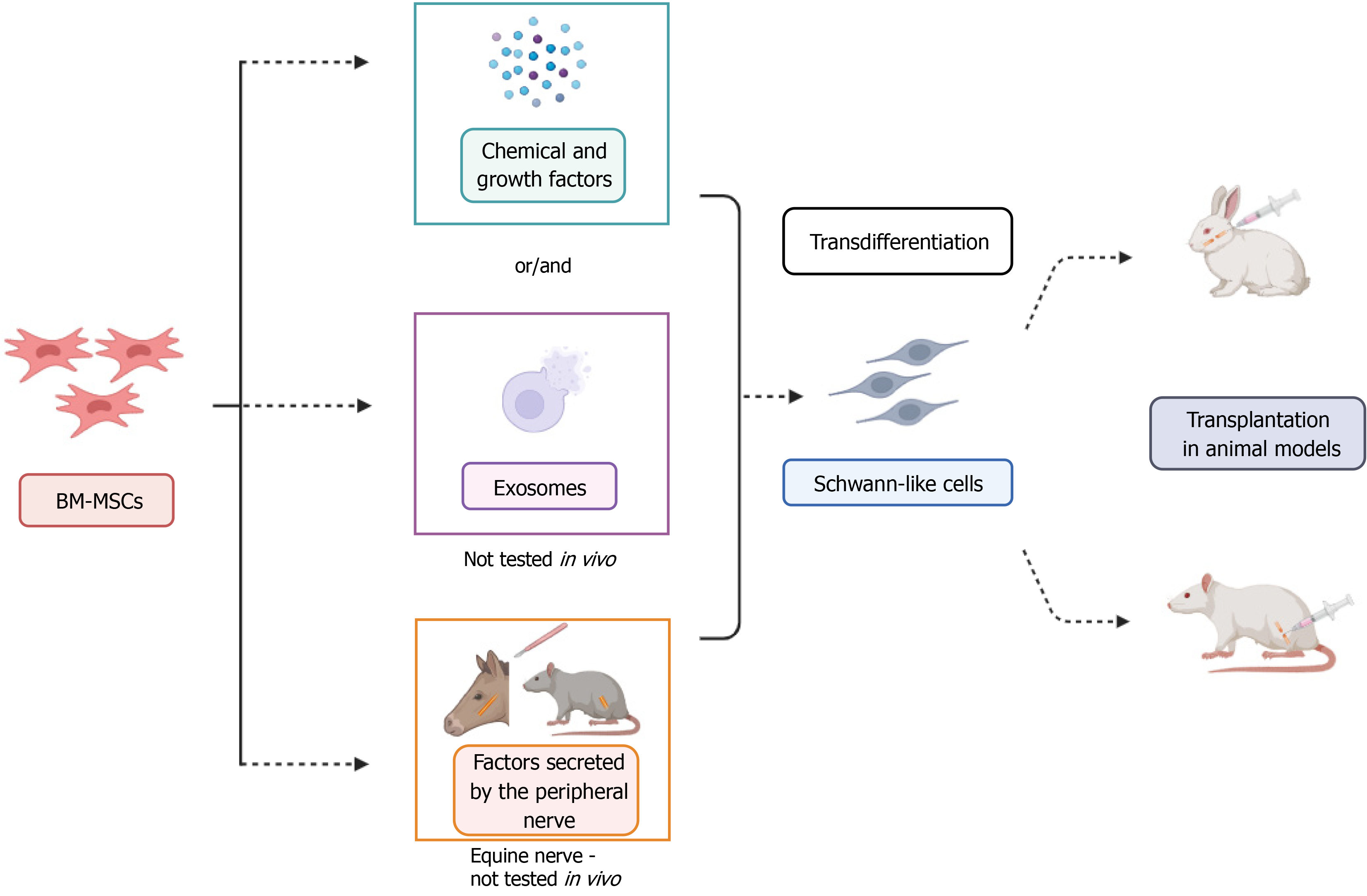
Mesenchymal stem cells (MSCs) have attracted considerable attention for peripheral nerve regeneration[9]. Their ease of expansion in culture, the ability to differentiate into various cell types[10], and their neuroprotective, anti-inflammatory, immunomodulatory, and pro-angiogenic properties highlight MSCs as a strategy with great therapeutic potential, as reported by Zou et al[1]. The transplantation of BM-MSCs in PNI has been investigated in small and large animal models, such as rats[11], dogs[12], rabbits[13], sheep[14], and horses[15]. Furthermore, studies have demonstrated the potential for the transdifferentiation of BM-MSCs into SLCs, as highlighted by Zou et al[1].
Different protocols are described for performing transdifferentiation and exploring the potential of the transplantation of SLCs derived from BM-MSCs into animal models (Figure 1). In this manuscript, we also present in vitro approaches that utilize exosomes derived from different cell sources[16] and conditioned medium containing secreted factors from the peripheral nerves of both rats[17] and horses[18]. The method using conditioned medium enhances the gene expression of neurotrophic factors following transdifferentiation[18]. This recent approach still needs to be further explored to determine which factors specifically trigger the transdifferentiation process, which could open new avenues for research and experimental methodologies.
Schwann cells (SCs) are the main glial cells in the peripheral nervous system and play a crucial role in neuroregeneration through proliferation, formation of Büngner bands, secretion of neurotrophic factors, phagocytosis of myelin debris, and recruitment of macrophages[19]. While protocols for SCs isolation are well-established and their transplantation has shown benefits in treating PNI[20,21], this approach has limitations. These include the need to sacrifice a functional nerve for cell harvesting and the extended time required for cell expansion, which can delay treatment[22]. In this context, SLCs have emerged as a promising alternative to mimic SCs. The generation of SLCs from MSCs reduces complications associated with donor nerve harvesting. However, potential challenges in translating SLC therapies to clinical applications must be addressed. Issues related to cell quality, phenotypic instability, immune compatibility, and long-term safety remain significant concerns. Moving forward, it will be essential to reach a consensus on the criteria to characterize these cells, ensuring reproducibility and reliability across different studies. Although various methodologies and protocols have been developed, offering promising strategies for nerve regeneration, further investigations are necessary. Studies focusing on BM-MSC-derived SLCs and comparative analyses of different approaches are crucial to optimize and enhance the therapeutic potential of SLCs.
Studies evaluating the transplantation of SLCs derived from BM-MSCs in PNI have demonstrated significant therapeutic benefits (Table 1). There is considerable evidence that the use of these cells can assist in nerve regeneration, underscoring their therapeutic potential. However, further investigations are still needed to establish effective protocols for clinical application. Although MSCs are considered immune evasive[23], the use of immunosuppressants such as tacrolimus and cyclosporine has been adopted in some studies to prevent immune rejection in xenogeneic transplants[24,25]. In addition, many studies have combined cell transplantation with biomaterials, optimizing treatment efficacy by creating a favorable microenvironment for regeneration[26,27].
| Model | Cells/grafts | Method of transdifferentiation | Outcome | Limitations | Ref. |
| Rat sciatic nerve (12 mm gap) | SLCs (rats). Hollow fiber | Chemical and growth factors | Improvements in motor conduction, sciatic nerve function index, regeneration of the nodes of Ranvier, and remyelination. No tumor formation was detected 6 months post-transplantation | Lack of detailed sensory functional analysis and gene expression evaluation of SLCs and nerve regeneration markers | [35] |
| Rat sciatic nerve (10 mm gap) | SLCs (humans). Transpermeable tube. Immunosuppressants used | Chemical and growth factors | Improvements in nerve regeneration and functional recovery | Lack of detailed sensory functional analysis, gene expression evaluation of SLCs and nerve regeneration markers, no electroneuromyography, assessed for three weeks | [25] |
| Rat sciatic nerve (12 mm gap) | SLCs (rats). Chitosan conduits | Neurosphere induction, incubation with growth factors and co-culture | Improvements in remyelination and axonal growth. No significant difference was observed compared to the transplantation of Schwann cells derived from the sciatic nerve | No undifferentiated BM-MSC transplantation group, lack of detailed sensory functional analysis and gene expression evaluation of SLCs and nerve regeneration markers | [26] |
| Buccal branch of the facial nerve in rabbits (1 cm gap) | SLCs (rabbits). Vein graft | Chemical and growth factors | Acceleration of axonal regeneration and improvement in remyelination | Lack of detailed sensory functional analysis of the facial nerve and gene expression evaluation of SLCs markers | [27] |
| Rat sciatic nerve (12 mm gap) | SLCs (humans). Chitosan conduits. Immunosuppressants used | Neurosphere induction, incubation with growth factors and co-culture | Improvements in axonal regeneration and myelination | No undifferentiated BM-MSC transplantation group, lack of detailed motor and sensory functional analysis, electroneuromyography, and gene expression evaluation of SLCs and nerve regeneration markers | [24] |
Biomaterials have been widely studied for the construction of nerve guidance conduits (NGCs), which may be natural or synthetic, exhibiting a wide range of characteristics that play crucial roles in nerve regeneration[28]. NGCs are designed to provide structural support and create a favorable microenvironment for axonal growth[3]. Physical pro
Despite evidence suggesting that SLCs are a promising alternative for nerve regeneration, several challenges remain to be addressed. Issues such as the quantity of cells to be used, the most efficient methodology for transdifferentiation, the application frequency, long-term safety, potential immune responses, dedifferentiation after the removal of the inducing medium, and efficacy in different types of injuries require further investigation.
To advance in this field, it is essential to standardize transdifferentiation protocols and establish a consensus on the characterization of SLCs. This will assist in ensuring reproducibility and clinical applicability. Preclinical studies utilizing large animal models to evaluate the safety and effectiveness of SLC-based therapies, followed by clinical trials, are also crucial to translate these findings into clinical practice. The combination of cellular therapy with biomaterials and other technologies has the potential to further enhance neuroregeneration. In this context, future studies should investigate the 3D bioprinting of NGCs incorporated with BM-MSCs, as demonstrated by Liu et al[34], or with SLCs, aiming to develop better therapeutic approaches for PNI. It is vital to create an optimal microenvironment that promotes cell survival and integration after transplantation. Moreover, incorporating and distributing stimulating factors that influence the phenotype of SLCs will be crucial for the therapeutic success of these approaches. Thus, we would like to highlight future perspectives toward therapies based on SLCs derived from BM-MSCs, which have great potential for the treatment of PNI.
| 1. | Zou XF, Zhang BZ, Qian WW, Cheng FM. Bone marrow mesenchymal stem cells in treatment of peripheral nerve injury. World J Stem Cells. 2024;16:799-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (3)] |
| 2. | Gouveia D, Cardoso A, Carvalho C, Oliveira AC, Almeida A, Gamboa Ó, Lopes B, Coelho A, Alvites R, Varejão AS, Maurício AC, Ferreira A, Martins Â. Early Intensive Neurorehabilitation in Traumatic Peripheral Nerve Injury-State of the Art. Animals (Basel). 2024;14:884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Lopes B, Sousa P, Alvites R, Branquinho M, Sousa AC, Mendonça C, Atayde LM, Luís AL, Varejão ASP, Maurício AC. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int J Mol Sci. 2022;23:918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 4. | Alvites R, Lopes B, Coelho A, Maurício AC. Peripheral nerve regeneration: a challenge far from being overcome. Regen Med. 2024;19:155-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Klimovich P, Rubina K, Sysoeva V, Semina E. New Frontiers in Peripheral Nerve Regeneration: Concerns and Remedies. Int J Mol Sci. 2021;22:13380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Kuffler DP, Foy C. Restoration of Neurological Function Following Peripheral Nerve Trauma. Int J Mol Sci. 2020;21:1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Bassilios Habre S, Bond G, Jing XL, Kostopoulos E, Wallace RD, Konofaos P. The Surgical Management of Nerve Gaps: Present and Future. Ann Plast Surg. 2018;80:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Manoukian OS, Baker JT, Rudraiah S, Arul MR, Vella AT, Domb AJ, Kumbar SG. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J Control Release. 2020;317:78-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Martins Amorim R, Vinícius de Oliveira Ferreira L. Schwann-like cells derived from mesenchymal stem cells: Their potential for peripheral nerve regeneration. In: Stem Cell Transplantation. United Kingdom: IntechOpen, 2024. |
| 10. | Barberini DJ, Freitas NP, Magnoni MS, Maia L, Listoni AJ, Heckler MC, Sudano MJ, Golim MA, da Cruz Landim-Alvarenga F, Amorim RM. Equine mesenchymal stem cells from bone marrow, adipose tissue and umbilical cord: immunophenotypic characterization and differentiation potential. Stem Cell Res Ther. 2014;5:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmüller GJ, Yuan N, Pang J, Sarkar K, Lee WP, Brandacher G. Mesenchymal Stem Cells Enhance Nerve Regeneration in a Rat Sciatic Nerve Repair and Hindlimb Transplant Model. Sci Rep. 2016;6:31306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Daradka MH, Bani Ismail ZA, Irsheid MA. Peripheral nerve regeneration: A comparative study of the effects of autologous bone marrow-derived mesenchymal stem cells, platelet-rich plasma, and lateral saphenous vein graft as a conduit in a dog model. Open Vet J. 2021;11:686-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Sivanarayanan TB, Bhat IA, Sharun K, Palakkara S, Singh R, Remya 5th, Parmar MS, Bhardwaj R, Chandra V, Munuswamy P, Kinjavdekar P, Pawde AM, Amarpal, Sharma GT. Allogenic bone marrow-derived mesenchymal stem cells and its conditioned media for repairing acute and sub-acute peripheral nerve injuries in a rabbit model. Tissue Cell. 2023;82:102053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 14. | Casañas J, de la Torre J, Soler F, García F, Rodellar C, Pumarola M, Climent J, Soler R, Orozco L. Peripheral nerve regeneration after experimental section in ovine radial and tibial nerves using synthetic nerve grafts, including expanded bone marrow mesenchymal cells: morphological and neurophysiological results. Injury. 2014;45 Suppl 4:S2-S6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Cruz Villagrán C, Schumacher J, Donnell R, Dhar MS. A Novel Model for Acute Peripheral Nerve Injury in the Horse and Evaluation of the Effect of Mesenchymal Stromal Cells Applied In Situ on Nerve Regeneration: A Preliminary Study. Front Vet Sci. 2016;3:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Zhang X, Zhang W, Sun H, Wang H. The effects of exosomes originating from different cell sources on the differentiation of bone marrow mesenchymal stem cells into schwann cells. J Nanobiotechnology. 2024;22:220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 17. | Galhom RA, Hussein Abd El Raouf HH, Mohammed Ali MH. Role of bone marrow derived mesenchymal stromal cells and Schwann-like cells transplantation on spinal cord injury in adult male albino rats. Biomed Pharmacother. 2018;108:1365-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Ferreira LVO, Kamura BDC, Oliveira JPM, Chimenes ND, Carvalho M, Santos LAD, Dias-Melicio LA, Amorim RL, Amorim RM. In Vitro Transdifferentiation Potential of Equine Mesenchymal Stem Cells into Schwann-Like Cells. Stem Cells Dev. 2023;32:422-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 19. | Jessen KR, Mirsky R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front Cell Neurosci. 2019;13:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 20. | Han GH, Peng J, Liu P, Ding X, Wei S, Lu S, Wang Y. Therapeutic strategies for peripheral nerve injury: decellularized nerve conduits and Schwann cell transplantation. Neural Regen Res. 2019;14:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Levi AD, Burks SS, Anderson KD, Dididze M, Khan A, Dietrich WD. The Use of Autologous Schwann Cells to Supplement Sciatic Nerve Repair With a Large Gap: First in Human Experience. Cell Transplant. 2016;25:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Piovesana R, Faroni A, Tata AM, Reid AJ. Schwann-like adipose-derived stem cells as a promising therapeutic tool for peripheral nerve regeneration: effects of cholinergic stimulation. Neural Regen Res. 2021;16:1218-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 1131] [Article Influence: 102.8] [Reference Citation Analysis (0)] |
| 24. | Cai S, Tsui YP, Tam KW, Shea GK, Chang RS, Ao Q, Shum DK, Chan YS. Directed Differentiation of Human Bone Marrow Stromal Cells to Fate-Committed Schwann Cells. Stem Cell Reports. 2017;9:1097-1108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun. 2007;359:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, Shum DK. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Wang X, Luo E, Li Y, Hu J. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 2011;1383:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Mankavi F, Ibrahim R, Wang H. Advances in Biomimetic Nerve Guidance Conduits for Peripheral Nerve Regeneration. Nanomaterials (Basel). 2023;13:2528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 29. | Huang Y, Wu W, Liu H, Chen Y, Li B, Gou Z, Li X, Gou M. 3D printing of functional nerve guide conduits. Burns Trauma. 2021;9:tkab011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020;106:54-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 31. | Soman SS, Vijayavenkataraman S. Perspectives on 3D Bioprinting of Peripheral Nerve Conduits. Int J Mol Sci. 2020;21:5792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Pedde RD, Mirani B, Navaei A, Styan T, Wong S, Mehrali M, Thakur A, Mohtaram NK, Bayati A, Dolatshahi-Pirouz A, Nikkhah M, Willerth SM, Akbari M. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv Mater. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 237] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 33. | Zhu W, Tringale KR, Woller SA, You S, Johnson S, Shen H, Schimelman J, Whitney M, Steinauer J, Xu W, Yaksh TL, Nguyen QT, Chen S. Rapid continuous 3D printing of customizable peripheral nerve guidance conduits. Mater Today (Kidlington). 2018;21:951-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 34. | Liu J, Zhang B, Li L, Yin J, Fu J. Additive-lathe 3D bioprinting of bilayered nerve conduits incorporated with supportive cells. Bioact Mater. 2021;6:219-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 35. | Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg. 2004;101:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |